Abstract
Aims:
Growing evidence suggests that vascular endothelial growth factor (VEGF) may be involved in the neuronal mechanisms underlying both depression aetiology and the response to ketamine treatments. The aim of this study was to examine whether changes in plasma VEGF levels are associated with the antidepressant effects of repeated ketamine infusions in patients with depression.
Methods:
Ninety-six patients with depression were enrolled and received six ketamine infusions during a 12-day period. Depressive symptom severity and plasma VEGF levels were measured by the Montgomery–Åsberg Depression Rating Scale (MADRS) and an enzyme-linked immunosorbent assay (ELISA) respectively, at baseline, 13 days and 26 days.
Results:
Despite a significant improvement in MADRS scores after patients received six ketamine infusions (p < 0.001), no changes in plasma VEGF levels were observed at 13 days when compared with baseline. Moreover, no significant difference in plasma VEGF levels at baseline and 13 days was found between ketamine responders and nonresponders. No association was found between the antidepressant effects of repeated ketamine treatments and plasma VEGF levels.
Conclusion:
This study indicated that VEGF may not be a potential predictor of antidepressant response to repeated intravenous administration of ketamine in patients with depression.
Keywords: depression, ketamine, plasma, vascular endothelial growth factor
Introduction
Major depression is one of the most widespread psychiatric illnesses and is commonly correlated with a high mortality rate, resulting in enormous personal and socioeconomic consequences.1 Although numerous antidepressant drugs are available for the therapy of major depression, issues with existing antidepressant treatments include limited efficacy and delayed onset of action.2 For example, the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study reported that approximately one-third of subjects suffering from major depression failed to respond to drug therapy and developed treatment-resistant depression (TRD).2
As an antagonist of glutamatergic N-methyl-D-aspartate receptors, ketamine has been approved for clinical use in anaesthesia and analgesia for decades.3 Numerous open-label studies,4–7 randomized controlled studies8–11 and meta-analyses12,13 have demonstrated that ketamine produces rapid antisuicidal and antidepressant effects in subjects with TRD. Ketamine’s antidepressant efficacy after a single infusion are transient, lasting nearly 1 week,10,14 but this can be extended to approximately 18 days by adding five subsequent infusions.7,15 However, the mechanism of the rapid antidepressant actions of ketamine and biomarkers identifying the responding and nonresponding subjects who receive repeated ketamine treatments remain unknown.
Vascular endothelial growth factor (VEGF) is a multifunctional cytokine that is thought to be involved in neuronal processes regulating growth, survival, neuroprotection and regeneration.16,17 Several studies have found that VEGF can affect hippocampal synaptic plasticity, including improved cognitive function and increased neurogenesis,18–21 and the synaptic transmission’s brain mechanism.22 Furthermore, Heine et al.23 reported that chronic stress reduces cell proliferation near the vasculature and expression of VEGF.
Both major depression and chronic stress, a precipitating factor in major depression, can decrease VEGF expression in the prefrontal cortex (PFC) and hippocampus.23–25 According to Isung et al.’s study26 cerebrospinal fluid VEGF levels were significantly lower in patients with suicide attempts than in healthy controls. In contrast, treatment with typical antidepressants, such as selective serotonin-reuptake inhibitors (SSRIs), increases expression of VEGF,27–29 and blockade of VEGF signalling attenuates the antidepressant response to these treatments.27 Importantly, an animal study found neuronal VEGF–Flk-1 signalling in the medial PFC to be associated with the antidepressant actions of a single ketamine infusion.29 Nonetheless, the role of plasma levels of VEGF in the antidepressant actions of repeated intravenous administration of ketamine remains unclear.
Thus, the purpose of the current study was to examine putative modulations in plasma levels of VEGF induced by repeated ketamine treatments in subjects with depression and to investigate whether VEGF can predict ketamine treatment response.
Methods
Inclusion criteria and study procedure
Adult patients (18–65 years) with suicidal ideation or TRD were recruited from the Affiliated Brain Hospital of Guangzhou Medical University between November 2016 and December 2017. The study design and clinical findings of ketamine as an adjunctive therapy for patients with unipolar and bipolar depression have been described in detail in a previous study.30 Ninety-six patients suffered from suicidal ideation and TRD, which were defined as a score of two or more on the first five items of the Scale for Suicidal Ideations31 and a poor or unsatisfactory response to at least two adequate trials of antidepressant medications during the current episode, respectively.30 The enrolled participants had no positive urine toxicology, no medical or neurological diseases (e.g. dementia) or history of drug or alcohol abuse. All recruited subjects suffering from unipolar and bipolar depression received a thrice-weekly infusion regimen of ketamine at a subanaesthetic dose of 0.5 mg/kg for 2 weeks. All participants continued to receive the same psychotropic agents throughout the infusion period (2 weeks). The protocol of this study was approved by the Affiliated Brain Hospital of Guangzhou Medical University’s Ethics Committee (Ethical Application Ref: 2016-030). The clinical trial registration number was ChicCTR-OOC-17012239. Written informed consent was provided by all subjects.
Antidepressant response
The severity of depression at baseline and 1 days and 14 days after the last ketamine infusion (13 days and 26 days) was measured with the Montgomery–Åsberg Depression Rating Scale (MADRS).32,33 The response was defined as a 50% or greater reduction in MADRS scores at 13 days, which has frequently been used in previous studies.30,34
Plasma levels of VEGF
Fasting blood samples were collected from depressed patients at baseline, 13 days and 26 days between 8:00 and 10:00 after an overnight fast and immediately stored at −80°C until analysis. In line with the manufacturer’s instructions, plasma VEGF levels were measured using a commercially available enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, USA).
Statistical analysis
Comparisons of demographic and clinical variables and plasma VEGF levels between antidepressant responders and nonresponders at 13 days were analysed by using the Chi-square test or Fisher’s exact test for categorical data, the Mann–Whitney U test for non-normally distributed continuous data and Student’s t-tests for normally distributed continuous data. MADRS scores and plasma VEGF levels were compared at baseline, 13 days, and 26 days between antidepressant responders and nonresponders using linear mixed models. Correlation analysis was also conducted to examine the association of plasma VEGF levels and the antidepressant effects of six ketamine infusions. An additional analysis focusing on patients with TRD was conducted in this study. SPSS version 24.0 software was used for all data analyses in this study based on the intent-to-treat (ITT) or modified ITT sample, with a p-value less than 0.05 considered statistically significant.
Results
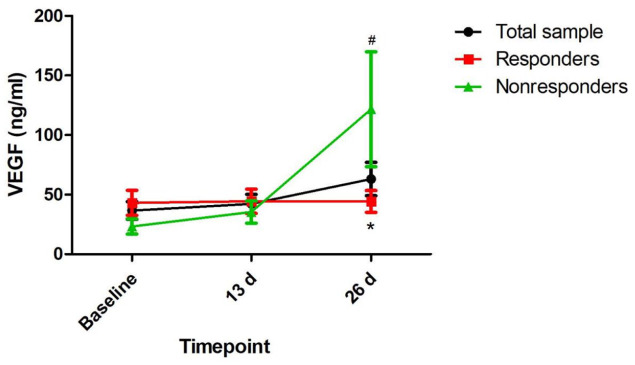
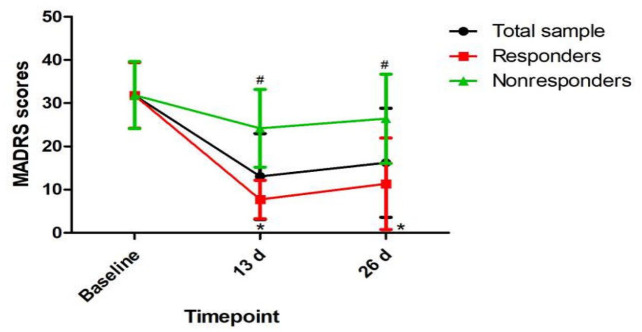
Ninety-six subjects with depression provided a fasting blood sample at baseline to allow a measurement of plasma VEGF levels in this study. Table 1 summarizes the demographic and clinical characteristics of the total sample participating in the study and the responder and nonresponder subgroups. Sixty-five (67.7%) patients with depression had a clinical response to ketamine following the sixth infusion. Plasma VEGF levels at baseline and 13 days did not differ significantly between ketamine responders and nonresponders (Table 1 and Figure 1). Ketamine nonresponders than responders had a significantly higher plasma VEGF levels at 26 days (p = 0.017). The comparison of MADRS scores between responders and nonresponders at the indicated times is presented in Figure 2.
Table 1.
Comparison of baseline sample characteristics between responders and nonresponders after six ketamine infusions.
| Variables | Total sample (n = 96) | Responder (n = 65) | Nonresponder (n = 31) | Statistics | ||
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | χ2 | df | p | |
| Female | 51 (53.1) | 35 (53.8) | 16 (51.6) | 0.04 | 1 | 0.84 |
| Employment | 39 (40.6) | 30 (46.2) | 9 (29.0) | 2.5 | 1 | 0.11 |
| Married | 51 (53.1) | 36 (55.4) | 15 (48.4) | 0.4 | 1 | 0.52 |
| TRD | 78 (81.3) | 53 (81.5) | 25 (80.6) | 0.01 | 1 | 0.92 |
| Depression | 0.06 | 1 | 0.81 | |||
| Unipolar | 76 (79.2) | 51 (67.1) | 25 (32.9) | |||
| Bipolar | 20 (20.8) | 14 (70.0) | 6 (30.0) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | T/Z | df | p | |
| Age (years) | 34.4 (11.6) | 35.2 (11.1) | 33.0 (12.5) | 0.9 | 94 | 0.38 |
| Education (years) | 12.4 (3.3) | 12.8 (3.2) | 11.5 (3.4) | 1.8 | 94 | 0.07 |
| BMI (kg/m2) | 22.3 (3.6) | 22.5 (3.5) | 22.1 (3.7) | 0.4 | 94 | 0.68 |
| Duration of illness (months) | 101.8 (97.5) | 105.9 (100.9) | 93.0 (90.8) | —a | —a | 0.48 |
| Baseline MADRS scores | 31.8 (7.6) | 31.8 (7.6) | 31.9 (7.8) | −0.03 | 94 | 0.97 |
| Baseline plasma VEGF levels (ng/ml) | 36.7 (72.8) | 43.0 (84.6) | 23.3 (35.2) | —a | —a | 0.60 |
Bolded values are p < 0.05.
Mann–Whitney U test.
BMI, Body Mass Index; MADRS, the Montgomery-Åsberg Depression Rating Scale; SD, standard deviation; TRD, treatment-resistant depression; VEGF, vascular endothelial growth factor.
Figure 1.
Change in plasma VEGF levels in patients with depression.
#Significant difference when comparing baseline to the indicated times (p < 0.05).
*Significant difference between responders and nonresponders at the indicated times (p < 0.05).
VEGF, vascular endothelial growth factor.
Figure 2.
Change in depressive symptoms in patients with depression.
#Significant difference when comparing baseline to the indicated times (p < 0.05).
*Significant difference between responders and nonresponders at the indicated times (p < 0.05).
MADRS, the Montgomery–Åsberg Depression Rating Scale.
A linear mixed model was applied to compare MADRS scores and plasma VEGF levels over time between ketamine responders and nonresponders (Table 2). MADRS scores showed a statistically significant time main effect (F = 230.63, p < 0.001), group main effect (F = 56.13, p < 0.001) and group-by-time interaction (F = 62.25, p < 0.001). Plasma VEGF levels showed a statistically significant time main effect (F = 4.78, p = 0.011) and group-by-time interaction (F = 4.89, p = 0.01) but no group main effect (F = 1.58, p = 0.211). When compared with baseline, significantly higher plasma VEGF levels were found at 26 days but not at 13 days (Figure 1). Similar results were observed for patients with TRD (Supplemental Table 1, Supplemental Figures 1 and 2).
Table 2.
Comparison of MADRS scores and plasma VEGF levels between responders and nonresponders and between remitters and nonremitters in patients with unipolar and bipolar depression using linear mixed model analysis.
| Variables | Group-by-time interaction | Time main effect | Group main effect | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| MADRS scores | 62.25 | <0.001 | 230.63 | <0.001 | 56.13 | <0.001 |
| Plasma VEGF levels (ng/ml) | 4.89 | 0.010 | 4.78 | 0.011 | 1.58 | 0.211 |
Bolded values are p < 0.05.
MADRS, the Montgomery–Åsberg Depression Rating Scale; VEGF, vascular endothelial growth factor.
No significant association was detected between baseline plasma VEGF levels and changes in MADRS scores in all patients with depression (Table 3) or among only TRD patients (Supplemental Table 2).
Table 3.
Correlation of baseline plasma VEGF levels and changes in MADRS scores at 13 days or 26 days in patients with depression.
| Variables | Changes in MADRS scores at 13 days | Changes in MADRS scores at 26 days |
|---|---|---|
| Baseline plasma VEGF levels (ng/ml) | r = −0.018 | r = 0.004 |
| p = 0.863 | p = 0.969 |
Bolded values are p < 0.05.
MADRS, the Montgomery–Åsberg Depression Rating Scale; r, Pearson coefficient of correlation; VEGF, vascular endothelial growth factor.
Discussion
This is the first study to determine whether plasma VEGF levels are correlated with ketamine’s antidepressant effects in Chinese patients with depression. The main findings focusing on patients suffering from unipolar and bipolar depression included the following: (1) ketamine has a rapid and robust antidepressant response, in line with the findings of previous studies;6,7 (2) plasma VEGF levels showed no changes from baseline after the last ketamine infusion but increased at 2 weeks after the end of six ketamine infusions; and (3) plasma VEGF levels showed no significant correlation with the improvement of depressive symptoms, as measured by MADRS. Similar results were also found among only TRD patients.
Numerous studies have reported that the behavioural and neurogenic actions of other antidepressants, including nonselective serotonin-reuptake inhibitors and SSRIs, may result from an increase in hippocampal VEGF expression.16,35 Similarly, VEGF in the rat hippocampus was strongly regulated by electroconvulsive therapy (ECT).36 These findings indicate that VEGF may be a common downstream target of both pharmacological and nonpharmacological antidepressant therapies. However, our data failed to demonstrate that plasma VEGF levels are involved in the rapid antidepressant effects of ketamine in patients with depression. Similarly, Choi et al.37 found that in rats, hippocampal VEGF is necessary for antidepressant-like behaviours but not sufficient for the antidepressant-like effects of ketamine.
In this study, plasma VEGF levels did not increase on the day following the last ketamine infusion when compared with baseline but did increase at 2 weeks after the end of six ketamine infusions, despite a relevant improvement in depressive symptoms by the completion of six ketamine infusions. These results indicate that increases in plasma VEGF levels, if they truly occur secondary to repeated ketamine infusions, are not precocious but may require some time to occur after the last ketamine infusion and may follow the improvement of depressive symptoms. Surprisingly, in this study increased plasma VEGF levels at 26 days appears to be mostly driven by the nonresponder group, but the interpretation of this phenomenon was unclear. Early studies found that plasma VEGF levels are reduced in untreated patients with depression and that patients with TRD have normal VEGF levels after receiving antidepressants28 and ECT,17 indicating that pharmacological and nonpharmacological therapy might increase plasma VEGF levels. Thus, changes in plasma VEGF levels may occur before starting ketamine infusion, secondary to the chronic use of medication. Regardless, it has been suggested that increased plasma VEGF concentrations may occur earlier than improvement of depressive symptoms.
Although ketamine has novel future potential as an effective antidepressant drug and esketamine has been approved by the US Food and Drug Administration for TRD,38 issues involving the safety and toxicity of ketamine, such as adverse cognitive effects, abuse potential and psychotomimetic effects,39 might limit its widespread clinical use. Identifying potential biomarkers that predict response to repeated ketamine infusions will be invaluable for selecting individuals who are suitable for this therapy.40 Zhou et al.40 reported that early changes in serum kynurenic acid levels and the kynurenic acid/kynurenine ratio are potential predictors of antidepressant response to six ketamine infusions. Yang et al.41 reported that serum levels of interleukin-6 appeared to be a predictive biomarker for the antidepressant response of a single intravenous administration of ketamine in subjects with TRD. Conversely, our results show that plasma VEGF levels at baseline were not significantly associated with the improvement of depressive symptom following six ketmaine treatments.
Several limitations should be addressed. First, the lack of a placebo may impact subjective evaluation. However, the primary aim of this project was to examine the potential gene targets of ketamine in treating depressed patients, and a control group was not included in the design of this project. Second, as a medication wash-out phase before starting the study was not conducted, the potential impact on plasma VEGF levels by continuingly existing psychotropic agents could not be excluded. Therefore, the observed changes in plasma VEGF levels might be attributed to a combination of the regulatory effects of ketamine and the psychotropic agents that the recruited subjects were already taking. Nevertheless, the psychotropic agents were not changed during the study period, reducing the influence of changing drug levels on plasma VEGF. Third, the sample size was relatively small. Finally, the generalizability of the current results was limited to Chinese patients with depression.
Conclusions
This study indicates that plasma VEGF levels are not a potential predictor of antidepressant response to repeated intravenous administration of ketamine in patients with depression.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253211014320 for Association of plasma VEGF levels and the antidepressant effects of ketamine in patients with depression by Wei Zheng, Yan-Ling Zhou, Cheng-Yu Wang, Xiao-Feng Lan, Bin Zhang, Su-Miao Zhou, Su Yan, Ming-Zhe Yang, Sha Nie and Yu-Ping Ning in Therapeutic Advances in Psychopharmacology
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grant numbers 81801343 and 81801345), Guangdong Basic and Applied Basic Research Foundation (grant number 2019A1515011366), the National Key Research and Development Program of China (grant number 2016YFC0906300), Science and Technology Department of Guangdong Province major science and technology (grant number 2016B010108003) and Guangzhou Municipal Psychiatric Disease Clinical Transformation Laboratory (grant number 201805010009).The funding source had no role in the study design, analysis or interpretation of data or in the preparation of the report or decision to publish.
ORCID iDs: Wei Zheng  https://orcid.org/0000-0003-2371-4789
https://orcid.org/0000-0003-2371-4789
Yu-Ping Ning  https://orcid.org/0000-0002-5727-2782
https://orcid.org/0000-0002-5727-2782
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wei Zheng, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Yan-Ling Zhou, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Cheng-Yu Wang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Xiao-Feng Lan, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Bin Zhang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Su-Miao Zhou, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Su Yan, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Ming-Zhe Yang, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Sha Nie, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China.
Yu-Ping Ning, The Affiliated Brain Hospital of Guangzhou Medical University (Guangzhou Huiai Hospital), Guangzhou, China; The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong 510370, China.
References
- 1. Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry 2015; 76: 155–162. [DOI] [PubMed] [Google Scholar]
- 2. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs 2009; 23: 627–647. [DOI] [PubMed] [Google Scholar]
- 3. Andrade C. Ketamine for depression, 3: does chirality matter? J Clin Psychiatry 2017; 78: e674–e677. [DOI] [PubMed] [Google Scholar]
- 4. Zheng W, Zhou YL, Liu WJ, et al. Investigation of medical effect of multiple ketamine infusions on patients with major depressive disorder. J Psychopharmacol 2019; 33: 494–501. [DOI] [PubMed] [Google Scholar]
- 5. Zhan Y, Zhang B, Zhou Y, et al. A preliminary study of anti-suicidal efficacy of repeated ketamine infusions in depression with suicidal ideation. J Affect Disord 2019; 251: 205–212. [DOI] [PubMed] [Google Scholar]
- 6. Rasmussen KG, Lineberry TW, Galardy CW, et al. Serial infusions of low-dose ketamine for major depression. J Psychopharmacol 2013; 27: 444–450. [DOI] [PubMed] [Google Scholar]
- 7. Murrough JW, Perez AM, Pillemer S, et al. Rapid and longer-term antidepressant effects of repeated ketamine infusions in treatment-resistant major depression. Biol Psychiatry 2013; 74: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ionescu DF, Bentley KH, Eikermann M, et al. Repeat-dose ketamine augmentation for treatment-resistant depression with chronic suicidal ideation: a randomized, double blind, placebo controlled trial. J Affect Disord 2019; 243: 516–524. [DOI] [PubMed] [Google Scholar]
- 9. Murrough JW, Soleimani L, DeWilde KE, et al. Ketamine for rapid reduction of suicidal ideation: a randomized controlled trial. Psychol Med 2015; 45: 3571–3580. [DOI] [PubMed] [Google Scholar]
- 10. Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 2006; 63: 856–864. [DOI] [PubMed] [Google Scholar]
- 11. Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry 2010; 67: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kishimoto T, Chawla JM, Hagi K, et al. Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: a meta-analysis of efficacy, safety and time trajectories. Psychol Med 2016; 46: 1459–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wilkinson ST, Ballard ED, Bloch MH, et al. The effect of a single dose of intravenous ketamine on suicidal ideation: a systematic review and individual participant data meta-analysis. Am J Psychiatr 2018; 175: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 2000; 47: 351–354. [DOI] [PubMed] [Google Scholar]
- 15. Shiroma PR, Johns B, Kuskowski M, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord 2014; 155: 123–129. [DOI] [PubMed] [Google Scholar]
- 16. Greene J, Banasr M, Lee B, et al. Vascular endothelial growth factor signaling is required for the behavioral actions of antidepressant treatment: pharmacological and cellular characterization. Neuropsychopharmacology 2009; 34: 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minelli A, Zanardini R, Abate M, et al. Vascular endothelial growth factor (VEGF) serum concentration during electroconvulsive therapy (ECT) in treatment resistant depressed patients. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1322–1325. [DOI] [PubMed] [Google Scholar]
- 18. Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet 2004; 36: 827–835. [DOI] [PubMed] [Google Scholar]
- 19. Storkebaum E, Lambrechts D, Carmeliet P. VEGF: once regarded as a specific angiogenic factor, now implicated in neuroprotection. Bioessays 2004; 26: 943–954. [DOI] [PubMed] [Google Scholar]
- 20. Fournier NM, Lee B, Banasr M, et al. Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling. Neuropharmacology 2012; 63: 642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calvo CF, Fontaine RH, Soueid J, et al. Vascular endothelial growth factor receptor 3 directly regulates murine neurogenesis. Genes Dev 2011; 25: 831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCloskey DP, Croll SD, Scharfman HE. Depression of synaptic transmission by vascular endothelial growth factor in adult rat hippocampus and evidence for increased efficacy after chronic seizures. J Neurosci 2005; 25: 8889–8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heine VM, Zareno J, Maslam S, et al. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci 2005; 21: 1304–1314. [DOI] [PubMed] [Google Scholar]
- 24. Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 25. Karege F, Vaudan G, Schwald M, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 2005; 136: 29–37. [DOI] [PubMed] [Google Scholar]
- 26. Isung J, Aeinehband S, Mobarrez F, et al. Low vascular endothelial growth factor and interleukin-8 in cerebrospinal fluid of suicide attempters. Transl Psychiatry 2012; 2: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci USA 2007; 104: 4647–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nowacka MM, Obuchowicz E. Vascular endothelial growth factor (VEGF) and its role in the central nervous system: a new element in the neurotrophic hypothesis of antidepressant drug action. Neuropeptides 2012; 46: 1–10. [DOI] [PubMed] [Google Scholar]
- 29. Deyama S, Bang E, Kato T, et al. Neurotrophic and antidepressant actions of brain-derived neurotrophic factor require vascular endothelial growth factor. Biol Psychiatry 2019; 86: 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zheng W, Zhou YL, Liu WJ, et al. Rapid and longer-term antidepressant effects of repeated-dose intravenous ketamine for patients with unipolar and bipolar depression. J Psychiatr Res 2018; 106: 61–68. [DOI] [PubMed] [Google Scholar]
- 31. Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: the scale for suicide ideation. J Consult Clin Psychol 1979; 47: 343–352. [DOI] [PubMed] [Google Scholar]
- 32. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry J Ment Sci 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 33. Zhong BL, Wang Y, Chen HH, et al. Reliability, validity and sensitivity of Montgomery-Åsberg Depression Rating Scale for patients with current major depressive disorder [in Chinese]. Chin J Behav Med Brain Sci 2011; 20: 85–87. [Google Scholar]
- 34. Zimmerman M, Posternak MA, Chelminski I. Derivation of a definition of remission on the Montgomery-Asberg depression rating scale corresponding to the definition of remission on the Hamilton rating scale for depression. J Psychiatr Res 2004; 38: 577–582. [DOI] [PubMed] [Google Scholar]
- 35. Warner-Schmidt JL, Duman RS. VEGF as a potential target for therapeutic intervention in depression. Curr Opin Pharmacol 2008; 8: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Altar CA, Laeng P, Jurata LW, et al. Electroconvulsive seizures regulate gene expression of distinct neurotrophic signaling pathways. J Neurosci 2004; 24: 2667–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Choi M, Lee SH, Chang HL, et al. Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim Biophys Acta 2016; 1862: 1247–1254. [DOI] [PubMed] [Google Scholar]
- 38. Zheng W, Cai DB, Xiang YQ, et al. Adjunctive intranasal esketamine for major depressive disorder: a systematic review of randomized double-blind controlled-placebo studies. J Affect Disord 2020; 265: 63–70. [DOI] [PubMed] [Google Scholar]
- 39. Hong YL, Yee CH, Tam YH, et al. Management of complications of ketamine abuse: 10 years’ experience in Hong Kong. Hong Kong Med J 2018; 24: 175–181. [DOI] [PubMed] [Google Scholar]
- 40. Zhou YL, Zheng W, Liu WJ, et al. Antidepressant effect of repeated ketamine administration on kynurenine pathway metabolites in patients with unipolar and bipolar depression. Brain Behav Immun 2018; 74: 205–212. [DOI] [PubMed] [Google Scholar]
- 41. Yang JJ, Wang N, Yang C, et al. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry 2015; 77: e19–e20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253211014320 for Association of plasma VEGF levels and the antidepressant effects of ketamine in patients with depression by Wei Zheng, Yan-Ling Zhou, Cheng-Yu Wang, Xiao-Feng Lan, Bin Zhang, Su-Miao Zhou, Su Yan, Ming-Zhe Yang, Sha Nie and Yu-Ping Ning in Therapeutic Advances in Psychopharmacology