Abstract
The identification of NADPH oxidase (NOX) isoforms in tissues is essential for interpreting experiments and for next step decisions regarding cell lines, animal models, and targeted drug design. Two basic methods, immunoblotting and reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR), are important to monitor NOX protein and messenger RNA (mRNA) levels, respectively, for a range of investigations from understanding cell signaling events to judging NOX inhibitor efficacies. For many other genes that are expressed in high abundance, these methods may seem rather simple. However, detecting the low expression levels of endogenous NOX/DUOX is difficult and can be frustrating, so some guidelines would be helpful to those who are facing difficulties. One reason why detection is so difficult is the limited availability of vetted NOX/DUOX antibodies. Many of the commercial antibodies do not perform well in our hands, and dependable antibodies, often generated by academic laboratories, are in limited supply. Another problem is the growing trend in the NOX literature to omit end-user validation of antibodies by not providing appropriate positive and negative controls. With regard to NOX mRNA levels, knockdown of NOX/DUOX has been reported in cell lines with very low endogenous expression (Cq values ≥30) or in cell lines devoid of the targeted NOX isoform (e.g., NOX4 expression in NCI-60 cancer cell panel cell line 786–0). These publications propagate misinformation and hinder progress in understanding NOX/DUOX function. This chapter provides overdue guidelines on how to validate a NOX antibody and provides general methodologies to prepare samples for optimal detection. It also includes validated methodology to perform RT-qPCR for the measurement of NOX mRNA levels, and we suggest that RT-qPCR should be performed prior to embarking on NOX protein detection.
Keywords: Immunoblot, NOX antibodies, NOX qPCR primers
1. Introduction
NADPH oxidases (NOX) are the major producers of purpose-driven ROS production. Unlike other enzymes that produce ROS as a side reaction during the course of catalysis, the NADPH oxidase enzymes use NADPH to reduce molecular oxygen to form superoxide and hydrogen peroxide as their sole known enzymatic function. NOX enzyme expression is complex and dependent upon factors such as species, tissue, cell type, and physiological or pathological status. Thus, the cataloguing of NOX expression has often been reported by specific tissue type. While immunoblotting and PCR are seemingly simple methods, NOX detection presents a special case requiring knowledge of certain precautions and preparations prior to embarking on these methods. Membrane proteins, in general, are more difficult to detect, and NOX proteins have adorned themselves with an “invisibility cloak” [a la Harry Potter] which may be due to their low abundance, glycosylation, resistance to standard extraction methods, susceptibility to proteolytic degradation, and perhaps a variety of other factors. Many manufacturers market “anti-NOX isoform antibodies,” but the majority of their validation data (and/or literature referenced in their product data sheets) are incomplete (e.g., lack of appropriate positive and negative controls, no information about isoform specificity). In our hands many of these antibodies fail to identify the claimed NOX isoform. Experts in the field are frustrated by the poor results obtained when using such antibodies. Astonishingly, a large number of manuscripts are accepted without proper antibody validation. Thus, guidelines are needed in order to properly evaluate immunoblot data to avoid misleading new investigators in this field who are searching for appropriate reagents.
A number of validated NOX antibodies have emerged from academic laboratories. Some of them are listed in Tables 5, 6, 7, 8, 9, 10, and 11, and additional ones are described in [1]. NOX and DUOX antibodies listed in Tables 5, 6, 7, 8, 9, 10, and 11 are not all-inclusive, and the reader is advised to judge other antibodies in the literature based on the methods of validation described in these guidelines. Exact dilutions for each antibody are not provided since it will vary depending on the characteristics of the sample.
Table 5.
NOX1 and NOXA1 antibodies
| NOX1 source/catalog # | Host and clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| Dr. J. Doroshow | Mouse Monoclonal Clone 22 |
224–564 | Yesa (IF, IHC) |
H | [11, 12] |
| Dr. C. Yabe-Nishimura | Rabbit Polyclonal |
139–152 (mouse) |
Yesb (IF, IHC) |
M | [7] |
| Dr. C. Yabe-Nishimura | Rat Monoclonal Clone 31C1 |
139–152 (mouse) |
Yesb (IF, IHC) |
M | Unpublished |
| Dr. J. David Lambeth | Mouse Monoclonal Clone E39 |
3rd extracellular loop | Yesc (IF, IHC) |
H | [13] |
| OriGene Technologies #TA349080 | Rabbit Polyclonal |
Proprietary | Nod | H | |
| Novus #NBP1–31546 | Rabbit Polyclonal |
Proprietary | Nod | H, M, R | |
| NOXA1 subunit Santa Cruz sc-398873 | Mouse Monoclonal Clone H-6 |
1–200 | Yese | H, M, R |
Evaluation of academic and commercial NOX1 antibodies
Specificity in cell lines is shown in Ref. [11]; specific in CHO cells overexpressing NOX1 (Dr. V. Jaquet, pers. communication)
Authors stated that the polyclonal antibody detected Nox1 in mouse colon but not in mouse small intestine or lung. Their mAb detects Nox1 in mouse colon, small intestine, and lung (personal communication Dr. M. Matsumoto) (see Fig. 8)
There was a limited quantity of this antibody available for WB verification (see Fig. 5d). This antibody was made by diaDexus (out of business). The specific epitope was not disclosed, only the location (3rd extracellular loop)
Unable to differentiate between NOX1 stably overexpressed in HEK 293 cells and empty vector-transfected controls
Product datasheet indicates that NOXA1 was detected in HEK 293T cells overexpressing NOXA1, but not in vector control
Table 6.
NOX2 antibodies
| NOX2 source/atalog # | Host and Clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| Dr. A. Jesaitis Abcam #ab80897 Santa Cruz #sc-130543 |
Mouse Monoclonal Clone 54.1 |
383–390 | Yesa (IF) | H, M | [14] |
| Dr. D. Roos, LSbio #LS-C85347 | Mouse Monoclonal Mo 48 |
Purified cytochrome b558 | Yes (IHC) | H | [17, 18] |
| Dr. A. Jesaitis Santa Cruz #sc-130549 |
Mouse Monoclonal Clone CL5 |
135–147 | Yesc | H | [19] |
| BD Transduction Laboratories, #BD61141 | Mouse Monoclonal Clone 53 |
450–556 | Yesd (IF rat) | H, M, R | [2, 20] |
| LifeSpan Biosciences #LS-C179418 | Mouse Monoclonal Clone 7D5 |
143–151 | Yesb (Flow) | H [15, 16] | |
Evaluation of academic and commercial NOX2 antibodies
Cross-reactivity with NOX1–4 and GRP58
Cross-reactivity with gelsolin
Isoform specificity not tested. Other applications not tested
According to the manufacturer, a 58 kDa band is detected in mouse macrophages
Table 7.
NOX3 antibodies
| NOX3 source/catalog # | Host and clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| Bioss bs-3683R | Rabbit Polyclonal | 70–120 | Yesa | H, M,R | Unpublished |
At this time there is little information on NOX3 antibodies and their validation. Several NOX3 antibodies are on the market (see www.abcam.com and www.antibodies-online.com), but none of these have, thus far, been validated nor tested for isoform specificity
Worked well in detecting NOX3 overexpression in HEK 293 cells for one group but not for another (Dr. Ulla Knaus, Dr. Vincent Jaquet, pers. communication), and specificity over other isoforms has not been tested
Table 8.
NOX4 antibodies
| NOX4 source/catalog # | Host and clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| Dr. A. Shah | Rabbit Polyclonal |
556–568 | Yesa (IF) (see Fig. 5a) | H, M | [21] |
| Dr. P. Jansen-Dürr | Rabbit Monoclonal (27–6) |
335–578 | Yesb (see Fig. 5f) | H, M | [22] |
| Dr. U. Knaus | Rabbit Polyclonal (7843) |
251–266 | Yesc (IF, Flow) | H | [23] |
| Dr. U. Knaus | Rabbit Monoclonal (20.1, 37.7) |
251–266 | Yesc (IF, Flow) | H | [23] |
| Dr. J. Doroshow | Rabbit Monoclonal (47–6) |
242–262 | Yesc (IHC) (see Fig. 5b) | H | [12] |
| Abcam ab133303 | Rabbit Monoclonal |
Unknown | Nod, e (see Fig. 5h) | H, R | |
| Abcam ab109225 | Rabbit Monoclonal |
500–578 | Nod,e | H, R | |
| Millipore ABC459 |
Rabbit Polyclonal |
C-terminus | Nod, f (see Fig. 5g) | H, M | |
| Novus Biologicals #NB110–58851 |
Rabbit Polyclonal |
500–578 | Nod, g (see Fig. 5c) | H, M, R |
Evaluation of academic and commercial NOX4 antibodies
Excellent sensitivity for mouse and human NOX4 although many bands are detected, some of which may be NOX4 variants; does not cross-react with NOX1 in HEK 293 cells stably overexpressing NOX1 (see Fig. 5a)
Detects human NOX4 in Trex cells overexpressing NOX4; detects Nox4 in mouse kidney cortex, multiple bands
NOX4-specific (epitope is absent in other NOX isoforms) but only detects human NOX4
Does not distinguish between HEK293 cells expressing an empty vector vs. HEK293 cells stably overexpressing NOX4
Identical results were obtained by two independent laboratories (Dr. J.D. Lambeth and Dr. J. Doroshow)
Website states that application is not for WB, but for IHC and ICC
Unspecific in mouse; older lots may have been specific for NOX4 in overexpressing cells, but recent lots are not specific
Table 9.
NOX5 antibodies
| NOX5 source/catalog # | Host and Clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (rodents lack NOX5 gene) | Reference |
|---|---|---|---|---|---|
| Dr. W. Nauseef | Rabbit Polyclonal |
1–169 | Yesa (IF, IP) |
H | [6, 24, 25] |
| Dr. J. D. Lambeth | Rabbit Polyclonal (#8579) |
491–506 and 577–588 | Yesb (IHC) |
H | [4] |
| Dr. J. Doroshow | Mouse Monoclonal |
600–746 | Yesb (IF, IHC) |
H | [26] |
Evaluation of NOX5 antibodies
Reference [24] shows a MW of 75 kDa in K562 cells stably transfected with NOX5β. Detects a 70 kDa band and a smaller band at 43kDa (degradation product) in HEK cells expressing NOX5. Antibody works for immunoprecipitation
Reference [4] shows a MW of 75kDa in HEK 293 cells transiently transfected with NOX5
Table 10.
DUOX antibodies
| DUOX1, DUOX2, DUOXA1, DUOXA2 source/catalog # | Host and clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| DUOX1 and 2 Dr. U. Knaus |
Rabbit Polyclonal |
DUOX1 775–1026 |
Yesa (Flow, IHC) |
H, M | [27] |
| DUOX2 specific Dr. U. Knaus |
Rabbit Polyclonal |
634–648 | Yes (Flow, IHC) |
H | [28] |
| DUOXA1 specific Dr. U. Knaus |
Rabbit Polyclonal |
280–299 | Yes | H | [28] |
| DUOX1 and 2 Dr. X. De Deken |
Rabbit Polyclonal I2 |
DUOX1 618–1004 |
Yesa | H, M | [29] |
| DUOX1 and 2 Dr. C. Dupuy |
Rabbit Polyclonal |
DUOX2 410–423 |
Yesa | H, M | [30] |
| DUOX1 specific Dr. C. Dupuy |
Rabbit Polyclonal |
DUOX1 988–1011 |
Yes | H, M | [31] |
| DUOX1 and 2 Dr. C. Dupuy |
Mouse Monoclonal Clone |
DUOX2 21–589 |
Yesa | H | [32] |
| DUOX1 and 2 Dr. J. Doroshow |
Mouse Monoclonal Clone S-40 |
DUOX2 131–540 |
Yesa | H, M | [33] |
| DUOX1 Bioss bs-11431R |
Rabbit Polyclonal |
Unspecified | Nob | H, M, R | |
| DUOX2 Santa Cruz (Y-15) sc-49939 |
Goat Polyclonal |
Unspecified | Yesa | H, M, R | |
| DUOX2 Santa Cruz (E-8) sc-398681 |
Mouse Monoclonal |
DUOX2 626–680 |
Not testedc | H, M, R | |
| DUOX1 Sigma-Aldrich HPA023544 |
Polyclonal | 910–1011 | Yesa (IHC) |
H | |
| DUOXA1 specific Sigma-Aldrich HPA041578 |
Polyclonal | 270–326 | Yesd (IHC) |
H |
Evaluation of DUOX1, DUOX2, DUOXA1, and DUOXA2 antibodies
Cross-reactivity between DUOX1 and DUOX2. Dr. Dupuy’s monoclonal antibody has ~tenfold preference for DUOX2, and in porcine tissues this antibody does not cross-react with Duox1 (see reference [32])
Detects a non-specific band at a similar molecular weight as DUOX in non-transfected cells
Recommended by Santa Cruz as a replacement for Santa Cruz (Y-15) sc-49939. See website for images
Detects additional non-specific bands
Table 11.
Antibodies for subunits (p47phox, p67phox) and for p22phox
| Subunits and p22phox source/catalog # | Host and Clonality | Immunogen (aa) | Is the target detected in WB (other applications)? | Species detected (human, mouse, rat) | Reference |
|---|---|---|---|---|---|
| p47phox Dr. M. Quinn Millipore 07–001 |
Rabbit Polyclonal |
Full-length human p47phox | Yesa, b (IHC, IP) |
H, M, R | [34] |
| p47phox Dr. M. Quinn |
Mouse Monoclonal Clone 43.12 |
Full-length human p47phox | Yes (others not tested) | H, M, R | Unpublished |
| p67phox Dr. M. Quinn Millipore 07–002 |
Rabbit Polyclonal |
Full-length human p67phox | Yesa, b (IHC, IP) |
H, M, R | [34] |
| p67phox Dr. M. Quinn |
Mouse Monoclonal Clone 81.1 |
Full-length human p67phox | Yes (IHC) | H, M, R | Unpublished |
| p47phox Santa Cruz sc-17844 | Mouse Monoclonal Clone A-7 |
196–390 | Yesa (others not tested) | H, M, R. | |
| p67phox Santa Cruz sc-374510 | Mouse Monoclonal Clone D-6 |
Full-length p67phox | Yesa (others not tested) | H, M, R | |
| p40phox Dr. M. Quinn |
Mouse Monoclonal Clone 1.9 |
Full-length human p40phox | Yes (IHC) | H | |
| p22phox Dr. A. Jesaitis Dr. M. Quinn Santa Cruz sc-130550 |
Mouse Monoclonal Clone 44.1 |
29–33 and 182–188 |
Yesa, b (IP) |
H, M, R | [14] |
Many academic laboratories have published antibodies generated for these subunits. We have used antibodies generated in the laboratory of Dr. Mark Quinn
Also listed are commercial antibodies that worked in our conditions for immunoblots
Tested with human neutrophil cytosolic fractions
Tested in HEK 293 cells overexpressing cytosolic components
Given the lack of dependable commercial antibodies, many researchers have relied upon PCR as a surrogate for the detection of NOX expression. Yet even with the specificity of NOX primers, conflicting results can be obtained regarding the presence of specific NOX isoforms in identical tissues under basal conditions. Thus, this chapter also includes a section describing one method for RT-qPCR. The basal levels of NOX mRNA are often low, indicated by quantification cycle (Cq) values in the range of 30–32. Thus, relying solely on reverse transcriptase PCR (RT-PCR) without performing reverse transcriptase-quantitative PCR (RT-qPCR) does not provide a secure sense of the quantity of NOX mRNA present. In addition, the high Cq values, indicating very low mRNA quantities, should heighten the level of concern about false-positive results arising from trace contamination from genomic DNA (gDNA), NOX-bearing plasmids, and other sources. Thus, the inclusion of appropriate negative controls is crucial, and the handling of samples requires rigorous protocols to avoid contamination. In addition, several NOX isoforms have variants due to alternative splicing. Thus, an added layer of precision is required in designing primers that detect the full-length form. We recommend that RT-qPCR be performed prior to immunoblotting to obtain an indication of whether the level of protein is likely to be detectable by immunoblot. Here, we share what we have learned and hope that these guidelines will improve the validity of published NOX immunoblots and PCR data in the future.
2. Materials
2.1. RNA Isolation and Reverse Transcriptase-Quantitative PCR (RT-qPCR)
RNaseZap™ RNase decontamination solution.
Decontaminated workspace and equipment free of NOX plasmids.
Nuclease-free water.
Nuclease-free pipette tips with barrier filters.
RNeasy Plus Kit containing gDNA eliminator columns.
70% ethanol prepared in DEPC-treated water.
DEPC-treated water.
β-mercaptoethanol.
1.5 mL micro-centrifuge tubes.
Phosphate-buffered saline (PBS).
Cell scrapers.
200-proof ethanol (100%) to prepare 70% ethanol in DEPC-treated water.
1 mL syringes and 21-gauge needles for homogenization or Qiashredder columns.
qScript XLT™ cDNA SuperMix.
0.2 mL PCR tubes.
A cold 4 °C cube designed to hold small tubes and 96-well plates for PCR setup.
Mini-centrifuge capable of briefly spinning down 0.2 mL PCR tubes.
RT-qPCR instrument capable of accepting 96-well plates such as Bio-Rad’s CFX96.
96-well PCR plate suggested by the manufacturer of the PCR instrument.
Adhesive sealing film for PCR plates.
A paddle to apply the adhesive sealing film.
Primers for the reference gene and for the specific NOX isoform(s).
PerfeCta® SYBR® Green PCR SuperMix or similar.
A centrifuge capable of spinning 96-well plates.
2.2. SDS-PAGE and Immunoblotting
SDS-PAGE, appropriate percentage or gradient gels.
Standard running buffer: 0.025 M Tris-base, do not adjust pH, 0.192 M glycine, 0.1% SDS.
Power supply capable of attaining 200 volts.
Pre-stained MW protein ladder.
Polyvinylidene fluoride (PVDF) membrane, filter papers, sponges, and transfer cassettes and tanks (standard wet transfer).
Standard transfer buffer: 0.025 M Tris-base, do not adjust pH, 0.192 M glycine, 20% (v/v) of 100% methanol, chilled to 4 °C.
100% methanol.
TBST: 10 mM Tris–HCl, pH 7.4, 0.15 M NaCl. 0.1% Tween-20.
Blocking buffer: 5% nonfat dry milk in TBST. Store at 4 °C.
Primary antibodies: See Tables 5, 6, 7, 8, 9, 10, and 11 for recommendations.
Secondary antibodies: HRP-conjugated goat anti-rabbit, HRP-goat anti-mouse, or fluorescent-labeled antibodies.
ECL reagent such as SuperSignal West Pico Chemiluminescent Substrate.
Plastic sheets to encase the immunoblot.
Autoradiography cassette equipped with intensifying screen son each side.
Autoradiography film and processor (or other film-less detection system).
Optional: LI-COR Odyssey fluorescent near-infrared (IR) scanner.
Optional: LI-COR Odyssey Blocking buffer (TBS).
Optional: LI-COR Odyssey IRDye 680LT (red)-labeled secondary antibody.
Optional LI-COR Odyssey IRDye 800Cw (green)-labeled secondary antibody.
Optional LI-COR Odyssey fluorescent IRDye-labeled protein ladder.
Plasmids for positive control antigens: Mammalian expression plasmid containing the NOX cDNA sequence for a specific NOX isoform.
Negative control plasmid: Same plasmid as above without the NOX sequence.
Human embryonic kidney cell line (HEK 293 cells) or any other cell line with very low or no endogenous expression of NOX/DUOX enzymes.
DMEM medium, high glucose (4.5 g/L), containing 10% FBS, or similar.
Cell line to be tested, appropriate culture medium.
Transfection reagent, such as X-tremeGENE 9, Lipofectamine, etc.
OptiMEM medium without any supplements.
RIPA buffer: 50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.5%sodium deoxycholate, 0.1% SDS, 1.0% Triton X-100.
Protease and phosphatase inhibitor cocktail or tablets.
Sonicator equipped with a pencil-tip probe or 1 mL syringes and 21-gauge 1-in. needles for homogenization.
Micro-centrifuge, cooled to 4 °C.
4× Laemmli’s sample buffer: 200 mM Tris–HCl, pH 6.8, 20% glycerol, 0.4% SDS, 4% β-mercaptoethanol.
Protein determination kit, such as the BCA protein determination kit.
3. Methods
3.1. RNA Isolation
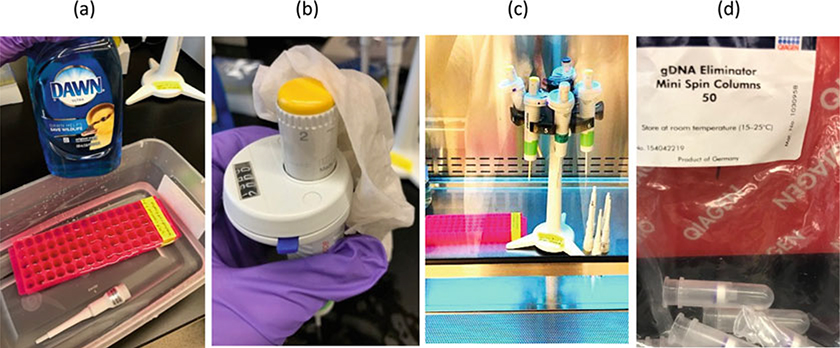
Use a PCR-clean workspace and tools that have been washed with soap and have been decontaminated from DNA (e.g., NOX plasmids and amplicons) by treatment with UV light. Spray the workspace, tools, and your gloves with ZAP spray. Wipe off any excess with a paper towel (see Note 1, Fig. 1).
Use barrier-filtered, PCR-clean pipet tips for all the following steps. Spray the surface of the pipette tip boxes with RNase Zap spray, especially where the thumb is placed.
Follow the protocol from Qiagen RNeasy Plus Kit described briefly below for cell lines. Refer to the manual for isolation of RNA from other sources such as tissues. Be sure to wipe the interior and exterior surfaces of the micro-centrifuge with Zap spray, especially knobs, dials, buttons, and the lid where the thumb is placed. Clean any rings of precipitated material from previous spins from the interior walls of the micro-centrifuge.
Wash adherent cells twice with PBS (see Note 2).
Add 600 μL of resuspension buffer containing fresh 5% β-mercaptoethanol to a 10-cm plate of rinsed cells (about 2.5 × 106 cells).
Scrape the cells off the plate, and transfer to the next plate and scrape again. A total of 1 × 107 cells can be processed in 600 μL. Transfer to a 1.5 mL micro-centrifuge tube.
Homogenize the cells using a Qiashredder column (Qiagen),or manually homogenize by using a 1-mL syringe and 21-gauge needle, 5 passes. Take 600 μL and add it to the gDNA eliminator column. Spin 10,000 rpm (8000 × g) for 30 s in a micro-centrifuge. Collect the flow through. Dispose of the used gDNA column, and reapply the flow through to a fresh gDNA column, and spin again to ensure that the gDNA is removed (see Note 3).
Precipitate the RNA by adding an equal volume (600 μL) of 70% ethanol prepared in DEPC-treated water.
Take 700 μL of the 1200 μL sample and add it to the RNA-binding column. Spin as before. Discard the flow through. Add the remainder of the sample to the same column and spin again.
Discard the flow through, and wash the column with the wash buffers as directed by the kit.
Dry the column by performing the optional spin at 13,200 rpm (15,000 × g), 1 min without buffer added to the column. Elute with 30–50 μL of nuclease-free water to keep the RNA concentrated. Determine the RNA concentration using a Nanodrop spectrophotometer or by other standard methods (see Note 4).
Store RNA samples at −70 to −80 °C or proceed to cDNA synthesis on the same day (see Note 5).
Fig. 1.
Preventing contamination in RT-qPCR. (a) Wash the bench and surfaces of tools and equipment with a paper towel soaked in diluted detergent and water. If possible unscrew the lower parts of the pipette barrel and wash in soapy water. (b) The dial and top of the pipette where the thumb is placed is a large source of contamination. (c) Treat the washed pipettes and test tube racks with UV light in a biosafety cabinet or in a UV light box for 1 h. (d) Use a gDNA eliminator column twice to ensure gDNA removal
3.2. cDNA First-Strand Synthesis
On the day of cDNA first-strand synthesis, thaw the RNA and keep it on ice. Place 2 μg of RNA into each 0.2 mL PCR tube (or well of a 96-well PCR plate for many samples). Prepare the negative control (Tube 1) first by adding nuclease-free water to the RNA to 20 μL total. To the test sample (Tube 2), add nuclease-free water to the RNA for a final volume of 16 μL. Add 4 μL of 5× qScript XLT cDNA SuperMix only to Tube 2. Each tube should have 20 μL total volume. Of these two tubes, 10 μL will be used for one NOX PCR master mix of 100 μL (4 replicate wells) and 10 μL will be used for one reference gene PCR master mix (4 replicate wells). Prepare enough cDNA first strand (and negative controls) if additional conditions are intended. Spin the PCR tubes briefly in a mini-centrifuge to bring the liquid to the bottom of the tubes before placing them in the thermocycler. If using a 96-well plate for many cDNA first-strand samples, seal the plate and centrifuge the plate at 400 × g for 5 min at 4 °C. Place in a standard thermocycler (it is not necessary to use a real-time PCR instrument at this step), and set the program according to the manufacturer’s instructions. If using the qScript XLT cDNA SuperMix, set the program for 5 min at 25 °C, 50 min at 42 °C, 5 min at 85 °C, and hold at 4 °C (see Note 6).
Return the RNA to the −70 °C freezer. Plan the experiment so that the first-strand cDNA can be used immediately, but if necessary freeze the first-strand cDNA tubes at −20 °C overnight (see Note 7).
3.3. Setting Up the RT-qPCR Master Mix and 96-Well Plate
Set up the following master mix RT-qPCR reactions (100 μL total volume in 1.5 mL tubes or in a 96-well plate if many samples are being processed). Avoid working on ice since ice may accidently contaminate the samples. Instead use a 4 °C test tube rack (cube) designed to hold PCR tubes or plates. If using a SuperMix containing a hot-start DNA polymerase, then the setup may be performed at 25 °C. Each 100 μL of master mix reaction will be pipetted into 4 replicate wells of the PCR plate (20 μL/well plus 20 μL extra).
Always start with the no template control (NTC) tubes first, then the negative control (NC) tubes, and finally the unknown (UNK) tubes (see Note 8).
No template controls (NTC): Add 50 μL of 2× PerfeCtA® SYBR® Green SuperMix (see Note 9). Add 30 μL of nuclease-free water. Add 10 μL of 10× (2 μM) NOX forward primer and 10 μL of 10× (2 μM) NOX reverse primer to NTC-1. The NOX primers should be isoform-specific and able to detect the full-length variant. Table 1 lists some of the variants of human NOX isoforms, their main differences, and their accession numbers. Table 2 lists the human NOX primers that we use. All of them detect the full-length isoform as well as all the variants known to date (see Note 10). Table 3 lists mouse NOX primers from a reference [2]. Add 10 μL of 10× (2 μM) reference gene forward primer and 10 μL of 10× (2 μM) reference reverse primer to NTC-2. Do not add any cDNA or RNA to these NTC tubes. Table 4 lists a selection of human primers for reference genes that have been suggested by Integrated DNA Technologies’ RT-qPCR tutorial manual available on their website (see Note 11). Primers for other species can be purchased from Integrated DNA Technologies or other companies and validated as we have done for human NOX RT-qPCR primers.
Negative controls (NC): Add 50 μL of 2× PerfeCtA® SYBR® Green SuperMix. Add 20 μL of nuclease-free water. Add 10 μL 10× of forward and 10 μL of 10× reverse NOX primers to NC-1. Add 10 μL (1 ug RNA) of the negative control first-strand cDNA synthesis tube prepared during the cDNA synthesis step. Those tubes only contain RNA and no cDNA. Each tube will now have 100 μL total volume. Continue with NC-2, which will have the reference gene primers instead of NOX primers.
Unknowns (UNK’s): Add 50 μL of 2× PerfeCtA® SYBR® Green SuperMix. Prepare one UNK tube for each set of primers to be tested. Add 20 μL of water, 10 μL of 10× forward and 10 μL of 10 × reverse primer, and 10 μL of cDNA (1 ug RNA equivalents) that was synthesized in the presence of first-strand cDNA synthesis SuperMix. The total volume is 100 μL.
Use a clean, decontaminated vortex to gently vortex all the PCR master mix tubes for a few seconds, and then spin in a clean, decontaminated micro-centrifuge for 30 s at 10,000 rpm (8000 × g) to bring the liquid to the bottom of the tubes. Starting with the NTC tubes, pipet 20 μL into each of 4 wells of a 96-well PCR plate. There will not be enough volume for a fifth well. Proceed then with the NC tubes; pipet 20 μL into 4 wells. Pipet the UNK wells last, and then change gloves again.
Seal the PCR plate with a clear adhesive film made for PCR plates. Use a plastic PCR paddle, and drag it over the surface of the film to ensure that each well is sealed. Use the edge of the paddle and drag it in between column, rows, and all along the edge of the outer rows and columns to ensure that each individual well is sealed tightly (see Note 12 and Fig. 2). Centrifuge the sealed plate at 400 × g (2000 rpm) for 5 min at 4 °C to bring all the liquid to the bottom of the wells.
Table 1.
Human NOX variants, accession numbers, and main characteristics
| NOX isoform | Variant number (V) and NCBI accession # | Notes about the variant (see accession # for full details) |
|---|---|---|
| NOX1 | (V1) NM_007052.4 (V2) NM_013955.2 (V3) NM-001271815 |
Full length Deletion of 1503–1610 (exon 10) Deletion of 459–543 (exon 4) |
| NOX2 (CYBB) | NM_000397.3 | Full length |
| NOX3 | NM_015718.2 | Full length |
| NOX4 | (V1) NM_016931.4 (V2) NM_001143836.2 (V3) NM_001143837 (V4) NM_001291926 (V5) NM_001291927.1 (V6) NM_001291929.1 (V7) NR_120406.1 (V8) NM_001300995.1 |
Full length Deletion of 1546–1656 (exon 14) Deletion of 1–375 (exon 1) Deletion of 472–475 (part of exon 3) Deletion of 1–375 (exon 1) Deletion of 1–471 (exon 1 and 2) Non-coding Deletion of 1–375 (exon 1) and of nucleotides corresponding to exon 14 of full length |
| NOX5 [8, 9] | (α) NM_001184779.1 (β) NR_033672.1 (γ) NM_024505.3 (δ) NR_033671.2 (ε) AF317889 (ζ) NM_001184780.1 |
NOX5α; 18 aa extension at the start of the N-terminus (exon 3); deletion of 27 aa in exon 6; predominant isoform in spleen NOX5β; deletion of 27 aa in exon 6; predominant isoform in testis NOX5γ complete exon 6 without the 18 aa extension of the N-terminus NOX5δ complete exon 6 with the 18 aa extension of the N-terminus NOX5ε (a.k.a. NOX5-S); truncated from exon 3 through most of exon 7 NOX5ζ: contains an extra 25 aa at the start of the N-terminus |
| DUOX1 NM_017434.4 | Full length | |
| DUOX2 NM_014080.4 | Full length | |
Table 2.
Primers used in the detection of human NOX isoforms by RT-qPCR
| NOX isoform | Human sequence of primer | Exon location and Tm | Integrated DNA technologies Cat. # |
|---|---|---|---|
| NOX1 | GGTCAACACGAGGAGAGC CAAGGATCCACTTCCAAGACTC |
E7F, 60.7 E8R, 61.4 |
Hs.PT.49a.435525.4 |
| NOX2 (CYBB) | CCCAATCCCTCAGTTTGCT CCTTCTGTTGAGATCGCCAA |
E7F, 61.1 E8R, 61.2 |
Hs.PT.51.3983106 |
| NOX3 | ACCTTCTGTAGAGACCGCTAT TCACATGCATACAAGACCACA |
E7F, 61.2 E8R, 61.1 |
Hs.PT.51.3065606 |
| NOX4 | CTGTGGTGTTACTATCTGTATTTTCTC CTTGCTGCATTCAGTTCAACA |
E4F, 60.7 E5R, 60.8 |
Hs.PT.51.22695360.g |
| NOX5 | GCCAGTGCCTCAACTTCG CCACTACCACGTAGCCCATA |
E7F, 62.1 E8R, 62.1 |
Hs.PT.58.40874887 |
| DUOX1 | GCGTCTACATGAGAAATGCCA GCAGCAGTGCATCCACAT |
E10F, 61.8 E12R, 61.9 |
Hs.PT.51.21346453 |
| DUOX2 | CGCCACCTACCAGAACATC GGTAGAGAAGAACTGC TCAGAG |
E7F, 61.1 E9R, 61.3 |
Hs.PT.51.20690545 |
Primers from PrimeTime™ qPCR Pre-designed Assays (without probes) were purchased from Integrated DNA Technology (IDT) and may have been modified slightly to obtain desired Tm. Primers are listed in the 5′ to 3′ direction (R = reverse and F = forward). The exon number may be different for different accession numbers. The Tm values were determined using IDT’s OligoAnalyzer tool (http://www.idtdna.com/calc/analyzer) using the following final concentrations in the qPCR reaction: [primers] = 200 nM, [Na+] = 55 mM, [Mg2+] = 1.5 mM, and [dNTP] = 0.2 mM. The actual annealing temperatures were determined by performing a gradient RT-qPCR for each primer set using the methods described in the text. These primers detect variant 1 (the full-length variant). To determine whether these primers detect other variants, the user should check the accession number of the variant, some of which are provided in Table 1
Table 3.
Primers used in the detection of mouse Nox isoforms by RT-qPCR
| Nox isoform | Mouse primer sequence | Exon location and Tm | Accession no. |
|---|---|---|---|
| Nox1 | CCCAGCAGAAGGTCGTGATT GCTAAAGCCTCGCTTCCTCAT |
E8F, 63.2 E9R, 63.5 |
NM_172203.2 |
| Nox2 (Cybb) | CAGGAACCTCACTTTCCATAAGAT AACGTTGAAGAGATGTGCAATTGT |
E4F 61.5 E5R 63.0 |
NM_007807.5 |
| Nox3 | CGACGAATTCAAGCAGATTGC AAGAGTCTTTGACATTGCTTTGG |
E12F. 61.3 E13R, 61.0 |
NM_198958.2 |
| Nox4 | CCGGACAGTCCTGGCTTATCT TGCTTTTATCCAACAATCTTCTTGTT |
E3F, 64.3 E4R, 61.7 |
NM_015760.5 |
| Duox1 | CACCAGGAACGGATTGTTCT CCTGCAAGCCAAAAGAAGAC |
E12–13F, 61.2 E13R, 60.7 |
NM_001099297.1 |
| Duox2 | TGCAACAGCTACTGGATTCG ATCCTGTCCTCCAGCTCTGA |
E9F, 61.4 E10R, 63.3 |
NM_001362755.1 |
These primers were selected from [2]. Primers are listed in the 5′ to 3′ direction (R = reverse and F = forward). The exon number may be different for different accession numbers. The Tm values were determined using IDT’s OligoAnalyzer tool (http://www.idtdna.com/calc/analyzer) using the following final concentrations in the qPCR reaction: [primers] = 200 nM, [Na+] = 55 mM, [Mg2+] = 1.5 mM, and [dNTP] = 0.2 mM. These primers detect the full-length variant 1 sequence. The listed accession numbers contain details of existing variants
Table 4.
Some suggested human reference primers
| Gene | Primer sequence | Exon location ID | Accession #T Cat. # |
|---|---|---|---|
| HMBS | GCAACTGTACCTGACTGGA TCAGGGCCATCTTCATGC |
E12–13F, 60.1 E14R, 60.7 |
NM_000190.3 Hs. PT.58.242481164 |
| HPRT1 | CATCAAAGCACTGAATAGAAATAGTGA CCAATTACTTTTATGTCCCCTGTT |
E3F, 60.5 E4R, 61.1 |
NM_000194 Hs. PT.53a.20881146 |
| HPRT1 | AGGTATGCAAAATAAATCAAGGTCAT TTCTCCTGAGCAGTCAGC |
E1F E2R |
NM_000194 Hs. PT.53a.2145446 |
| YWHAZ | TCTGATCCCCAATGCTTCAC TGGTATGCTTGTTGTGACTGA |
E8F, 60.9 E9R, 60.9 |
NM_003406 Hs. PT.53a.20437075 |
A discussion of reference gene choice can be found in Refs. [3, 10]. Primer sequences are 5′ to 3′. Catalog numbers are for IDT’s Prime Time Pre-Designed qPCR Assays. The exon number may be different for different accession numbers. The Tm values were determined using IDT’s OligoAnalyzer tool using the following final concentrations in the qPCR reaction: [primers] = 200 nM, [Na+] = 55 mM, [Mg2+] = 1.5 mM, and [dNTP] = 0.2 mM. The actual annealing temperatures were determined by performing a gradient RT-qPCR for each primer set using the methods described in the text. Primers for mouse or rat can be obtained by entering the name of the gene and desired species on the IDT website
Fig. 2.
Correct manual application of the adhesive seal to PCR plates. A plastic paddle is available commercially to assist in the proper application of the adhesive seal to PCR plates. Large standard deviations result from evaporation from individual wells that are not sealed completely
3.4. Running the RT-qPCR Program and Obtaining the Data
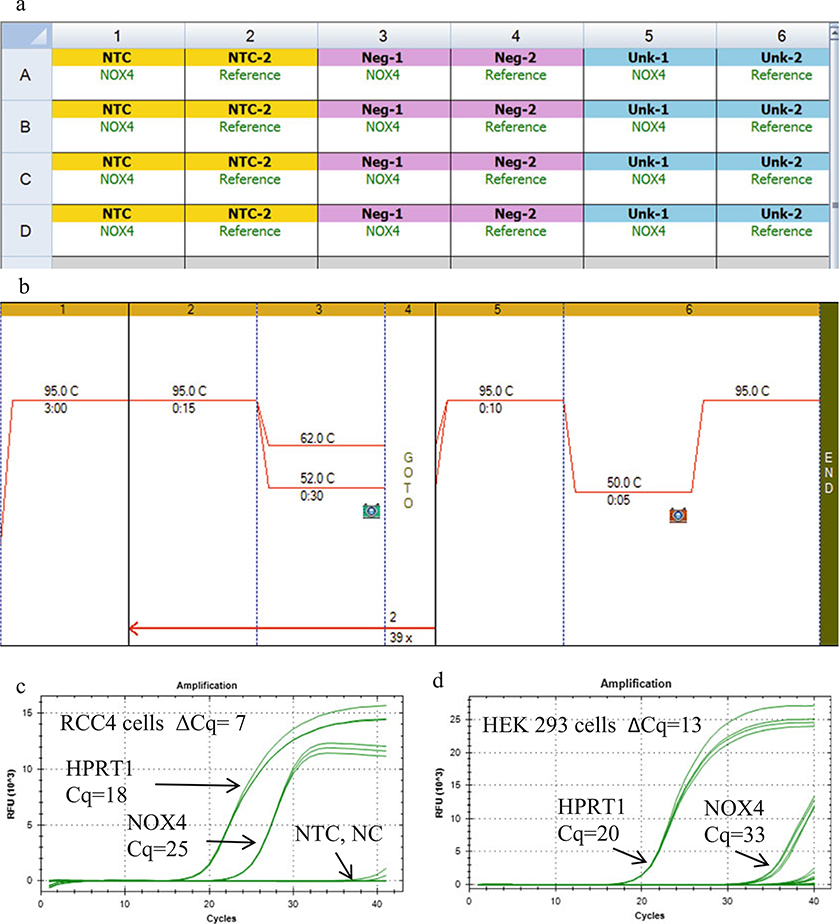
Insert the 96-well plate into a RT-qPCR instrument, and enter the plate layout using the software of the instrument. Figure 3a shows an example of a 96-well PCR plate layout using Bio-Rad’s CFX 96 instrument and CFX Manager software. Choose a two-step PCR program followed by a melt curve. An example of a two-step program that we use is 3 min at 95 °C for 1 cycle (hot start), then 40 cycles of 95 °C for 15 s and 55 °C for 30 s, plus a melt curve (Fig. 3b). The optimal annealing temperature is determined by performing a gradient RT-qPCR as explained next.
To optimize the annealing temperature for each primer set, perform a gradient RT-qPCR by inserting a gradient step in the program such that each row represents a different annealing temperature (Fig. 3b). Then prepare a master mix for 25 identical reactions, and place 8 samples in 1 column of the plate, or if tubes, place the tubes in one column of the instrument. Replicates are recommended and should be placed in additional columns. Run the two-step program with the gradient step inserted. The annealing temperature yielding the lowest Cq value should be chosen. For the human primers in Table 2, the optimal annealing temperatures are within 55–58 °C using the reagents and instrument we used. Examples of the amplification curves obtained using the human NOX4 primer set E4F, E5R in Table 2 and reference gene HPRT1 primer set E3F, E4R in Table 4 for RCC4 and HEK 293 cells are shown in Fig. 3c, d, respectively.
The software of the instrument calculates the threshold cycle (formerly known as “Ct”). The correct terminology according to the MIQE rules is quantification cycle (Cq) [3]. The Cq values are dependent upon the value of the threshold level which is either set by the user or determined by the instrument. Consult the instrument’s manual for directions. Preferably, obtain the Cq values by linear regression of the amplification curves which does not depend on the user’s input of the threshold level. Consult the instrument’s manual on how to choose this setting. Observe the software’s calculated mean and standard deviation of the replicate wells for each sample. If necessary, eliminate one outlier out of the four replicates. If more than one well is an outlier, or the standard deviation is too large (>0.5), it is recommended that additional replicates be used and that the experiment repeated until technical errors (pipetting, mixing, sealing) are minimized as determined by the standard deviation. If a Cq value <35 is obtained in the NTC or NC wells, this indicates either a primer-dimer problem, or DNA contamination. In this case, the melting temperature of the amplicons of the NTC and NC wells should be examined to see if the values are due to NOX contamination or primer-dimers. For NOX DNA contamination, the melting temperature will be identical to that obtained from a positive control (plasmid containing the NOX isoform). The PCR products should be run on an agarose gel to check that the anticipated amplicon size is correct. Primer-dimers run less than 100 bp.
Sequencing of the PCR products from the UNK wells adds another layer of confidence that the products are correct.
Obtain the ΔCq values for each well by subtracting the Cq value of the reference sample from the Cq value of the NOX sample. The mean ΔCq value can be used to compare relative NOX mRNA levels between different samples if the reference gene Cq value does not differ significantly between samples. These values can also be used as an indication of whether or not protein detection will be feasible (see Note 13).
If two conditions are being compared, e.g., treated vs. untreated, then the software will also calculate the relative fold change, R, using the ΔΔCq (Livak) method. If the efficiency of the NOX primers is determined to be quite different (10% or more) from the reference gene, then the Pfaffl method should be used as well and the results compared using the two methods. The primer efficiency values can be entered into the dialogue box (see Notes 14 and 15).
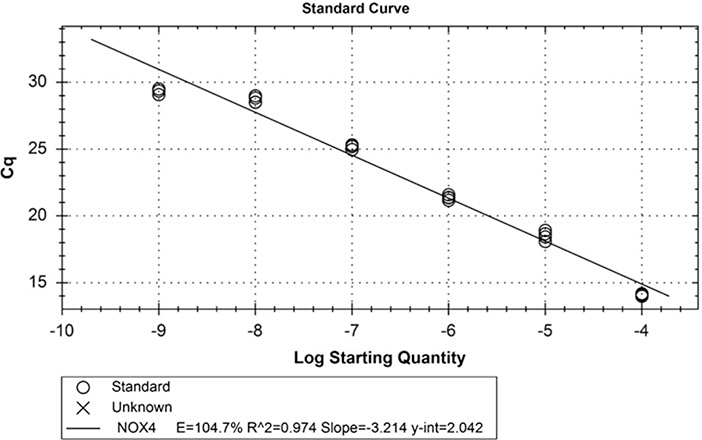
To determine the efficiency of the NOX and reference gene primer sets, generate a RT-qPCR standard curve using linearized plasmids that contain the NOX cDNA or the reference gene. Be careful not to use the PCR-designated bench and tools to make a series of ten 10-fold dilutions of the linearized plasmid in nuclease-free water starting with a known amount of the plasmid, about 1 μg/μL. Leave the dilutions at the non-PCR bench. Change gloves and go to the PCR-designated bench. Set up ten master mix PCR tubes for one primer set without cDNA. Add 10 μL of one of the plasmid dilutions per tube. Vortex gently and centrifuge the tubes briefly. Pipet four replicate wells per column of the PCR plate (40 wells). Seal the plate well. It is not necessary to mix the wells since centrifugation of the sealed plate (400 × g, 2000 rpm, 5 min, 4 °C) will induce mixing. Freeze the diluted stocks at −20 °C so that they can be used again. Run the qPCR program under the same conditions, but label each well as STD-1, STD-2, STD-3, etc., and enter the dilution-fold values associated with each set of wells. The software will generate a standard curve of Cq values vs. dilution-fold as shown in Fig. 4. The efficiency is calculated from the slope, which ideally would be −3.32 (the binary exponent required for a tenfold change in Cq). The standard curve can also be used to estimate copy number by converting the mass of the plasmid (plus insert) to moles and then to the number of molecules, which is the number of copies since each plasmid molecule contains one copy of the gene (see Note 16).
Fig. 3.
Example of a basic qPCR setup using a Bio-Rad CFX 96 instrument and CFX manager software. (a) Plate layout of the no template control (NTC), negative control (NC) and unknown (UNK) wells, 4 replicate wells each for the NOX primers, and for the reference primers. (b) Example of a two-step PCR program with an annealing temperature gradient inserted. (c) Representative amplification curves (3–4 replicate wells per set of curves) for the reference gene (HPRT1, left set of curves) and NOX4 (right set of curves) for untreated RCC4 clear cell renal cell carcinoma cells. (The cells were a kind gift from Dr. William Kaelin, Harvard University). (d) Amplification curves for untreated HEK 293 cells. Note that for the negative controls (NC) and no template controls (NTC) wells, the curves should resemble the baseline (flat lines) up to cycles 35–40. Primers (200 nM) for NOX4 (E4F and E5R) from Table 2 and HPRT1 (E3F and E4R) from Table 4 were used. Each UNK well contained 200 ng of cDNA (RNA equivalents) or 200 ng RNA in place of cDNA in the NC wells. The optimal annealing temperature was determined from a gradient RT-qPCR experiment
Fig. 4.
Example of a RT-qPCR standard curve used for the determination of primer set efficiency. Ten tenfold serial dilutions were made from pcDNA3.1-NOX4 variant 1 at a starting concentration of 1 μg/μL. Primers (200 nM) for NOX4 (E4F and E5R) from Table 2 were used. The Cq values were plotted vs. log (dilution-fold), and the efficiency of the primer set was determined from the slope
3.5. Immunoblotting: Preparing Lysates of Positive and Negative Control Cells
The overexpression of NOX isoforms in a cell line having little or no NOX expression is used as a positive control, and the overexpression of the corresponding empty vector in the same cell line is used as the negative control. NOX1–4 require p22phox for maturation and expression, so use a cell line that expresses p22phox for these isoforms. We recommend HEK 293 cells because they contain sufficient endogenous p22phox protein to support stable NOX isoform expression [4], and they have a transfection efficiency close to 100%. If using a different cell line, the presence of p22phox protein should be confirmed. If the cell line does not express p22phox protein (e.g., COS7 cells), then the CYBA gene that codes for p22phox should be co-transfected along with NOX plasmids. DUOX1 and DUOX2 require the co-transfection of their maturation proteins, DUOXA1 and DUOXA2, respectively. Harvest HEK 293 cells from freshly seeded master plates prepared 1–2 days before the day of seeding. Seed the HEK 293 cells at 2 × 106 cells (high confluency for X-tremeGENE 9, ~80%) in 10 mL of DMEM containing 10% FBS per 10 cm dish without addition of antibiotics the day before transfection.
On the day of transfection, add a total of 6 μg of positive control plasmid (e.g., pcDNA3.1-NOX) to 600 μL of OptiMEM in a 1.5 mL micro-centrifuge tube and vortex gently. The DNA concentration should be kept at 0.01 μg/μL. Prepare a second tube in the same way for the negative control plasmid (e.g., pcDNA3.1 plasmid lacking the NOX cDNA as a negative control). If desired, a GFP plasmid (0.5 μg/10 cm plate) can be co-transfected to estimate transfection efficiency (see Note 17).
Transfect the cells following the manufacturer’s directions. A number of transfection reagents are suitable such as Lipofectamine, but we use the X-tremeGENE 9 reagent because it does not require any media changes after transfection. If using X-tremeGENE 9, it should be at room temperature and gently vortexed for a few seconds just prior to use. For a 10-cm dish of cells containing 10 mL of media (no antibiotics), add 18 μL of X-tremeGENE 9 to 6 μg of DNA in 600 μL OptiMEM, keeping the ratio of X-tremeGENE 9:DNA at a ratio of 3 μL:1 μg DNA. Be sure not to touch the walls of the tube while depositing the transfection reagent directly into the liquid. Vortex the mixture gently, and spin briefly in a micro-centrifuge to collect all the liquid to the bottom of the tube. Incubate at 25 °C for 15 min to allow lipid micelles to incorporate the DNA.
Add the mixture dropwise to the 10 cm plate while swirling the plate. Swirl or rock the plate a few more times prior to placing the plate(s) back in the incubator. Incubate for 24–48 h for protein expression. No media change is required if using XtremeGENE 9 (see Note 18).
Remove the medium from the plates containing the transfected HEK 293 cells. Wash the cells twice with PBS. Remove as much of the wash as possible. Place the plates on ice. To the first 10 cm plate, add a minimal volume (300–400 μL) of ice-cold RIPA buffer containing protease and phosphatase inhibitors. Scrape the plate, collect the scraped cells, and keep on ice. If multiple plates are used per condition, then add the collected cells from the first plate to the next plate, and scrape using the same 300–400 μL of RIPA buffer (see Note 19).
Sonicate or homogenize both the control and test sample lysates gently by placing the micro-centrifuge tube in a beaker of ice, and administer two 1 s bursts from a sonicator equipped with a pencil-tip probe. Avoid foam by using a low-output setting of 30%. If a sonicator is not available, homogenize 5 times by using a 1-mL syringe and a 21-gauge needle, avoiding foam formation.
Clarify the lysate by centrifuging the samples at 13,000 rpm (15,000 × g), 10 min, 4 °C.
Collect the clarified lysate, and determine the protein concentration using the BCA protein determination kit or similar methods.
Storage of samples: Do not use all of the lysate for SDS-PAGE gel sample preparation because the NOX proteins degrade within 1 week if stored in Laemmli’s sample buffer. Prepare only as much gel sample as needed on the day of use. Aliquot and store the lysates at −20 °C for up to 2 weeks or for longer storage at −70 °C. If the protein precipitates after thawing, re-sonicate or homogenize, re-clarify, and determine the protein concentration again for positive and negative controls. For test samples, fresh lysates should be prepared each time and stored for no more than 1–2 weeks or as determined by each user.
To prepare concentrated SDS-PAGE gel samples, use a concentrated recipe for Laemmli’s sample buffer, for example, 4× sample buffer. Take three parts of sample (e.g., 75 μL of the cell lysate), and add one part (25 μL) of 4× Laemmli’s sample buffer. The protein concentration of the SDS-PAGE sample is now 75% of the original lysate. For a 4 mg/mL sample, it is now 3 mg/mL. For a 3 mg/mL sample, load 33 μL which is 100 μg of the test sample on the SDS-PAGE gel. For the positive and negative control lysates, load 0.1, 1, and 10 μg initially, and then determine the best amount to use in your conditions (exposure time, ECL reagent strength, etc.).
For NOX, do not boil the samples (see Note 20).
3.6. Preparing Lysates of Cells with Endogenous NOX
For the cells to be tested (e.g., RCC4 renal clear cell carcinoma cells), grow enough cells to obtain 2–3 × 107 cells/mL of lysis buffer for each test condition.
If desired, treat the cells with a stimulus that can induce or upregulate NOX isoform expression (e.g., TGFβ1, 5 ng/mL final in complete media for 24 and 48 h). Leave some plates untreated or treated with the vehicle.
Harvest the cells, and prepare the lysates from the test plates as done in steps 5–11 in Subheading 3.5 above.
3.7. SDS-PAGE and Transfer
Use an appropriate percentage SDS-PAGE gel. A 1.5-mm-thick gel and comb will permit large sample volumes to be loaded (e.g., 100 μg protein in 30–50 μL) (see Note 21).
Load the pre-stained or fluorescently labeled protein ladder and 100 μg of test samples (30–50 μL). Also load the positive and negative control cell lysates (between 0.1 and 10 μg, determined by user). Run the 1.5-mm-thick mini-gels (about 8 × 10 cm plus the stacking gel) at 25 °C using constant amperage (30 mA/gel) for 15 min to allow stacking, and then increase the amperage to 40 mA/gel for about 1–1.5 h until the dye front reaches the very bottom edge of the gel to obtain optimal separation of the bands. After the run, rinse the gels briefly by dipping them in transfer buffer, and place them on the PVDF membrane. The PVDF membrane should be pre-wetted in 100% methanol and equilibrated in transfer buffer. Remove any bubbles by smoothing with a gloved finger or roller tool.
Transfer the proteins to the PVDF membrane. For a standard wet transfer, set the voltage at 90 V for 1 h and transfer at room temperature or in a cold room, use chilled transfer buffer containing 20% methanol, add a small magnetic stir bar to the tank, and place the tank on a magnetic stir plate.
Block the PVDF membrane after the transfer for 1 h at RT on a rocking platform. Use 5% nonfat dry milk in TBST, or use LI-COR Odyssey Blocking Buffer without Tween-20. Incubations of up to 3 days (over the weekend) at 4 °C on a rocker should not result in high background if PVDF membranes are used. If using 5% nonfat dry milk in TBST as a blocking buffer, wash the blot with TBST 4 times (5 min each) before adding a primary antibody that is diluted in LI-COR Odyssey Blocking Buffer plus 0.1% Tween-20 (see Note 22).
For most antibodies, dilute the antibodies in 3–5% nonfat dry milk in TBST or use LI-COR Odyssey’s Blocking Buffer (TBS) plus 0.1% Tween 20 which works for both fluorescent and non-fluorescent antibodies. A dilution of 1000-fold to 2000-fold (0.5–1 μg/mL final) is a good starting point for immunoblot optimization (see Note 22).
Incubate the blot with the primary antibody overnight or longer at 4 °C on a rocking platform. Detection of endogenous NOX proteins may require 72 h for detection (see Note 23).
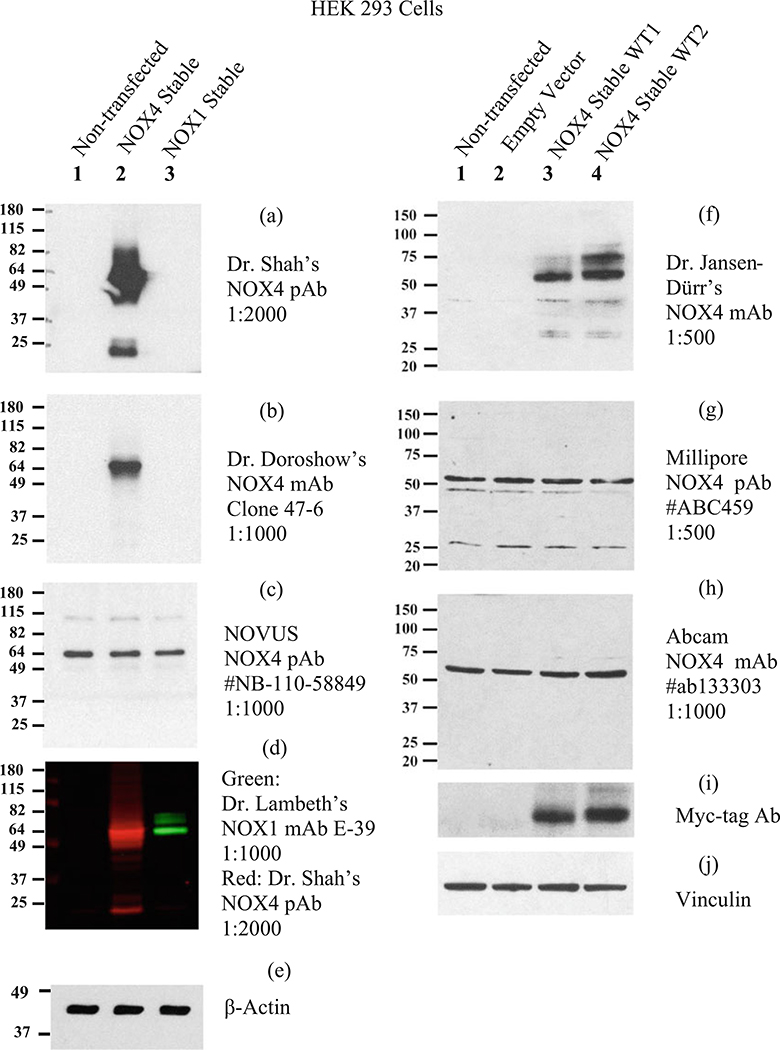
Choice of primary antibody: Some recommended antibodies are listed in Tables 5, 6, 7, 8, 9, 10, and 11. Some commercial antibodies that did not pass validation under the conditions we used (HEK 293 cells overexpressing NOX isoforms) are also listed. Figures 5, 6, 7, and 8 show representative immunoblots using some of the selected antibodies from Tables 5, 6, 7, 8, 9, 10, and 11. Additional information about specific antibodies is provided in the footnotes of Tables 5, 6, 7, 8, 9, 10, and 11.
Recover and save the primary antibody and wash the blot 4 times, 5 min each with TBST.
Add the secondary antibody to the blot. Dilute HRP-conjugated goat anti-rabbit or mouse antibodies according to the manufacturer’s instructions (see Note 22). Incubate the blot with the secondary antibody solution for 1 h at room temperature. If using LI-COR Odyssey’s fluorescent secondary antibodies, dilute them 20,000-fold in LI-COR blocking buffer plus 0.1% Tween 20 and 0.01% SDS as recommended by LI-COR. Protect the solution from light during incubation. Incubate the blot with the secondary antibody solution for 1 h at room temperature.
Discard the used solution of HRP-conjugated secondary antibody. Save fluorescent secondary antibody solutions in a foil-wrapped tube at 4 °C, and reuse until the signal fades (~3 repeats).
Wash the blots 4 times with TBST, 5 min per wash, and then add the ECL reagent or proceed to LI-COR Odyssey scanning.
Prepare the ECL reagent as directed by the manufacturer. For SuperSignal West Pico Enhanced Chemiluminescent Substrate, we do not dilute the reagent. For an 8 × 10 cm blot, add 2 mL of the SuperSignal West Pico Enhanced Chemiluminescent Substrate, and leave it on for 5 min on a platform rocker (or rock occasionally manually).
Remove the blot from the ECL detecting solution, and blot lightly between two pieces of filter paper so that the ECL solution is not dripping. Tape the blot in a sheet protector that has been cut (with the folded edge intact) to the shape of a (used) autoradiography film. Open the sheet protector and tape the blots between the plastic sheets. Close the sheet protector over the blots, and insert it into the autoradiography cassette containing intensifying screens on both sides.
Expose the membrane for 1, 10, and 30 s and then 1, 3, 5, and 10 min. If no bands appear by 30 min, then remove the blots and place them in an ECL reagent with higher sensitivity such as SuperSignal West Dura solution. No rinsing of the blot is required before going to the next level of SuperSignal West ECL reagent. SuperSignal West Femto usually results in high background during long exposures.
For LI-COR Odyssey scanning, the blot can be wet or lightly dried between two filter papers. Place the wet blot facedown on the glass bed of the scanner. For dried blots, place a glass gel plate or similar weight on top of the membrane to keep it flat. Be sure to choose the membrane mode (169 μm thickness). Preview under an initial intensity of 3.0 for each IRDye wavelength. If the preview looks fine, then proceed to scan the membrane, and adjust the signal to noise ratio as desired using the settings of the instrument. Consult the instrument’s manual for further instructions. If necessary, scan again at a higher intensity for increased sensitivity. The membranes can be re-scanned up to 6 months if stored in the dark.
Fig. 5.
Examples of using positive and negative control lysates for the validation of NOX4 antibodies. Two laboratories tested several NOX4 antibodies from Table 8. Left column: NOX4 antibodies tested in the laboratory of Dr. J. D. Lambeth. Right column: NOX4 antibodies tested in the laboratory of Dr. J. Doroshow. Left column: Identical PVDF immunoblots consisting of 10 μg of the following non-heated cell line lysates. Lane (1) non-transfected HEK 293 cells, lane (2) HEK 293 cells stably transfected with pcDNA3.1-NOX4, untagged (a kind gift from Dr. R. Brandes (Goethe-University Frankfurt), lane (3) HEK 293 cells stably transfected with pcDNA3.1-NOX1, untagged (also a gift from Dr. Brandes). The blots were probed with the following NOX4 antibodies from Table 8 (a) Dr. A. Shah’s NOX4 rabbit pAb, 1:2000 (a kind gift from Dr. A. Shah, University College London); (b) Dr. J. Doroshow’s NOX4 rabbit mAb, clone 47–6, 1 μg/mL (1:1000); (c) NOVUS NOX4 rabbit pAb #NB110–58849 Lot F1 (1:1000, 1 μg/mL); (d) green, Dr. J. D. Lambeth’s NOX1 E39 mAb, 1:1000, as a positive control for NOX1; red, Dr. Shah NOX4 rabbit pAb, 1:2000. (e) β-actin mouse mAb (Sigma-Aldrich), 1:10,000. Blocking buffer, 5% nonfat dry milk in TBST; antibody diluent, LI-COR Odyssey blocking buffer +0.1% Tween-20; primary antibodies incubated overnight; secondary antibodies (HRP-conjugated goat anti-rabbit or anti-mouse), 1 h. ECL reagent: SuperSignal West Pico, 1 s. Right column: identical nitrocellulose membranes consisting of 40 μg of the following cell lysates. Lane (1) non-transfected HEK 293 cells, lane (2) HEK 293 transfected with pCMV6-empty vector, lane (3) HEK 293 stable clone (WT1) overexpressing N-terminal tagged-Myc-DDK-NOX4, lane (4) HEK 293 stable clone (WT2) overexpressing N-terminal tagged-Myc-DDK-NOX4. The following antibodies from Table 8 were tested: (f) Dr. P. Jansen-Dürr’s NOX4 mAb 1:500; (g) Millipore NOX4 pAb (#ABC549), 1:500; (h) Abcam NOX4 antibody (ab133303), 1:1000; (i) anti-Myc antibody; (j) anti-vinculin antibody. Conditions: similar to left panel, except the antibody was diluted in 5% milk in TBST and the ECL reagent was ECL Plus reagent (GE Healthcare)
Fig. 6.
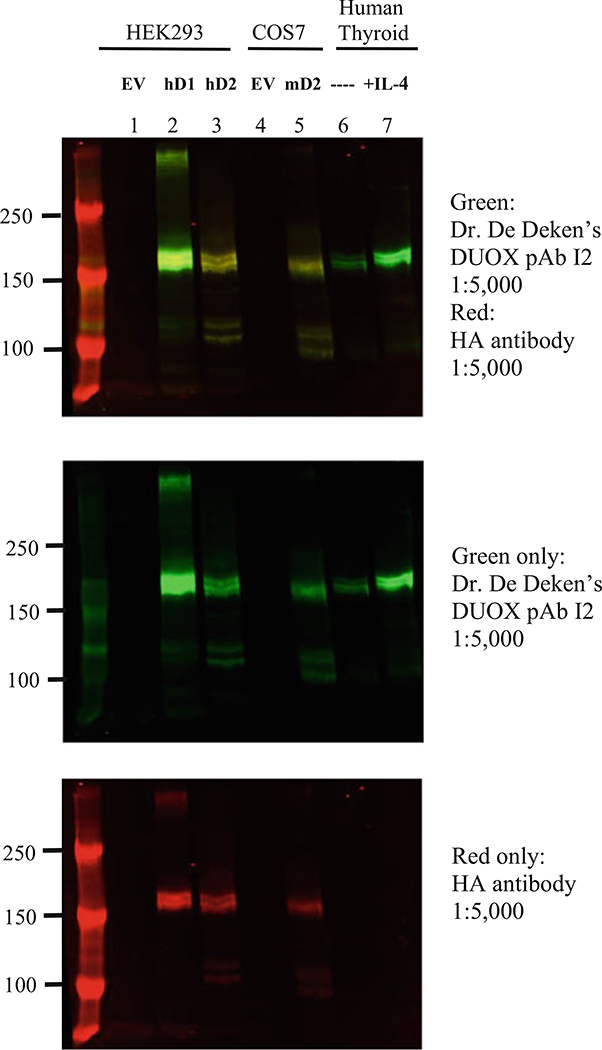
Validation of DUOX antibodies. HEK293 cells were transfected with pcDNA3 HA- DUOX1(hD1) or DUOX2(hD2) and pcDNA3 V5-DUOXA1,2 constructs or empty vectors (EV) for the control condition. COS7 cells were transfected with pcDNA3 HA-mouse Duox2 (mD2) and pcDNA3 V5-mDuoxa2. Primary human thyroid cells were incubated 48 h with or without 14 ng/mL human IL-4. Lysis buffer: 50 mM phosphate buffer, pH 7.5, containing 20 mM EDTA, 1% Triton X-100 plus protease inhibitors (Complete; Roche Applied Science) and phosphatase inhibitors (1 mM vanadate, 100 mM NaF, and 50 nM okadaic acid). DUOX proteins (20–30 μg) in Laemmli buffer were heated at 80 °C for 5 min and were separated on a 6% gel and transferred to a low-fluorescence PVDF membrane (Immobilon-FL, Millipore, Billerica, USA). Blocking: LI-COR Odyssey blocking buffer, 2 h RT. Primary antibody: Dr. De Deken’s anti-DUOX pAb I2 1:5000 or anti-HA-mAb 1:5000 (Covance) overnight at 4 °C. Secondary antibodies: anti-rabbit IRDye800 (green) and anti-mouse IRDye680 (red) from LI-COR, 1 h RT. This DUOX antibody recognizes both human DUOX1 and DUOX2 as well as mouse Duox2 in overexpressing cell lines. DUOX proteins were also detected in human thyrocyte extracts, with higher expression after IL-4 treatment
Fig. 7.
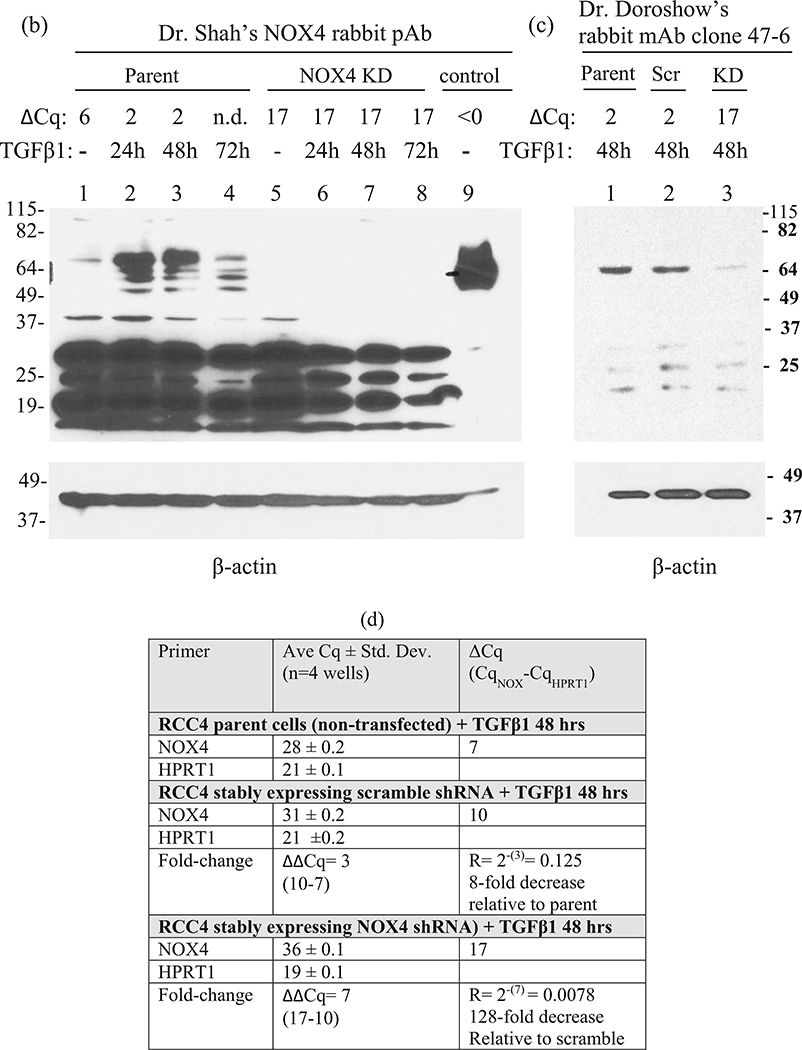
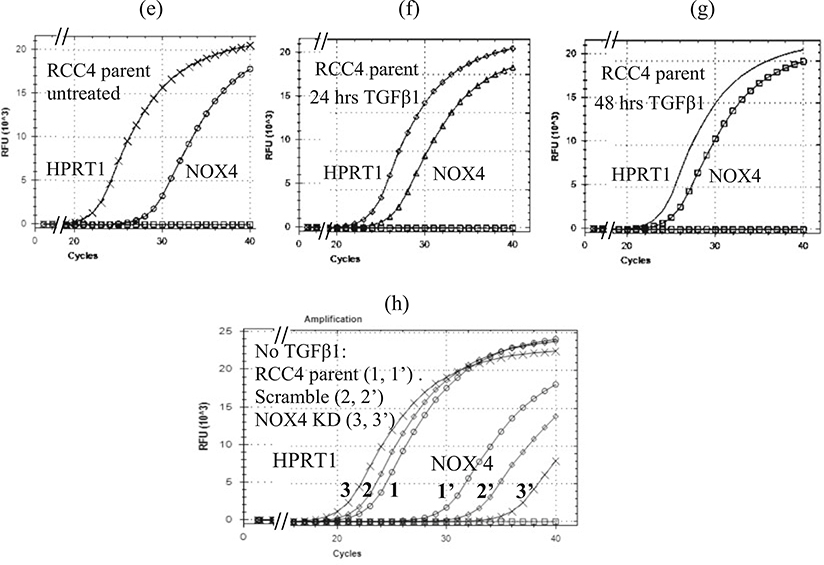
Correlation between ΔCq values and endogenous NOX4 protein detection in RCC4 cells. (a) Cq, ΔCq, and R (fold-change) values for untreated RCC4 parent cells and for TGFβ1-treated cells (5 ng/mL in complete medium). (b) NOX4 immunoblot probed with Dr. Shah’s NOX4 antibody (1:2000 for 72 h at 4 °C), then developed with SuperSignal West Pico Chemiluminescent reagent, and exposed for 30 min. Lanes (1–4) lysates of parent RCC4 cells (100 μg) untreated or treated with TGFβ1 for 0–72 h as indicated. Lanes (5–8) lysates of a representative RCC4 stable NOX4 knockdown (KD) clone untreated or treated with TGFβ1 for 0–72 h as indicated. Lane (9) positive NOX4 control (1.0 μg) of HEK293 cells stably expressing NOX4. The ΔCq values corresponding to each condition are shown above each lane, except for lane 4 (72 h) for which the Cq was not determined (n.d.). (c) Immunoblot using Dr. Doroshow’s NOX4 rabbit mAb clone 47–6 (1:1, 000 for 72 h at 4 °C). Lane (1) 48 h, TGFβ1-treated parent RCC4 cells; lane (2) 48 h treated, representative RCC4 stable scramble shRNA clone; lane (3) 48 h treated, representative RCC4 stable NOX4 knockdown (KD) clone. The ΔCq values corresponding to each condition are shown above each lane. NOX4 protein could not be detected in untreated RCC4 cells in these conditions using Dr. Doroshow’s NOX4 antibody. (e–g) Amplification curves for untreated and 24–72 h TGFβ1-treated parent cells corresponding to the Cq values in panel a; (h) amplification curves for untreated parent and representative clones of scramble shRNA and NOX4 shRNA (KD) corresponding to the Cq values in panel d.The scramble shRNA plasmid had an effect of decreasing NOX4 mRNA expression by eightfold, but the effect on protein expression could not be evaluated without TGFβ1 treatment. When the scramble clone was treated with TGFβ1 for 48 h, the NOX4 protein expression was similar to that of the parent cells treated for 48 h (lane 1 vs. 2, panel c). The NOX4 primer sets were NOX4 E4F/E5R and HPRT1 E3F/E4R as indicated in Table 2. The NOX4 shRNA (KD, retroviral) plasmid (OriGene Technologies, #TG302911) contained the target sequence 5′CACTGAACTATGAGGTCAGCCTCTGCGTG. The scramble (Scr) shRNA (retroviral) plasmid (OriGene Technologies, #TR30013) contained the non-targeting sequence: 5′ GCACTACCAGAGCTAACTCAGATAGTACT 3′
Fig. 8.
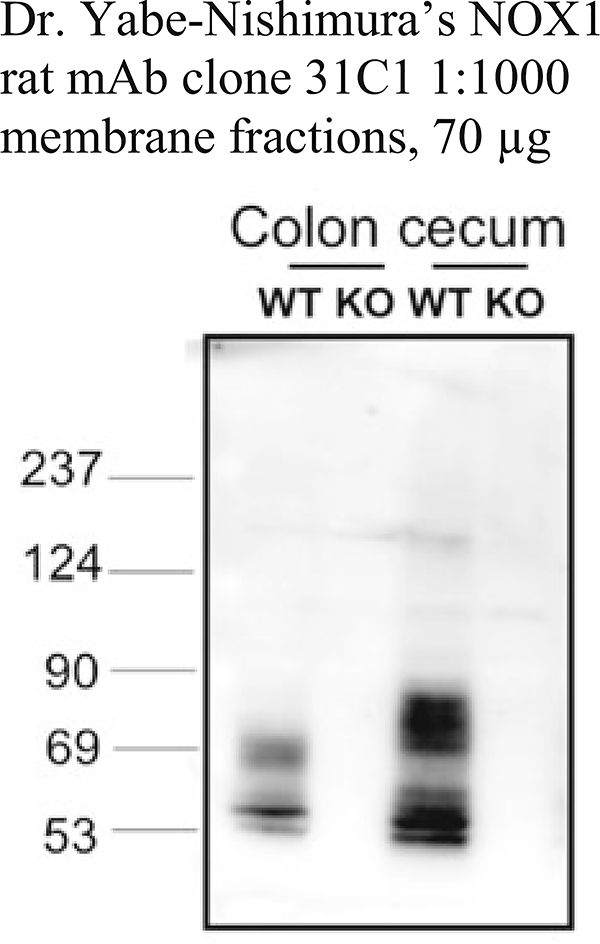
Validation of a Nox1 rat monoclonal antibody. A rat monoclonal antibody against mouse Nox1 (clone 31C1) detects Nox1 in wild-type (WT) mouse colon (lane 1) and cecum (lane 3) membrane fractions (70 μg) solubilized in 2% n-dodecyl β-D-maltoside in 10 mM Hepes pH 7.4, 10 mM NaCl, 100 mM KCl, 1 mM EDTA, and 1 mM PMSF. This monoclonal antibody is more specific and sensitive than a previous polyclonal antibody to the same epitope (see Table 5) [7]. The Nox1-specific bands are observed diffusely at 56–80 kDa due to differently glycosylated forms of Nox1 (lane 1). The specificity for Nox1 was confirmed using solubilized membrane fractions from the colon from Nox1-deficient mice (KO) as a negative control (lane 2, 4). This rat monoclonal antibody also detects Nox1 in solubilized membrane fractions of mouse small intestine and mouse lung (unpublished, personal communication from Dr. M. Matsumoto). However, Nox1 protein was not detected in other mouse tissues, possibly due to its low abundance
Fig. 9.
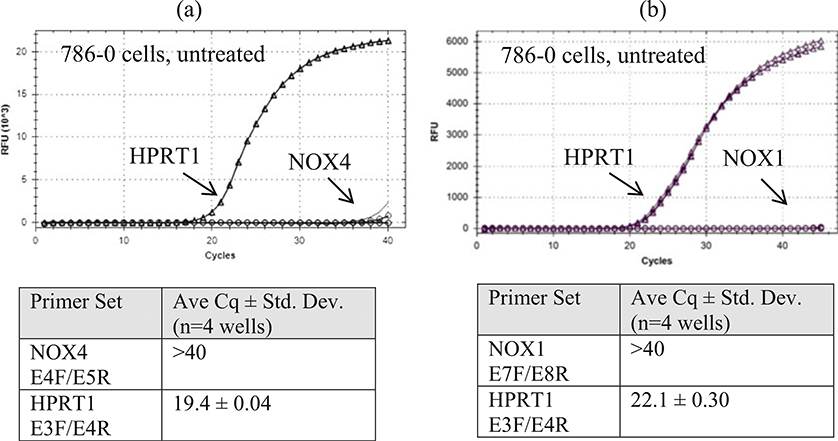
786–0 cells do not express NOX4 and NOX1. 786–0 cells have been used as a model for studying NOX4 and NOX1, but this cell line does not express NOX4 mRNA (a) or NOX1 mRNA (b) using the methods in this chapter. The y-axis scales differ in (a) and (b) due to the use of two different commercial SYBR Green reagents from Quanta in (a) and Bio-RAD in (b)
Acknowledgments
We thank for their generous support of this work: Dr. Ajay Shah and Dr. Pidder Jansen-Dürr for the NOX4 polyclonal rabbit antibodies; Dr. William Kaelin for the RCC4 parent cell line; Dr. Ralf Brandes for HEK 293 cells stably expressing NOX4 or NOX1; and Dr. Vincent Jaquet, Dr. Mark Quinn, Dr. Corinne Dupuy, Dr. Chihiro Yabe-Nishimura, Dr. Misaki Matsumoto, Dr. Ulla Knaus, and Dr. William Nauseef for contributing specific information for some of the NOX/DUOX antibodies and reviewing the manuscript. We also thank the editors, Dr. Ulla Knaus and Dr. Tom Leto, for the invitation to contribute a chapter to this book.
This chapter is dedicated to the late Dr. Gary Bokoch (The Scripps Research Institute, La Jolla, CA, USA), who made many contributions to the field of NADPH oxidases and served as a post-doctoral mentor of both Dr. Becky Diebold and Dr. Ulla Knaus.
This work was made possible by funding from the National Institute of Health, USA, and the American Heart Association. XDD is supported by the “Fonds de la Recherche Scientifique” (FRS-FNRS), the “Fonds Docteur J.P. Naets” managed by the “Fondation Roi Baudouin,” and the “Fondation Tournay-Solvay.”
Footnotes
To minimize contamination from plasmids containing NOX cDNA sequences, the surface of the workspace and all tools should be washed with diluted detergent. We use household dish detergent. Do not spray the surfaces with ethanol, which can precipitate DNA. Rinse off any soap with a wet paper towel 2–3 times, dry, and spray with Zap spray. Wipe off the excess Zap spray solution without rinsing. Do this for all the items on the workspace such as pipettes, pipette aids, pipette racks, pipette tip boxes, micro-centrifuge, vortex, tube racks, pens, and calculator. We use pipettes and dual filter tips (Eppendorf) designated for RNA and PCR setup. No amplified PCR products (amplicons) should be brought to this workspace. After washing the surface of the pipettes, treat them with UV light for 1 h on a UV light box or in a biosafety cabinet with the UV light turned on. Other equipment (e.g., tube racks) can be treated overnight with UV light in the biosafety cabinet. It is especially important to clean the top of the pipette where the thumb is placed and the dial of the pipet. These areas should be exposed to the UV light. If it is easy to separate the pipette’s lower barrel, wash and treat with UV light along with the other parts of the pipette. It is not necessary to do this washing protocol before every PCR experiment (although we do), but if contamination (Cq values ≤35) appears in the negative controls or no template controls, then washing and UV treatment is strongly recommended. If contamination is still evident, this may be due to traces of genomic cDNA (overloading of the gDNA eliminator column) or other non-NOX products such as primer-dimers. Thus, always include negative controls and a melting curve to check if the contamination is due to gDNA, primers, or an unknown source. If it is due to plasmid DNA, the melting temperature of the contaminating amplicon will be identical or close to that of the desired NOX amplicon.
Washing the cells twice removes phenol red which distorts the UV spectrum of RNA, thus interfering with the measurement of RNA concentration.
Removal of gDNA has been simplified by Qiagen’s gDNA eliminator columns, which are only available in Qiagen’s RNeasy Plus kits. This allows results to be more consistent since DNase I digestion is not performed by the user. It may be necessary to repeat this step twice (as we do) when processing large quantities of cells (5–10 million per column).
Some cell lines have a low yield of RNA, so we elute the first column with 50 μL and use the eluant to elute a second column. We aim for an RNA concentration of 0.5–1.0 μg/μ L. We check the quality of our RNA preparations by sending a small sample to a core facility to be analyzed by a bioanalyzer. The analyzer measures the sizes of the rRNA bands and determines an RNA Integrity Number (RIN) that can be used to normalize RNA samples. For cell lines, the RIN number is usually 10, indicating a very good quality.
The RNA preparation should be kept at −70 to −80 °C. It will deteriorate if stored at −20 °C.
We prefer qScript XLT cDNA SuperMix (Quanta Biosciences) because it features a unique blend of oligo (dT) and random primers that captures unbiased representation of all RNA sequences into cDNA product (including the 5′ end, 3′-end, or central regions of long RNAs). This reagent is highly sensitive and easy to use for two-step RT-PCR and RT-qPCR (up to 1 kb). The engineered M-MLV reverse transcriptase has reduced RNase H activity and improved activity and stability at higher temperatures and over a wide dynamic range of input RNA, with up to eightfold higher sensitivity than cDNA synthesis kits utilizing RNaseH(+) reverse transcriptase (RT). The 5× concentrated master mix provides all necessary components for first-strand synthesis including buffer, dNTPs, MgCl2, primers, RNase inhibitor protein, qScript XLT reverse transcriptase, and stabilizers.
The best results (low standard deviation, clean negative controls, and lowest Cq values) are obtained if the cDNA is used on the day of synthesis. If the cDNA is stored overnight, store at −20 °C.
It is better to start with the NTC (no template control) tubes first, followed by the NC (negative control) tubes to avoid contaminating these tubes with cDNA from the UNK (unknown). The thumb used to open cDNA tubes is a large source of contamination. Change gloves after setting up the UNK tubes.
We use Quanta Biosciences PerfeCtA SYBR Green SuperMix because it provides a high level of specificity to reduce the occurrence of primer-dimers and other non-specific artifacts associated with the binding of SYBR Green I dye to any dsDNA generated. The AccuStart Taq DNA polymerase contains monoclonal antibodies that bind to the polymerase and keep it inactive prior to the initial PCR denaturation step. Upon heat activation (3 min at 95 °C), the antibodies denature irreversibly, releasing fully active, unmodified TaqDNA polymerase. This enables specific and efficient primer extension with the convenience of room temperature reaction assembly. We also found that Bio-Rad’s Universal iTaq SYBR Green SuperMix performs equally well. To increase specificity, use a fluorescently labeled probe (instead of SYBR Green technology) that anneals to the target sequence and emits fluorescence due to FRET when displaced by the primer set. Consult your primer manufacturer’s website for descriptions on the types of probe technologies available.
Upon receipt of freeze-dried primers, original primer stocks (10 μM or 100 μM) are prepared in TE buffer (10 mM Tris-Cl, pH 8, 0.1 mM EDTA). To avoid contamination of the original primer stocks, aliquots are prepared by one designated person and given to other users. The final primer concentration in the PCR reaction is 200 nM, so a 10× solution is prepared in nuclease-free water for setting up the PCR reactions. Higher final concentrations may be used such as 500 nM, but one should check that the Cq values of the NTC wells are not lower than 35, which is an indication of primer-dimer formation. We purchase pre-designed primers from IDT (Integrated DNA Technology) and verify their exon docking site, variant specificity as well as isoform specificity, and anticipated amplicon sizes using appropriate accession numbers available in the National Center for Biotechnology Information (NCBI) (see Table 1). For example, for human NOX4 exon 14 is present in variant 1, the full-length form, but is absent in variant 2.
There are several reference genes that are often used in the literature. However, we do not recommend using β-actin or GAPDH because there are about 60 pseudogenes for actin and about 197 for GAPDH [5]. However, we emphasize that with any reference gene, one should confirm that the expression level (Cq value) does not vary with the experimental conditions, although it may vary by 1–2 cycles due to the accuracy of the amount of cDNA pipetted into the well. We used HPRT1 (hypoxanthine-guanine phosphoribosyltransferase (HGPRT), which is an enzyme encoded by the HPRT1 gene. In mouse, it only has one pseudogene and in humans three, but there are several regions in the exon sequences that are not homologous in the pseudogenes.
Applying the adhesive seal is tricky and if not done well can lead to large standard deviation values. We noticed variation in the standard deviation values of the mean Cq values of identical plates when different students applied the adhesive seal. We discovered that each student had a different technique to do this. An automated plate sealer is better for consistency if available. However, a paddle that is supplied with the adhesive seals (Bio-Rad) (Fig. 2) is adequate if used correctly. First drag the paddle on its edge across the entire surface of the seal. Then, drag it between each column and row and all along the perimeter of the outer wells to ensure that each well is sealed to avoid evaporation and cross-contamination between wells. This greatly decreases the standard deviation values. Be sure to centrifuge the plate as directed before proceeding.
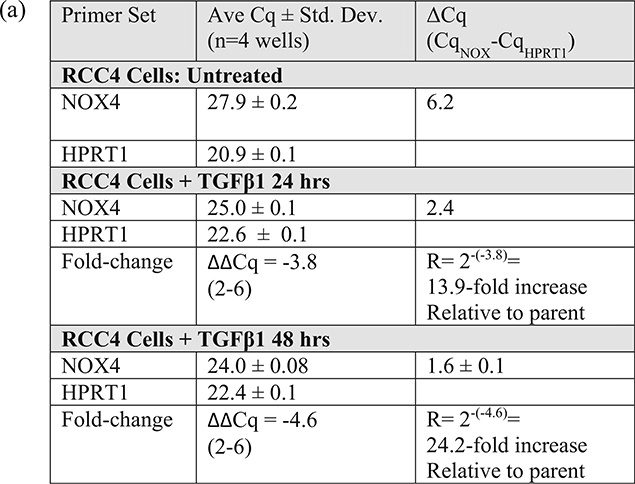
Figure 7 shows that the detection of endogenous NOX4 protein in untreated RCC4 cells (ΔCq ~ 6), using the methods in this chapter, requires extreme conditions such as 72 h of incubation with the primary antibody (Dr. Shah’s NOX4 antibody, 1:2000 and SuperSignal West Chemiluminescent Substrate, 30 min) (Fig. 7a, b). It is possible that lower dilutions might shorten the incubation time. This antibody detects several lower MW bands (likely variants or degradation products) as major bands. Treatment with TGFβ1 (5 ng/mL in complete medium) for 24–72 h induced NOX4 expression (ΔCq ~ 2), and this treatment can be helpful when judging NOX4 protein knockdown. Dr. Doroshow’s NOX4 rabbit mAb (1:1000, SuperSignal West Chemiluminescent Substrate, 2 min exposure) also requires about 72 h of incubation (Fig. 7c). It is less sensitive than Dr. Shah’s antibody since endogenous NOX4 in RCC4 cells could not be detected without TGFβ1 treatment. However, it appears to be more specific since less of the lower MW bands were observed. Importantly, this example shows that endogenous NOX4 protein from a cell line with a high basal level of mRNA expression (Cq ~ 27 and ΔCq ~ 6) requires extreme conditions to be detected by immunoblot, suggesting that for cell lines with lower mRNA expression levels (Cq >27), detection of NOX protein may be very difficult or impossible given our current tools. Here, RCC4 cells were used because they have a high basal level of NOX4 mRNA (Cq ~ 27), possibly due to a mutation of the von Hippel Lindau gene, VHL, and the subsequent impaired protein degradation of hypoxia inducible factor-1-alpha, HIF1-α. Figure 9 shows that untreated 786–0 cells, one of the NCI-60 panel cell lines (renal clear cell carcinoma cell line), do not express NOX4 (or NOX1) mRNA. Thus, 786–0 cells should not be used to determine the effects of deleting NOX4 (or NOX1) in renal cancer.
Then, the relative fold change, R, of NOX mRNA levels with respect to the reference mRNA levels is calculated by inserting the ΔΔCq value into the following equation:
. If there is an increase in the experimental NOX mRNA levels (a decrease in the experimental ΔCq), then ΔΔCq will be a negative number. If there is a one cycle decrease (ΔΔCq = −1), then R is twofold. Thus, it is important to take into consideration whether the standard deviations of the mean Cq values are much less than 1 when interpreting qPCR data that report twofold changes. Even for a tenfold change in mRNA levels, the change between the experimental and control values for Cq (ΔΔCq) is only 3.32 cycles. Thus, the Cq values and standard deviations should be included when reporting RT-qPCR data, especially for low fold changes.
From the total number of base pairs of the plasmid plus insert that is used to generate the standard curve, it is possible to estimate the molecular weight of the plasmid using the average MW of a base pair (650 g/mol) and hence the molarity of the initial 1 μg/μL stock of linearized plasmid. From the number of moles, the number of molecules (copies) of plasmid can be calculated using Avogadro’s number. The copy number can be estimated by plotting copy number rather than dilution-fold on the x-axis. Thus the standard curve could be used as an estimation of copy number.
Using a C-terminal tagged NOX/DUOX isoform for transfection will abolish catalytic activity, while N-terminal tags may be tolerated (certain tags for NOX4, DUOX) or decrease activity (NOX1). Epitope tags (e.g., Myc-DDK-NOX4) may also distort localization. Thus, tagged NOX/DUOX constructs should not be used when NOX activity and/or localization will be investigated or need to be validated stringently. However, for purposes of testing antibodies using lysates, a tagged NOX isoform may be used.
NOX1–5 require 24 h for initial expression, but 48 h yield higher expression levels when activity measurements are intended. DUOX1 and DUOX2 require co-transfection of DUOXA1 and DUOXA2, respectively, and at least 48 h for optimal expression in HEK 293 cells.
Remove as much of the rinse solution as possible so that the rinse solution does not add volume to the RIPA buffer. This is important to keep the protein concentration high for endogenous proteins (2–3 × 107 cells/mL; 4–6 mg/mL) as generally 100 μg is loaded in a volume of about 30 μL. For overexpressed NOX proteins, it is not as important, and a protein concentration of about 1 mg/mL (5 × 106 cells/mL) should be sufficient to load 10–20 μg. Hold the plate at an angle for 10–15 s and aspirate any remaining rinse solution. Repeat for all the plates before adding RIPA buffer. For the positive control (overexpressed NOX) and empty vector negative controls, one 10 cm plate of transfected cells should yield abundant control protein. However, for cell lines to be tested for endogenous NOX protein, at least 3–4 full plates of cells should be scraped in a total volume of 400 μL to obtain a concentrated protein sample of about 4 mg/mL. After dilution with 4× Laemmli’s sample buffer, the protein concentration will be 3 mg/mL, and 33 μL is loaded for 100 μg of protein.
Boiling samples compromises detection of integral membrane proteins, including NOX proteins. Boiling NOX proteins will result in bands below 25 kDa or in a high MW band as insoluble material that cannot enter the gel. We find it unnecessary to heat NOX samples, but some laboratories apply heat. For example, heating NOX5 samples at 37 °C for 15 min improves solubility without adverse effects when using Dr. Nauseef’s NOX5 antibody (Dr. W. Nauseef, pers. communication) [6]. For DUOX samples, Dr. De Deken applies 80 °C for 5 min due to the high number of glycosylation sites (Fig. 6). It is best to test whether heating will improve your results or whether it is unnecessary. Also a number of reducing agents can be tested in addition to β-mercaptoethanol, such as DTT. Smeared high MW bands may be avoided by preparing lysates fresh and adding reducing agent immediately (Dr. U. Knaus, pers. communication).
In our hands, freshly poured gels give better resolution than pre-cast gels. However, this may depend on the manufacturer, the ratio of acrylamide and BIS, and buffer system.
We prefer LI-COR blocking buffer (TBS-based) without Tween-20 and also 5% nonfat dry milk in TBST from Sigma-Aldrich as blocking buffers. We normally compare both buffers for new antibodies. We also prefer to dilute our antibodies (non-fluorescent as well as fluorescent) in the LI-COR blocking buffer +0.1% Tween 20 because the antibody performs well for over a year in this buffer, whereas antibodies stored in milk lose sensitivity due to degradation. We prepare fresh dilutions of antibody in milk after 2 weeks of storage. When using the LI-COR Odyssey blocking buffer, add 0.1% Tween when using it as antibody diluent, and also add 0.01% SDS for secondary antibodies as recommended by the manufacturer.
For NOX antibodies, an overnight incubation is adequate for 10 μg of overexpressed NOX proteins; for endogenous NOX protein detection, incubate for 24–72 h at 4 °C while rocking (to be determined by the end user; depends on tissue, antibody dilution of primary and secondary antibodies, and ECL reagent). For secondary antibodies and antibodies used for loading controls (e.g., β-actin), an incubation period of 1 h at 25 °C is sufficient.
References
- 1.Altenhofer S, Kleikers PW, Radermacher KA, Scheurer P, Rob Hermans JJ, Schiffers P, Ho H, Wingler K, Schmidt HH (2012) The NOX toolbox: validating the role of NADPH oxidases in physiology and disease. Cell Mol Life Sci 69(14):2327–2343. 10.1007/s00018-012-1010-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seredenina T, Nayernia Z, Sorce S, Maghzal GJ, Filippova A, Ling SC, Basset O, Plastre O, Daali Y, Rushing EJ, Giordana MT, Cleveland DW, Aguzzi A, Stocker R, Krause KH, Jaquet V (2016) Evaluation of NADPH oxidases as drug targets in a mouse model of familial amyotrophic lateral sclerosis. Free Radic Biol Med 97:95–108. 10.1016/j.freeradbiomed.2016.05.016 [DOI] [PubMed] [Google Scholar]
- 3.Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55(4):611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 4.Kawahara T, Lambeth JD (2008) Phosphatidylinositol (4,5)-bisphosphate modulates Nox5 localization via an N-terminal polybasic region. Mol Biol Cell 19(10):4020–4031. 10.1091/mbc.E07-12-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun Y, Li Y, Luo D, Liao DJ (2012) Pseudogenes as weaknesses of ACTB (Actb) and GAPDH (Gapdh) used as reference genes in reverse transcription and polymerase chain reactions. PLoS One 7(8):e41659. 10.1371/journal.pone.0041659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dho SH, Kim JY, Kwon ES, Lim JC, Park SS, Kwon KS (2015) NOX5-L can stimulate proliferation and apoptosis depending on its levels and cellular context, determining cancer cell susceptibility to cisplatin. Oncotarget 6 (36):39235–39246. 10.18632/oncotarget.5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto M, Katsuyama M, Iwata K, Ibi M, Zhang J, Zhu K, Nauseef WM, Yabe-Nishimura C (2014) Characterization of N-glycosylation sites on the extracellular domain of NOX1/NADPH oxidase. Free Radic Biol Med 68:196–204. 10.1016/j.freeradbiomed.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Jaquet V, Krause KH (2012) NOX5: from basic biology to signaling and disease. Free Radic Biol Med 52(4):725–734. 10.1016/j.freeradbiomed.2011.11.023 [DOI] [PubMed] [Google Scholar]
- 9.Chen F, Wang Y, Barman S, Fulton DJ (2015) Enzymatic regulation and functional relevance of NOX5. Curr Pharm Des 21 (41):5999–6008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob F, Guertler R, Naim S, Nixdorf S, Fedier A, Hacker NF, Heinzelmann-Schwarz V (2013) Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS One 8(3):e59180. 10.1371/journal.pone.0059180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doroshow JH, Juhasz A, Ge Y, Holbeck S, Lu J, Antony S, Wu Y, Jiang G, Roy K (2012) Antiproliferative mechanisms of action of the flavin dehydrogenase inhibitors diphenylene iodonium and di-2-thienyliodonium based on molecular profiling of the NCI-60 human tumor cell panel. Biochem Pharmacol 83 (9):1195–1207. 10.1016/j.bcp.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meitzler JL, Makhlouf HR, Antony S, Wu Y, Butcher D, Jiang G, Juhasz A, Lu J, Dahan I, Jansen-Durr P, Pircher H, Shah AM, Roy K, Doroshow JH (2017) Decoding NADPH oxidase 4 expression in human tumors. Redox Biol 13:182–195. 10.1016/j.redox.2017.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laurent E, McCoy JW 3rd, Macina RA, Liu W,Cheng G, Robine S, Papkoff J, Lambeth JD (2008) Nox1 is over-expressed in human colon cancers and correlates with activating mutations in K-Ras. Int J Cancer 123 (1):100–107. 10.1002/ijc.23423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burritt JB, Quinn MT, Jutila MA, Bond CW, Jesaitis AJ (1995) Topological mapping of neutrophil cytochrome b epitopes with phagedisplay libraries. J Biol Chem 270 (28):16974–16980 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura M, Murakami M, Koga T, Tanaka Y, Minakami S (1987) Monoclonal antibody 7D5 raised to cytochrome b558 of human neutrophils: immunocytochemical detection of the antigen in peripheral phagocytes of normal subjects, patients with chronic granulomatous disease, and their carrier mothers. Blood 69 (5):1404–1408 [PubMed] [Google Scholar]
- 16.Kawai C, Yamauchi A, Kuribayashi F (2018) Monoclonal antibody 7D5 recognizes the R147 epitope on the gp91(phox), phagocyte flavocytochrome b558 large subunit. Microbiol Immunol 62(4):269–280. 10.1111/1348-0421.12584 [DOI] [PubMed] [Google Scholar]
- 17.Verhoeven AJ, Bolscher BG, Meerhof LJ, van Zwieten R, Keijer J, Weening RS, Roos D (1989) Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils. Blood 73(6):1686–1694 [PubMed] [Google Scholar]
- 18.Seredenina T, Sorce S, Herrmann FR, Ma Mulone XJ, Plastre O, Aguzzi A, Jaquet V, Krause KH (2017) Decreased NOX2 expression in the brain of patients with bipolar disorder: association with valproic acid prescription and substance abuse. Transl Psychiatry 7(8):e1206. 10.1038/tp.2017.175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor RM, Maaty WS, Lord CI, Hamilton T, Burritt JB, Bothner B, Jesaitis AJ (2007) Cloning, sequence analysis and confirmation of derived gene sequences for three epitope-mapped monoclonal antibodies against human phagocyte flavocytochrome b. Mol Immunol 44(4):625–637. 10.1016/j.molimm.2005.10.022 [DOI] [PubMed] [Google Scholar]
- 20.Sorce S, Nuvolone M, Keller A, Falsig J, Varol A, Schwarz P, Bieri M, Budka H, Aguzzi A (2014) The role of the NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog 10(12):e1004531. 10.1371/journal.ppat.1004531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anilkumar N, Weber R, Zhang M, Brewer A, Shah AM (2008) Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling responses to agonist stimulation. Arterioscler Thromb Vasc Biol 28(7):1347–1354. 10.1161/ATVBAHA.108.164277 [DOI] [PubMed] [Google Scholar]
- 22.Holl M, Koziel R, Schafer G, Pircher H, Pauck A, Hermann M, Klocker H, Jansen-Durr P, Sampson N (2016) ROS signaling by NADPH oxidase 5 modulates the proliferation and survival of prostate carcinoma cells. Mol Carcinog 55(1):27–39. 10.1002/mc.22255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Lohneysen K, Noack D, Jesaitis AJ,Dinauer MC, Knaus UG (2008) Mutational analysis reveals distinct features of the Nox4-p22 phox complex. J Biol Chem 283 (50):35273–35282. 10.1074/jbc.M804200200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Jamali A, Valente AJ, Lechleiter JD, Gamez MJ, Pearson DW, Nauseef WM, Clark RA (2008) Novel redox-dependent regulation of NOX5 by the tyrosine kinase c-Abl. Free Radic Biol Med 44(5):868–881. 10.1016/j.freeradbiomed.2007.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzaioli V, Hurtado-Nedelec M, Pintard C, Tlili A, Marie JC, Monteiro RC, Gougerot-Pocidalo MA, Dang PM, El-Benna J (2017) NOX5 and p22phox are 2 novel regulators of human monocytic differentiation into dendritic cells. Blood 130(15):1734–1745. 10.1182/blood-2016-10-746347 [DOI] [PubMed] [Google Scholar]
- 26.Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, Doroshow JH (2013) Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 65:497–508. 10.1016/j.freeradbiomed.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pacquelet S, Lehmann M, Luxen S, Regazzoni K, Frausto M, Noack D, Knaus UG (2008) Inhibitory action of NoxA1 on dual oxidase activity in airway cells. J Biol Chem 283(36):24649–24658. 10.1074/jbc.M709108200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, Knaus UG (2009) Heterodimerization controls localization of Duox-DuoxA NADPH oxidases in airway cells. J Cell Sci 122. (Pt 8:1238–1247. 10.1242/jcs.044123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F (2000) Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275 (30):23227–23233. 10.1074/jbc.M000916200 [DOI] [PubMed] [Google Scholar]
- 30.Caillou B, Dupuy C, Lacroix L, Nocera M, Talbot M, Ohayon R, Deme D, Bidart JM, Schlumberger M, Virion A (2001) Expression of reduced nicotinamide adenine dinucleotide phosphate oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid tissues. J Clin Endocrinol Metab 86(7):3351–3358. 10.1210/jcem.86.7.7646 [DOI] [PubMed] [Google Scholar]
- 31.Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot F, Diallo I, de Vathaire F, Schlumberger M, Dupuy C (2015) NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A 112(16):5051–5056. 10.1073/pnas.1420707112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A, Dupuy C (2005) Dual oxidase 2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288(5):G933–G942. 10.1152/ajpgi.00198.2004 [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Antony S, Hewitt SM, Jiang G, Yang SX, Meitzler JL, Juhasz A, Lu J, Liu H, Doroshow JH, Roy K (2013) Functional activity and tumor-specific expression of dual oxidase 2 in pancreatic cancer cells and human malignancies characterized with a novel monoclonal antibody. Int J Oncol 42(4):1229–1238. 10.3892/ijo.2013.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Leo FR, Ulman KV, Davis AR, Jutila KL, Quinn MT (1996) Assembly of the human neutrophil NADPH oxidase involves binding of p67phox and flavocytochrome b to a common functional domain in p47phox. J Biol Chem 271(29):17013–17020 [DOI] [PubMed] [Google Scholar]