Abstract
Purpose:
To evaluate the results of the randomized, double-blind, placebo-controlled phase II clinical trial of ICT-107 in patients with newly diagnosed glioblastoma.
Patients and Methods:
We conducted a double-blinded randomized phase II trial of ICT-107 in newly diagnosed patients with glioblastoma (GBM) and tested efficacy, safety, quality of life (QoL), and immune response. HLA-A1+ and/or -A2+–resected patients with residual tumor ≤1 cm3 received radiotherapy and concurrent temozolomide. Following completion of radiotherapy, 124 patients, randomized 2:1, received ICT-107 [autologous dendritic cells (DC) pulsed with six synthetic peptide epitopes targeting GBM tumor/stem cell–associated antigens MAGE-1, HER-2, AIM-2, TRP-2, gp100, and IL13Rα2] ormatching control (unpulsed DC). Patients received induction ICT-107 or control weekly × 4 followed by 12 months of adjuvant temozolomide. Maintenance vaccinations occurred at 1, 3, and 6 months and every 6 months thereafter.
Results:
ICT-107 was well tolerated, with no difference in adverse events between the treatment and control groups. The primary endpoint, median overall survival (OS), favored ICT-107 by 2.0 months in the intent-to-treat (ITT) population but was not statistically significant. Progression-free survival (PFS) in the ITT population was significantly increased in the ICT-107 cohort by 2.2 months (P = 0.011). The frequency of HLA-A2 primary tumor antigen expression was higher than that for HLA-A1 patients, and HLA-A2 patients had higher immune response (via Elispot). HLA-A2 patients achieved a meaningful therapeutic benefit with ICT-107, in both the MGMT methylated and unmethylated prespecified subgroups, whereas only HLA-A1 methylated patients had an OS benefit.
Conclusions:
PFS was significantly improved in ICT-107–treated patients with maintenance of QoL. Patients in the HLA-A2 subgroup showed increased ICT-107 activity clinically and immunologically.
Introduction
Glioblastoma is the most common malignant primary brain tumor with over 12,500 new cases diagnosed annually in the United States (1). Despite optimal therapy, the prognosis remains poor with median overall survival (OS) of approximately 14–18 months (2–5).
ICT-107, is an autologous dendritic cell (DC) immunotherapy targeting six antigens on both tumor and cancer stem cells (6–8). These antigens are the HLA-A1–restricted, melanoma-associated antigen-1 (MAGE-1) and antigen isolated from immunoselected melanoma-2 (AIM-2), and the HLA-A2–restricted, human EGFR-2 (HER2/neu), tyrosinase-related protein-2 (TRP-2), glycoprotein 100 (gp100), and IL13 receptor alpha 2 (IL13Rα2). They were selected on the basis of studies that included an analysis of 46 primary glioblastoma (GBM) tumors (7, 9–15). Three or more of the selected antigens were expressed on all of the tumors, four or more antigens on 97% of tumors, and five or more antigens on 93% of tumors. All six antigens were expressed in 83% of tumors. The highest expression was observed for AIM-2, TRP-2, HER2/neu, and IL13Rα2, with MAGE-1 and gp100 showing weaker expression. Given the heterogeneous cell population in GBM, targeting multiple antigens rather than a single antigen should decrease the probability of tumor escape.
In a prior phase I study, intradermal administration of ICT-107 to 21 patients with GBM was well tolerated (8). Immunologic studies showed that 33% of patients developed systemic type I cytokine responses IFNγ and TNFα and were associated with a trend toward improved survival (8). In patients who underwent reoperation, analysis of tumor specimens suggested preferential elimination of CD133+ cancer stem cells by ICT-107 (8). At a median follow-up of 40.1 months, 6 of 16 newly diagnosed patients with GBM showed no evidence of tumor recurrence. This study suggested that ICT-107 was well tolerated and associated with promising preliminary evidence of activity. Here, we report the results of a randomized, double-blind, placebo-controlled phase IIb trial evaluating the safety and efficacy of ICT-107 in newly diagnosed patients with GBM following resection and radiotherapy with concomitant temozolomide.
Patients and Methods
ICT 107
ICT-107 production involves harvesting monocytes from each subject through plasmapheresis, driving the monocytes to differentiate into DCs, and then activating the new DCs by incubating them with peptides present on GBM stem and tumor cells (6, 16). Monocyte differentiation is triggered by culturing these cells with recombinant human GM-CSF and IL4, followed by IFNγ and bacterial lipopolysaccharide. The resulting DCs are then incubated with 20 μg/mL of each of six preselected synthetic peptides, 9–10 amino acids in length, derived from antigens associated with cancer stem cells in GBM (Supplementary Table S1). These synthetic peptides were manufactured at Polypeptide Laboratories. Manufacturing of ICT-107 and control was originally done at the University of Pennsylvania Clinical Cell and Vaccine Production (CVPF) facility (UPenn) and subsequently replaced by PCT Cell Therapy Services (PCT). Lots manufactured at PCT were shown to be comparable with lots made at UPenn. ICT-107 vaccination consisted of 1mL of pulsed DCs (1.1 × 107 cells/mL). Control consisted of 1 mL of DCs unpulsed with antigen (3.6 × 106 cells/mL).
Patients
Eligible patients were ≥ 18 years with histologically confirmed GBM and had undergone gross total resection resulting in no residual gadolinium enhancement, or subtotal resection consisting of enhancement measuring less than 1cm3 total volume or 100 mm2 of tumor in cross-sectional area (by central radiologic review). Other inclusion criteria included Karnofsky performance status (KPS) ≥ 70%, HLA-A1+ or HLA-A2+, and adequate bone marrow, liver, and renal function. Patients with recurrent disease, clinically significant systemic disease, autoimmune disorders, >4 mg/day of dexamethasone within 7 days of first administration of study treatment, or contraindication to MRI were excluded. Approval from institutional review boards and/or independent ethics committees was obtained at each site. The studies were conducted in accordance with recognized Federal ethical guidelines. All patients provided written, informed consent. This study was registered on ClinicalTrials.gov (NCT01280552).
Treatment and study design
This was a randomized, double-blind, multicenter phase II placebo-controlled study that enrolled patients between February 2009 and June 2011 (CONSORT diagram; Fig. 1). Patients underwent initial screening following diagnosis and then apheresis. Following completion of radiotherapy and concomitant temozolomide, additional eligibility confirmation was conducted to ensure that the patients had ≤ 1 cm3 of residual tumor and adequate organ function. Eligible patients must have completed 6 weeks of radiotherapy and at least 50% of the concomitant temozolomide chemotherapy and not have developed progressive disease.
Figure 1.
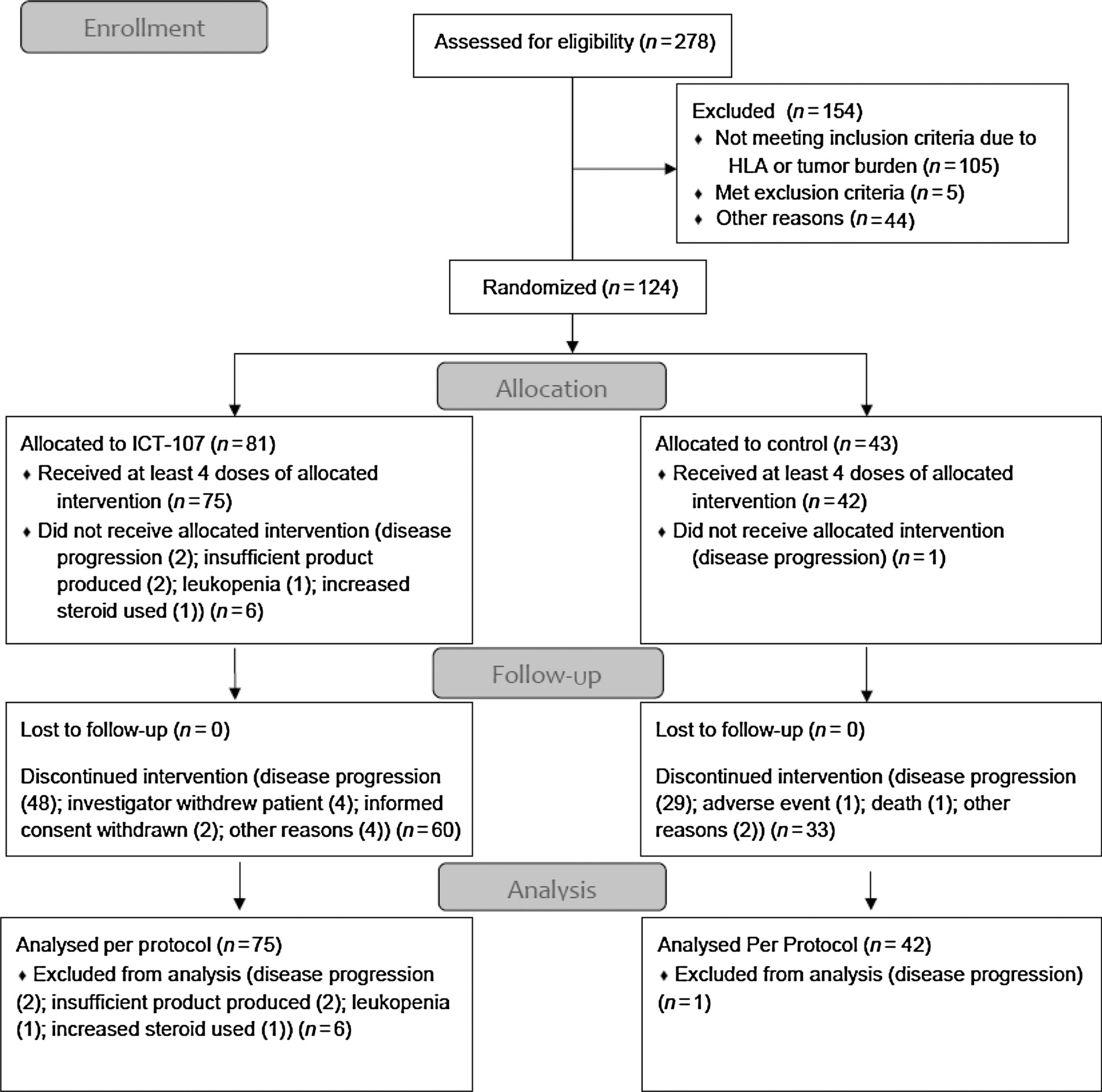
CONSORT diagram.
Following standard radiotherapy (60 Gy/30 fractions) with concomitant temozolomide (75 mg/m2/day × 42 days), there was a 1-week rest period during which patient eligibility was reconfirmed. Two-hundred seventy-eight patients underwent screening of which 182 patients were eligible based on HLA requirement. One-hundred twenty-four patients were eligible based on radiologic, clinical, and laboratory parameters and were randomly assigned in a 2:1 ratio to receive ICT-107 or placebo and adjuvant temozolomide beginning 1 week after completion of radiochemotherapy. Patients were stratified by study site and age (<50 years/≥50 years). ICT-107 vaccination consisted of 1mL of activated, mature, pulsed DCs (1.1 × 107 cells/mL). Control consisted of 1 mL of activated, mature, unpulsed DCs (3.6 × 10 the6 cells/mL). completion In the of 4-radiotherapyweek interval and after the the beginning rest period, of between adjuvant temozolomide (vaccine induction phase), patients received vaccination once weekly intradermally in the axilla. Patients then began the maintenance phase and received standard adjuvant temozolomide at 150 mg/m2/d × 5 days for cycle 1, and then 200 mg/m2/day × 5 days on cycles 2 to 12. Additional vaccinations were administered day 21 of cycles 1, 3, 6, 10 and then every 6 months until depletion of study treatment or confirmation of progressive disease (PD), whichever occurred first (Fig. 2).
Figure 2.

Study flow chart.
SOC = standard of care; TMZ = temozolomide
Treatment scheme is the same for all patients throughout the course of the study, regardles of treatment group (ICT-107 or Control)
*Chemoradiation for 6 weeks: induction TMZ (75 mg/m2/day for 42 days) and focal radiotherapy (60 Gy in 30 fractions, 5 days/week for 6 weeks)
§ Administered 5 days per week for 12 months. Cycle 1: 150 mg/m2/day × 5. Cycles 2 −12: 200 mg/m2/day × 5
β Maintenance administration on day 21 of cycles 1, 3, 6, 10, coupled with maintenance TMZ. if TMZ is delayed, these vaccinations will also be delayed to ensure TMZ and vaccination cycles remain synchronized. Extended maintenance administration at cycles 16, 22 and continuing every 6 months until depletion or confirmation of PD
Patients were evaluated every 2 weeks during the “induction” phase and every 4 weeks during the maintenance phase. MRI was obtained prior to initiation of study treatment, prior to cycle 1 of adjuvant temozolomide, and then every 8 weeks thereafter.
The primary objective was OS compared with control. Secondary objectives included progression-free survival (PFS), safety and tolerability, immune response in patients treated with ICT-107 and predictors of response. Exploratory objectives included quality-of-life (QoL) evaluation.
Toxicity was graded according to the NCI Common Toxicity Criteria (NCI CTC), version 4.03. Radiographic assessments were based on the Response Assessment in Neuro-Oncology (RANO) criteria (17).
Statistical considerations
The trial planned to enroll approximately 200 patients for screening with the goal of randomizing at least 102 patients with a treatment allocation of 2:1 (68 patients to ICT-107:34 patients to control). The trial significance was α= 0.025 (one-sided). The median expected OS was 24 months for the ICT-107 group and 15 months for the control group, and assumed a Weibull distribution with a shape parameter of κ= 1.4 for both groups. The HR was calculated as .
This group sequential adaptive design included one interim analysis after 32 events, using an O’Brien Fleming alpha-spending boundary, and Hwang and colleagues (γ = −3) beta-spending boundary (18). From sample size reestimation, the number of trial subjects was not planned to exceed 50% of the original trial design. The inverse normal method was employed to preserve overall type I error at α = 0.025, testing the null hypothesis H0: HR ≥ 1. Simulations estimated that an average of 70 deaths needed to be observed to provide approximately 83% power. On the basis of lost-to follow-up estimates, up to 10% more patients could be randomized.
A z-test under the proportional hazards model utilizing the inverse normal method was used to test for treatment significance with age, O6-methylguanine-DNA-methyltransferase (MGMT) methylation status, and treatment as covariates (19).
The intent-to-treat (ITT) population was defined as all patients who were randomized. Per Protocol Population included all randomized patients who received their first four (induction) treatments. Stratification factors included MGMT methylation status and age.
During analyses, the data was noted to not fit a Weibull distribution and this method was therefore not applied. As specified in the statistical analyses plan, the stratified log-rank test was used for the hypothesis test.
After a review of interim data, the Data Monitoring Committee recommended accrual of patients continue to increase the likelihood of reaching the number of required events in a timely manner. Ultimately, 278 patients were enrolled and 124 were randomized into the ITT population, with 81 patients treated with ICT 107 and 43 patients treated with control. Stratified log-rank analysis was performed from the time of randomization, which occurred after diagnosis and surgical resection. There were a number of prospectively defined subgroup analyses. These included age, gender, MGMT methylation status, HLA haplotype, resection status, KPS, and manufacturing site.
Immunologic testing
The ex vivo (without previous in vitro stimulation) immune response of all patients in this trial for which there was sufficient number of cells was determined for each antigen associated with their HLA status by IFNγ ELISpot using previously reported methodology (20). HLA-A2 patients from both treatment and control groups with a sufficient number of cells available were tested for each of the associated antigens using multimer testing, employing an approach derived from a previously reported harmonized protocol (21). Monte Carlo simulation of a fusion approach incorporating multiple response metrics was performed and used to determine thresholds for response, as outlined in the appendix.
QoL methods
KPS and steroid dosing, as well as patient-reported QoL using the Functional Assessment of Cancer Therapy-Brain (FACT-BR) were evaluated during the progression-free period. The FACT-BR was completed by patients at baseline, and longitudinally during the maintenance phase and at the end of study visit. Corticosteroid dosing and KPS were evaluated throughout the progression-free period at monthly time points for 12 months and every 6 months thereafter. Between arm differences were evaluated using Fisher test or ANOVA statistics. The sign-rank test was used to compare KPS score differences between the ICT-107 group and the control group at prevaccine and postvaccine time points. The stratified log-rank test was used to compare the initiation of steroids between the ICT-107 group and the control group.
Results
A total of 124 patients were randomized to standard-of-care plus either ICT-107 or placebo. There were 43 control patients and 81 active patients representing a 1:2 randomization. Seventy-five (60.5%) were men. The median age was 59.2 (range, 22.9–81.8); median KPS was 90 (range, 70–100; Table 1).
Table 1.
Patient demographics
| ICT-107 | Control DC | Total | |
|---|---|---|---|
| # Subjects randomized | 81 | 43 | 124 |
| # Subjects completed treatment | 75 | 42 | 117 |
| Subjects by age group | |||
| Median (years) | 59.0 | 59.8 | 59.2 |
| Mean (years) | 57.4 | 57.5 | 57.4 |
| <50 years | 20 (24.7%) | 12 (27.9%) | 32 (25.8%) |
| ≥50 years | 61 (75.3%) | 31 (72.1%) | 92 (74.2%) |
| ≥50 and <65 years | 39 (48.2%) | 17 (39.55) | 56 (45.2%) |
| ≥65 years | 22 (27.2%) | 14 (32.6%) | 36 (29.0%) |
| Gender | |||
| Males | 44 (54.3%) | 31 (72.1%) | 75 (60.5%) |
| Females | 37 (45.7%) | 12 (27.9%) | 49 (39.5%) |
| HLA haplotype | |||
| HLA-A1 (HLA-A2−) | 33 (40.7%) | 14 (32.6%) | 47 (37.9%) |
| HLA-A2 (HLA-A1−) | 42 (51.9%) | 22 (51.2%) | 64 (51.6%) |
| HLA-A1, A2 | 6 (7.4%) | 7 (16.3%) | 13 (10.5%) |
| All HLA A2+ | 48 (59.3%) | 29 (67.4%) | 77 (62.1%) |
| MGMT statusa | |||
| Methylated | 28 (34.6%) | 18 (41.9%) | 46 (37.1%) |
| Unmethylated | 47 (58.0%) | 24 (55.8%) | 71 (57.3%) |
| KPS categoryb | |||
| 100 | 24 (29.6%) | 8 (18.6%) | 32 (25.8%) |
| 90 | 36 (44.4%) | 18 (41.9%) | 54 (43.5%) |
| <90 | 20 (24.7%) | 17 (39.5%) | 37 (29.8%) |
| Resection status | |||
| Complete resection | 58 (71.6%) | 32 (74.4%) | 90 (72.6%) |
| Subtotal resection | 23 (28.4%) | 11 (25.6%) | 34 (27.4%) |
Six patients in the ICT-10 group and 1 patient in the control group did not have MGMT methylation data.
KPS data missing from 1 patient in ICT-107 group.
Toxicity
Overall the vaccine was well tolerated (Table 2). The most frequent adverse events were fatigue, convulsions, and nausea. There was no difference in adverse events between ICT-107 and the control DC group.
Table 2.
Safety—common adverse events
| Adverse event grade | 1CT-107 (N = 80) |
Control DC (N = 43) |
||||
|---|---|---|---|---|---|---|
| Grade 2 | Grade 3 | Grade 4 | Grade 2 | Grade 3 | Grade 4 | |
| Nervous system disorders | 27 (33%) | 14 (17.5%) | 21 (48.8%) | 7 (16.3%) | ||
| Headache | 2 (2.5%) | 7 (16.3%) | ||||
| Convulsion | 7 (8.8%) | 4 (5.0%) | 6 (14.0%) | 1 (2.3%) | ||
| Hemiparesis | 4 (5.0%) | 2 (4.7%) | ||||
| Partial seizures | 5 (6.3%) | 1 (2.3%) | ||||
| General disorders and administration site conditions | 16 (20.0%) | 6 (7.5%) | 12 (27.9%) | 5 (11.6%) | ||
| Fatigue | 9 (11%) | 3 (3.8%) | 8 (18.6%) | 3 (7.0%) | ||
| Gastrointestinal disorders | 11 (13.8%) | 6 (14.0%) | ||||
| Nausea | 6 (7.5%) | 4 (9.3%) | ||||
| Musculoskeletal and connective tissue disorders | 10 (12.5%) | 1 (1.3%) | 8 (18.6%) | 3 (7.0%) | ||
| Muscular weakness | 2 (2.5%) | 3 (7.0%) | 3 (7.0%) | |||
| Investigations | 5 (6.3%) | 4 (5%) | 4 (9.3%) | 2 (4.7%) | ||
| Skin and subcutaneous tissue disorders | 7 (8.8%) | 7 (16.3%) | ||||
| Blood and lymphatic system disorders | 9 (11.3%) | 6 (7.5%) | 6 (14.0%) | 4 (9.3%) | ||
| Thrombocytopenia | 3 (3.8%) | 3 (7.0%) | ||||
| WBC decrease | 4 (5.0%) | |||||
| Infections | 13 (16.3%) | 2 (2.5%) | 11 (25.6%) | 3 (7.0%) | ||
| Urinary tract infection | 5 (6.3%) | 2 (4.7%) | ||||
| Upper respiratory tract infection | 3 (7.0%) | |||||
| Metabolism and nutrition disorders | 5 (6.3%) | 3 (3.8%) | 7 (16.3%) | 4 (9.3%) | ||
| Decreased appetite | 2 (2.5%) | 3 (7.0%) | ||||
| Psychiatric disorders | 9 (11.3%) | 6 (14.0%) | ||||
| Insomnia | 3 (3.8%) | 4 (9.3%) | ||||
| Injury and procedural complications | 9 (11.3%) | 4 (9.3%) | ||||
| Vascular disorders | 6 (7.5%) | 3 (7.0%) | ||||
| Reproductive system and breast disorders | 3 (7.0%) | |||||
NOTE: Adverse events by body system with an incidence >5%.
Efficacy
At the 67-event trial completion point, the primary efficacy endpoint of OS for the ICT-107 group in the ITT population was 17.0 months (CI: 13.68–20.61) and was not significantly increased compared with the 15.0 months (CI: 12.33–23.05) for the control group (HR = 0.87; P = 0.58). However, the secondary endpoint, PFS, for ICT-107 of 11.2 months (CI: 8.22–13.05) was statistically significantly increased compared with 9.0 months (CI: 5.52–10.29) for the control group (HR = 0.57; P = 0.011; Fig. 3). The median OS had not been reached by this point. At 99 events, median OS favored ICT-107 by 1.6 months (HR = 0.85; P= 0.44 two-sided). PFS, for ICT-107 of 11.4 months was statistically significantly increased compared with the 10.1 months for the control group (HR = 0.64; P = 0.033).
Figure 3.
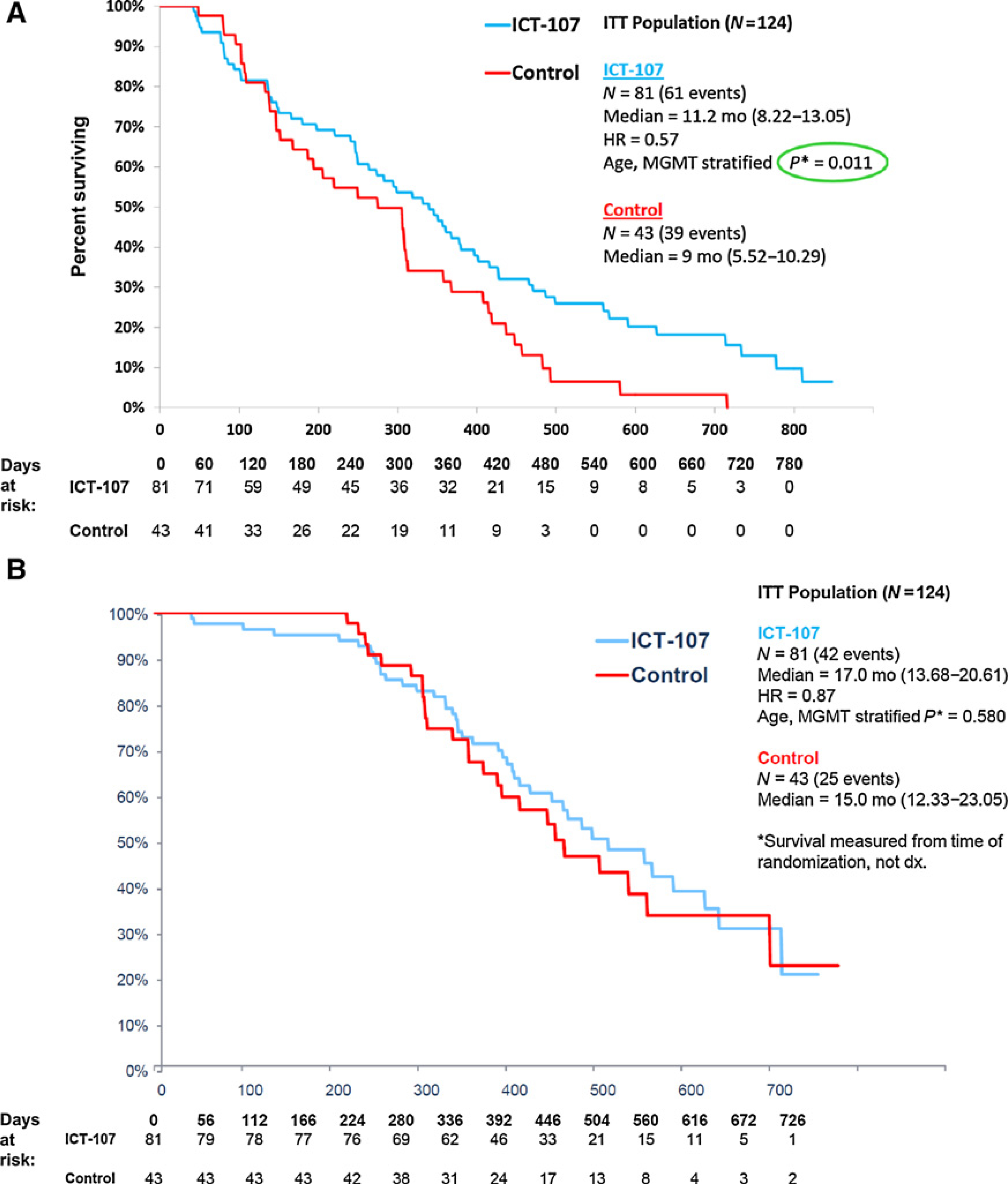
PFS (A) and OS (B) Kaplan-Meier Curves.
Analysis of the tumor specimens showed that while over 90% of patients expressed all the HLA-A2 antigens (gp100, HER2/neu, IL13Rα2, TRP-2), only 37.8% of patients expressed the HLA-A1 antigens (MAGE-1 and AIM-2), (Supplementary Fig. 1). This suggested that the patient population most likely to benefit from this therapy were HLA-A2+ patients. For HLA-A2+ patients with MGMT promoter methylation, median PFS for the ICT-107 group was 24.1 months compared with a median PFS of 8.5 months for the control group (HR = 0.26; P = 0.004), while median OS for the ICT-107 group was 33.7 months compared with 23.9 months for the control group (HR = 0.80; P = 0.65). For HLA-A2+ patients with unmethylated MGMT promoter, median PFS for the ICT-107 group was 10.5 months compared with 6.0 months for the control group (HR = 0.72; P = 0.37), while median OS was 15.8 months for the ICT-107 group compared with 11.8 months for the control group (HR = 0.70; P = 0.33). HLA-A1+ patients with unmethylated MGMT promoter treated with ICT-107 had a median OS of 14.3 months compared with 15.1 months for the control group (P = 0.52). The HLA-A1+ patients with MGMT methylation treated with ICT-107 had a median OS of 47.6 months compared with 25.8 months for the control group (P = 0.049).
Patient immune response and association to survival
Mature monocyte–derived DCs were activated in vitro with IFNγ and lipopolysaccharide to become DCs that secrete IL12p70. This preconditioned DCs for a second burst of IL12 if these cells subsequently encounter CD40 ligand (CD40L) expressed on T cells. IL12 secretion in response to CD40L was evaluated in DCs prior to pulsing with peptides and measured using a Quantikine ELISA kit from R&D Systems. We sought to determine the impact of the function of DC on survival of patients. The magnitude of IL12 secretion by DCs was measured in response to CD40L. Patient survival in this trial was associated with IL12 secretion in vitro in response to CD40L stimulation. Data (Supplementary Table S2) demonstrate that those patients that secreted IL12 levels above the median had improved survival compared with those with IL12 levels below the median. Although this was observed in both ICT-107 and control groups, the OS benefit was statistically significant for the ICT-107 group.
One-hundred and eight patients were tested for immune response using IFNγ ELISpot, and 47 were classified as responders. Of the 68 patients tested within the treatment group (ICT-107), 34 (50%) of them were classified as responders, while 13/50 (33%) patients in the placebo group were responders. This association approached significance at α = 0.10the median (P = 0.058 Fisher exact test). Within the treatment group the median OS for a responder was 433 days, while for a nonresponder it was 407 days (P = 0.050, log-rank test; Supplementary Fig. S2). Supplementary Table S3 provides a complete summary of all responder counts and associations and additional discussion.
QoL results
FACT-BR assessments were collected with 97% compliance prior to treatment and 84% and 68% completing end of cycle 1 and end-of-study assessments, respectively. Change from baseline in QoL reporting was not different between the experimental and control arms during the maintenance phase, at progression, or at treatment completion. ICT-107 patients maintained QoL longer as they progressed later. The findings were limited by small sample size and attrition at end of study.
KPS was significantly higher during the treatment period in those receiving ICT-107 (cycles 1–4 or maintenance phase). Treated patients also maintained a higher KPS after the initiation of vaccination, whereas there was no significant difference prior to vaccination. The sign-rank test P value was less than 0.05 for each of four KPS assessments between at the postvaccination time points (Supplementary Data Fig. S3A). There was a trend toward less initiation of steroids over time in the ICT-107 arm versus the control (P= 0.1395, log-rank; Supplementaryl Data Fig. S3B). On the basis of these initial analyses of clinical benefit, increased PFS time with ICT-107 was not associated with a detrimental impact on self-reported QoL, and was associated with maintenance of KPS and a longer corticosteroid-free period than those in the control arm.
Discussion
In this randomized, double-blind, placebo-controlled phase IIb trial of ICT-107 in newly diagnosed patients with GBM following resection and radiotherapy with concomitant temozolomide, the vaccine was very well tolerated. There was a statistically significantly increase in PFS in the ITT population for ICT-107 compared with the control group. The increased PFS was associated with preservation of QoL as measured by FACT-BR and improved KPS. The primary endpoint, median OS, was increased by 2 months in the treatment group compared with the control group but this difference was not statistically significant.
The vaccine was designed to target both HLA-A1 and A2 antigens and 47 patients (37.9%) were only HLA-A1+. However, analysis of the tumor specimens showed that only a small percentage of patients (37.8%) expressed the HLA-A1 antigens MAGE-1 and AIM-2, while over 90% of patients expressed all the HLA-A2 antigens (gp100, HER2/neu, IL13Rα2, TRP-2). Analysis of only the HLA-A2+ patients treated with ICT-107 showed an increase in median OS both in the methylated and unmethylated MGMT groups; although, this difference was not statistically significant with the small number of patients. PFS for HLA-A2+ patients with MGMT promoter methylation was significantly increased for the ICT-107 group (24.1 months) compared with the control group [8.5 months; HR = 0.26 (0.095–0.70); P = 0.004]. Overall survival for the HLA-A2+patients with methylated MGMT promoter and OS and PFS for HLA-A2+ patients with unmethylated MGMT promoter were greater in the ICT-107 group compared with controls but were not statistically significant with the small patient numbers.
Testing for immune response using IFNγ ELISpot in the ITT population showed an increase in responders treated with ICT-107 (50%) compared with control (33%; α = 0.10; P = 0.058). These responders had an improved OS compared with nonresponders. In the HLA-A2+ group, 86% of the patients treated with ICT-107 were responders by both ELISpot and multimer assays, compared with only 33% of the controls. These findings link the immune response to the clinical outcomes of PFS as well as survival. Control patients who received activated and mature DCs that were not pulsed with antigen may have mounted an immune response to peptides used in ICT-107 by processing free tumor antigen at the lymph nodes to prime T cells. This may explain the high immune response rate in the control population and may argue for a less active control agent in future trials.
This study had a number of limitations. The number of patients were small, limiting potential conclusions. The vaccine was designed to treat patients with both HLA-A1 and HLA-A2 and the study was powered for this combined population. However, the majority of the patients in the HLA-A1+ subgroup did not express the target antigens and most of the benefit was derived from patients in the HLA-A2+ subgroup. Nevertheless, the only subgroup with a significant OS benefit was the HLA-A1+ methylated group. Given the small numbers, this finding needs to be further validated in a larger randomized study. As a result of the small sample size, the study was not adequately powered to assess differences between HLA-A2+ patients, nor the patients with and without MGMT promoter methylation. Accurate determination of PFS for immunotherapies could be affected by pseudoprogression resulting in patients being taken off-study sooner than appropriate. No specific guidance was provided to the investigators to avoid this phenomenon as the iRANO criteria (22) had not been published. However, because pseudoprogression is theoretically more likely in the treatment group, the failure to account for pseudoprogressors should make it more difficult to demonstrate a PFS advantage in the ICT-107 group (greater risk of type II error).
Despite these limitations and the failure of ICT-107 to increase OS in the ITT population, there was improvement in PFS and an increased number of immune responders. Moreover, analysis of the HLA-A2 patients suggested potential benefit; although, the numbers were too small to reach statistical significance. This is the first study to demonstrate a clinical benefit in patients with newly diagnosed GBM following immunotherapy. These findings point in the direction of the use of immunomodulatory combinations to effect a synergistic and more potent clinical response. This study led to a phase III study evaluating the addition of ICT-107 to adjuvant temozolomide in newly diagnosed HLA-A2+ patients with GBM. The trial is currently on hold pending additional funding.
Supplementary Material
Translational Relevance.
We evaluated an autologous dendritic cell vaccine pulsed with six synthetic peptide epitopes targeting glioblastoma (GBM) tumor/stem cell–associated antigens MAGE-1, HER-2, AIM-2, TRP-2, gp100, and IL13Rα2 (ICT-107) in a double-blinded, randomized, phase II trial in newly diagnosed patients with GBM who were HLA-A1+ and/or -A2+. The vaccine was well tolerated and increased progression-free survival significantly by 2.2 months, (P = 0.011) with maintenance of quality-of-life; although, the primary endpoint of overall survival was not increased. The frequency of HLA-A2 primary tumor antigen expression was higher than that for HLA-A1 patients. HLA-A2 patients had higher immune response (via Elispot) and patients achieved a meaningful therapeutic benefit with ICT-107, in both the MGMT methylated and unmethylated prespecified subgroups. This is the first vaccine trial in GBM to show a clinical benefit and led to a phase III trial in HLA-A2+ newly diagnosed patients with GBM.
Acknowledgments
The study was supported by Immunocellular Therapeutics.
Disclosure of Potential Conflicts of Interest
P.Y. Wen reports receiving commercial research grants from Merck and VBI Vaccines; reports receiving speakers bureau honoraria from Merck, Prime Oncology, and Immunocellular; and is a consultant/advisory board member for Agios, Blue Earth Diagnostics, Roche, Kiyatec, Puma, Vascular Biogenics, Taiho, Deciphera, VBI Vaccines, and Karyopharm. D.A. Reardon is a consultant/ advisory board member for Abbvie, Advantagene, Agenus, Amgen, Bayer, Bristol-Myers Squibb, Celldex, DellMar, EMD Serono, Genentech/Roche, Inovio, Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, and Taiho Oncology. R.D. Aiken is a consultant/advisory board member for Tactical Therapeutics. J. Zhu reports receiving commercial research grants from Immuncellular Therapeutics. J.M.Markert isanemployee of UABHS and UABHSF; reports receiving commercial research grants from ICT-107; and holds ownership interest (including patents) in Aettis, Treovir, and Mustang Biotech. K. Fink reports receiving other commercial research support from Boston Biomedical, Abbvie, Orbus, Diffusion, Cantex, Vascular Biogenics, and Five Prime, and is a consultant/advisory board member for UCB Pharma. G.J. Lesser reports receiving commercial research grants from NewLink Genetics, Vascular Biogenics, Incyte, and Novartis; is a consultant/advisory board member for Monteris and BTG; and reports receiving other remuneration from Stemline Therapeutics. J.S. Yu is an employee of and holds ownership interest (including patents) in Immunocellular Therpaeutics. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010–2014. Neuro Oncol 2017;19:v1–v88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med 2008;359: 492–507. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dosedense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 2013;31:4085–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 2017;318:2306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu G, Yuan X, Zeng Z, Tunici P, Ng H, Abdulkadir IR, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer 2006;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saikali S, Avril T, Collet B, Hamlat A, Bansard JY, Drenou B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol 2007;81:139–48. [DOI] [PubMed] [Google Scholar]
- 8.Phuphanich S, Wheeler CJ, Rudnick JD, Mazer M, Wang H, Nuño MA, et al. Phase I trial of a multi-epitope-pulsed dendritic cell vaccine for patients with newly diagnosed glioblastoma. Cancer Immunol Immunother 2013; 62:125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang JG, Eguchi J, Kruse CA, Gomez GG, Fakhrai H, Schroter S, et al. Antigenic profiling of glioma cells to generate allogeneic vaccines or dendritic cell-based therapeutics. Clin Cancer Res 2007;13:566–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu G, Ying H, Zeng G, Wheeler CJ, Black KL, Yu JS. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells. Cancer Res 2004;64:4980–6. [DOI] [PubMed] [Google Scholar]
- 11.Liu G, Yu JS, Zeng G, Yin D, Xie D, Black KL, et al. AIM-2: a novel tumor antigen is expressed and presented by human glioma cells. J Immunother 2004;27:220–6. [DOI] [PubMed] [Google Scholar]
- 12.Liu G, Khong HT, Wheeler CJ, Yu JS, Black KL, Ying H. Molecular and functional analysis of tyrosinase-related protein (TRP)-2 as a cytotoxic T lymphocyte target in patients with malignant glioma. J Immunother 2003; 26:301–12. [DOI] [PubMed] [Google Scholar]
- 13.Harada M, Li YF, El-Gamil M, Ohnmacht GA, Rosenberg SA, Robbins PF. Melanoma-Reactive CD8+ T cells recognize a novel tumor antigen expressed in a wide variety of tumor types. J Immunother 2001;24:323–33. [DOI] [PubMed] [Google Scholar]
- 14.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Ralpha2 is a glioma-restricted receptor for interleukin-13. Neoplasia 2002;4:388–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A0201-restricted, cytotoxic T lymphocyte epitope in a human glioma-associated antigen, interleukin 13 receptor alpha2 chain. Clin Cancer Res 2002;8:2851–5. [PubMed] [Google Scholar]
- 16.Xu Q, Liu G, Yuan X, Xu M, Wang H, Ji J, et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 2009;27:1734–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28:1963–72. [DOI] [PubMed] [Google Scholar]
- 18.Hwang IK, Shih WJ, De Cani JS. Group sequential designs using a family of type I error probability spending functions. Stat Med 1990;9:1439–45. [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld D The asymptotic properties of nonparametric tests for comparing survival distributions. Biometrika 1981;68:316–9. [Google Scholar]
- 20.Santos R, Buying A,Sabri N, Yu J,Gringeri A,Bender J, et al. Improvement of IFNγ ELISPOT performance following overnight resting of frozen PBMC samples confirmed through rigorous statistical analysis. Cells 2014;4: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britten CM, Janetzki S, Ben-Porat L, Clay TM, Kalos M, Maecker H, et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother 2009;58:1701–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 2015;16:e534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


