Summary
Reactive oxygen species (ROS) are important signaling molecules for physiologic processes such as acute response to hypoxia. However, reliable real-time ROS measurement in cells has been a long-standing methodological challenge. Here, we present a protocol to record acute changes in ROS production in sensory cells from mouse carotid body (CB) slices using redox-sensitive green fluorescent protein probes and microfluorimetry. This protocol provides sensitive and reproducible quantification of ROS during acute hypoxia in different subcellular compartments of CB glomus cells.
For complete details on the use and execution of this protocol, please refer to Fernández-Agüera et al. (2015) and Arias-Mayenco et al. (2018).
Subject areas: Cell Biology, Metabolism, Microscopy, Molecular/Chemical Probes
Graphical Abstract

Highlights
-
•
ROS are signaling molecules in the carotid body (CB) during acute hypoxia
-
•
Fluorescent roGFP probes are used to monitor acute changes of ROS in mouse CB slices
-
•
Genetically encoded roGFP probes target different mitochondrial compartments
-
•
Cellular basal redox status can be determined using roGFP targeted to cytosol
Reactive oxygen species (ROS) are important signaling molecules for physiologic processes such as acute response to hypoxia. However, reliable real-time ROS measurement in cells has been a long-standing methodological challenge. Here, we present a protocol to record acute changes in ROS production in sensory cells from mouse carotid body (CB) slices using redox-sensitive green fluorescent protein probes and microfluorimetry. This protocol provides sensitive and reproducible quantification of ROS during acute hypoxia in different subcellular compartments of CB glomus cells.
Before you begin
Solution preparation
Timing: 30 min
See “materials and equipment” for a complete list of recipes for each solution.
-
1.
Modified 0 Ca2+ Tyrode’s solution, which should be kept at 4°C up to 1 week until use.
-
2.
Low-melting point agarose solution, which needs to be prepared the same day of CB dissection and sectioning.
-
3.
Enzymatic digestion solution, which needs to be prepared fresh.
-
4.
Culture medium, which needs to be prepared the same day of experiment, equilibrated in a cell culture incubator with 5% CO2 at 37°C for at least 1 h before use, and maintained at the cell culture incubator until use.
-
5.
Recording solution, which is kept at 4°C up to 1 week and pre-warmed to 37°C before recording.
Vibratome calibration
Timing: 2 min
-
6.
Calibrate the vertical deflection of the blade (Z axis of the vibratome) using the Vibrocheck measurement device (Leica), following the manufacturer’s instruction. This can minimize vibration at this direction during sectioning, retaining viable cells on the section surface, where ROS production will be recorded.
Note: Researchers using vibratome from other manufacturer should consult with the company for the availability of a calibration tool similar to Vibrocheck measurement device (Leica).
Preparation of roGFP probes in aliquots
Timing: 5 min
-
7.
Aliquot roGFP probes, which have been incorporated into adenovirus vector, and store the aliquots at −80°C up to 1 year.
CRITICAL: To obtain optimal transduction efficiency, aliquots of roGFP-containing adenovirus should be prepared on ice and dark, and then kept at −80°C. Repeated freezing-thawing should be avoided.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| VQAd ro-GFP | ViraQuest | Cat#111414 |
| VQAd CMV GDP-roGFP | ViraQuest | Cat#022411 |
| VQAd CMV mito-roGFP | ViraQuest | Cat#122909 |
| Chemicals, peptides, and recombinant proteins | ||
| Sodium thiopental | Braun | 8470006355732 |
| Low melting-point agarose | FMC BioProducts | 50072 |
| Phosphate-buffered saline | Gibco | 10010-015 |
| Cyanoacrylate | Loctite | 8410436130431 |
| Dithiothreitol | Sigma | D0632 |
| Hydrogen peroxide | Sigma | H-1009 |
| MitoTracker (Deep Red FM) | Invitrogen | 12010156 |
| HEPES | Sigma | H-4034 |
| Collagenase II | Sigma | C6885 |
| Trypsin | Sigma | T8003 |
| Porcine elastase | Millipore | 324682 |
| DMEM (0 glucose) | Gibco | 11966-025 |
| DMEM/F-12 | Gibco | 21331-020 |
| Penicillin/Streptomycin | Gibco | 15140-122 |
| L-glutamine | Gibco | 25030-024 |
| Fetal bovine serum | Gibco | 10270-106 |
| Insulin | Novo Nordisk | Atrapid, EU/1/02/230/011 |
| Erythropoietin | Sandoz | Binocrit, EU1/07/410/028 |
| Experimental models: Organisms/strains | ||
| Wild-type C57BL/6 mouse, 8-week-old, male and female | Charles River | n/a |
| Other | ||
| Vibratome | Leica | VT1000S |
| Direct microscope | Olympus | BX51 |
| 60×/0.90w objective | Olympus | LUMPlanFI/IR |
| 150 W Xenon lamp | Ushio Inc. (Japan) | PX45 |
| Monochromator | T.I.L.L. Photonics | Polychrome V |
| CCD camera | Andor Technology | iXon3 |
| Live Acquisition software | T.I.L.L. Photonics | n/a |
| Dichroic | Semrock | Di02-R488 |
| Band-pass filter | Semrock | FF01-520/35 |
| Gas mixture (5% CO2 and 95% N2) | Air Liquide España, Spain | Mixture DOC45 |
| Gas mixture (20% O2, 5% CO2, and 75% N2) | Air Liquide España, Spain | Mixture DOC45R |
Materials and equipment
-
•
Modified 0 Ca2+ Tyrode’s solution:
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl (MW 58.44) | 148 mM | 1.73 g |
| KCl (MW 74.55) | 2 mM | 0.03 g |
| MgCl2 (1M) | 3 mM | 0.6 mL |
| HEPES (MW 238.3) | 10 mM | 0.477 g |
| Glucose (MW 180.16) | 10 mM | 0.36 g |
| ddH2O | - | up to 200 mL |
| pH 7.4 |
-
•
Low-melting point agarose solution:
| Reagent | Final concentration | Amount |
|---|---|---|
| Low-melting point agarose | 1% (w/v) | 30 mg |
| Phosphate-buffered saline | 1× | up to 3 mL/animal |
| pH 7.4 |
-
•
Enzymatic digestion solution:
| Reagent | Final concentration | Amount |
|---|---|---|
| CaCl2 (5 mM) | 50 μM | 30 μL |
| Collagenase II (60.24 mg/mL) | 0.6 mg/mL | 30 μL |
| Trypsin (26.7 mg/mL) | 0.27 mg/mL | 30 μL |
| Porcine elastase (125 U/mL) | 1.25 U/mL | 30 μL |
| Phosphate-buffered saline | 1× | up to 3 mL |
| pH 7.4 |
-
•
Culture medium:
| Reagent | Final concentration | Amount |
|---|---|---|
| DMEM (0 glucose)/DMEM-F12 (3:1) | - | up to 10 mL |
| Penicillin (10000 U/mL/ Streptomycin (1 mg/mL) | 100 U/mL / 10 μg/mL | 100 μL |
| L-glutamine (200 mM) | 2 mM | 100 μL |
| Fetal bovine serum | 10% | 1 mL |
| Insulin (100 U/mL) | 84 U/L | 8.4 μL |
| Erythropoietin (2000 U/mL) | 1.2 U/mL | 6 μL |
Note: Insulin and erythropoietin are added to culture medium, in addition to other supplements. In our experience, CB slices are healthier when cultured with medium supplemented with insulin and erythropoietin. In general, the appearance of glomeruli in the surface of the slice and secretory responses to stimuli are favored by this treatment.
-
•
Recording solution:
| Reagent | Final concentration | Amount |
|---|---|---|
| NaCl (MW 58.44) | 117 mM | 13.675 g |
| KCl,(MW 74.55) | 4.5 mM | 0.67 g |
| NaHCO3 (MW 84.01) | 23 mM | 3.864 g |
| MgCl2 (1 M) | 1 mM | 2 mL |
| CaCl2 (1 M) | 2.5 mM | 5 mL |
| Glucose (MW 180.16) | 5 mM | 1.801 g |
| Sucrose (MW 342.3) | 5 mM | 3.423 g |
| ddH2O | - | up to 2 L |
Step-by-step method details
Acute cellular and systemic responses are essential for mammals to adapt to environmental, physiological, and pathophysiological conditions causing oxygen (O2) deficiency (hypoxia). The carotid body (CB), located in the carotid bifurcation, is the main arterial chemoreceptor responsible for detecting decreases in blood O2 tension (hypoxemia) in order to trigger hyperventilation and sympathetic activation. The CB is organized in clusters of cells (glomeruli) and each glomerulus is composed of O2-sensitive glomus cells and other cell types. Recently, a signaling role of reactive oxygen species (ROS) has been reported underlying molecular mechanisms of acute O2 sensing (Ortega-Sáenz and López-Barneo, 2020). These findings would not have been possible without the development of redox-sensitive green fluorescent protein (roGFP) probes (Dooley et al., 2004; Cannon and Remington, 2008; Waypa et al., 2010). roGFP probes contain engineered cysteine residues, which form disulfide bonds in the presence of oxidants. This causes reciprocal changes in emission intensity when excited at two different wavelengths (see review by Cannon and Remington, 2008 for detailed biophysical properties of roGFPs). roGFP probes can react with several natural and synthetic redox reagents (Dooley et al., 2004), but are highly sensitive to H2O2, which is produced in mitochondria in the presence of superoxide dismutase. Using genetic targeting sequences, roGFP probes can be expressed in different intracellular compartments. These ratiometric redox probes allow us to detect rapid changes in intracellular redox status, with a time course of s/min within the dynamic range of the cellular (transmitter release) and systemic (increase in ventilation) physiological responses to hypoxia. In the current protocol, step-by-step method details are presented from CB sample preparation, roGFP probe transduction, to real-time measurement of ROS production by microfluorimetry. This method helps understand molecular mechanisms of acute O2 sensing by arterial chemoreceptors. Moreover, it may also be applied to study other physiological and pathological processes where acute ROS production plays a signaling role.
Preparation of CB slices and transduction of roGFP probes by adenoviral infection
Timing: 2 h
Mouse CBs are dissected and sectioned quickly (Ortega-Sáenz et al., 2018). roGFP probes incorporated in adenoviral vectors are transduced to CB glomus cells by adenoviral infection (Arias-Mayenco et al., 2018). CB slices are then cultured for 48 h before measurement of ROS production by microfluorimetry.
-
1.Carotid body dissection
-
a.Sacrifice mouse with intraperitoneal administration of a lethal dose of sodium thiopental (120 to 150 mg/kg).
-
b.Quickly dissect carotid bifurcation under a microscope and place it in ice-cooled and modified 0 Ca2+ Tyrode’s solution (Figures 1A and 1B).
-
c.Dissect CB from the carotid bifurcation by removing superior cervical ganglion, adjacent arteries, fat, and connective tissues (Figures 1C–1E).
-
a.
Note: Other anesthetic or ways of sacrifice can also be applied without interfering in the success of the experiment.
-
2.Carotid body slicing
-
a.Embed CB in 1% (wt/vol) low melting-point agarose in PBS, which has been pre-warmed to 42°C, and quickly place the agarose block on ice for solidification (Figures 1F and 1G). Agarose blocks are prepared using a fragment (about 1 cm in length) of 1 mL syringe, with both ends perfectly polished and one end sealed with parafilm (Figures 1F and 1G).
-
b.Remove the solidified agarose block from the syringe fragment, make a smaller agarose cylinder containing the CBs, and fix it to a specimen disc with cyanoacrylate. Place the specimen disc inside the buffer tray of a vibratome (Figure 1H). Fill the tray with cold, modified 0 Ca2+ Tyrode’s solution.
-
c.Make 150 μm-thick slices using a standard razor blade (Figure 1I). Transfer slices to a plastic container and wash them twice with cold sterile PBS.
-
a.
CRITICAL: The temperature of agarose solution for embedment should be maintained between 40°C–42°C. A temperature lower than 40°C may affect CB embedment and CB sectioning, whereas a temperature above 42°C may affect the viability of CB cells.
CRITICAL: The speed to section CB embedded in the agarose block needs to be maintained between 0.1 and 0.16 mm/s. CB may detach from agarose block if the speed is too high.
Alternatives: Modified 0 Ca2+ Tyrode’s solution can be replaced with PBS without affecting the quality of CB slices.
-
3.Brief enzymatic treatment
-
a.Incubate CB sections with enzymatic digestion solution for 5 min at 37°C in a water bath with shaking.
-
b.Transfer CB sections to a 35-mm petri dish and wash the sections with cold PBS twice.
-
a.
Note: Enzymatic treatment is performed to partially digest and loosen connective tissues in the surface of CB slices. This treatment seems to help the appearance of glomeruli on the surface of CB slices during culturing. This step is not necessary for rat CB.
-
4.Culture and transduction of roGFP probes to CB sections by adenoviral infection
-
a.Remove PBS completely and add culture medium, which has been pre-equilibrated in cell culture incubator of 21% O2, 5% CO2 at 37°C.
 CRITICAL: The culture medium should be equilibrated with pH and temperature in the incubator right before use. The correct pH (7.4) of culture medium is important for CB recovery from sectioning and enzymatic treatment, and for survival during the time of culture.
CRITICAL: The culture medium should be equilibrated with pH and temperature in the incubator right before use. The correct pH (7.4) of culture medium is important for CB recovery from sectioning and enzymatic treatment, and for survival during the time of culture. -
b.Transduce roGFP probes into CB cells by adenoviral infection. roGFP DNA construct has been incorporated previously in adenoviral vector. One μl of adenovirus with a stock concentration of 1–1.2 × 1012 viral particles/mL is added to 2 mL of complete culture medium.
 CRITICAL: The amount of adenovirus necessary for infection may vary from one lot of virus production to another. To maximize transduction efficiency, functional viral titration may be performed, and approximately 400,000 pfu/mL of adenovirus is recommended for each infection.
CRITICAL: The amount of adenovirus necessary for infection may vary from one lot of virus production to another. To maximize transduction efficiency, functional viral titration may be performed, and approximately 400,000 pfu/mL of adenovirus is recommended for each infection. -
c.Adenovirus with different roGFP constructs are available to target specific subcellular compartments: cytosol, cyto-roGFP (VQAd ro-GFP); mitochondrial intermembrane space (IMS), IMS-roGFP (VQAd CMV GDP-roGFP); and mitochondrial matrix, matrix-roGFP (VQAd CMV mito-roGFP) (Waypa et al., 2010).
-
d.Culture CB sections in a humidified incubator with 5% CO2 and 21% O2 at 37°C for 24 h. CB slices from each mouse (4–6 slices) are cultured in a 35-mm petri dish. Before putting in the incubator, shake the dish slightly and horizontally to avoid slice overlapping.
-
e.Replace the medium with adenovirus-free culture medium and incubate CB sections for an additional 24 h before microfluorimetric recording of ROS production.Alternatives: If necessary, cells may be incubated with adenovirus-containing culture medium for 48 h until microfluorimetric recording to allow maximum roGFP expression. This may be useful especially when the transduction efficiency is low.
 CRITICAL: The optimal culturing time of CB slices is 24 to 48 h, which allows the recovery of typical CB glomerulus morphology and response to physiological stimuli, such as hypoxia and hypercapnia. CB slices can be maintained in relatively good conditions for up to 4–5 days (Fernández-Agüera et al., 2015). However, the number of O2 responding cells in CB sections decreases with time after reaching a maximum number at about 48 h. It is essential to maintain CB slices healthy, since the response to hypoxia of CB glomus cells can be easily lost.Note: When possible, it is recommended to use young adult mice (2–3 months). Although feasible, older mice tend to contain more connective tissue, which may affect the quality of tissue sectioning and enzymatic treatment.
CRITICAL: The optimal culturing time of CB slices is 24 to 48 h, which allows the recovery of typical CB glomerulus morphology and response to physiological stimuli, such as hypoxia and hypercapnia. CB slices can be maintained in relatively good conditions for up to 4–5 days (Fernández-Agüera et al., 2015). However, the number of O2 responding cells in CB sections decreases with time after reaching a maximum number at about 48 h. It is essential to maintain CB slices healthy, since the response to hypoxia of CB glomus cells can be easily lost.Note: When possible, it is recommended to use young adult mice (2–3 months). Although feasible, older mice tend to contain more connective tissue, which may affect the quality of tissue sectioning and enzymatic treatment.
-
a.
Figure 1.
Mouse carotid body (CB) dissection and slicing
(A) Adult mouse after treatment with lethal dose of sodium thiopental.
(B) Dissection of carotid bifurcation with forceps and scissors. Scale bar, 2 mm.
(C) Carotid bifurcations before (left) and after (right) removal of superior cervical ganglion (SCG, highlighted on the left bifurcation). CB is highlighted on the right bifurcation. Scale bar, 500 μm.
(D) Magnified image of the carotid bifurcation without SCG in C (right). ICA, internal carotid artery; ECA, external carotid artery; CA, carotid artery; CS, carotid sinus. Scale bar, 500 μm.
(E) Two CBs dissected from one mouse. Scale bar, 200 μm.
(F) Two CBs (arrows) embedded within the agarose block inside the syringe fragment.
(G) Agarose block with two embedded CBs (arrows) removed from the syringe. Scale bar, 5 mm.
(H) Agarose block (ag) with embedded CBs fixed to a specimen disc inside the buffer tray of a vibratome. The buffer tray is filled with modified 0 Ca2+ Tyrode’s solution. rb, razor blade.
(I) Slices (150-μm thick) of the agarose block (circle) containing a CB section (arrow). The slices are floating in a small plastic tube filled with Modified 0 Ca2+ Tyrode’s solution. Scale bar, 2 mm.
Real-time recording of ROS production from CB slices using roGFP probes by microfluorimetry
Timing: 1–2 h
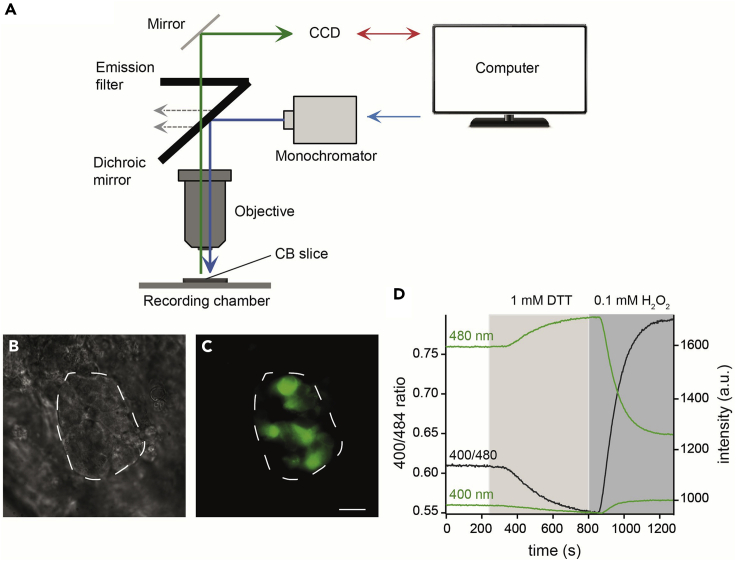
ROS production is recorded in real-time using a microfluorimetric setup 48 h after adenoviral infection. As shown in Figure 2A, our microfluorimetric setup consists of a direct microscope equipped with a 60×/0.90w objective, a filter wheel, a 150 W xenon lamp, a monochromator, a CCD camera and a computer. The monochromator allows to excite samples at different wavelength. The emitted fluorescence passes through a band-pass filter and is detected by a CCD camera. Monochromator, CCD camera, and image acquisition are controlled by Live Acquisition software.
Figure 2.
Microfluorimetric setup for measurement of reactive oxygen species production using redox-sensitive green fluorescent protein (roGFP)
(A) Schematic representation of microfluorimetric setup. See text for details.
(B and C) Representative carotid body (CB) slice 48 h after adenoviral infection in bright field (B) and with excitation of 480 nm and emission of 535 nm (C), demonstrating green fluorescent signal due to roGFP expression. A CB glomerulus is highlighted with a white discontinuous line. Scale bar, 10 μm.
(D) Representative recording of fluorescent signals excited at 400 nm and 480 nm with an emission at 535 nm and the ratio of fluorescent signals (400/480) of a CB glomus cell. DTT, dithiothreitol.
roGFP expression in CB section can be observed (Figures 2B and 2C). ROS production in different subcellular compartments, such as mitochondrial IMS, mitochondrial matrix, and cytosol, can be measured using roGFP probe targeted to each compartment (Waypa et al., 2010). roGFPs have two fluorescence excitation peaks (400 and 484 nm), which allows ratiometric measurements to determine reversible changes of redox state (Figure 2D). The presence of oxidants causes a disulfide bond formation between cysteine residues in roGFP and an increase in the fluorescent signal at the 400 nm excitation wavelength and a decrease at 480 nm wavelength. In addition, basal level redox state can be determined by in situ calibration of roGFP probes with excess reducing agent (dithiothreitol) to achieve maximum reduction and oxidant (H2O2) to achieve maximum oxidation. Effect of hypoxia on ROS production from CB glomus cells can be studied using recording solutions bubbled with gas mixtures containing different O2 content.
-
5.
Transfer a CB slice to the recording chamber of microfluorimetric setup, which has been filled with pre-warmed recording solution, and maintain a continuous flow of recording solution.
Note: The recording solution is pre-warmed to 37°C and kept in a water bath of 37°C during the experiment in order to maintain the recording temperature at 30°C–33°C.
Note: The volume of recording solution in the recording chamber is minimized to ≈ 0.2 mL during recording to facilitate fast changes of experimental condition. The flow rate of recording solution is about 4 mL/min.
-
6.
Use a dichroic Di02-R488 (Semrock) and a band-pass filter FF01-520/35 (Semrock) for recording.
-
7.
Select single glomus cell (region of interest, ROI) with strong green fluorescent signal, indicating high level of roGFP expression. Several ROIs can be selected in the same field for simultaneous recording.
CRITICAL: To avoid florescent signal bleaching during experiment, it is recommended to decrease exposure time right after ROI selection.
Note: Adenoviral infection results in transduction of roGFP probes not only in glomus cells, but also in other cell types in CB. Immunohistochemical analyses indicate that after 48 h in culture, most of the cells in the glomeruli that appear at the surface of the slice are glomus cells (tyrosine hydroxylase positive) (Pardal et al., 2000). These cells can be easily distinguished morphologically from other cell types in CB glomeruli under microscope. Glomus cells are round with big nucleus and form compact clusters. Glomus cell clusters without compact structure usually indicate a poor quality of culture and may not be suitable for experiment.
-
8.
Establish the acquisition protocol at the beginning of the experiment using Live Acquisition software (T.I.L.L. Photonics). A spatial resolution of 8 x 8 pixels, an excitation time of 20 milliseconds and an acquisition interval of 5 s are used. Two fluorescence excitation wavelengths (400 and 484 nm) are selected with the emission wavelength of 535 nm. The resulting 400/484 ratio is recorded to determine reversible changes of redox state in each selected ROI. Background fluorescent signal is subtracted from an area of CB section without green fluorescent signal.
-
9.
To study the effect of hypoxia on ROS production, recording solution is bubbled with gas mixtures containing different percentage of O2. For normoxic condition, the gas mixture is comprised of 20% O2, 5% CO2, and 75% N2 (O2 tension ~145 mmHg). For hypoxic treatment, a gas mixture with 5% CO2 and 95% N2 is used so that an O2 tension of ~10 to 15 mmHg is reached in the recording chamber.
CRITICAL: To avoid potential artifact, the switch from normoxic to hypoxic condition is achieved changing only the gas mixture used to bubble the recording solution instead of changing perfusing tube and recording solution. With the flow rate of 4 mL/s and the low volume (0.2 mL) of recording solution in the recording chamber, gas concentration in the recording chamber is equilibrated rapidly with the bubbled gas mixture.
Note: Oxygen tension in the recording solution can be monitored using an oxygen electrode, which is an insulated carbon or platinum fiber polarized to −700 mV. Redox currents generated at the tip of the electrode are proportional to the amount of dissolved O2 in the recording solution (Ganfornina and López-Barneo, 1992). In addition, gas mixtures with different O2 levels can be used to study dose-dependent effect of hypoxia on ROS production (Arias-Mayenco et al., 2018).
-
10.
Determine basal level redox state of CB glomus cells at the end of experiment. Record 400/484 ratio with cyto-roGFP. Perform in situ calibration of roGFP probes by treating CB slice with 1 mM dithiothreitol to achieve maximum reduction (0% oxidation) and 0.1 mM H2O2 to achieve maximum oxidation (100% oxidation).
CRITICAL: During experiment, ROIs may move away from the recording region due to subtle movements of the CB slice. Recording may be paused to re-localize ROIs before resuming the recording.
Expected outcomes
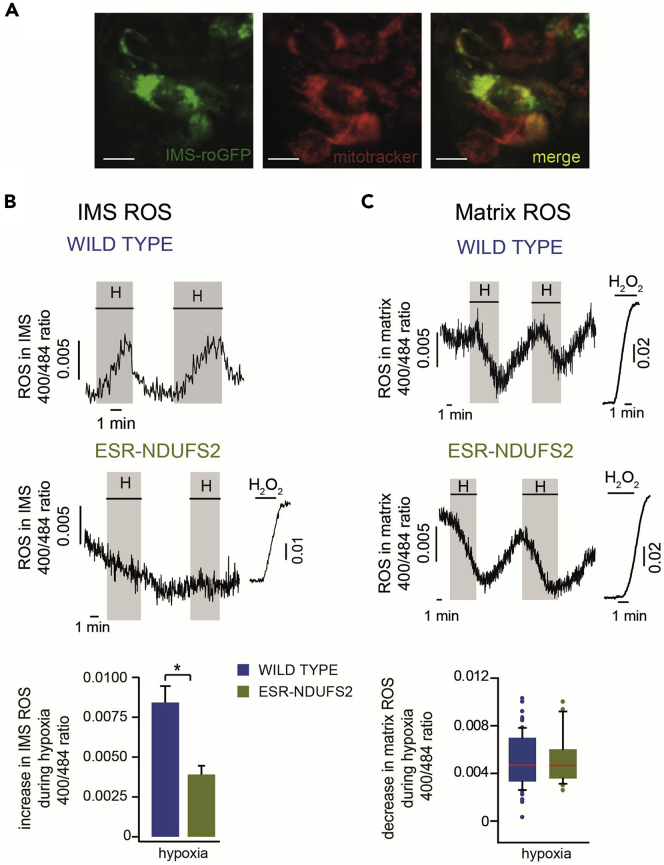
roGFP probes provide high spatial and temporal resolution to monitor dynamic intracellular redox modifications. Successful measurement of transient changes in ROS production in the range of seconds to minutes from different subcellular compartments of mouse CB glomus cells can be achieved using the protocol just mentioned above. This technique helped us understand molecular mechanisms underlying the physiological process of acute O2 sensing by arterial chemoreceptors (Fernández-Agüera et al., 2015, Arias-Mayenco et al., 2018). To study the effect of mitochondrial complex I (MCI) on acute O2 sensing, we have generated conditional knockout mice deficient in MCI subunit NDUFS2 (Fernández-Agüera et al., 2015). Systemic and cellular responses to hypoxia are lost in NDUFS2 knockout mice. Basal level redox state in CB glomus cells is determined using roGFP probe targeted to cytosol (Figures 3A and 3B). TH-NDUFS2 mice, in which NDUFS2 is deleted during embryonic development, present a more oxidized redox state compared to wild-type mice. This modification is not observed in the adult model of NDUFS2 knockout mice (ESR-NDUFS2), in which NDUFS2 is removed once mice reach adulthood (Fernández-Agüera et al., 2015). To study the role of mitochondrial ROS on acute O2 sensing, roGFPs targeted to different compartments of mitochondria are used. As shown in Figure 4A, green fluorescent signal due to roGFP expression at mitochondrial IMS is colocalized with the red fluorescent signal of MitoTracker, a mitochondrial marker, demonstrating the correct targeting of roGFP probe to mitochondria of glomus cells. Hypoxia induces differential changes of ROS in different subcellular compartments of CB glomus cells from wildtype mice (Figures 4B and 4C upper panels). Whereas a transient and reversible increase in ROS production, possibly H2O2, is observed in mitochondrial IMS, hypoxia causes a decrease in ROS content in mitochondria matrix. We have observed that hypoxia-induced changes in ROS production in mitochondrial IMS, but not in mitochondrial matrix, are lost in CB glomus cells of NDUFS2 knockout animals (Figures 4B and 4C lower panels). These results demonstrate that ROS from mitochondrial IMS with MCI (and possible also MCIII) origin, but not from mitochondrial matrix, serve as signaling molecules to trigger adaptive response during acute hypoxia.
Figure 3.
Basal redox level determined using redox-sensitive green fluorescent protein targeted to the cytosol (cyto-roGFP) in mouse carotid body glomus cells
Representative recordings of cytosolic ROS levels and changes in ROS production with treatment of 1 mM dithiothreitol (DTT) and 0.1 mM H2O2 in wild-type and NDUFS2-deficient mice (A, TH-NDUFS2; B, ESR-NDUFS2). Percentage of oxidation was obtained comparing 400/484 ratio to those ratios calibrated with DTT and H2O2.
On the right panels data are represented by mean ± standard error. ∗ p < 0.05. (Figure reprinted with permission from Fernández-Agüera et al., 2015; Arias-Mayenco et al., 2018)
Figure 4.
Hypoxia-induced reversible changes in ROS in mitochondrial intermembrane space (IMS) and mitochondrial matrix of mouse carotid body (CB) glomus cells
(A) Immunofluorescent images of cells in a CB slice transfected with a redox-sensitive green fluorescent protein probe targeted to the IMS (IMS-roGFP) and overlap between IMS-roGFP and MitoTracker (a mitochondrial marker) fluorescence. Scale bars, 10 μm.
(B) Representative recordings and quantifications of changes in ROS production in mitochondrial IMS induced by hypoxia (H) or 0.1 mM H2O2 in wild-type and NDUFS2-deficient (ESR-NDUFS2) glomus cells. On the bottom panel data are represented by mean ± standard error. ∗ p < 0.05.
(C) Representative recordings and box diagrams showing the quantifications of changes in ROS in mitochondrial matrix induced by hypoxia (H) or 0.1 mM H2O2 in wild-type and NDUFS2-deficient (ESR-NDUFS2) glomus cells. In the bottom panel, median (red lines) and quartiles are indicated.
(Figure reprinted with permission from Fernández-Agüera et al., 2015; Arias-Mayenco et al., 2018).
Changes in ROS production during acute hypoxia not only occurs in CB, but also in other tissues and organs. Indeed, hypoxia-induced modifications in subcellular ROS production have been detected in vascular smooth muscle cells using roGFPs (Waypa et al. 2010). Changes in redox status may also be involved in other physiological and pathological signaling processes. The protocol presented here requires tissue slices to be cultured for at least 48 h to express roGFP probes. Organotypic cultures of tissue slices fit perfectly for this requirement, as they can maintain cells alive for several days/weeks under a physiologically relevant condition. As a result, the protocol presented here may be adapted to determine changes in cell redox status in other type of tissue sections (such as brain slices).
Quantification and statistical analysis
A minimum of 3 or 4 mice need to be used for each experiment. Data are presented as mean ± SEM, unless specified otherwise. Shapiro-Wilk test is used to determine whether the data present a normality distribution. Data from two groups are analyzed with either a t test or a paired t test. Data with multiple groups are analyzed by ANOVA or RM-ANOVA followed by a post-hoc Tukey’s test. For data without a normal distribution, nonparametric tests (Mann-Whitney rank-sum test or ANOVA on ranks followed by a post-hoc Tukey’s test) are applied. A p < 0.05 is considered statistically significant.
The ratio of fluorescent intensities excited at 400 and 484 nm (400/484 ratio) is used to perform relative quantification of ROS production. The application of ratiometric value instead of fluorescent intensity excited with single wavelength minimizes measurement errors due to changes in illumination intensity, or probe concentration. To determine basal level redox state of glomus cells, in situ calibration of cyto-roGFP probe is performed treating CB slices with excess amount of dithiothreitol to achieve maximum reduction and H2O2 to achieve maximum oxidation. These peak ratios correspond to 0% and 100% oxidized forms of the probe, respectively. The ratio obtained from cells is compared with the ratios of calibration to calculated percentage of oxidation. To study the hypoxic effect on ROS production, the difference in 400/484 ratio between hypoxia and normoxia is obtained.
Limitations
Responses to hypoxia by CB glomus cells can be lost during sample preparation. The transduction efficiency of roGFPs depends on whether CB slices are healthy. In addition, changes in ROS production in CB glomus cells during hypoxia are small. Therefore, it is essential to keep CB sections healthy in order to measure ROS production successfully.
Another limitation is low transduction efficiency of CB glomus cells compared to those cells under constant division. It is necessary to test the amount of adenovirus needed for infection to reach sufficient transduction efficiency. Both female and male mice have been used and no difference in transduction efficiency has been observed between sex.
Troubleshooting
Problem 1
Very few glomus cells with green fluorescent signal are detected (steps 1 to 4).
Potential solution
This may result from bad preparation of CB slices (steps 1 to 3 of the protocol) or low transduction efficiency of roGFP probes (step 4 of the protocol). In the former case, CB slices may be prepared with new solutions and culture mediums. In the latter case, functional viral titration may be performed, and approximately 400,000 pfu/mL of adenovirus is recommended for each infection. Slices may be incubated with adenovirus-containing culture medium for 48 h to allow maximum roGFP expression. The amount of virus for infection may be increased if necessary.
Problem 2
The fluorescent signal is very high and no change in fluorescent signal with stimulus is detected (step 4).
Potential solution
The fluorescent signal is saturated. The amount of virus for infection may be decreased.
Problem 3
Adequate signal that does not change with stimulus (steps 1 to 4).
Potential solution
CB slices are not healthy. Response to hypoxia is highly sensitive to the quality of tissue sectioning and culturing. Prepare new CB slices.
Problem 4
Poor tissue viability 24–48 h after slicing and culturing (steps 1 to 4).
Potential solution
This is normally due to incorrect preparation of the solutions, tissue slicing, or slice culture. Prepare new solutions and repeat tissue slicing and culture.
Problem 5
Low signal to noise ratio from roGFP emission (step 4).
Potential solution
The amount of roGFP probe used is not optimal. Perform functional viral titration and use approximately 400,000 pfu/mL of adenovirus for each infection.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, José López-Barneo (lbarneo@us.es).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate/analyze [datasets/code].
Acknowledgments
This research was supported by the Botin Foundation, the Spanish Ministries of Science and Innovation and Health (SAF2012-39343, SAF2016-74990-R), and the European Research Council (ERC Advanced Grant PRJ201502629). We also thank members of our laboratory and IBiS staff for advice and technical assistance.
Author contributions
L.G. and J.L.-B. designed the manuscript and contributed to the writing of the paper. I.A.-M. and P.O.-S. performed the experiments and contributed to generating the draft of the manuscript. J.L.-B. coordinated the project.
Declaration of interests
The authors declare no competing interests.
References
- Arias-Mayenco I., Gonzalez-Rodriguez P., Torres-Torrelo H., Gao L., Fernandez-Aguera M.C., Bonilla-Henao V., Ortega-Saenz P., Lopez-Barneo J. Acute O2 sensing: role of coenzyme QH2/Q ratio and mitochondrial ROS compartmentalization. Cell Metab. 2018;28:145–158 e4. doi: 10.1016/j.cmet.2018.05.009. [DOI] [PubMed] [Google Scholar]
- Cannon M.B., Remington S.J. Redox-sensitive green fluorescent protein: probes for dynamic intracellular redox responses. A review. Methods Mol. Biol. 2008;476:51–65. doi: 10.1007/978-1-59745-129-1_4. [DOI] [PubMed] [Google Scholar]
- Dooley C.T., Dore T.M., Hanson G.T., Jackson W.C., Remington S.J., Tsien R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- Fernández-Agüera M.C., Gao L., Gonzalez-Rodriguez P., Pintado C.O., Arias-Mayenco I., Garcia-Flores P., Garcia-Perganeda A., Pascual A., Ortega-Saenz P., Lopez-Barneo J. Oxygen sensing by arterial chemoreceptors depends on mitochondrial complex I signaling. Cell Metab. 2015;22:825–837. doi: 10.1016/j.cmet.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Ganfornina M.D., López-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxygen. J. Gen. Physiol. 1992;100:401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Sáenz P., Caballero C., Gao L., Lopez-Barneo J. Testing acute oxygen sensing in genetically modified mice: plethysmography and amperometry. Methods Mol. Biol. 2018;1742:139–153. doi: 10.1007/978-1-4939-7665-2_13. [DOI] [PubMed] [Google Scholar]
- Ortega-Sáenz P., López-Barneo J. Physiology of the carotid body: from molecules to disease. Annu. Rev. Physiol. 2020;82:127–149. doi: 10.1146/annurev-physiol-020518-114427. [DOI] [PubMed] [Google Scholar]
- Pardal R., Ludewig U., Garcia-Hirschfeld J., Lopez-Barneo J. Secretory responses of intact glomus cells in thin slices of rat carotid body to hypoxia and tetraethylammonium. Proc. Natl. Acad. Sci. U S A. 2000;97:2361–2366. doi: 10.1073/pnas.030522297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waypa G.B., Marks J.D., Guzy R., Mungai P.T., Schriewer J., Dokic D., Schumacker P.T. Hypoxia triggers subcellular compartmental redox signaling in vascular smooth muscle cells. Circ. Res. 2010;106:526–535. doi: 10.1161/CIRCRESAHA.109.206334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not generate/analyze [datasets/code].