Abstract
Overexpression of ATP-binding cassette (ABC) transporters is one of the most important mechanisms responsible for the development of multidrug resistance (MDR). Selonsertib, a serine/threonine kinase inhibitor, targets apoptosis signal-regulating kinase 1 (ASK1) and is now in phase III clinical trial for the treatment of non-alcoholic steatohepatitis (NASH). In this study, we investigated whether selonsertib could reverse MDR-mediated by ABC transporters, including ABCB1, ABCG2, ABCC1 and ABCC10. The results showed that selonsertib significantly reversed ABCB1- and ABCG2-mediated MDR, but not MDR-mediated by ABCC1 or ABCC10. Mechanism studies indicated that the reversal effect of selonsertib was related to the attenuation of the efflux activity of ABCB1 and ABCG2 transporters, without the protein level decrease or change in the subcellular localization of ABCB1 or ABCG2. Selonsertib stimulated the ATPase activity of ABCB1 and ABCG2 in a concentration-dependent manner, and in silico docking study showed selonsertib could interact with the substrate-binding sites of both ABCB1 and ABCG2. This study provides a clue into a novel treatment strategy, which includes a combination of selonsertib with antineoplastic drugs to attenuate MDR-mediated by ABCB1 or ABCG2 in cancer cells overexpressing these transporters.
Keywords: Selonsertib, Multidrug resistance, ATP-Binding cassette transporter, ABCB1, ABCG2
1. Introduction
Chemotherapy is an important tool to combat a variety of cancers. However, multidrug resistance (MDR) in cancer cells remains a major challenge that contributes to the failure of cancer chemotherapy [1,2]. MDR in cancer leads to synchronous resistance of cancer cells to structurally unrelated anticancer drugs, and as a result, chemotherapy fails. There are several mechanisms contributing to cancer MDR, including reduced apoptosis, advanced DNA damage repair mechanisms, or altered drug metabolism. However, one important mechanism of MDR is mediated by the efflux pump protein, known as the ATP-binding cassette (ABC) transporters, which are located on the membrane of cancer cells [3,4].
The ABC transporters are one of the largest known protein families, which contain diverse groups of active membrane transporters with important physiological and pharmacological roles [5]. Divided into seven subfamilies from ABCA to ABCG, the human ABC protein family has 49 ABC proteins and 48 of them have functions [3,6]. Collectively, they transport and regulate levels of physiological substrates such as lipids, porphyrins and sterols [7], and are widely expressed in the placenta, blood-brain barrier (BBB), intestines, livers and kidneys to restrict the bioavailability of administered drugs [8,9]. The ABC transporters also play an important role in MDR, especially the ABCB1 (P-glycoprotein, P-gp), ABCG2 (breast cancer resistance protein, BCRP), ABCC1 (multidrug resistance-associated protein 1, MRP1), and ABCC10 (multidrug resistance-associated protein 7, MRP7). Briefly, the ABC transporters overexpressing on the membrane of cancer cells can pump out a series of chemotherapeutic drugs. For example, paclitaxel and doxorubicin are substrates of the ABCB1 transporter [10], while ABCG2 transporter can pump out mitoxantrone, SN-38, and topotecan [11,12]. By pumping out the substrate drugs of the cancer cells, the ABC transporters significantly decrease the intracellular concentration of certain anticancer drugs, and this becomes a major impediment to chemotherapy. It is well documented that the ABC transporters are highly associated with the level of chemotherapy and the progression of malignancy [13–17]. Thus, either decreasing the expression of ABC proteins or inhibiting the efflux function of ABC transporters by certain inhibitors is of great importance to reverse MDR in cancer cells [18].
Apoptosis signal-regulating kinase 1 (ASK1), a serine/threonine kinase that belongs to the mitogen-activated protein kinase kinase kinase (MAP3K) family, is involved in severe human diseases including neurodegenerative disorders, inflammatory diseases and cancer [19–23]. Selonsertib (GS-4997), a selective ASK1 inhibitor, has been found to significantly improve metabolic parameters associated with nonalcoholic steatohepatitis (NASH) and to reduce hepatic steatosis, inflammation, as well as fibrosis. Its phase III clinical trial has been initiated by the U.S. Food and Drug Administration (FDA) [24–26]. In this study, we discovered for the first time that selonsertib suppressed the efflux function of ABCB1 and ABCG2, which sensitized cancer cells to chemotherapeutic drugs.
2. Materials and methods
2.1. Chemicals
Selonsertib (GS-4997) was a gift from Chemie Tek (Indianapolis, IN). Bovine serum albumin (BSA), fetal bovine serum (FBS), Dulbecco’s modified Eagle’s Medium (DMEM), penicillin/streptomycin and 0.25% trypsin were purchased from Corning Incorporated (Corning, NY). GAPDH (MA5–15738), Alexa Fluor 488 conjugated goat anti-mouse IgG secondary antibody, SN-38 and MK571, were purchased from Thermo Fisher Scientific Inc ( Rockford, IL). The monoclonal antibodies for ABCG2 (BXP-21) were purchased from Millipore (Billerica, MA). The monoclonal antibodies for ABCB1 (F4), dimethylsulfoxide (DMSO), 3-(4,5-dimethylthiazol-yl)-2,5-diphenyltetrazolium bromide (MTT), Triton X-100, 4′,6-diam idino-2-phenylindole (DAPI), paraformaldehyde, paclitaxel, doxorubicin, cisplatin, vincristine, mitoxantrone and verapamil were purchased from Sigma-Aldrich (St. Louis, MO). Ko143 was a product from Enzo Life Sciences (Farmingdale, NY). [3H]-paclitaxel (15 Ci/mmol) and [3H]-mitoxantrone (2.5 Ci/mmol) were purchased from Moravek Biochemicals, Inc (Brea, CA). All other chemicals were purchased from Sigma Chemical Co (St. Louis, MO).
2.2. Cell lines and cell culture
We used the human epidermoid carcinoma cell line KB-3–1 and its colchicine-selected ABCB1-overexpressing KB-C2 cells, the human colon cancer cell line SW620 and its doxorubicin-selected ABCB1-overexpressing SW620/Ad300 cell line for ABCB1 reversal study. For ABCG2 reversal study, the non-small cell lung cancer (NSCLC) cell line NCI-H460 and its mitoxantrone-selected ABCG2-overexpressing NCI-H460/MX20 cells, as well as the human colon carcinoma cell line S1 and its mitoxantrone-selected derivative ABCG2-overexpressing S1-M1–80, were used. The KB-C2, SW620/Ad300, NCI-H460/MX20, and the S1-M1–80 cells were maintained as previously described [27,28]. HEK293/pcDNA3.1, HEK293/ABCG2–482-R2, HEK293/ABCG2–482-G2, and HEK293/ABCG2–482-T7 cells were transfected with either an empty vector pcDNA3.1 or a pcDNA3.1 vector containing a full length ABCG2 with Arginine, Glycine or Threonine at position 482. Transfected cells were selected with complete culture medium containing G418 (2 m g/m l).HEK293/ABCB1 and HEK293/ABCC1 transfected cells overexpressing ABCB1 and ABCC1, respectively, were obtained from Dr. Susan E. Bate’s lab (Columbia University, NY), and Dr. Michael Gottesman’s lab (NCI, NIH, Bethesda, MD), respectively [29,30]. HEK293/ABCC10 transfected cells overexpressing ABCC10 were selected with G418 (2 m g/m l) as previously described [31]. Each aforementioned cell line was cultured in DMEM medium containing 10% fetal bovine serum, 1% penicillin/streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. All cells were grown as an adherent monolayer and drug-resistant cell lines were grown in drug-free culture media for more than 2 weeks before assay.
2.3. Cell viability and reversal experiments
Cell viability and reversal fold were determined using MTT assay as described [32]. Briefly, for the reversal study, each type of cells was harvested and resuspended and seeded evenly into a 96-well plate at a final concentration of 5 × 103 cells per well in 160 μL medium. After incubating for 24 h, selonsertib was added 2 h prior to incubation with anticancer drugs. After 72 h of incubation, MTT solution (4 mg/ml) was added to each well and the cells were further incubated for 4 h. Then, the supernatant was discarded and 100 μL of DMSO was used to dissolve the formazan crystals. An accuSkanTM GO UV/Vis Microplate Spectrophotometer (Fisher Sci., Fair Lawn, NJ) was used to determine the absorbance at 570 nm. The concentration for 50% inhibition of cell viability (IC50) of the anticancer drug was calculated as previously described [33]. Verapamil (10 μM), Ko 143 (10 μM), MK571 (25 μM) and cepharanthine (10 μM) were used to reverse ABCB1-, ABCG2-, ABCC1- and ABCC10-mediated MDR, respectively as positive controls. Cisplatin, which is not a substrate of ABCB1, ABCG2, ABCC1, or ABCC10, was used as a negative control drug.
2.4. Western blotting analysis
Western blotting analysis was carried out as previously described [32,34]. In short, cells were incubated with or without selonsertib for varying amounts of time (0 h, 24 h, 48 h, and 72 h) before being lysed. Protein concentrations were determined with BCA Protein Assay Kit (Pierce, Rockford, IL). Equal amounts (20 μg) of proteins were subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to PVDF membranes (Millipore, Billerica, MA). The presence of ABCB1 and ABCG2 was determined using monoclonal antibody F4 (dilution 1:500) and BXP-21 (dilution 1:1000), respectively. GAPDH was used as a loading control. The resulting protein bands were analyzed using Image J software.
2.5. Immunofluorescence assay
The immunofluorescence assay was performed as we previously described [32]. After cultured overnight in 24-well plates, the cells (2 × 104) were treated with selonsertib for 0 h, 24 h, 48 h, and 72 h respectively. Then cells were fixed in 4% paraformaldehyde for 10 min and permeabilized by 0.1% Triton X-100 for 10 min before blocked with 6% BSA for 1 h. The presence of ABCB1 and ABCG2 was determined using monoclonal antibody F4 (dilution 1:100) and BXP-21 (dilution 1:150) respectively for incubation at 4 °C overnight. Alexa Fluor 488 conjugated secondary antibody (1:1000) was used after washing with iced PBS, DAPI was used to counterstain the nuclei. Immunofluorescence images were collected using an EVOS FL Auto fluorescence microscope (Life Technologies Corporation, Gaithersburg, MD).
2.6. ATPase assay
The vanadate-sensitive ATPase activity of ABCB1 and ABCG2 in membrane vesicles of High Five insect cells was measured as previously described [27,35]. In short, the membrane vesicles (10 μg) were incubated in ATPase assay buffer [50 mmoL/L MES (pH 6.8), 50 mmoL/L KCl, 5 mmoL/L sodium azide, 2 mmoL/L EGTA, 2 mmoL/L DTT, 1 mmoL/L ouabain, and 10 mmoL/L MgCl2] with or without 0.3 mmoL/L vanadate at 37 °C, followed by incubation with 0–40 μM of selonsertib at 37 °C for 3 min. Mg-ATP (5 mM) was used to start the ATPase reaction in 100 μL volume for 20 min at 37 °C and 100 μL of a 5% SDS solution was added to stop the reaction. The amount of inorganic phosphate (Pi) release was determined as described previously [36].
2.7. [3H]-Paclitaxel and [3H]-mitoxantrone accumulation assay
For the [3H]-paclitaxel accumulation assay, KB-3–1 and its drug resistance subline KB-C2, as well as SW620 and its drug resistance subline SW620/Ad300 were used. Briefly, 5 × 105 cells were cultured in 24-wells plates overnight before the assay, and selonsertib was added 2 h prior to the addition of [3H]-paclitaxel. After incubating with [3H]-paclitaxel with or without selonsertib for 2 h at 37 °C, cells were washed twice with iced PBS, and lysed with 0.25% trypsin before being placed in 5 mL scintillation fluid and radioactivity was measured in the Packard TRI-CARB 1900CA liquid scintillation analyzer (Packard Instrument, Downers Grove, IL).
NCI-H460, NCI-H46/MX20, S1 and S1-M1–80 cells were used for [3H]-mitoxantrone accumulation assay as previously described [37].
2.8. [3H]-Paclitaxel and [3H]-mitoxantrone efflux assay
For the efflux assay, cells were incubated with selonsertib for 2 h followed by incubation with [3H]-paclitaxel or [3H]-mitoxantrone with or without selonsertib for 2 h at 37 °C. The cells were washed with iced PBS twice and then lysed at various time point (0, 30, 60, and 120 min) with trypsin. Subsequently, cells were placed in 5 mL of scintillation fluid and radioactivity was measured in the Packard TRI-CARB 1900CA liquid scintillation analyzer (Packard Instrument, Downers Grove, IL). KB-3–1, KB-C2, SW620 and SW620/Ad300 cells were used for [3H]-paclitaxel efflux assay and NCI-H460, NCI-H46/MX20, S1, and S1-M1–80 cells were used for [3H]-mitoxantrone efflux assay [37].
2.9. Molecular modeling of human ABCB1 and ABCG2 and docking of selonsertib
All docking experiments were performed in Maestro v11.1 (Schrödinger, LLC, Cambridge, MA) as described previously [27,38]. Ligand preparation and protein preparation were essentially performed. Human ABCB1 homology model was established based on refined mouse ABCB1 (PDB ID: 4M1M), and the docking grid at drug-binding pocket was generated [39]. The grid of ABCG2 was generated by selecting residues at a substrate-binding pocket of ABCG2 (PDB ID: 5NJ3, selected residues: Phe432, Phe 439, Leu539, Ile543, Val546, and Met549) [40]. Glide XP docking was performed and the receptor grid for induced-fit docking (IFD) was generated by selecting the best scoring ligand. Then induced-fit docking was performed with the default protocol.
2.10. Statistical analysis
Data are expressed as the mean ± SD. For the efflux assays, data were analyzed using a two-way ANOVA, other data were analyzed using a one-way ANOVA by GraphPad Prism 7.00 software. All experiments were repeated at least three times. Differences were considered significant when p < 0.05.
3. Results
3.1. Selonsertib enhanced the sensitivity to anticancer drugs of cell lines overexpressing ABCB1 and ABCG2, but not cell lines overexpressing ABCC1 or ABCC10
First of all, to choose concentrations of selonsertib that would not significantly alter cell survival rate, we determined the toxicity of selonsertib in the cell lines used in this study. Concentrations of selonsertib below IC20 upon 72 h-incubation with cells were selected. Based on the results (Fig. 1 and Fig. 2), we conducted the following assays with selonsertib at concentration of 3 and 10 μM.
Fig. 1. Chemical structure of selonsertib and concentration-dependent viability curves for parental and ABCB1-overexpressing cell lines incubated with selonsertib.
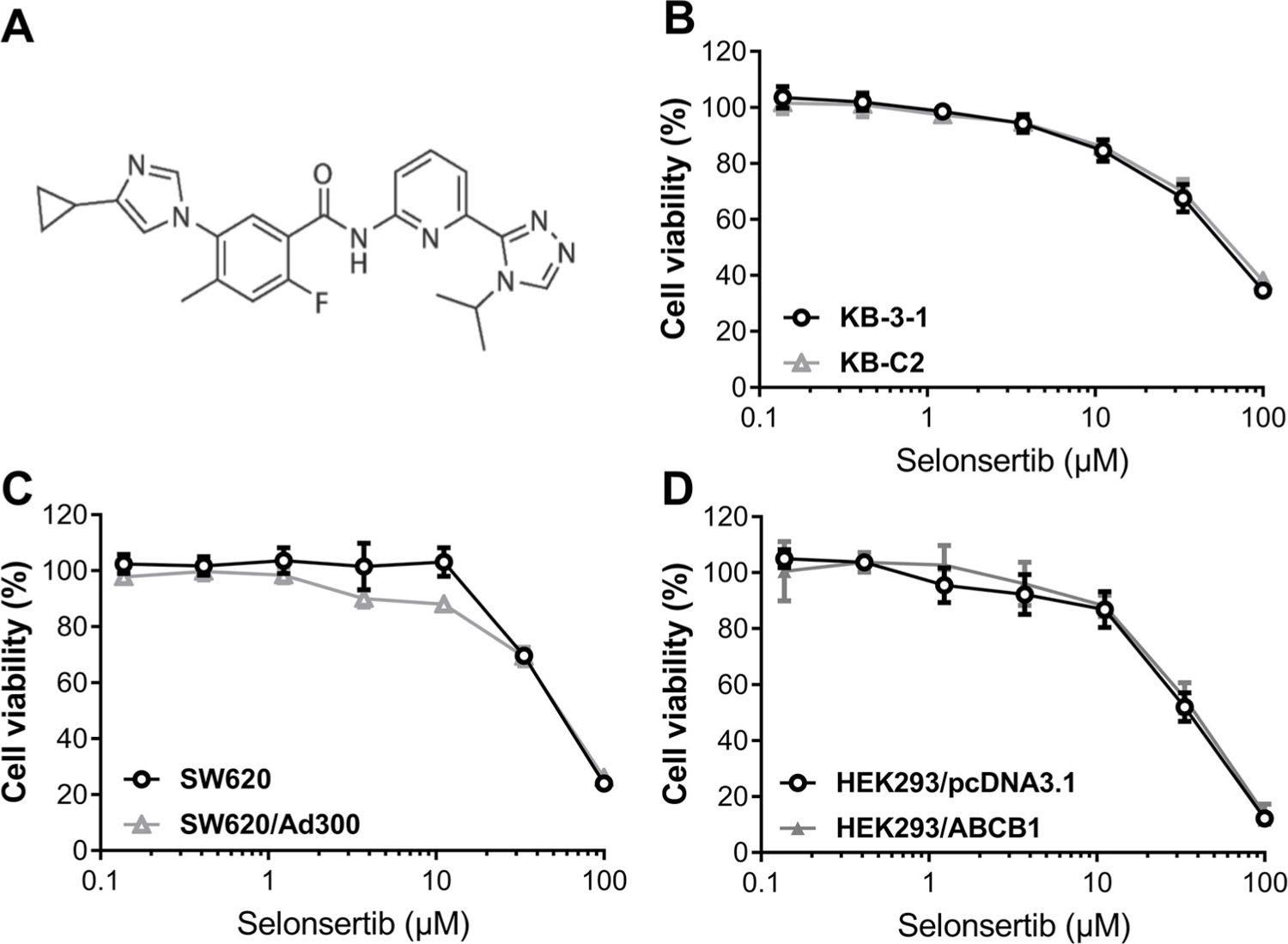
(A) Chemical structure of selonsertib. (B) Concentration-dependent viability curves for KB-3–1 and KB-C2 cell lines incubated with selonsertib for 72 h. (C) Concentration-dependent viability curves for SW620 and SW620/Ad300 cell lines incubated with selonsertib for 72 h. (D) Concentration-dependent viability curves for HEK293/pcDNA3.1 and HEK293/ABCB1 cell lines incubated with selonsertib for 72 h. The cell viability was determined by MTT assay. Data are expressed as mean ± SD, and representative of three independent experiments in triplicate are shown.
Fig. 2. Effect of selonsertib on the viability of parental and ABCG2-, ABCC1-, ABCC10-overexpressing cell lines.
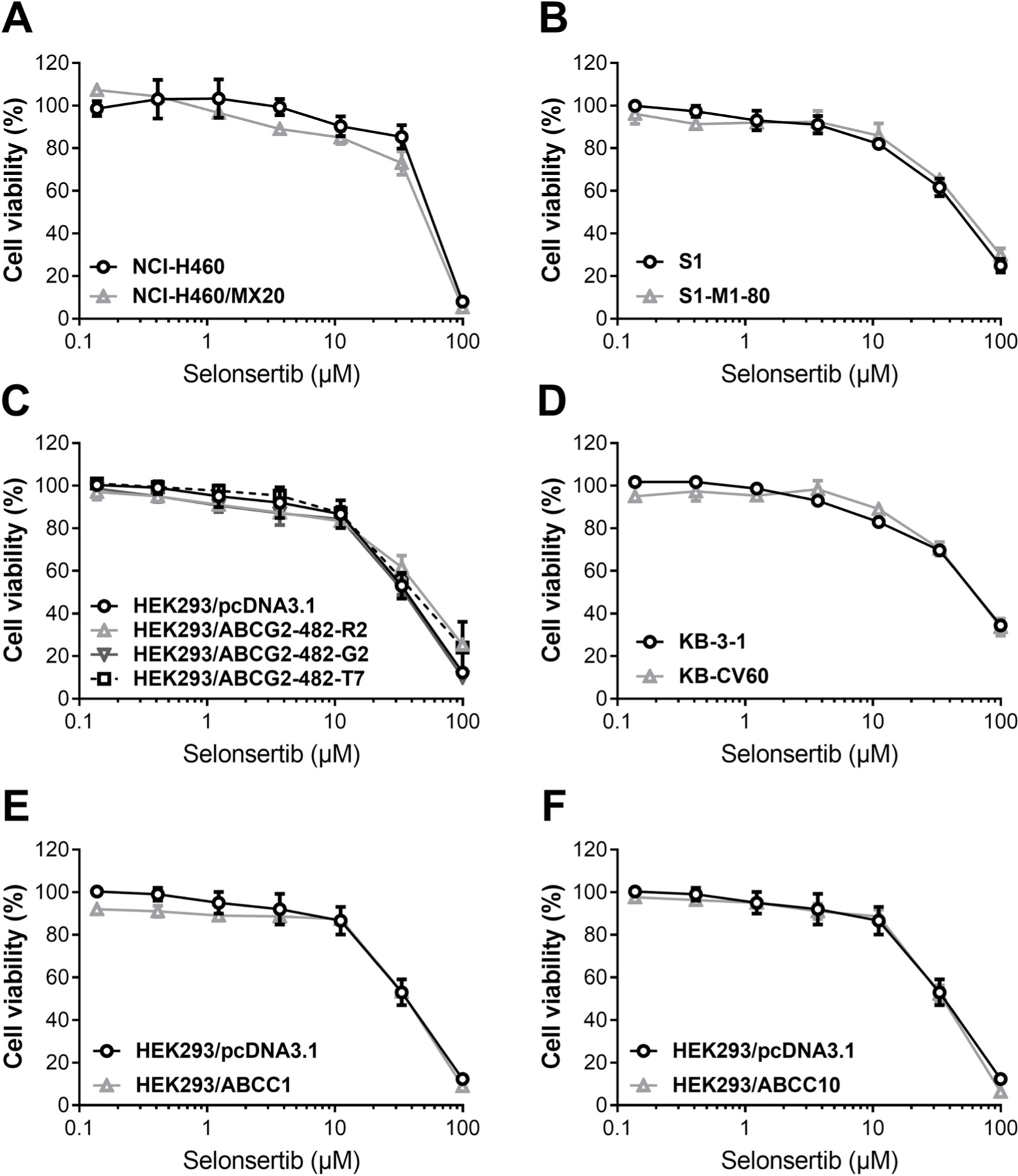
(A) Concentration-dependent viability curves for NCI-H460 and NCI-H460/MX20, (B) S1 and S1-M1–80, (C) HEK293/pcDNA3.1, HEK293/ABCG2–482-R2, HEK293/ABCG2–482-G2 and HEK293/ABCG2–482-T7, (D) KB-3–1 and KB-CV60, (E) HEK293/pcDNA3.1 and HEK293/ABCC1 and (F) HEK293/pcDNA3.1 and HEK293/ABCC10 cell lines incubated with selonsertib for 72 h. Other details same as given in the legend to Fig. 1.
As shown in Table 1, selonsertib significantly lowered the IC50 values of doxorubicin and paclitaxel to KB-C2 and SW620/Ad300 cells compared to their control cell lines in a dose-dependent manner. In Table 3, The IC50 values of mitoxantrone, topotecan and SN-38 to NCI-H460/MX20 and S1-M1–80 cells after treatment with selonsertib were much lower than those in untreated resistant cells. Similarly, selonsertib significantly increased the efficacy of doxorubicin and paclitaxel in HEK293/ABCB1 cell lines compared with that in the control resistant cells group (Table 2). Furthermore, the ABCG2-transfected cell lines ABCG2–482-R2, ABCG2–482-G2, and ABCG2–482-T7 were much more sensitive to mitoxantrone, topotecan and SN-38 after treatment with selonsertib compared with the control group (Table 4). However, selonsertib did not alter the sensitivity of KB-CV60, HEK293/ABCC1 or HEK293/ABCC10 to vincristine or paclitaxel (Tables 5–6). In addition, selonsertib did not significantly alter the cytotoxic effect of cisplatin, which is neither a substrate of ABCB1 nor ABCG2 (Tables 1–6). These results suggested that selonsertib could reverse ABCB1- and ABCG2-mediated MDR, but not MDR-mediated by ABCC1 or ABCC10.
Table 1.
Selonsertib sensitized ABCB1-substrate-selected resistant cell lines to ABCB1 substrates.
| Treatment | IC50 ± SDa (RFb) |
|||
|---|---|---|---|---|
| KB-3–1 (μM) | KB-C2 (μM) | SW620 (μM) | SW620/Ad300 (μΜ) | |
| Doxorubicin | 0.034 ± 0.004 (1.00) | 4.792 ± 0.809 (142.01) | 0.052 ± 0.011 (1.00) | 2.487 ± 0.785 (47.91) |
| +Selonsertib (3 μM) | 0.032 ± 0.002 (0.96) | 3.332 ± 0.616 (98.74) | 0.040 ± 0.002 (0.76) | 1.731 ± 0.103 (33.35) |
| +Selonsertib (10 μM) | 0.029 ± 0.002 (0.87) | 0.368 ± 0.077 (10.91)* | 0.055 ± 0.012 (1.07) | 0.367 ± 0.017 (7.07)* |
| +Verapamil (10 μM) | 0.020 ± 0.004 (0.60) | 0.166 ± 0.042 (4.91)* | 0.044 ± 0.013 (0.84) | 0.214 ± 0.044 (4.12)* |
| Paclitaxel | 0.004 ± 0.002 (1.00) | 3.007 ± 0.546 (799.31) | 0.026 ± 0.001 (1.00) | 1.587 ± 0.418 (60.44) |
| +Selonsertib (3 μM) | 0.003 ± 0.001 (0.82) | 2.291 ± 0.369 (609.04) | 0.023 ± 0.003 (0.88) | 1.059 ± 0.008 (40.34) |
| +Selonsertib (10 μM) | 0.003 ± 0.001 (0.70) | 0.125 ± 0.095 (33.23)* | 0.030 ± 0.002 (1.15) | 0.219 ± 0.174 (8.34)* |
| +Verapamil (10 μM) | 0.003 ± 0.002 (0.78) | 0.091 ± 0.004 (24.07)* | 0.021 ± 0.010 (0.78) | 0.092 ± 0.061 (3.51)* |
| Cisplatin | 1.381 ± 0.776 (1.00) | 2.267 ± 1.461 (1.64) | 1.159 ± 0.038 (1.00) | 1.303 ± 0.083 (1.12) |
| +Selonsertib (3 μM) | 1.734 ± 1.280 (1.26) | 2.405 ± 1.790 (1.74) | 1.134 ± 0.105 (0.98) | 1.588 ± 0.067 (1.37) |
| +Selonsertib (10 μM) | 1.917 ± 1.242 (1.39) | 2.546 ± 1.941 (1.84) | 1.488 ± 0.047 (1.28) | 1.140 ± 0.059 (0.98) |
| +Verapamil (10 μM) | 1.462 ± 0.681 (1.06) | 2.376 ± 1.846 (1.72) | 1.108 ± 0.031 (0.96) | 1.160 ± 0.176 (1.00) |
indicates p < 0.05 versus group treated with antineoplastic drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistant cells by the IC50 of parental cells in the absence of selonsertib or positive control inhibitor.
Table 3.
Selonsertib sensitized ABCG2-substrate-selected resistant cell lines to ABCG2 substrates.
| Treatment | IC50 ± SDa (RFb) |
|||
|---|---|---|---|---|
| NCI-H460 (μM) | NCI-H460/MX20 (μM) | S1 (μM) | S1-M1–80 (μΜ) | |
| Mitoxantrone | 0.038 ± 0.003 (1.00) | 5.139 ± 0.801 (135.01) | 0.033 ± 0.007 (1.00) | 2.700 ± 0.711 (81.52) |
| +Selonsertib (3 μM) | 0.031 ± 0.001 (0.82) | 0.557 ± 0.036 (14.63)* | 0.033 ± 0.014 (0.99) | 0.540 ± 0.521 (16.30)* |
| +Selonsertib (10 μM) | 0.027 ± 0.002 (0.71) | 0.385 ± 0.061 (10.12)* | 0.034 ± 0.009 (1.03) | 0.133 ± 0.082 (4.01)* |
| +Ko 143 (10 μM) | 0.030 ± 0.001 (0.80) | 0.428 ± 0.091 (11.24)* | 0.036 ± 0.015 (1.08) | 0.064 ± 0.018 (1.92)* |
| SN-38 | 0.039 ± 0.009 (1.00) | 3.493 ± 0.129 (88.80) | 0.047 ± 0.006 (1.00) | 2.336 ± 0.345 (50.04) |
| +Selonsertib (3 μM) | 0.021 ± 0.002 (0.53) | 0.463 ± 0.424 (11.77)* | 0.061 ± 0.005 (1.31) | 1.346 ± 0.674 (28.83)* |
| +Selonsertib (10 μM) | 0.023 ± 0.006 (0.58) | 0.178 ± 0.080 (4.51)* | 0.056 ± 0.013 (1.21) | 0.421 ± 0.359 (9.02)* |
| +Ko 143 (10 μM) | 0.018 ± 0.004 (0.46) | 0.104 ± 0.065 (2.65)* | 0.035 ± 0.014 (0.75) | 0.076 ± 0.014 (1.63)* |
| Topotecan | 0.066 ± 0.003 (1.00) | 4.113 ± 0.738 (62.72) | 0.065 ± 0.002 (1.00) | 2.492 ± 0.210 (38.54) |
| +Selonsertib (3 μM) | 0.049 ± 0.001 (0.75) | 0.909 ± 0.453 (13.86)* | 0.061 ± 0.004 (0.94) | 1.167 ± 0.733 (18.05)* |
| +Selonsertib (10 μM) | 0.046 ± 0.002 (0.70) | 0.311 ± 0.150 (4.74)* | 0.077 ± 0.023 (1.19) | 0.168 ± 0.184 (2.60)* |
| +Ko 143 (10 μM) | 0.047 ± 0.002 (0.71) | 0.212 ± 0.129 (3.23)* | 0.069 ± 0.004 (1.07) | 0.197 ± 0.075 (3.05)* |
| Cisplatin | 2.061 ± 0.500 (1.00) | 2.889 ± 0.861 (1.40) | 1.770 ± 0.285 (1.00) | 1.918 ± 0.462 (1.08) |
| +Selonsertib (3 μM) | 1.580 ± 0.281 (0.77) | 2.928 ± 1.224 (1.42) | 2.016 ± 0.262 (1.14) | 2.169 ± 0.304 (1.23) |
| +Selonsertib (10 μM) | 1.621 ± 0.165 (0.79) | 2.813 ± 0.695 (1.37) | 2.268 ± 0.212 (1.28) | 2.325 ± 0.283 (1.31) |
| +Ko 143 (10 μM) | 2.554 ± 1.115 (1.24) | 2.717 ± 1.453 (1.32) | 1.952 ± 0.191 (1.10) | 2.286 ± 0.255 (1.29) |
indicates p < 0.05 versus group treated with anticancer drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistance cells by the IC50 of parental cells in the of selonsertib or positive control inhibitor.
Table 2.
Selonsertib sensitized ABCB1-gene-transfected cell lines to ABCB1 substrates.
| Treatment | IC50 ± SDa (RFb) |
|
|---|---|---|
| HEK293/pcDNA3.1 (μM) | HEK293/ABCB1 (μM) | |
| Doxorubicin | 0.073 ± 0.007 (1.00) | 1.233 ± 0.511 (16.85) |
| +Selonsertib (3 μM) | 0.084 ± 0.001 (1.15) | 0.963 ± 0.275 (13.16) |
| +Selonsertib (10 μM) | 0.086 ± 0.011 (1.17) | 0.501 ± 0.236 (6.85)* |
| +Verapamil (10 μM) | 0.074 ± 0.004 (1.01) | 0.440 ± 0.113 (6.02)* |
| Paclitaxel | 0.047 ± 0.003 (1.00) | 2.684 ± 0.572 (57.62) |
| +Selonsertib (3 μM) | 0.053 ± 0.015 (1.13) | 1.716 ± 0.192 (36.85) |
| +Selonsertib (10 μM) | 0.039 ± 0.004 (0.84) | 0.116 ± 0.057 (2.48)* |
| +Verapamil (10 μM) | 0.037 ± 0.002 (0.79) | 0.106 ± 0.059 (2.28)* |
| Cisplatin | 1.209 ± 0.498 (1.00) | 1.283 ± 0.505 (1.06) |
| +Selonsertib (3 μM) | 0.991 ± 0.419 (0.82) | 1.469 ± 0.128 (1.22) |
| +Selonsertib (10 μM) | 0.906 ± 0.104 (0.75) | 1.581 ± 0.372 (1.31) |
| +Verapamil (10 μM) | 1.194 ± 0.595 (0.99) | 1.198 ± 0.329 (0.99) |
indicates p < 0.05 versus group treated with antineoplastic drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistance cells by the IC50 of parental cells in the absence of selonsertib or positive control inhibitor.
Table 4.
Selonsertib sensitized ABCG2-gene-transfected cell lines to ABCG2 substrates.
| Treatment | IC50 ± SDa (RFb) |
|||
|---|---|---|---|---|
| HEK293/pcDNA3.1 (μM) | HEK293/ABCG2–482-R2 (μM) | HEK293/ABCG2–482-G2 (μM) | HEK293/ABCG2–482-T7 (μM) | |
| Mitoxantrone | 0.032 ± 0.001 (1.00) | 0.513 ± 0.193 (15.99) | 0.693 ± 0.107 (21.58) | 0.520 ± 0.075 (16.19) |
| +Selonsertib (3 μM) | 0.034 ± 0.009 (1.06) | 0.299 ± 0.081 (9.30)* | 0.423 ± 0.055 (13.17)* | 0.310 ± 0.069 (9.67)* |
| +Selonsertib (10 μM) | 0.038 ± 0.004 (1.20) | 0.179 ± 0.079 (5.59)* | 0.043 ± 0.006 (1.32)* | 0.088 ± 0.025 (2.75)* |
| +Ko 143 (10 μM) | 0.043 ± 0.001 (1.35) | 0.054 ± 0.032 (1.69)* | 0.050 ± 0.010 (1.56)* | 0.033 ± 0.073 (1.04)* |
| SN-38 | 0.021 ± 0.006 (1.00) | 0.530 ± 0.070 (25.02) | 0.477 ± 0.043 (22.52) | 0.446 ± 0.064 (21.06) |
| +Selonsertib (3 μM) | 0.018 ± 0.010 (0.85) | 0.132 ± 0.282 (6.25)* | 0.266 ± 0.077 (12.55)* | 0.175 ± 0.100 (8.25)* |
| +Selonsertib (10 μM) | 0.026 ± 0.018 (1.23) | 0.103 ± 0.161 (4.89)* | 0.057 ± 0.028 (2.68)* | 0.068 ± 0.026 (3.21)* |
| +Ko 143 (10 μM) | 0.025 ± 0.015 (1.19) | 0.070 ± 0.297 (3.30)* | 0.075 ± 0.015 (3.54)* | 0.038 ± 0.008 (1.80)* |
| Topotecan | 0.044 ± 0.010 (1.00) | 0.670 ± 0.031 (15.27) | 0.617 ± 0.109 (14.05) | 0.747 ± 0.280 (17.01) |
| +Selonsertib (3 μM) | 0.046 ± 0.009 (1.04) | 0.244 ± 0.032 (5.55)* | 0.283 ± 0.013 (6.45)* | 0.265 ± 0.152 (6.02)* |
| +Selonsertib (10 μM) | 0.042 ± 0.009 (0.96) | 0.096 ± 0.038 (2.18)* | 0.031 ± 0.002 (0.71)* | 0.126 ± 0.100 (2.87)* |
| +Ko 143 (10 μM) | 0.047 ± 0.011 (1.07) | 0.058 ± 0.023 (1.32)* | 0.030 ± 0.011 (0.69)* | 0.043 ± 0.017 (0.98)* |
| Cisplatin | 1.230 ± 0.066 (1.00) | 1.329 ± 0.453 (1.08) | 1.367 ± 1.163 (1.11) | 1.831 ± 0.548 (1.49) |
| +Selonsertib (3 μM) | 1.170 ± 0.239 (0.95) | 1.355 ± 0.132 (1.10) | 1.613 ± 1.919 (1.31) | 1.384 ± 0.381 (1.13) |
| +Selonsertib (10 μM) | 1.385 ± 0.078 (1.13) | 1.729 ± 0.190 (1.41) | 2.064 ± 1.863 (1.68) | 1.773 ± 0.422 (1.44) |
| +Ko 143 (10 μM) | 1.400 ± 0.363 (1.14) | 1.838 ± 0.207 (1.50) | 1.787 ± 1.022 (1.45) | 2.574 ± 1.096 (2.09) |
indicates p < 0.05 versus group treated with antineoplastic drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistant cells by the IC50 of parental cells in the absence of selonsertib or positive control inhibitor.
Table 5.
Selonsertib did not affect ABCC1-mediated MDR.
| Treatment | IC50 ± SDa (RFb) |
|||
|---|---|---|---|---|
| KB-3–1 (μM) | KB-CV60 (μM) | HEK293/pcDNA3.1 (μM) | HEK293/ABCC1 (μM) | |
| Vincristine | 0.011 ± 0.003 (1.00) | 0.217 ± 0.012 (18.99) | 0.038 ± 0.007 (1.00) | 0.356 ± 0.169 (9.39) |
| +Selonsertib (3 μM) | 0.016 ± 0.006 (1.38) | 0.195 ± 0.003 (17.11) | 0.033 ± 0.001 (0.88) | 0.341 ± 0.130 (8.98) |
| +Selonsertib (10 μM) | 0.014 ± 0.002 (1.23) | 0.234 ± 0.079 (20.46) | 0.031 ± 0.002 (0.81) | 0.369 ± 0.062 (9.73) |
| +MK571 (25 μM) | 0.009 ± 0.003 (0.80) | 0.109 ± 0.003 (9.51)* | 0.035 ± 0.001 (1.09) | 0.045 ± 0.008 (1.18)* |
| Cisplatin | 1.681 ± 0.369 (1.00) | 2.421 ± 0.369 (1.44) | 1.343 ± 0.549 (1.00) | 1.646 ± 0.998 (1.23) |
| +Selonsertib (3 μM) | 2.332 ± 0.398 (1.39) | 2.232 ± 0.398 (1.33) | 1.369 ± 0.623 (1.02) | 1.554 ± 0.828 (1.16) |
| +Selonsertib (10 μM) | 2.138 ± 0.460 (1.27) | 2.392 ± 0.460 (1.42) | 1.279 ± 0.384 (0.95) | 1.474 ± 0.939 (1.10) |
| +MK571 (25 μM) | 1.647 ± 0.374 (0.98) | 2.056 ± 0.374 (1.22) | 1.162 ± 0.469 (0.87) | 1.306 ± 0.365 (0.97) |
indicates p < 0.05 versus group treated with antineoplastic drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistant cells by the IC50 of parental cells in the absence of selonsertib or positive control inhibitor.
Table 6.
Selonsertib did not affect ABCC10-mediated MDR.
| Treatment | IC50 ± SDa (RFb) |
|
|---|---|---|
| HEK293/pcDNA3.1 (μM) | HEK293/ABCC10 (μM) | |
| Paclitaxel | 0.041 ± 0.005 (1.00) | 0.457 ± 0.146 (11.15) |
| +Selonsertib (3 μM) | 0.043 ± 0.002 (1.05) | 0.611 ± 0.105 (14.90) |
| +Selonsertib (10 μM) | 0.037 ± 0.002 (0.90) | 0.460 ± 0.177 (11.22) |
| +Cepharanthine (10 μM) | 0.038 ± 0.003 (0.93) | 0.054 ± 0.005 (1.32)* |
| Cisplatin | 1.042 ± 0.645 (1.00) | 1.313 ± 0.731 (1.26) |
| +Selonsertib (3 μM) | 1.124 ± 0.728 (1.08) | 1.137 ± 0.540 (1.09) |
| +Selonsertib (10 μM) | 1.132 ± 0.635 (1.09) | 1.346 ± 0.649 (1.29) |
| +Cepharanthine (10 μM) | 1.153 ± 0.669 (1.11) | 1.238 ± 0.373 (1.19) |
indicates p < 0.05 versus group treated with antineoplastic drug only.
IC50 values were determined by MTT assay as described in “materials and methods”, and were obtained from three independent experiments in triplicate.
Resistance fold (RF) was calculated from dividing the IC50 values of parental cells or resistant cells by the IC50 of parental cells in the absence of selonsertib or positive control inhibitor.
3.2. Selonsertib did not affect the protein expression or subcellular localization of ABCB1 or ABCG2 transporters
Since selonsertib reversed ABCB1- and ABCG2-mediated MDR, the mechanisms may involve down-regulation of the protein expression and/or change of the subcellular localization of the transporter. Therefore, we performed Western blotting and immunofluorescence assay to determine whether selonsertib could alter the expression level and/or the subcellular localization of ABCB1 and ABCG2 transporters. As shown in Fig. 3, when cells were incubated for 24, 48, and 72 h, selonsertib did not significantly alter the expression level of ABCB1 protein (170 kDa) in ABCB1-overexpressing KB-C2 or SW620/Ad300 cell lines. Likewise, the expression level of ABCG2 protein (72 kDa) in ABCG2-overexpressing cell lines NCI-H460/MX20 and S1-M1–80 was not altered significantly by selonsertib up to 72 h (Fig. 3). As shown in Fig. 4, ABCB1 and ABCG2 transporters were located on the membrane of KB-C2 and NCI-H460/MX20 cells after treatment with selonsertib for 24–72 h, indicating that selonsertib did not alter subcellular localization of the ABCB1 or ABCG2 transporters. In this study, KB-3–1 and SW620, NCI-H460 and S1 cells were used as negative controls that did not express ABCB1 and ABCG2 transporters (Figs. 3 and 4).
Fig. 3. Selonsertib did not affect the protein expression of ABCB1 or ABCG2 transporters in ABCB1 or ABCG2 overexpressing cell lines.
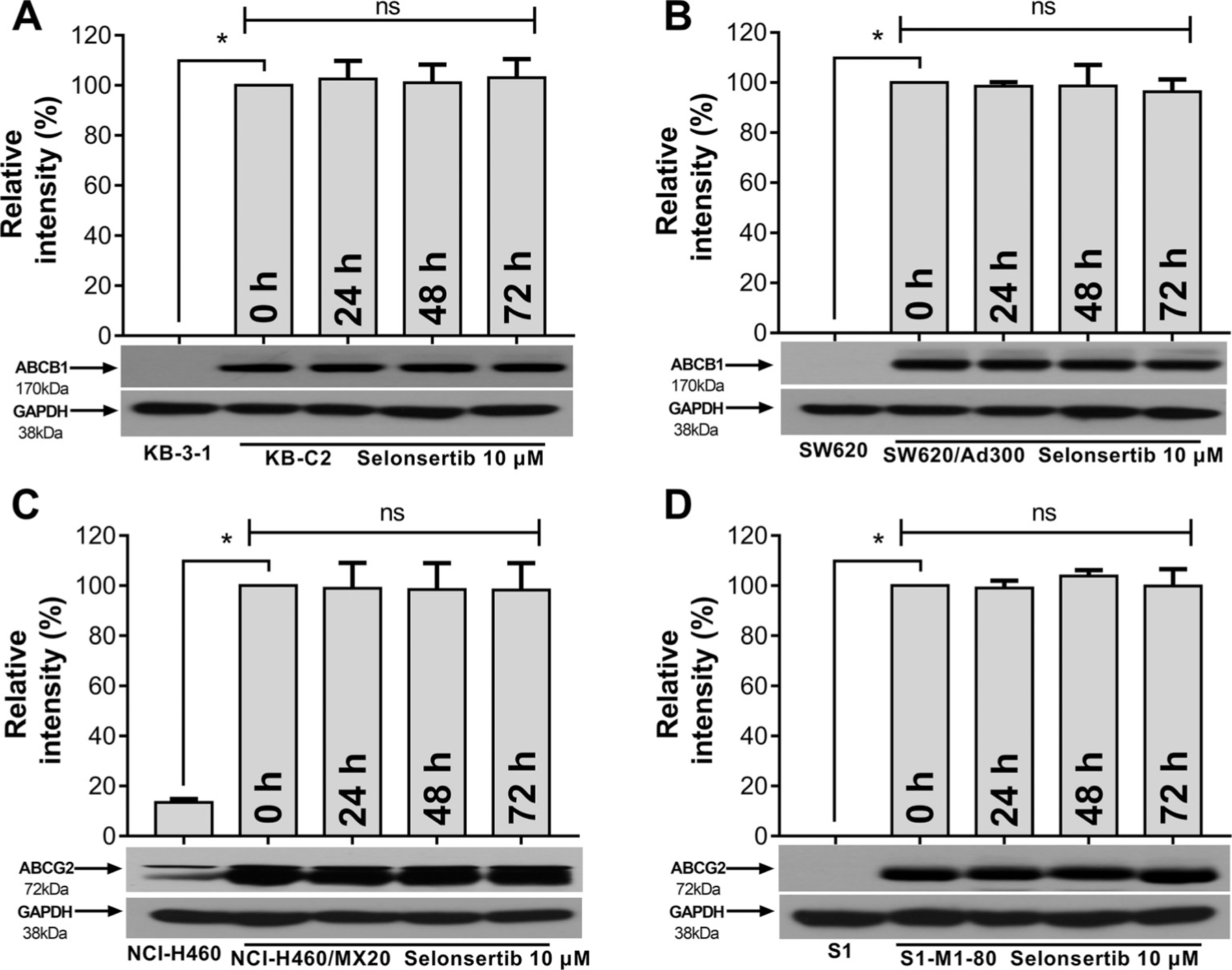
Detection and relative intensity of ABCB1 expression in KB-C2 (A) and SW620/Ad300 (B) cells incubated with 10 μM of selonsertib for 0, 24, 48, and 72 h. Detection and relative intensity of ABCG2 expression in NCI-H460/MX20 (C) and S1-M1–80 (D) cells incubated with 10 μM of selonsertib for 0, 24, 48, and 72 h. Data are mean ± SD, representative of three independent experiments. *p < 0.05, compared with control group (KB-3–1 [A], SW620 [B], NCI-H460 [C] and S1 [D]).
Fig. 4. Selonsertib did not affect the localization of ABCB1 or ABCG2 transporters in ABCB1 or ABCG2 overexpressing cell lines.
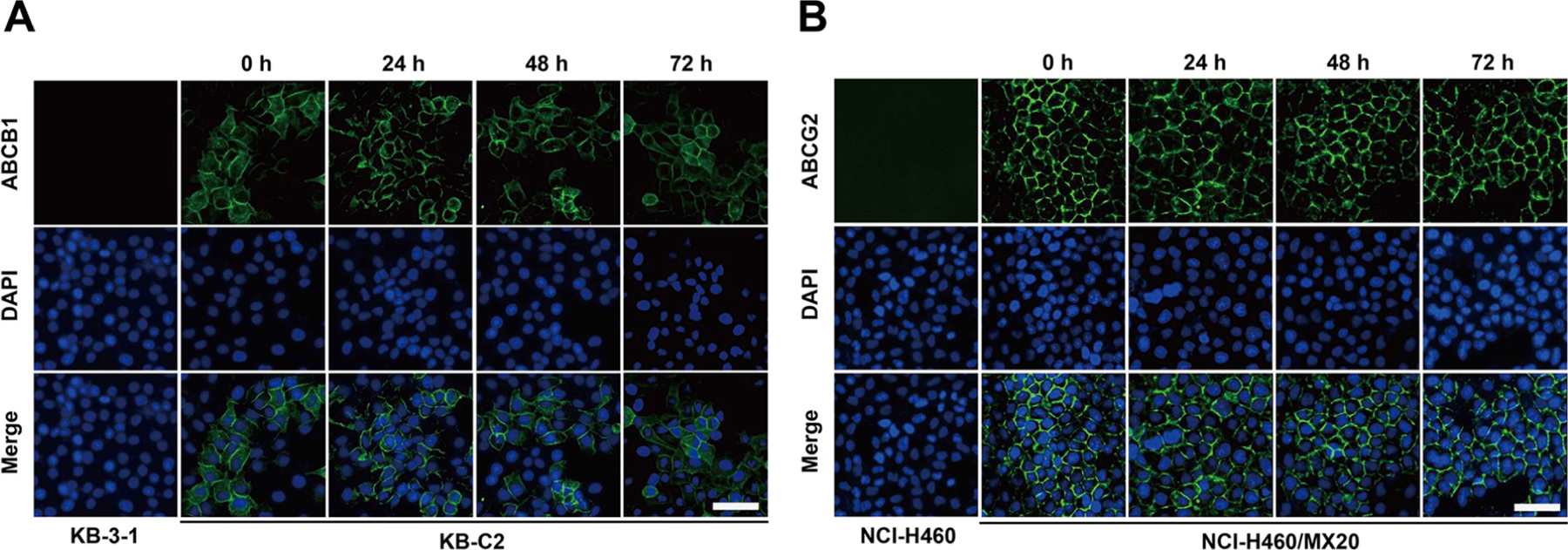
Sub-cellular localization of ABCB1 expression in KB-C2 (A) and ABCG2 expression in NCI-H460/MX20 (B) cells incubated with 10 μM of selonsertib for 0, 24, 48, and 72 h. ABCB1, green and ABCG2, blue. DAPI counterstains the nuclei. KB-3–1 and NCI-H460 represented the control group; Scale bar: 200 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
3.3. Selonsertib increased the intracellular drug accumulation in cancer cell lines overexpressing ABCB1 and ABCG2
The above results demonstrate that selonsertib significantly reversed ABCB1- and ABCG2-mediated MDR without altering their protein expression or subcellular localization. To gain insight into the mechanisms of action of selonsertib, drug accumulation assays were performed. The intracellular level of [3H]-paclitaxel and [3H]-mitoxantrone were measured respectively in cell lines overexpressing ABCB1 and ABCG2 transporters in the presence or absence of selonsertib. As shown in (Fig. 5A and B), selonsertib significantly increased the intracellular levels of [3H]-paclitaxel in KB-C2 and SW620/Ad300 cell lines, which overexpress ABCB1 transporters, but not in their parental cell lines KB-3–1 or SW620. Similarly, the intracellular level of [3H]-mitoxantrone in ABCG2-overexpressing cell lines NCI-H460/MX20 and S1-M1–80 significantly increased after treatment with selonsertib, compared to their parental cell lines NCI-H460 and S1 which showed a negative result (Fig. 5C and D). In short, these results suggested that selonsertib may increase the intracellular accumulation of anticancer drugs by inhibiting the function of ABCB1 and ABCG2 transporters.
Fig. 5. Selonsertib increased the intracellular [3H]-drug accumulation in cancer cells overexpressing ABCB1 and ABCG2.
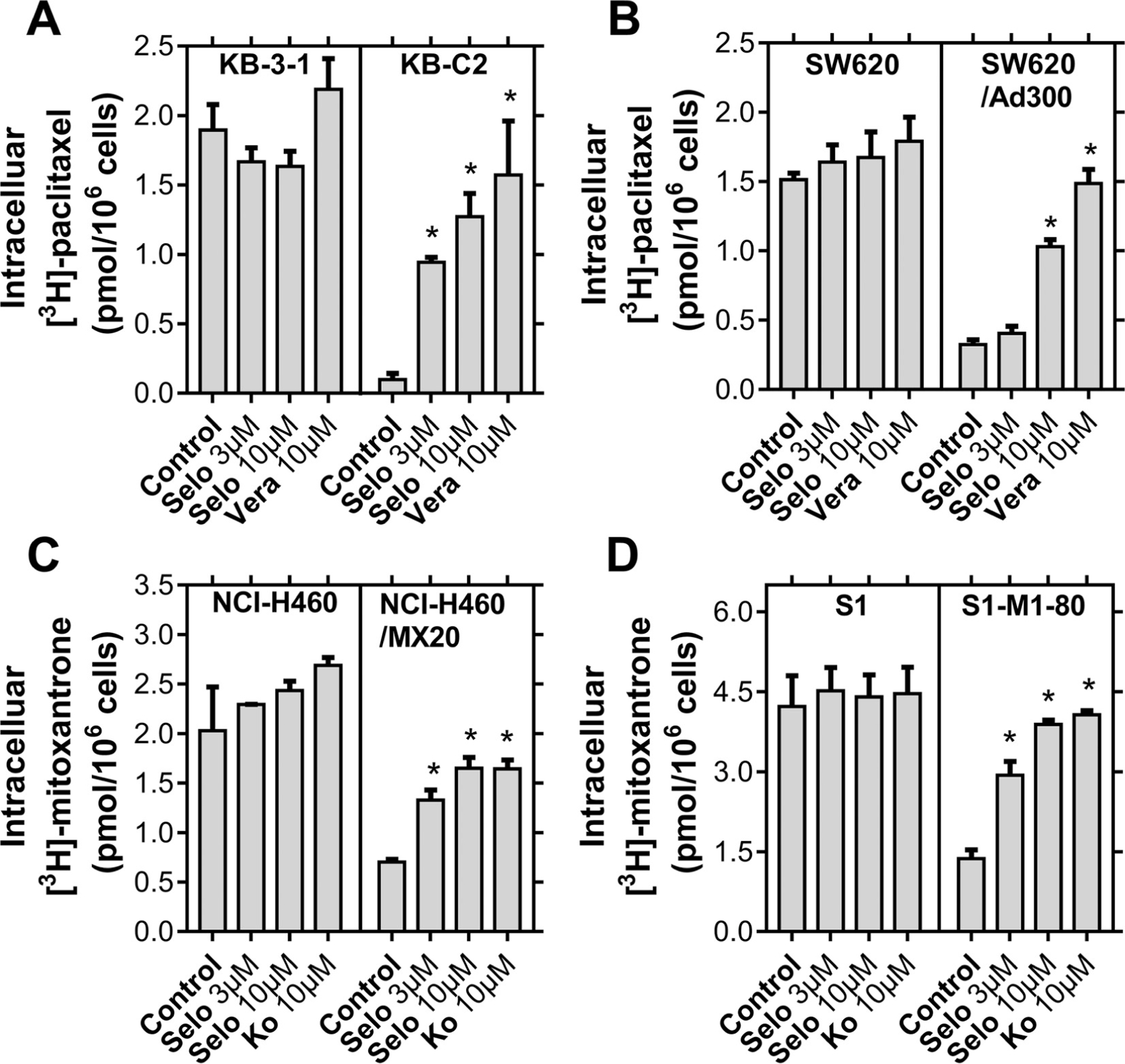
(A) The effect of selonsertib on the accumulation of [3H]-paclitaxel in KB-3–1 and KB-C2 cell lines. (B) The effect of selonsertib on the accumulation of [3H]-paclitaxel in SW620 and SW620/Ad300 cell lines. (C) The effect of selonsertib on the accumulation of [3H]-mitoxantrone in NCI-H460 and NCI-H460/MX20 cell lines. (D) The effect of selonsertib on the accumulation of [3H]-mitoxantrone in S1 and S1-M1–80 cell lines. Verapamil (10 μM) and Ko 143 (10 μM) were used as positive controls for ABCB1 and ABCG2 overexpressing cells respectively. Data are mean, representative of three independent experiments. *p < 0.05, compared with control group. Selo, selonsertib; Vera, verapamil and Ko, Ko 143.
3.4. Selonsertib inhibited the efflux function mediated by ABCB1 and ABCG2 transporters in cancer cell lines
To further understand the mechanism of selonsertib in reversing ABCB1- and ABCG2-mediated MDR, we performed the efflux assay to determine the effect of selonsertib on the efflux function of ABCB1 and ABCG2 transporters. As shown in Fig. 6B, D, F, and H, selonsertib significantly decreased the efflux of [3H]-paclitaxel in ABCB1-overexpressing cell lines KB-C2 and SW620/Ad300, and [3H]-mitoxantrone efflux in ABCG2 overexpressing cell lines NCI-H460/MX20 and S1-M1–80. However, selonsertib did not significantly alter the efflux of [3H]-paclitaxel or [3H]-mitoxantrone in parental cell lines KB-3–1, SW620, NCI-H460, or S1 (Fig. 6A, C, E, G). These results suggested that selonsertib could increase the accumulation of anticancer drugs by impeding the efflux function mediated by ABCB1 and ABCG2.
Fig. 6. Selonsertib inhibited the efflux function of ABCB1 and ABCG2 transporters.
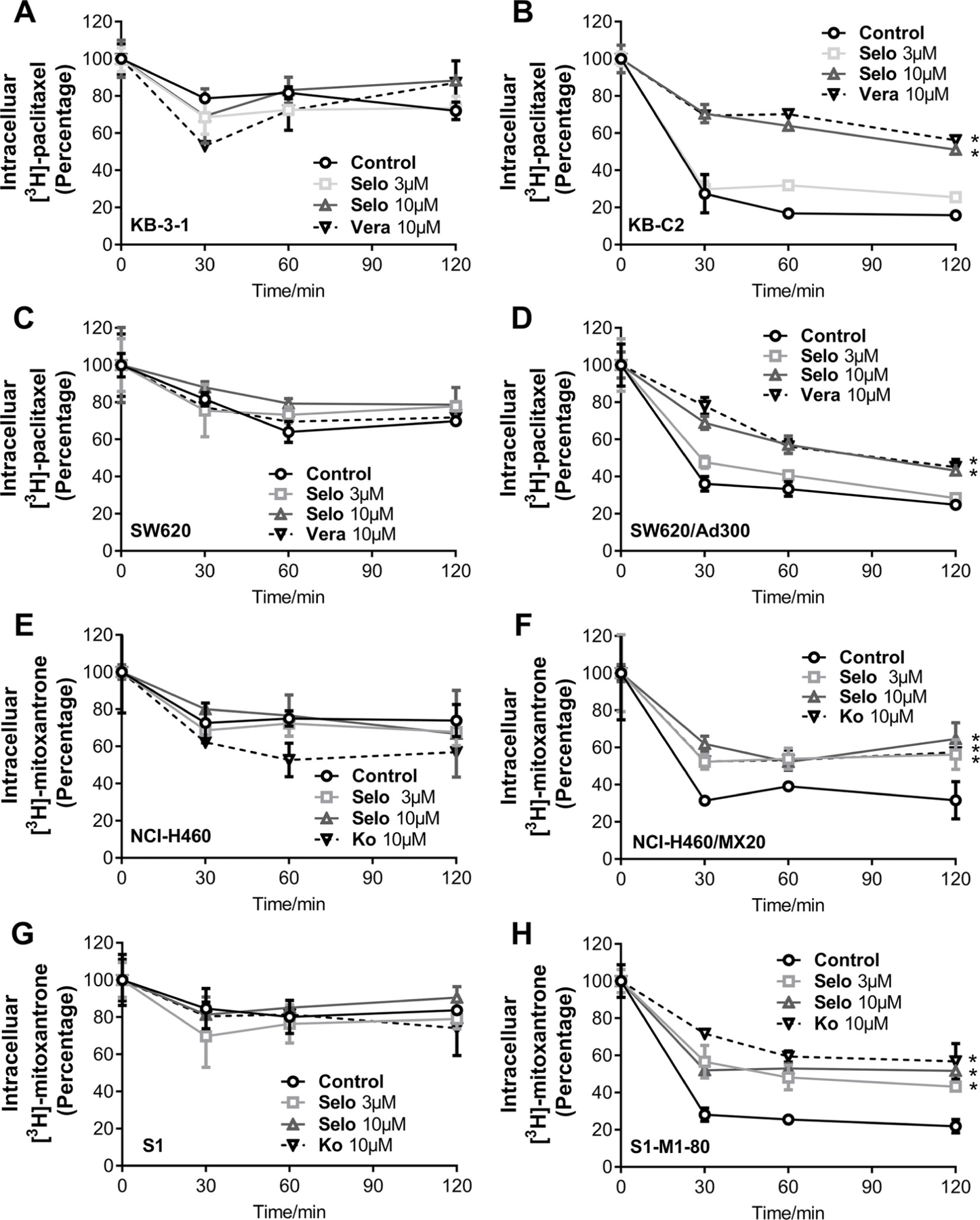
The effect of selonsertib on efflux of [3H]-paclitaxel in KB-3–1 and KB-C2 (A, B) and SW620 and SW620/Ad300 (C, D) cells. The effect of selonsertib on efflux of [3H]-mitoxantrone in NCI-H460 and NCI-H460/MX20 (E, F) and S1 and S1-M1–80 (G, H) cells. Data are mean ± SD, representative of three independent experiments. *p < 0.05, compared with control group. Selo, selonsertib; Vera, verapamil and Ko, Ko 143.
3.5. Selonsertib stimulated the ATPase activity of ABCB1 and ABCG2
To determine the effect of selonsertib on the ATPase activity of ABCB1 and ABCG2 transporters, we measured ABCB1- and ABCG2-mediated ATP hydrolysis in the presence or absence of selonsertib (0–40 μM). As shown in Fig. 7A, selonsertib stimulated the ATPase activity of ABCB1 transporters in a dose-dependent manner. The concentration of selonsertib required to obtain 50% of maximal stimulation (EC50) was 4.29 μM and the maximum of stimulation was 4.22-fold. Similarly, selonsertib also stimulated the ATPase activity of ABCG2 transporters (Fig. 7B), the concentration of selonsertib required to obtain EC50 was 2.58 μM, with 4.72-fold of maximum stimulation. These results suggested that selonsertib stimulates the ATPase activity of ABCB1 and ABCG2 transporters by interacting at the drug-binding pocket of these transporters.
Fig. 7. Selonsertib stimulated the ATPase activity of ABCB1 and ABCG2.
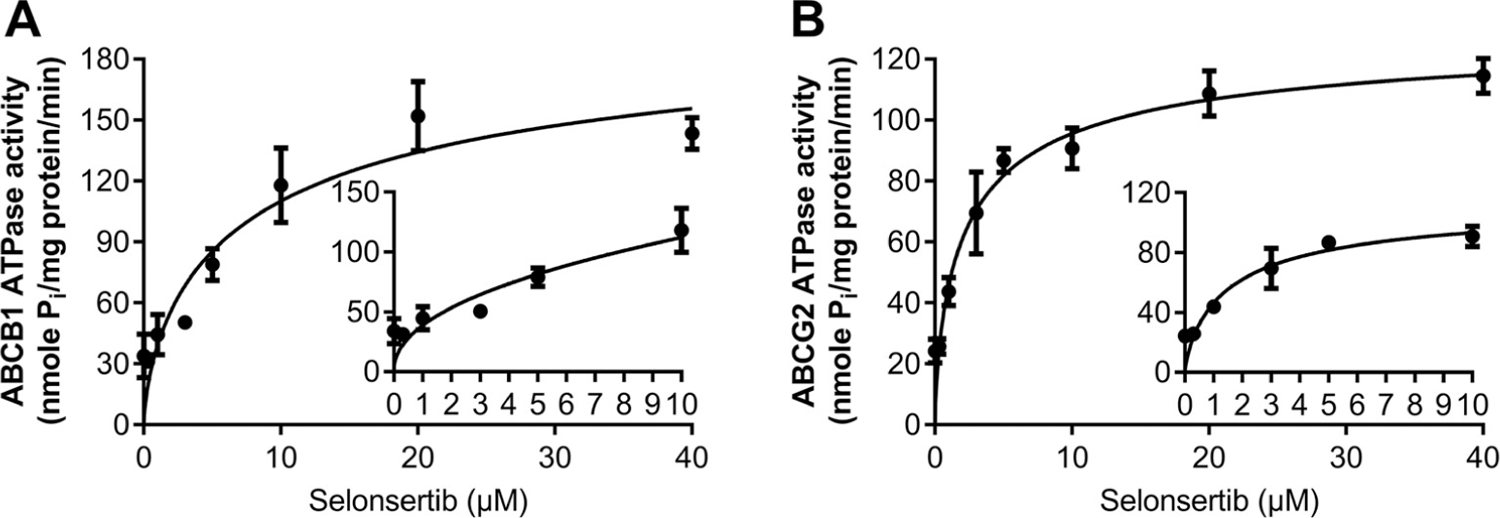
Effect of various concentrations of selonsertib on the ATPase activity of ABCB1 (A) and ABCG2. The inset graphs illustrate the effect of 0–10 μM selonsertib on the ATPase activity of ABCB1 (A) and ABCG2 (B). Data are mean ± SD, representative of three independent experiments.
3.6. Docking analysis of the binding of selonsertib with human ABCB1 homology model and ABCG2 model
The best-scored docked position of selonsertib within the drug-binding pocket in the transmembrane region of homology model of human ABCB1 and cryo-EM structure of human ABCG2 (PDB.5NJ3) are shown in Fig. 8. The docking score of the binding of selonsertib to ABCB1 is −11.094 kcaL/mol and that of the binding of selonsertib to the drug-binding pocket of ABCG2 is −12.278 kcaL/mol, suggesting that selonsertib shows good affinity to both ABCB1 and human ABCG2. Fig. 8A shows that there are two π-π interactions between selonsertib and residues in the drug-binding pocket of ABCB1. Both the phenol ring and the imidazole ring of selonsertib have π-π interactions with Phe957 of ABCB1. Interestingly, the nitrogen in imidazole group of selonsertib was ionized and formed a π-cation bond with Phe72 in the transmembrane helix 1. Tumor has a major feature of acidic microenvironment [41], so acidic extracellular pH could ionize selonsertib and generate the π-cation bond with ABCB1. Besides the interactions mentioned above, selonsertib could be stabilized in the drug-binding pocket of ABCB1 by residues including Leu65, Met68, Met69, Phe72, Ile340, Phe336, Tyr950, Gln946, Phe983, Phe978, Phe983 (Fig. 8C). In Fig. 8B, there are both hydrogen bonding interaction and π-π interaction in the binding of selonsertib with ABCG2. The imidazole ring and phenyl ring of selonsertib have π-π interaction with Phe439 in the monomers A and B, respectively. Residue Asn436 in the B chain have hydrogen bonding interaction with the triazole group (C=N⋯H2N-Asn436) and amide group (O=C-N-H⋯O=C-Asn436), respectively. Moreover, selonsertib has both hydrophobic and hydrophilic effect with the residues in the substrate-binding pocket of ABCG2 (Fig. 8D), such as Gln398, Thr401, Phe431, Thr435, Asn436, Ile543, Val546 and Met549.
Fig. 8. In silico docking of selonsertib with homology model of human ABCB1 and human ABCG2.
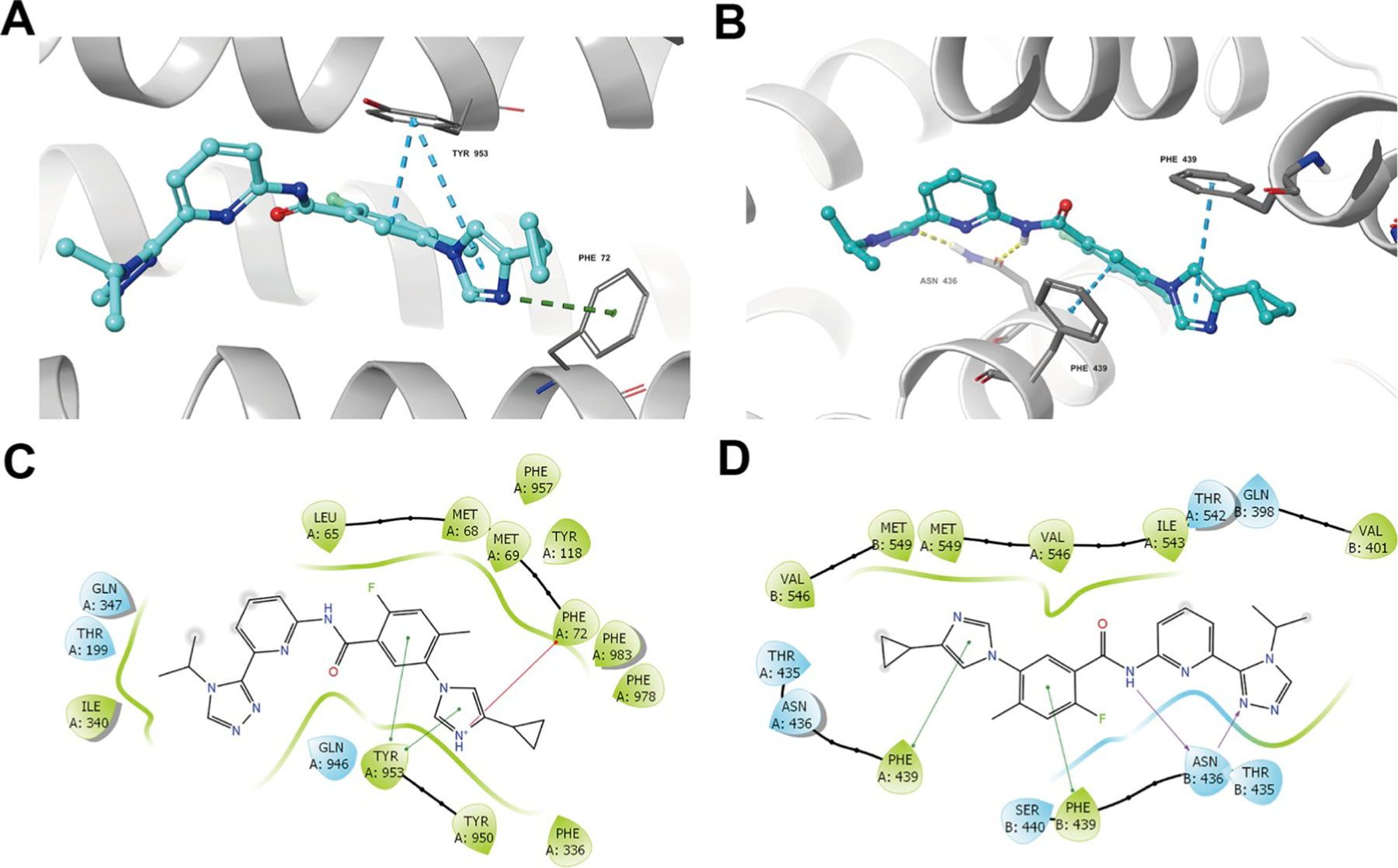
(A) Docked position of selonsertib within the drug-binding site of ABCB1. (B) Docked position of selonsertib within the drug-binding site of human ABCG2. Selonsertib is shown as ball and stick mode with the atoms colored: carbon-cyan, hydrogen-white, nitrogen-blue, oxygen-red, fluorine-green, hydrogen-white. Important residues are shown as sticks with orange color. π-π stacking interactions are indicated with cyan dotted short line. Hydrogen bonds are shown by the yellow dotted line. The two-dimensional ligand-receptor interaction diagram of selonsertib and ABCB1 (C) and ABCG2 (D). The amino acids within 3 Å are shown as colored bubbles, cyan indicates polar residues, and green indicates hydrophobic residues. π-π stacking interactions are indicated with green short line. Hydrogen bonds are shown by the purple arrow. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
ABC transporters play an important role in restricting the bioavailability of administered drugs. Unfortunately, abundant research has shown that the ABC transporters expressed on the plasma membrane of cancer cells are responsible for MDR, which finally leads to the failure of chemotherapy [1–3,5,6,18]. Hence, reversing MDR by a combination of a chemotherapeutic drug and a reversal agent against the function of ABC transporters is a potential pharmacological approach for increasing the efficiency of chemotherapy in cancer patients. In recent years, research has shown that a series of small-molecule targeted drugs have the capacity to reverse ABC transporter-mediated MDR, including EGFR inhibitor gefitinib, erlotinib, AG1478, PD153035 and dacomitinib, an EGFR and HER-2 inhibitor lapatinib, a pan-HER inhibitor canertinib, a BCR-ABL inhibitor imatinib, a Bruton tyrosine kinase (BTK) inhibitor ibrutinib and certain multi-kinase inhibitor such as sunitinib [27,30,42–46]. However, there is hardly any research about the potential reversal activity of ASK1 inhibitors against ABC transporter-mediated MDR.
Selonsertib (GS-4997) is a highly selective and potent ASK1 inhibitor with potential anti-inflammatory, antineoplastic and anti-fibrotic activities, and is currently in a phase III clinical trial for the treatment of NASH. In a multicenter phase II trial study, higher rates of fibrosis improvement and lower rates of fibrosis progression in selonsertib-treated patients were observed than patients treated with simtuzumab alone over a 24-week treatment period, suggesting that selonsertib may reduce liver fibrosis in patients with NASH and moderate to severe fibrosis [24]. However, we did not find any clinical information about the antitumor effect of selonsertib alone, or in combination with anticancer drugs. Here we report for the first time that selonsertib shows a significant effect ABCB1- and ABCG2-mediated MDR at a non-toxic concentration.
In this study, our main finding was that selonsertib, at a non-toxic concentration, significantly sensitized ABCB1- and ABCG2-verexpressing cancer cells to their substrates, respectively, in a dose-dependent manner. However, the re-sensitizing effects were not observed in drug-resistant cells overexpressing ABCC1 or ABCC10. To avoid possible bias caused by selonsertib induced-cytotoxicity in evaluating its reversal effects, MTT assays were performed to get the relatively non-toxic concentration of selonsertib for the cells used in this work. Based on the cytotoxicity results, 3 and 10 μM of selonsertib were selected for the reversal studies. Our results indicated that selonsertib significantly increased the efficacy of doxorubicin and paclitaxel to the ABCB1 overexpressing KB-C2 and SW620/Ad300 cells compared to untreated control resistant cells in a dose-dependent manner. Likewise, selonsertib also significantly reduced the IC50 values of substrate-drugs in HEK293/ABCB1 cells in a dose-dependent manner. In addition, selonsertib sensitized ABCG2 overexpressing cell lines NCI-H460/MX20, and S1-M1–80, and the ABCG2-transfected HEK293 subline ABCG2–482-R2, ABCG2–482-G2, and ABCG2–482-T7 to mitoxantrone, topotecan, and SN-38 in a concentration-dependent manner. However, selonsertib, up to 10 μM, did not significantly sensitize the parental cells such as KB-3–1, SW620, NCI-H460, S1, or HEK293/pcDNA3.1 cells. Moreover, there was no significant alteration in sensitivity of cancer cells to cisplatin, which was neither an ABCB1 nor ABCG2 substrate. Furthermore, at 3 and 10 μM, selonsertib did not significantly alter the IC50 value of substrate drug to ABCC1-overexpressing KB-CV60 cells or ABCC1-transfected HEK293 subline or ABCC10-transfected HEK293 subline. These findings suggested that the reversal effect of selonsertib was specific to ABCB1- and ABCG2-mediated MDR.
The reversal of ABC transporter-mediated MDR may due to the down-regulation of ABC protein expression level and/or change of subcellular localization, so we performed Western blotting and immunofluorescence assay to investigate the potential mechanisms. However, there was no significant decrease in the protein level of ABCB1 or ABCG2 transporters after treatment with selonsertib (10 μM) up to 72 h. Likewise, selonsertib at 10 μM did not significantly change the ABCB1 or ABCG2 transporters subcellular localization after incubating for up to 72 h, suggesting that the reversal effects of selonsertib on MDR were not related to alteration of the protein level or subcellular localization of ABC transporters. However, it has been reported that the MAPK pathways, which is the target of selonsertib is involved in the regulation of ABCB1 and ABCG2 expression [47,48]. Further studies are required to determine the indirect effect of selonsertib on the expression of ABCB1 and ABCG2 at a higher concentration and a longer incubation time. Moreover, we could not exclude the possibility that part of the reversal effect of selonsertib could involve its effect on some other proteins and/or cross-talk with other proteins, which may affect the function of ABCB1 and ABCG2, this also needs to be studied further.
To gain insight into the mechanism of selonsertib on ABC transporter-mediated MDR attenuation, drug accumulation and efflux assays were performed. We found that selonsertib significantly increased the intracellular [3H]-paclitaxel concentration in ABCB1-overexpressing KB-C2 and SW620/Ad300 cells, and [3H]-mitoxantrone in ABCG2-overexpressing NCI-H460/MX20 and S1-M1–80 cells, in a dose-dependent manner, while no significant [3H]-drugs alteration was found in their corresponding parental cells. Furthermore, selonsertib significantly prevented [3H]-drugs being pumped out of ABCB1- and ABCG2-overexpressing cells in a dose-dependent manner, while no significant change of efflux was observed in their corresponding parental cells. The results of the substrate accumulation and efflux experiments were congruent with the reversal effects of selonsertib shown in anti-cancer efficacy testing when co-administered with substrate-drugs, demonstrating that selonsertib increased the accumulation of substrate-drugs in ABCB1- and ABCG2-overexpressing cancer cells by inhibiting the efflux activity of ABCB1- and ABCG2. These results are also consistent with our studies of other small-molecule reversal reagents [42,49,50].
It is known that the function of ABC transporters requires the energy from the hydrolysis of ATP by the transporter, which can be modulated by the presence of substrates or inhibitors [51,52]. Our results indicated that selonsertib stimulated the ATPase activity of both ABCB1 and ABCG2, to the maximal level of 4.22 and 4.72-fold for ABCB1 and ABCG2, respectively.
Although selonsertib modulated ATPase activity of ABCB1 and ABCG2, its binding site on these transporters still remain unclear, so molecular docking of selonsertib to the substrate-binding sites of ABCB1 and ABCG2 was performed. Modeling study suggested that selonsertib could interact with the drug-binding pocket in the transmembrane domain (TMD) of both ABCB1 and ABCG2 with docking scores of −11.094 kcaL/mol and −12.278 kcaL/mol respectively, and hydrogen bonding and π-π interactions were predicted between selonsertib and residues lining the drug-binding pocket of ABCB1 and ABCG2. In summary, these results, suggest that selonsertib acts as a potential competitive substrate or modulator that displaces chemotherapeutic drugs from the drug-binding pocket of ABCB1 and ABCG2 transporters, thereby inhibiting the efflux function of ABCB1 and ABCG2, resulting in increased accumulation of anticancer substrate-drugs and sensitization of drug-resistant cancer cells.
Clinical resistance to chemotherapy in a series of cancers is strongly associated with the overexpression of some of the ABC transporters. The overexpression of ABCB1 and ABCG2 in cancers may come with poor prognosis and high risk of death [53–57]. It has been reported that the genetic polymorphisms in ABCB1 and ABCG2 significantly increased the risk of death in patients with colorectal cancer or NSCLC [58,59]. Doxorubicin is used for treatment of a wide range of cancers including colorectal cancer [60], and topotecan is used for treatment of NSCLC relapse [61]. Furthermore, in a clinical study, patients with increased ABCG2-expressing colorectal cancer were significantly less sensitized to SN-38 compared to patients who had lower expression level of ABCG2 in their tumor [62]. Our study provides a clue that the combination of selonsertib with ABCB1 or ABCG2 substrate-drugs, like doxorubicin or topotecan as well as SN-38, could be a novel treatment strategy to attenuate the drug resistance in cancer patients.
In conclusion, this study demonstrates that selonsertib reverses ABCB1- and ABCG2-mediated MDR by inhibiting the efflux of anticancer drugs by ABC drug transporters such as ABCB1 and ABCG2. Thus, the combination of selonsertib with substrate-drugs of ABCB1 and ABCG2 for cancer treatment could be useful to evade MDR.
Acknowledgements
We would like to thank Chemie Tek (Indianapolis, IN) for providing us the selonsertib compound. We would like to thank Dr. Stephen Aller (The University of Alabama at Birmingham, Birmingham) for kindly providing the human ABCB1 homology model. We thank Tanaji T. Talele (St. John’s University, New York, NY) for providing the computing resources for the docking analysis. We thank Drs. Susan E. Bates and Robert W. Robey (NCI, NIH, Bethesda, MD) for providing the cell lines. We thank Dr. Yangmin Chen for editorial assistance. The first author thanks China Scholarship Council for providing financial assistance for stay in America.
Funding
This work was supported by St. John’s University Research Seed Grant (No.579–1110-7002) (ZSC), grant from National Natural Science Foundation of China (81673464), grant for Major Project of Tianjin for New Drug Development (17ZXXYSY00050) (DK), the Postgraduate Innovation Fund of '13th Five-Year comprehensive investment', Tianjin Medical University (YJSCX201712) (NJ), and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (SS and SVA).
Footnotes
Conflicts of interest
The authors have declared no potential conflicts of interest.
References
- [1].Kartal-Yandim M, Adan-Gokbulut A, Baran Y, Molecular mechanisms of drug resistance and its reversal in cancer, Crit. Rev. Biotechnol 36 (2016) 716–726. [DOI] [PubMed] [Google Scholar]
- [2].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Targeting multidrug resistance in cancer, Nature reviews, Drug Discov 5 (2006) 219–234. [DOI] [PubMed] [Google Scholar]
- [3].Eckford PD, Sharom FJ, ABC efflux pump-based resistance to chemotherapy drugs, Chem. Rev 109 (2009) 2989–3011. [DOI] [PubMed] [Google Scholar]
- [4].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat. Rev. Canc 2 (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [5].Dassa E, Bouige P, The ABC of ABCS: a phylogenetic and functional classification of ABC systems in living organisms, Res. Microbiol 152 (2001) 211–229. [DOI] [PubMed] [Google Scholar]
- [6].Stavrovskaya AA, Stromskaya TP, Transport proteins of the ABC family and multidrug resistance of tumor cells, Biochem. Biokhimiia 73 (2008) 592–604. [DOI] [PubMed] [Google Scholar]
- [7].Wu CP, V.A. S, The pharmacological impact of ATP-binding cassette drug transporters on vemurafenib-based therapy, Acta Pharm. Sin. B 4 (2014) 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Linton KJ, Higgins CF, Structure and function of ABC transporters: the ATP switch provides flexible control, Pflueg. Arch. Eur. J. Physiol 453 (2007) 555–567. [DOI] [PubMed] [Google Scholar]
- [9].Linton KJ, Structure and function of ABC transporters, Physiology 22 (2007) 122–130. [DOI] [PubMed] [Google Scholar]
- [10].Kim SW, Kwon HY, Chi DW, Shim JH, Park JD, Lee YH, Pyo S, Rhee DK, Reversal of P-glycoprotein-mediated multidrug resistance by ginsenoside Rg(3), Biochem. Pharmacol 65 (2003) 75–82. [DOI] [PubMed] [Google Scholar]
- [11].Lemos C, Jansen G, Peters GJ, Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors, Br. J. Canc 98 (2008) 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Marchetti S, de Vries NA, Buckle T, Bolijn MJ, van Eijndhoven MA, Beijnen JH, Mazzanti R, van Tellingen O, Schellens JH, Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/− (triple-knockout) and wild-type mice, Mol. Canc. Therapeut 7 (2008) 2280–2287. [DOI] [PubMed] [Google Scholar]
- [13].Liu YS, Hsu HC, Tseng KC, Chen HC, Chen SJ, Lgr5 promotes cancer stemness and confers chemoresistance through ABCB1 in colorectal cancer, Biomed. Pharmacother.= Biomed. Pharmacotherapie 67 (2013) 791–799. [DOI] [PubMed] [Google Scholar]
- [14].Ali MA, Elsalakawy WA, ABCB1 haplotypes but not individual SNPs predict for optimal response/failure in Egyptian patients with chronic-phase chronic myeloid leukemia receiving imatinib mesylate, Med. Oncol 31 (2014) 279. [DOI] [PubMed] [Google Scholar]
- [15].Xie ZY, Lv K, Xiong Y, Guo WH, ABCG2-meditated multidrug resistance and tumor-initiating capacity of side population cells from colon cancer, Oncol. Res. Treat 37 (2014) 666–668 670–662. [DOI] [PubMed] [Google Scholar]
- [16].Liu L, Zuo LF, Guo JW, ABCG2 gene amplification and expression in esophageal cancer cells with acquired adriamycin resistance, Mol. Med. Rep 9 (2014) 1299–1304. [DOI] [PubMed] [Google Scholar]
- [17].Yang B, Ma YF, Liu Y, Elevated expression of Nrf-2 and ABCG2 involved in multidrug resistance of lung cancer SP cells, Drug Res 65 (2015) 526–531. [DOI] [PubMed] [Google Scholar]
- [18].Shukla S, Wu CP, Ambudkar SV, Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges, Expet Opin. Drug Metabol. Toxicol 4 (2008) 205–223. [DOI] [PubMed] [Google Scholar]
- [19].Nishida T, Hattori K, Watanabe K, The regulatory and signaling mechanisms of the ASK family, Adv. Biol. Regul 66 (2017) 2–22. [DOI] [PubMed] [Google Scholar]
- [20].Ryuno H, Naguro I, Kamiyama M, ASK family and cancer, Adv. Biol. Regul 66 (2017) 72–84. [DOI] [PubMed] [Google Scholar]
- [21].Takeda K, Noguchi T, Naguro I, Ichijo H, Apoptosis signal-regulating kinase 1 in stress and immune response, Annu. Rev. Pharmacol. Toxicol 48 (2008) 199–225. [DOI] [PubMed] [Google Scholar]
- [22].Hayakawa R, Hayakawa T, Takeda K, Ichijo H, Therapeutic targets in the ASK1-dependent stress signaling pathways, Proc. Jpn. Acad. B Phys. Biol. Sci 88 (2012) 434–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fujisawa T, Therapeutic application of apoptosis signal-regulating kinase 1 inhibitors, Adv. Biol. Regul 66 (2017) 85–90. [DOI] [PubMed] [Google Scholar]
- [24].Loomba R, Lawitz E, Mantry PS, Jayakumar S, Caldwell SH, Arnold H, Diehl AM, Djedjos CS, Han L, Myers RP, Subramanian GM, McHutchison JG, Goodman ZD, Afdhal NH, Charlton MR, Investigators G-U, The ASK1 Inhibitor Selonsertib in Patients with Nonalcoholic Steatohepatitis: a Randomized, Phase 2 Trial, Hepatology, 2017. [DOI] [PMC free article] [PubMed]
- [25].Younossi ZM, Stepanova M, Lawitz E, Charlton M, Loomba R, Myers RP, Subramanian M, McHutchison JG, Goodman Z, Improvement of hepatic fibrosis and patient-reported outcomes in non-alcoholic steatohepatitis treated with selonsertib, Liver Int.: Offic. J. Int. Assoc. Stud. Liver 38 (10) (2018. October) 1849–1859. [DOI] [PubMed] [Google Scholar]
- [26].Gawrieh S, Chalasani N, Emerging treatments for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis, Clin. Liver Dis 22 (2018) 189–199. [DOI] [PubMed] [Google Scholar]
- [27].Fan YF, Zhang W, Zeng L, Lei ZN, Cai CY, Gupta P, Yang DH, Cui Q, Qin ZD, Chen ZS, Trombetta LD, Dacomitinib antagonizes multidrug resistance (MDR) in cancer cells by inhibiting the efflux activity of ABCB1 and ABCG2 transporters, Cancer Lett. 421 (2018) 186–198. [DOI] [PubMed] [Google Scholar]
- [28].Zhao XQ, Dai CL, Ohnuma S, Liang YJ, Deng W, Chen JJ, Zeng MS, Ambudkar SV, Chen ZS, Fu LW, Tandutinib (MLN518/CT53518) targeted to stem-like cells by inhibiting the function of ATP-binding cassette subfamily G member 2, Eur. J. Pharmaceut. Sci.: Offic. J. Eur. Fed. Pharmaceut. Sci 49 (2013) 441–450. [DOI] [PubMed] [Google Scholar]
- [29].Newman MJ, Rodarte JC, Benbatoul KD, Romano SJ, Zhang C, Krane S, Moran EJ, Uyeda RT, Dixon R, Guns ES, Mayer LD, Discovery and characterization of OC144–093, a novel inhibitor of P-glycoprotein-mediated multidrug resistance, Cancer Res 60 (2000) 2964–2972. [PubMed] [Google Scholar]
- [30].Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B, Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters, Drug Resist. Updates: Rev. Comment. Antimicrob. Anticancer Chemothe 8 (2005) 15–26. [DOI] [PubMed] [Google Scholar]
- [31].Chen ZS, Hopper-Borge E, Belinsky MG, Shchaveleva I, Kotova E, Kruh GD, Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10), Mol. Pharmacol 63 (2003) 351–358. [DOI] [PubMed] [Google Scholar]
- [32].Zhang XY, Zhang YK, Wang YJ, Gupta P, Zeng L, Xu M, Wang XQ, Yang DH, Chen ZS, Osimertinib (AZD9291), a mutant-selective EGFR inhibitor, reverses ABCB1-mediated drug resistance in cancer cells, Molecules (2016) 21. [DOI] [PMC free article] [PubMed]
- [33].Zhang YK, Zhang H, Zhang GN, Wang YJ, Kathawala RJ, Si R, Patel BA, Xu J, Chen ZS, Semi-synthetic ocotillol analogues as selective ABCB1-mediated drug resistance reversal agents, Oncotarget 6 (2015) 24277–24290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shi Z, Peng XX, Kim IW, Shukla S, Si QS, Robey RW, Bates SE, Shen T, Ashby CR Jr., Fu LW, Ambudkar SV, Chen ZS, Erlotinib (Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B member 1 and ATP-binding cassette subfamily G member 2-mediated drug resistance, Cancer Res 67 (2007) 11012–11020. [DOI] [PubMed] [Google Scholar]
- [35].Wang YJ, Zhang YK, Zhang GN, Al Rihani SB, Wei MN, Gupta P, Zhang XY, Shukla S, Ambudkar SV, Kaddoumi A, Shi Z, Chen ZS, Regorafenib overcomes chemotherapeutic multidrug resistance mediated by ABCB1 transporter in colorectal cancer: in vitro and in vivo study, Cancer Lett 396 (2017) 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ambudkar SV, Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammaliancells, Methods Enzymol 292 (1998) 504–514. [DOI] [PubMed] [Google Scholar]
- [37].Sun YL, Kathawala RJ, Singh S, Zheng K, Talele TT, Jiang WQ, Chen ZS, Zafirlukast antagonizes ATP-binding cassette subfamily G member 2-mediated multidrug resistance, Anti Canccer Drugs 23 (2012) 865–873. [DOI] [PubMed] [Google Scholar]
- [38].Zhang YK, Zhang GN, Wang YJ, Patel BA, Talele TT, Yang DH, Chen ZS, Bafetinib (INNO-406) reverses multidrug resistance by inhibiting the efflux function of ABCB1 and ABCG2 transporters, Sci. Rep 6 (2016) 25694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li J, Jaimes KF, Aller SG, Refined structures of mouse P-glycoprotein, Protein Sci.: Publ. Protein Soc 23 (2014) 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP, Structure of the human multidrug transporter ABCG2, Nature 546 (2017) 504–509. [DOI] [PubMed] [Google Scholar]
- [41].Kato Y, Ozawa S, Miyamoto C, Maehata Y, Suzuki A, Maeda T, Baba Y, Acidic extracellular microenvironment and cancer, Cancer Cell Int 13 (2013) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang GN, Zhang YK, Wang YJ, Gupta P, Ashby CR Jr. , Alqahtani S, Deng T, Bates SE, Kaddoumi A, Wurpel JND, Lei YX, Chen ZS, Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: in vitro and in vivo, Cancer Lett. 424 (2018) 19–29. [DOI] [PubMed] [Google Scholar]
- [43].Shukla S, Robey RW, Bates SE, Ambudkar SV, Sunitinib (Sutent, SU11248), a small-molecule receptor tyrosine kinase inhibitor, blocks function of the ATP-binding cassette (ABC) transporters P-glycoprotein (ABCB1) and ABCG2, Drug Metabol. Dispos.: Biol Fate Chem 37 (2009) 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR Jr., Chen X, Chen ZS, Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the ac-tivity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters, Biochem. Pharmacol 78 (2009) 153–161. [DOI] [PubMed] [Google Scholar]
- [45].Erlichman C, Boerner SA, Hallgren CG, Spieker R, Wang XY, James CD, Scheffer GL, Maliepaard M, Ross DD, Bible KC, Kaufmann SH, The HER tyrosine kinase inhibitor CI1033 enhances cytotoxicity of 7-ethyl-10-hydro-xycamptothecin and topotecan by inhibiting breast cancer resistance protein-mediated drug efflux, Cancer Res 61 (2001) 739–748. [PubMed] [Google Scholar]
- [46].Dai CL, Tiwari AK, Wu CP, Su XD, Wang SR, Liu DG, Ashby CR Jr., Huang Y, Robey RW, Liang YJ, Chen LM, Shi CJ, Ambudkar SV, Chen ZS, Fu LW, Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2, Cancer Res 68 (2008) 7905–7914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wang X, Campos CR, Peart JC, Smith LK, Boni JL, Cannon RE, Miller DS, Nrf2 upregulates ATP binding cassette transporter expression and activity at the blood-brain and blood-spinal cord barriers, J. Neurosci.:Offic. J. Soc. Neurosci 34 (2014) 8585–8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tomiyasu H, Watanabe M, Sugita K, Goto-Koshino Y, Fujino Y, Ohno K, Sugano S, Tsujimoto H, Regulations of ABCB1 and ABCG2 expression through MAPK pathways in acute lymphoblastic leukemia cell lines, Anticancer Res 33 (2013) 5317–5323. [PubMed] [Google Scholar]
- [49].Gupta P, Zhang YK, Zhang XY, Wang YJ, Lu KW, Hall T, Peng R, Yang DH, Xie N, Chen ZS, Voruciclib, a potent CDK4/6 inhibitor, antagonizes ABCB1 and ABCG2-mediated multi-drug resistance in cancer cells, cellular physiology and biochemistry, Int. J. Exp. Cell. Phys., Biochem., Pharmacol 45 (2018) 1515–1528. [DOI] [PubMed] [Google Scholar]
- [50].Zhang H, Patel A, Wang YJ, Zhang YK, Kathawala RJ, Qiu LH, Patel BA, Huang LH, Shukla S, Yang DH, Ambudkar SV, Fu LW, Chen ZS, The BTK inhibitor ibrutinib (PCI-32765) overcomes paclitaxel resistance in ABCB1- and ABCC10-overexpressing cells and tumors, Mol. Canc. Therapeut 16 (2017) 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gottesman MM, Ambudkar SV, Overview: ABC transporters and human disease, J. Bioenerg. Biomembr 33 (2001) 453–458. [DOI] [PubMed] [Google Scholar]
- [52].Wilkens S, Structure and mechanism of ABC transporters, F1000prime Rep 7 (2015) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Marsh S, Somlo G, Li X, Frankel P, King CR, Shannon WD, McLeod HL, Synold TW, Pharmacogenetic analysis of paclitaxel transport and metabolism genes in breast cancer, Pharmacogenomics J 7 (2007) 362–365. [DOI] [PubMed] [Google Scholar]
- [54].Litviakov NV, Cherdyntseva NV, Tsyganov MM, Denisov EV, Garbukov EY, Merzliakova MK, Volkomorov VV, Vtorushin SV, Zavyalova MV, Slonimskaya EM, Perelmuter VM, Changing the expression vector of multidrug resistance genes is related to neoadjuvant chemotherapy response, Cancer Chemother. Pharmacol 71 (2013) 153–163. [DOI] [PubMed] [Google Scholar]
- [55].Campa D, Muller P, Edler L, Knoefel L, Barale R, Heussel CP, Thomas M, Canzian F, Risch A, A comprehensive study of polymorphisms in ABCB1, ABCC2 and ABCG2 and lung cancer chemotherapy response and prognosis, Int. J. Canc 131 (2012) 2920–2928. [DOI] [PubMed] [Google Scholar]
- [56].Bartholomae S, Gruhn B, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D, Steinbach D, Coexpression of multiple ABC-transporters is strongly associated with treatment response in childhood acute myeloid leukemia, Pediatr. Blood Canc 63 (2016) 242–247. [DOI] [PubMed] [Google Scholar]
- [57].Hlavata I, Mohelnikova-Duchonova B, Vaclavikova R, Liska V, Pitule P, Novak P, Bruha J, Vycital O, Holubec L, Treska V, Vodicka P, Soucek P, The role of ABC transporters in progression and clinical outcome of colorectal cancer, Mutagenesis 27 (2012) 187–196. [DOI] [PubMed] [Google Scholar]
- [58].Wu H, Kang H, Liu Y, Xiao Q, Zhang Y, Sun M, Liu D, Wang Z, Zhao H, Yao W, Jia T, Wang E, Zheng Z, Wei M, Association of ABCB1 genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis, Pharmacogenomics 14 (2013) 897–911. [DOI] [PubMed] [Google Scholar]
- [59].Wang F, Huang Z, Zheng K, Zhao H, Hu W, Two SNPs of ATP-binding cassette B1 gene on the risk and prognosis of colorectal cancer, Int. J. Clin. Exp. Pathol 8 (2015) 3083–3089. [PMC free article] [PubMed] [Google Scholar]
- [60].Frytak S, Moertel CG, Schutt AJ, Hahn RG, Reitemeier RJ, Adriamycin (NSC-123127) therapy for advanced gastrointestinal cancer, Cancer Chemother. Rep 59 (1975) 405–409. [PubMed] [Google Scholar]
- [61].Powell SF, Beitinjaneh A, Tessema M, Bliss RL, Kratzke RA, Leach J, Dudek AZ, Phase II study of topotecan and bevacizumab in advanced, refractory non–small-cell lung cancer, Clin. Lung Canc 14 (2013) 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Tuy HD, Shiomi H, Mukaisho KI, Naka S, Shimizu T, Sonoda H, Mekata E, Endo Y, Kurumi Y, Sugihara H, Tani M, Tani T, ABCG2 expression in colorectal adenocarcinomas may predict resistance to irinotecan, Oncol. Lett 12 (2016) 2752–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]


