Abstract
Background
Skeletal muscle wasting, or sarcopenia, affects a significant proportion of patients undergoing transcatheter aortic valve replacement (TAVR). However, its influence on post-TAVR recovery and 1-year health-related quality of life (HR-QOL) remains unknown. We examined the relationship between skeletal muscle index (SMI), post-TAVR length of hospital stay (LOS), and 1-year QOL.
Methods
The study sample consisted of 300 consecutive patients undergoing TAVR from 2012 to 2018 who had pre-TAVR computed tomographic scans suitable for analysis of body composition. Skeletal muscle mass was quantified as cm2 of skeletal mass per m2 of body surface area from the cross-sectional computed tomographic image at the third lumbar vertebra. Sarcopenia was defined using established sex-specific cutoffs (women: SMI < 39 cm2/m2; men: < 55 cm2/m2). Multivariable linear regression analysis was used to determine the relationship between SMI, LOS, and HR-QOL using the Kansas City Cardiomyopathy Questionnaire.
Results
Sarcopenia was present in most (59%) patients and associated with older age (82 vs 76 years; P < .001) and lower body mass index (27 vs 33 kg/m2; P < .001). There were no other differences in baseline clinical or echocardiographic characteristics among the 4 quartiles of SMI. SMI was positively correlated with LOS and 1-year QOL. After adjusting for age, gender, race, and body mass index, SMI remained a significant predictor of both LOS (P = .01) and 1-year QOL (P = .012). For every 10 cm2/m2 higher SMI, there was an 8-point increase in Kansas City Cardiomyopathy Questionnaire score, a difference that is clinically meaningful.
Conclusions
Sarcopenia is prevalent in TAVR patients. Higher SMI is associated with shorter LOS and better 1-year HR-QOL. To achieve optimal TAVR benefits, further study into how body composition influences post-TAVR recovery and durable improvement in QOL is warranted.
Transcatheter aortic valve replacement (TAVR) is the standard of care for intermediate- and high-risk patients with severe symptomatic aortic stenosis.1–4 Although favorable immediate and long-term outcomes have been established,5–8 geriatric syndromes continue to limit the overall effectiveness of TAVR in clinical practice.9–11 Of these, sarcopenia, a progressive and generalized skeletal muscle disorder, is associated with increased risk of adverse outcomes including falls, fractures, physical disability, and mortality.10,12 Sarcopenia is also inter-twined with the development and progression of frailty, which has been linked to poor outcome and reduced functional capacity in cancer, chronic obstructive pulmonary disease, diabetes mellitus, cirrhosis, rheumatoid arthritis, and congestive heart failure.13–19 Reduced skeletal muscle quantity/mass and strength are clinical characteristics of sarcopenia that can be readily measured. Whether skeletal muscle mass and strength can be used to stratify older patients undergoing TAVR procedures into those who are most likely to derive benefit as it relates to health-related quality of life (HR-QOL) remains unknown.
By providing precise segmentation of individual muscle and adipose tissue components, computed tomography (CT) can be used to assess body composition and quantify skeletal muscle mass and quality.20–23A single cross-sectional image at the third lumbar vertebrae (L3) correlates well with whole-body skeletal muscle and adipose tissue, providing an accurate assessment of whole-body tissue composition.13–15 We have previously shown that a measure of skeletal muscle quantity, skeletal muscle index (SMI) (skeletal muscle cross-sectional area [cm2]/height2 [m2]), predicts post-TAVR length of stay (LOS).24 Given that TAVR patients routinely undergo a preoperative CT scan of the chest, abdomen, and pelvis, body composition can be readily assessed. The aim of this study was to examine the association between sarcopenia and HR-QOL 1 year after TAVR procedure in a large consecutive cohort of older patients.
Methods
Study design
The study sample was derived from 346 consecutive outpatients who underwent TAVR at 1 large academic hospital system from September 2012 to June 2018. The study was approved by the Tallahassee Memorial Hospital Institutional Review Board. All patients presented with severe symptomatic aortic valve stenosis (aortic valve area of ≤1.0 cm2 and/or mean transvalvular gradient of ≥40 mm Hg) and were deemed by the multidisciplinary heart team to be at high or intermediate risk for surgical aortic valve replacement due to a predicted Society of Thoracic Surgery (STS) operative mortality of ≥3%, major comorbidities/contraindications for surgery, and/or frailty. All patients underwent a pre-TAVR CT scan of the chest, abdomen, and pelvis; pulmonary function tests; and standard 2-dimensional echocardiography with Doppler assessment.
Of the 346 consecutive patients treated during this time frame, the 300 (87%) whose CT scans were suitable for interpretation of body composition served as the final study sample. Baseline patient demographics, clinical characteristics, and frailty measures were recorded prospectively using essential frailty toolset.9 Muscle strength testing included dominant handgrip strength (kg) and 5-m walk test (seconds). Albumin levels (g/dL) were measured prospectively for all study participants. Moderate to severe chronic obstructive lung disease was defined as a forced expiratory volume in 1 second of less than 1.5 L. Prolonged respiratory failure was defined as the need for mechanical ventilation for more than 72 hours post-TAVR. Mitral and tricuspid regurgitations were deemed significant if reported as moderate or severe by transthoracic echocardiography and Doppler assessment. Pulmonary arterial hypertension was defined as an estimated right ventricular systolic pressure of at least 50 mm Hg from the baseline echocardiogram. Major complications were recorded using established Valve Academic Research Consortium definitions for death, major bleeding, myocardial infarction, stroke, vascular complications, valve-related dysfunction, and acute kidney injury.25 Hospital LOS was defined as the number of days from the date of procedure to the date of being discharged home or to a rehabilitation facility. All patients were evaluated at 30 days and 1-year post TAVR.
The Kansas City Cardiomyopathy Questionnaire score
The Kansas City Cardiomyopathy Questionnaire (KCCQ) is a validated HR-QOL instrument which was developed to assess the health status among patient with heart failure.26,27 The questionnaire is self-administered and can be completed on average within 4–6 minutes. Within each domain of the health status, the questionnaire is scored by assigning each response to an ordinal value, with 1 beginning for the response related to the lowest value.26 The questionnaire includes 12 items which quantify physical limitation, symptom frequency, quality of life, and social limitation.27 Scores are aggregated under an overall summary score.26 Symptom burden as measured by KCCQ predicts recurrent heart failure, death, and major cardiovascular events in patients with chronic congestive heart failure and a variety of cardiovascular disoders.27 The KCCQ questionnaire is a scale that measures HR-QOL and overall health status, and it has undergone psychometric testing in patients with severe symptomatic aortic stenosis. KCCQ was determined at baseline and at 30 days and 1-year post-TAVR.
Body composition assessment by CT
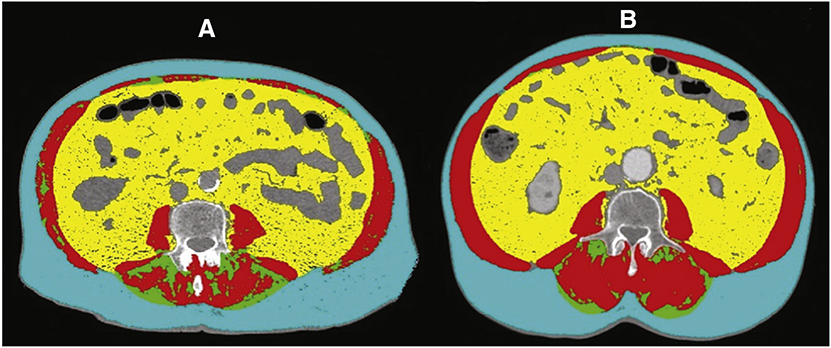
Using predefined methods, muscle quantity was evaluated from the CT cross section at L3.13,28 At this level, using Hounsfield units (HU) thresholds, the cumulative area of all abdominal skeletal muscles (psoas and paraspinal muscles, erector spinae, quadratus lumborum, transversus abdominus, external and internal oblique abdominals, and the rectus abdominus) was quantified in cm2.13,28,29 The following HU thresholds were used to characterize and differentiate tissue types: −29 to 150 for muscle, −150 to −50 for visceral adipose tissue, and −190 to −30 for intermuscular adipose tissue and subcutaneous adipose tissue.30 All CT images were analyzed by a single trained observer (G. R.) using Slice-O-Matic software, version 5.0 (Tomovision, Montreal, Quebec, Canada). A brief overview of this technique can be found here: https://www.youtube.com/watch?v=KJrsQ_dg5mM. Examples of CT-assessed body composition are shown in Figure 1. The independent observer analyzed the images retrospective to the TAVR procedure and was blinded to the patients’ demographics and all other clinical information including LOS and QOL. For skeletal muscle mass, the intraobserver coefficient of variability was less than 2%.
Figure 1.
Computed tomographic analysis of body composition. CT images (A and B) using Slice-O-Matic software, version 5.0 (Tomovision). A brief overview of this analysis can be found here: https://www.youtube.com/watch?v=KJrsQ_dg5mM. Skeletal muscle (red), visceral adipose tissue (yellow), subcutaneous adipose tissue (blue), and intramuscular adipose tissue (green). A, An 83-year-old male sarcopenic patient with a BMI of 30 kg/m2 and an SMI of 33 cm2/m2. B, A 77-year-old male nonsarcopenic patient with a BMI of 31 kg/m2 and an SMI of 65 cm2/m2.
Using the above established method, the L3 skeletal muscle cross-sectional area (cm2) was measured and then indexed to the square of the patient’s height2 (m2) and reported as a skeletal muscle mass index (SMI; cm2/m2). Very low skeletal muscle mass (ie, sarcopenia) was defined using Baumgartner’s previously established sex-specific cutoff values in elderly individuals: <55 cm2/m2 for men and <39 cm2/ m2 for women.15 These values were converted to CT measurements using previously established prediction equations.31 We also evaluated muscle attenuation (HU), as reduced muscle attenuation is reflective of intramuscular adipose tissue or poor “quality” skeletal muscle.32
Statistical analysis
Descriptive statistics for the study population baseline characteristics were presented by quartiles of SMI (cm2/m2). Categorical variables were summarized as frequencies and proportions; and continuous variables, as means and SDs. Differences between the 4 quartiles of SMI (cm2/m2) were compared using the Wilcoxon rank sum, Student t, Pearson χ2, and Fisher exact tests, as appropriate. To determine the distribution of SMI by gender and the prevalence of CT-defined sarcopenia,12 we plotted SMI according to patient gender and used the previously established binary cutoffs (<55 cm2/m2 for men and <39 cm2/m2) to depict those with severely reduced muscle mass (sarcopenia).12 To examine the relationship between SMI and clinical outcomes and HR-QOL, we compared the following across SMI quartiles: in-hospital mortality, 30-day mortality, hospital LOS, and KCCQ at 30 days and at 1 year. To determine the relationship between HR-QOL and SMI, we fitted a linear regression of HR-QOL as assessed by the KCCQ instrument (ie, Yi) on indicator variables for quartiles of SMI with the reference is being quartile (Q) 1, i.e. β0= mean Y in lowest quartile of SMI). For other quartiles, mean Y is given by:
where j represents the quartile of the ith individual minus 1. Thus, each β1, β2, β3 coefficient represents the expected mean difference in 1-year HR-QOL (as measured by the KCCQ) between patients within SMI quartiles Q2 to Q4 compared to the lowest quartile (Q1). To address confounding, we constructed the following 4 multiple linear regression analyses: (1) a model adjusting for age, gender, and race; (2) a model including variables in model 1 + body mass index (BMI); (3) a model including the variables adjusted for in model 2 in addition to total adipose tissue; and (4) a model adjusted for all of these variables in addition to the following cardiovascular risk factors: hypertension, dyslipidemia, diabetes, and prior stroke. In each of the models above, we evaluated normality by plotting histograms and q-normal plots. Figure 2 represents the q-normal plot for the multivariable linear regression that includes demographic characteristics, BMI, total adipose tissue, hypertension, dyslipidemia, diabetes mellitus, and prior stroke.
Figure 2.
The q-normal plot for multivariable linear regression that includes demographic characteristics, BMI, total adipose tissue, hypertension, dyslipidemia, diabetes mellitus, and prior stroke illustrating normality assumption is met.
We then reproduced the same results with SMI as a continuous variable. In a similar fashion, normality was evaluated using histograms, q-normal plots, and residual plotted against each continuous covariate. Using an adjusted variable plot, we visualized the multiple linear regression coefficient of SMI. Using a forward stepwise additive approach, multivariable linear regression was used to determine the independent predictors of 1-year QOL. All statistical analyses were performed using STATA version 15 MP (State Corp, College Station, TX). We considered a P value < .05 as significant, and all tests were 2-sided.
Results
Baseline clinical characteristics
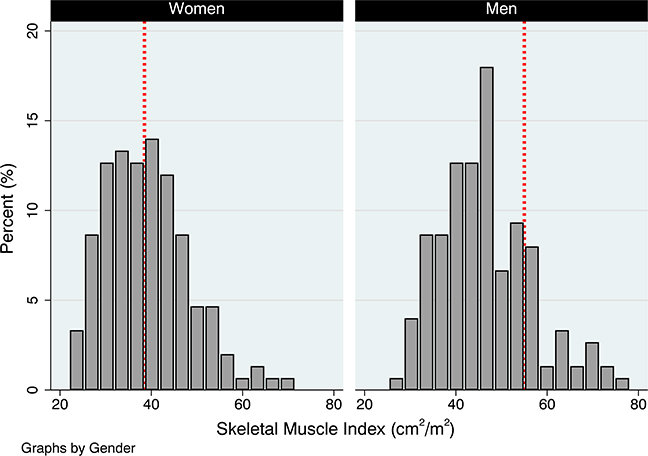
Baseline patient demographics and characteristics are shown in Table 1 for the overall sample (n = 300) and according to SMI quartile. Mean age was 79 years, 49% were women, and mean BMI was 29 kg/m2. Mean SMI was higher in men compared to women (46 vs 39 cm2/m2; P < .001). For most patients, hand grip strength was greater in the right versus left hand. Most patients had hypertension (86%) and dyslipidemia (62%), and approximately one third had diabetes mellitus. Peripheral arterial disease and prior stroke were present in approximately one quarter of patients, a third had a history of prior percutaneous coronary intervention (PCI), and fewer had prior coronary artery bypass graft (CABG). Most procedures were transfemoral (93%), with lower rates of transapical (7%) and transaortic (<1%) access. Mean aortic valve area was 0.74 cm2, and mean left ventricular ejection fraction was 54%. At least moderate mitral and tricuspid regurgitation was present in over half, but only a quarter of patients had significant pulmonary hypertension. Mean creatinine clearance was 40 mL/min, and mean STS score was 6%. The distribution of SMI ([cm2]/height2 [m2]) based on the gender of the participant is presented in Figure 3. Using preidentified cut points, the prevalence of low muscle quantity (ie, sarcopenia) was higher in men than women (men: 61% vs women: 39%, P < .001). The mean age for patients who did not have SMI ([cm2]/height2 [m2]) data available for analysis (n = 46) was 77 years, 46% were women, and the mean BMI was 30 kg/m2.
Table 1.
Baseline demographic, clinical, and echocardiographic characteristics by SMI quartile
| Variable name* | Total (N = 346) | Q1 (n = 75) | Q2 (n = 75) | Q3 (n = 75) | Q4 (n = 75) | P value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, y | 79 (9) | 83 (8) | 81 (9) | 79 (9) | 75 (9) | <.001 |
| Female gender | 171 (49) | 56 (75) | 42 (56) | 30 (40) | 22 (29) | <.001 |
| BMI, kg/m2 | 29 (7) | 26 (6) | 27 (6) | 29 (6) | 33 (6) | <.001 |
| Baseline KCCQ | 39 (23) | 39 (23) | 40 (23) | 45 (23) | 39 (23) | .883 |
| Right grip strength, kg | 15 (12) | 12 (6) | 14 (7) | 15 (7) | 20 (7) | <.001 |
| Left grip strength, kg | 13 (8) | 10 (5) | 12 (6) | 14 (6) | 16 (6) | <.001 |
| 5-m walk test, min | 9.1 (5) | 9.3 (3) | 9.7 (4) | 8.7 (5) | 8.5 (6) | .208 |
| Albumin, mg/dL | 3.5 (0.5) | 3.5 (0.6) | 3.5 (0.4) | 3.6 (0.5) | 3.6 (0.5) | .112 |
| Medical history | ||||||
| Diabetes mellitus | 121 (35) | 24 (32) | 25 (33) | 26 (35) | 33 (44) | .408 |
| Hypertension | 298 (86) | 61 (81) | 64 (85) | 66 (88) | 66 (88) | .611 |
| Dyslipidemia | 216 (62) | 49 (65) | 52 (69) | 43 (57) | 46 (61) | .460 |
| Atrial fibrillation/flutter | 116 (34) | 25 (33) | 24 (32) | 26 (35) | 27 (36) | .961 |
| Stroke | 31 (9) | 11 (15) | 5 (7) | 3 (4) | 9 (12) | .096 |
| PVD | 80 (23) | 15 (20) | 22 (29) | 18 (24) | 17 (23) | .601 |
| COPD | 52 (17) | 6 (8) | 21 (28) | 15 (20) | 10 (13) | .008 |
| CrCl, mL/min | 40 (18) | 38 (14) | 40 (17) | 44 (17) | 39 (17) | .439 |
| Previous PCI | 107(31) | 13 (17) | 26 (35) | 24 (32) | 28 (37) | .037 |
| Previous CABG surgery | 71 (21) | 12 (16) | 17 (23) | 15 (20) | 16 (22) | .753 |
| Previous pacemaker | 53 (15) | 10 (13) | 10 (13) | 13 (17) | 13 (18) | .807 |
| Echocardiographic data | ||||||
| Mean AV gradient, mm Hg | 40 (14) | 36 (12) | 39 (14) | 43 (14) | 43 (14) | .001 |
| AV area, cm2 | 0.74 (0.3) | 0.75 (0.3) | 0.75 (0.2) | 0.75 (0.3) | 0.73 (0.2) | <.001 |
| Ejection fraction, % | 54 (14) | 55 (13) | 54 (15) | 53 (15) | 55 (15) | .823 |
| Pulmonary hypertension† | 94 (27) | 16 (21) | 26 (35) | 20 (27) | 19 (25) | .312 |
| Mitral regurgitation‡ | 207 (60) | 44 (59) | 50 (67) | 43 (57) | 39 (53) | .374 |
| Tricuspid regurgitation‡ | 231 (67) | 51 (68) | 52 (69) | 45 (60) | 50 (67) | .632 |
| CT-derived body composition | ||||||
| SMI, cm2/m2 | 42.8 (10) | 30.5 (3) | 38.9 (2) | 45.1 (1.9) | 56.7 (7) | <.001 |
| Muscle attenuation, HU | 32.9 (17) | 28.8 (12) | 30.8 (14) | 34.2 (17) | 37.9 (21) | .004 |
| Total adipose tissue, cm2 | 345 (220) | 319 (157) | 361 (169) | 440 (169) | 458 (169) | <.001 |
| STS predicted mortality, % | 6 (4) | 7 (5) | 6 (3) | 6 (3) | 5 (3) | .072 |
>Percentage may not add to 100 because of rounding.
PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease; CrCl, creatinine clearance; AV, aortic valve.
Estimates are presented as either mean (SD) or percent of total; missing CT data = 46 patients.
Defined as right ventricular systolic pressure > 50 mm Hg.
Defined as either moderate or severe regurgitation.
Figure 3.
The distribution of SMI ([cm2]/height [m2]) based on the gender, which shows the prevalence of sarcopenia by the gender of participant.
Muscle quantity, strength, and performance
Patients with the lowest quartile of muscle quantity/mass (Q1) were older and more likely to be female and to have lower BMI and weaker right- and left-hand grip strength and 5-m walk time (Table 1). However, cardiovascular risk factors, comorbidities, renal function, STS score, and echocardiographic data were similar across all quartiles of SMI. Although patients in the lower quartile of SMI were less likely to have had prior PCI, the rate of prior coronary artery bypass surgery and pacemaker insertion was similar across SMI quartiles. Total adipose tissue measured in cm2 and muscle attenuation measured in HU were lowest in patients with lowest quartile of SMI.
Clinical outcomes, hospital LOS, and HR-QOL
In-hospital, 30-day, and 1-year mortality rates were low (2%, 7%, and 11%, respectively), with no differences across quartiles of SMI. Median hospital LOS was 3 days, and there was an inverse relationship between LOS and SMI (SMI Q1 LOS: 5.0 days vs SMI Q4 LOS: 3.9 days, P = .042). In a multivariable model, for every 10-unit increase in SMI ([cm2]/height [m2]), there was a 0.4-day mean reduction in LOS (β = − 0.42, CI −0.75 to −0.09, P = .012).
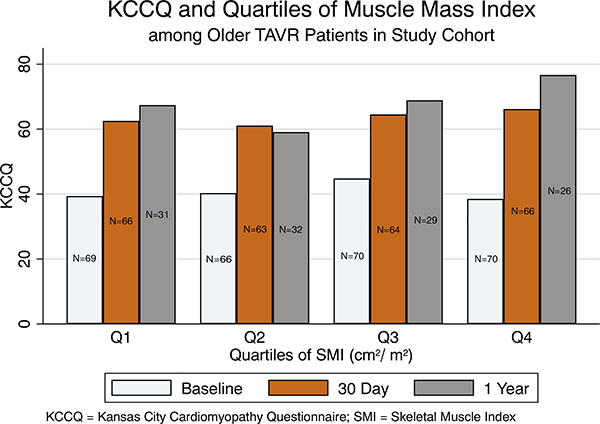
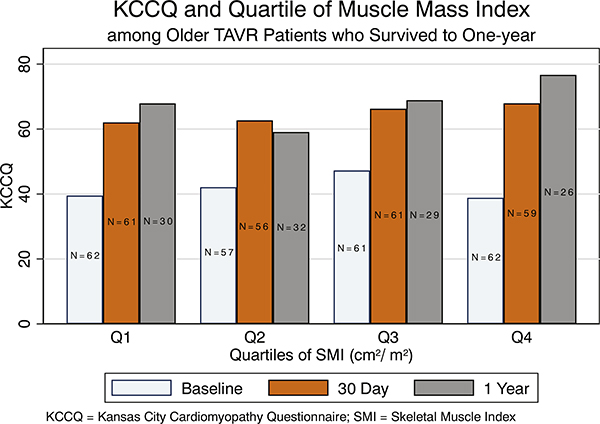
At baseline, the HR-QOL was low (mean KCCQ = 39), consistent with the pre-TAVR state. Health-related QOL was similar across all 4 quartiles of baseline SMI (Pearson P = .891). Similarly, there was no difference in KCCQ scores at 30 days across all quartiles of SMI (Pearson P = .351). However, at 1 year, patients in the highest quartile of SMI had the highest HR-QOL scores in KCCQ post-TAVR (Figure 4). When restricting the analysis to only those patients who survived at 1-year follow-up, a similar pattern emerged (Figure 5).
Figure 4.
In-hospital, 30-day, and 1-year HR-QOL as measured by KCCQ, which showed markedly improved scores at 1 year among those with the highest quartile of muscle mass index.
Figure 5.
In-hospital, 30-day, and 1-year HR-QOL as measured by KCCQ only among patients who survived to 1-year follow-up, which showed similar markedly improved scores at 1 year among those with the highest quartile of muscle mass index.
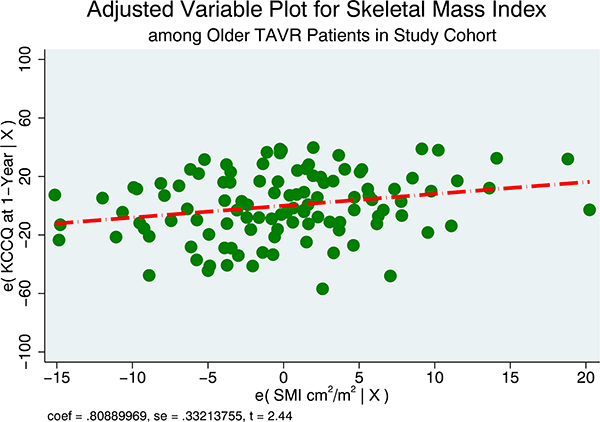
In a simple linear regression model with indicator variables for SMI, patients with the highest SMI (Q4) had no difference in KCCQ score at 1 year compared to the lowest quartile (Q1). After adjusting for age, gender, and race, this difference was larger and statistically significant (18-point difference, P = .02). After adjusting for BMI, adipose tissue, and other cardiovascular comorbidities (hypertension, dyslipidemia, diabetes mellitus, and prior stroke), the KCCQ difference between SMI Q4 versus Q1 increased to 23 points (P = .019) (Table 2). In the multivariable model, after adjusting for age, gender, race, BMI, total adipose tissue, and cardiovascular risk factors, there was on average an 8-point increase in KCCQ score per 10-unit increase of SMI ([cm2]/height [m2]) (β1 = 0.81, 95% CI 0.14–1.5, P = .017) (Figure 6).
Table 2.
Simple and multivariable linear regression of the association between quartiles of skeletal mass index (cm2/m2) and KCCQ at 1 year post-TAVR
| KCCQ Model 1* |
β Coefficient | SD | t | P>|t| | 95% CI | |
|---|---|---|---|---|---|---|
| Q1 | Ref | – | – | – | – | – |
| Q2 | −8.318024 | 6.012576 | −1.38 | .169 | −20.23725 | 3.601206 |
| Q3 | 1.476674 | 6.163706 | 0.24 | .811 | −10.74215 | 13.6955 |
| Q4 | 9.274805 | 6.951419 | 1.33 | .185 | −4.505573 | 23.05518 |
| Model 2† | ||||||
| Q1 | Ref | – | – | – | – | – |
| Q2 | −4.616871 | 6.110662 | −0.76 | .452 | −16.73454 | 7.500802 |
| Q3 | 6.983177 | 6.70859 | 1.04 | .300 | −6.320209 | 20.28656 |
| Q4 | 18.81687 | 8.028083 | 2.34 | .021 | 2.896883 | 34.73686 |
| Model 3‡ | ||||||
| Q1 | Ref | – | – | – | – | – |
| Q2 | −4.176543 | 6.230354 | −0.67 | .504 | −16.53298 | 8.179894 |
| Q3 | 7.912338 | 7.113436 | 1.11 | .269 | −6.195484 | 22.02016 |
| Q4 | 20.39908 | 8.952165 | 2.28 | .025 | 2.644569 | 38.15358 |
| Model 4§ | ||||||
| Q1 | Ref | – | – | – | – | – |
| Q2 | −3.918549 | 6.247991 | −0.63 | .532 | −16.31141 | 8.474312 |
| Q3 | 8.35313 | 7.144687 | 1.17 | .245 | −5.818324 | 22.52458 |
| Q4 | 22.49985 | 9.322309 | 2.41 | .018 | 4.009091 | 40.9906 |
| Model 5∥ | ||||||
| Q1 | Ref | – | – | – | – | – |
| Q2 | −.5522827 | 6.44249 | −0.09 | .932 | −13.33884 | 12.23428 |
| Q3 | 9.85715 | 7.39358 | 1.33 | .186 | −4.817058 | 24.53136 |
| Q4 | 22.66924 | 9.464635 | 2.40 | .019 | 3.884557 | 41.45392 |
Model 1: simple linear regression; Q1 is the reference group; Q1 is the quartile with lowest skeletal muscle index.
Model 2: adjusted for age, gender, and race.
Model 3: adjusted for variables in model 2 + BMI
Model 4: adjusted for variables in model 3 + total adipose tissue.
Model 5: adjusted for variables in model 4 + hypertension, dyslipidemia, diabetes, and prior stroke.
Figure 6.
Adjusted variable plot that shows the influence of skeletal muscle index on KCCQ at 1 year. There was on average an 8-point increase in KCCQ score per 10-unit increase of SMI ([cm2]/height [m2]). The model was adjusted for age, gender, race, BMI, total adipose tissue, and cardiovascular risk factors.
For patients who did not have SMI ([cm2]/height [m2]) data available for analysis, in-hospital (2% vs 2%, P = .645) and 1-year mortality rates were similar (17% vs 11%, P = .241). The median hospital LOS was also similar (3 vs 3 days, P = .818). Similarly, KCCQ scores at 30 days and 1 year did not differ between patients with missing SMI.
Discussion
In this study, we reported on the prevalence of sarcopenia in a consecutive cohort of older TAVR patients and determined the relationship between CT-derived SMI, hospital LOS, and 1-year HR-QOL. The key findings include the following: (1) sarcopenia was highly prevalent in the study population and more common in men versus women; (2) pre-TAVR muscle strength was positively correlated with SMI; (3) SMI was inversely and independently associated with hospital LOS; and (4) SMI independently predicted 1-year HR-QOL. These findings are relevant because they provide further insight into the factors that govern post-TAVR recovery and QOL and reveal patients in whom nutrition and exercise interventions could have the most impact.
Sarcopenia is widely recognized as a clinical disorder that disproportionately affects older adults12 and is characterized by low muscle quantity/mass, strength, and quality. For patients undergoing TAVR, muscle strength (often measured as hand grip strength) and CT-derived muscle mass have both been used to assess for sarcopenia.24,33,34 In our study, patients with low muscle quantity/mass were more likely to have low muscle strength and overall muscle strength fell below the gender and age standards. Compared to the frailty AVR study,9 the mean hand grip strength was on average 10 kg lower in our study.9 We believe that these differences are due to the fact that our TAVR study population had lower muscle quantity/mass and strength, suggesting a more extensive degree of muscle disorder. Interestingly and despite this finding, the 30-day and 1-year post-TAVR mortality outcomes in our study were favorable and independent of muscle quantity/mass and strength. This lack of association persisted when we examined SMI as (1) continuous variable, (2) categorical ordinal variable (ie, quartiles of SMI), or (3) as a binary variable with gender-specific cutoffs. These findings are similar to results reported by Nemec et al, who also failed to show an association between skeletal muscle mass and in-hospital, 30-day, and 1-year mortality35; however, this was in contrast to other studies that have shown higher post-TAVR mortality in patients with low muscle mass.33,34 We suspect that the lack of association between SMI and mortality in our study was due to the low overall mortality observed at both 30 days (4%) and 1 year (11%) in our study compared to others. These low rates of mortality compromise any ability to detect differences in outcomes across quartiles of muscle quantity/mass. To that end, it appears that short- and intermediate-term outcomes both appear to be favorable and relatively unaffected by sarcopenia, implying that contemporary TAVR appears to be safe across the broad spectrum of patients with varying degrees of progressive muscle mass loss.
We also observed that the sarcopenia (as measured by SMI) was more common in older men than in older women (61% vs 39%, respectively; P < .001).24,33 This is similar to Mok et al33 who, using similar criteria, reported sarcopenia to be present in the vast majority of men (80%) versus only approximately half of women (47%) undergoing TAVR .33 In a smaller TAVR study, using L3 CT-derived SMI measurements but slightly different cutoffs (sarcopenia: men <52.4 cm2/m2; women <38.9cm2/m2), Nemec et al35 also reported a higher prevalence of sarcopenia in men versus women (52% vs 15%, respectively; P < .001). Whether the higher prevalence of sarcopenia in men suggests that they are more prone to muscle wasting from advanced valvular heart disease or another cause merits further investigation. In a TAVR cohort, Heidari et al found no difference in hospital mortality and 30-day adverse outcomes in patients with sarcopenia versus those without sarcopenia.10 Similar to their findings, our study did not find an association with hospital mortality. This could partially be explained by the lack of statistical power to detect a difference in a fairly low mortality rate after TAVR. This association may surface with more patients or longer follow-up. However, not all geriatric syndromes in older adults with cardiovascular disease affect mortality. Some important geriatric syndromes impair the quality of life of older adults (eg, disability, functional decline); these are important outcomes for older patients as they live into their older years.36
In our study, skeletal muscle mass (SMI) predicted both LOS and HR-QOL. These findings are consistent with those reported in other clinical arenas. In a systematic review of the literature, sarcopenia was associated with worse overall HR-QOL in both older men and women.37 Furthermore, involuntary weight loss combined with low muscle quantity/mass has been shown to be associated with worse HR-QOL as measured using EuroQol-5 instrument.38 The natural age-related decline in muscle strength and HR-QOL37,39 may be mitigated by exercise, in particular, resistance training.40 We have previously shown a relationship between SMI and LOS24; however, to our knowledge, this is the first study to examine the relationship between SMI and 1-year HR-QOL post-TAVR. We found that patients with the highest baseline SMI showed the greatest improvement in 1-year HR-QOL, and this relationship between SMI and 1-year QOL strengthened after adjustment for age, gender, BMI, and other risk factors. In the multivariable model, there was on average an 8-point increase in KCCQ score per 10-unit increase of SMI which translated into a 23-point difference in KCCQ score between the highest and lowest SMI quartiles (P = .019). Given that as little as a 5-point change in KCCQ is considered clinically meaningful, this magnitude of difference is large and would be associated with major impact on a patient’s life, symptoms, and functional status.41,42 Interestingly, SMI did not predict 30-day QOL, suggesting that there may be a time dependency for the SMI-dependent improvements in QOL post-TAVR. Taken together, these findings suggest that the intermediate-term QOL of an elderly patient with aortic stenosis likely depends not only on successful TAVR but also on the effective identification and treatment of sarcopenia. Our findings and those of others suggest that sarcopenic patients, who are less likely to note an improvement in HR-QOL following TAVR, may be readily identified from the preprocedure CT scan.24,33,34 The implication is that these patients could in the future be targeted for either pre- or post-TAVR nutrition and rehabilitation interventions aimed at improving muscle mass and strength.43–46 The question of whether sarcopenia is a modifiable risk factor is an important gap in knowledge. Although aging is not modifiable, sarcopenia can potentially be actionable. For example, the PERFORM-TAVR trial (Protein and Exercise to Reverse Frailty in OldeR Men and women undergoing Transcatheter Aortic Valve Replacement) is an ongoing multicenter randomized clinical trial with a parallel-group design aimed to examine the influence of protein supplementation and exercise training on short physical performance and battery scale and quality of life, physical function, sarcopenia, and frailty during follow-up (ClinicalTrials.gov Identifier: NCT03522454). The trial highlights the importance of the findings presented in our study that raise the valid question of the association between sarcopenia a health-related quality of life after TAVR procedures. Further studies aimed at improved nutritional protein intake combined with physical therapy, medications to increase muscle mass, prehabilitation, or rehabilitation for TAVR are needed to assess the impact on long-term patient reported outcomes in sarcopenic patients (Clinical Trials: NCT03117296).
Limitations
There are important limitations to our study. First, the mortality rate in our cohort was quite low (7% at 30 days). However, the primary purpose of our study was to examine the relationship between SMI and HR-QOL rather than mortality. A systematic review of literature has already suggested that sarcopenia may be associated with hospital mortality.47 However, because of this low event rate during hospital admission, at 30 days, and at 1-year follow-up, evaluating the biasing effects of death in this study cannot be achieved with high confidence, and a study with a larger sample size may help uncover such biases. Second, we showed that patients with sarcopenia had longer hospital LOS as compared to nonsarcopenic patients. Patients with geriatric syndromes, including sarcopenia, may exhibit differences in the patterns of hospital discharge. For example, older patients who exhibit signs of sarcopenia may trigger higher likelihood of discharge to a rehabilitation facility, but those without sarcopenia can potentially be discharged home. Although this limitation can introduce bias, we did not find a difference in the proportion of patients discharged home or to a rehabilitation facility among sarcopenic versus nonsarcopenic group. Third, survival bias can potentially exist in which patients who survived were those on a better HR-QOL trajectory as measured using the KCCQ instrument as compared to those who died. Although this is a potential limitation, we found no difference in mortality rate during index admission and at 30-day or 1-year follow-up by different definitions of muscle disorder. We also had similar KCCQ score trajectories when restricting the analysis to only those patients who survived to 1-year follow-up. Outcomes after TAVR procedure have improved significantly over that time. However, we doubt that this would significantly influence the association between baseline sarcopenia and 1-year quality of life because such improvement was mainly related to advances in device technologies which would primarily influence vascular and TAVR-related complication. Fourth, differential selection bias may exist because of death at 1 year, missing data, and loss to follow-up. However, the number of patients represented within each quartile of skeletal muscle mass index at baseline, 30 days, and 1 year was relatively constant. Fifth, the definition of sarcopenia in the published literature remains variable and is dependent on the instrument used to measure muscle strength, quantity, and function. 12 This variability in definition of sarcopenia and its component make it more difficult to draw comparisons across other study populations. To address this problem, a recent European consensus on the definition of sarcopenia was developed.12 In that statement, an operational definition of sarcopenia was proposed to make it easier for clinicians to diagnose muscle disease in practice. The presence of low muscle strength alone indicates a probable sarcopenia; the addition of low muscle quantity or quality confirms the diagnosis; and if it is complicated by low physical performance, sarcopenia is considered severe.12 As we integrate many of the geriatric principles into interventional cardiology practice, well-accepted and standardized assessment tools for muscle strength, quantity, and performance are needed for research and clinical practice. Finally, we did not have extensive data on frailty testing. Our study predated the routine measurement of the Essential Frailty Test which has recently been shown to be an excellent predictor of both mortality and debilitation post-TAVR.9
Conclusions
Sarcopenia is prevalent in TAVR patients, especially among men. Higher SMI is associated with more favorable in hospital LOS and 1-year HR-QOL. To achieve optimal TAVR benefits, further study into how body composition influences post-TAVR recovery and long-term improvement is warranted.
Acknowledgements
The authors would like to acknowledge the Dudley Family for their continued contributions and support of the INOVA Dudley Family Center for Cardiovascular Innovation.
Funding and financial disclosures: Dr Damluji receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334. Dr Brown is supported in part by K24AI120834. Presentations: This work is part of a PhD for Dr Damluji at Johns Hopkins University’ GTPCI program. It was presented at the National Claude D. Pepper Older Americans Independence Centers funded by the National Institute on Aging, National Institutes of Health, and the 2019 Society of Cardiovascular Angiography and Intervention 2019 Scientific Sessions in Las Vegas, NV.
Footnotes
Disclosures
None
References
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187–98. [DOI] [PubMed] [Google Scholar]
- 3.Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. [DOI] [PubMed] [Google Scholar]
- 4.Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. [DOI] [PubMed] [Google Scholar]
- 5.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med 2012;366:1686–95. [DOI] [PubMed] [Google Scholar]
- 6.Svensson LG, Blackstone EH, Rajeswaran J, et al. Comprehensive analysis of mortality among patients undergoing TAVR: results of the PARTNER trial. J Am Coll Cardiol 2014;64:158–68. [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA 2013;310:2069–77. [DOI] [PubMed] [Google Scholar]
- 8.Genereux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol 2012;59:2317–26. [DOI] [PubMed] [Google Scholar]
- 9.Afilalo J, Lauck S, Kim DH, et al. Frailty in older adults undergoing aortic valve replacement: the FRAILTY-AVR study. J Am Coll Cardiol 2017;70:689–700. [DOI] [PubMed] [Google Scholar]
- 10.Heidari B, Al-Hijji MA, Moynagh MR, et al. Transcatheter aortic valve replacement outcomes in patients with sarcopaenia. EuroIntervention 2019;15:671–7. [DOI] [PubMed] [Google Scholar]
- 11.Kleczynski P, Dziewierz A, Bagienski M, et al. Impact of frailty on mortality after transcatheter aortic valve implantation. Am Heart J 2017;185:52–8. [DOI] [PubMed] [Google Scholar]
- 12.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Writing Group for the European Working Group on Sarcopenia in Older P and the Extended Group for E. Sarcopenia: revised European consensus on definition and diagnosis Age Ageing 2019;48:16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mourtzakis M, Prado CM, Lieffers JR, et al. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab 2008;33:997–1006. [DOI] [PubMed] [Google Scholar]
- 14.Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–63. [DOI] [PubMed] [Google Scholar]
- 16.Sheean PM, Peterson SJ, Gomez Perez S, et al. The prevalence of sarcopenia in patients with respiratory failure classified as normally nourished using computed tomography and subjective global assessment. JPEN J Parenter Enteral Nutr 2014;38:873–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Staal-van den Brekel AJ, Schols AM, Dentener MA, ten Velde GP, Buurman WA and Wouters EF. Metabolism in patients with small cell lung carcinoma compared with patients with non–small cell lung carcinoma and healthy controls. Thorax. 1997;52:338–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE,Bain VG and Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–73, 173 e1. [DOI] [PubMed] [Google Scholar]
- 19.Roubenoff R Sarcopenic obesity: does muscle loss cause fat gain? Lessons from rheumatoid arthritis and osteoarthritis. Ann N Y Acad Sci 2000;904:553–7. [DOI] [PubMed] [Google Scholar]
- 20.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61:1059–64. [DOI] [PubMed] [Google Scholar]
- 21.Sternfeld B, Ngo L, Satariano WA, et al. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol 2002;156: 110–21. [DOI] [PubMed] [Google Scholar]
- 22.Evans WJ, Campbell WW. Sarcopenia and age-related changes in body composition and functional capacity. J Nutr 1993;123:465–8. [DOI] [PubMed] [Google Scholar]
- 23.Roubenoff R Sarcopenia: effects on body composition and function. J Gerontol A Biol Sci Med Sci 2003;58:1012–7. [DOI] [PubMed] [Google Scholar]
- 24.Dahya V, Xiao J, Prado CM, et al. Computed tomography-derived skeletal muscle index: a novel predictor of frailty and hospital length of stay after transcatheter aortic valve replacement. Am Heart J 2016;182:21–7. [DOI] [PubMed] [Google Scholar]
- 25.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438–54. [DOI] [PubMed] [Google Scholar]
- 26.Green CP, Porter CB, Bresnahan DR, et al. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35: 1245–55. [DOI] [PubMed] [Google Scholar]
- 27.Mishra RK, Yang W, Roy J, et al. Kansas City Cardiomyopathy Questionnaire score is associated with incident heart failure hospitalization in patients with chronic kidney disease without previously diagnosed heart failure: chronic renal insufficiency cohort study. Circ Heart Fail 2015;8:702–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J,Heymsfield SB and Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985). 2004;97:2333–8. [DOI] [PubMed] [Google Scholar]
- 29.Kleczynski P, Tokarek T, Dziewierz A, et al. Usefulness of psoas muscle area and volume and frailty scoring to predict outcomes after transcatheter aortic valve implantation. Am J Cardiol 2018;122: 135–40. [DOI] [PubMed] [Google Scholar]
- 30.Miller KD, Jones E, Yanovski JA, et al. Visceral abdominal-fat accumulation associated with use of indinavir. Lancet 1998;351: 871–5. [DOI] [PubMed] [Google Scholar]
- 31.Shen W, Punyanitya M, Wang Z, Gallagher D, St.-Onge M-P, Albu J, Heymsfield SB and Heshka S. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. Journal of Applied Physiology. 2004;97:2333–2338. [DOI] [PubMed] [Google Scholar]
- 32.Prado CM, Cushen SJ, Orsso CE, et al. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proceedings of the Nutrition Society 2016;75:188–98. [DOI] [PubMed] [Google Scholar]
- 33.Mok M, Allende R, Leipsic J, et al. Prognostic value of fat mass and skeletal muscle mass determined by computed tomography in patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2016;117:828–33. [DOI] [PubMed] [Google Scholar]
- 34.Foldyna B, Troschel FM, Addison D, et al. Computed tomography–based fat and muscle characteristics are associated with mortality after transcatheter aortic valve replacement. J Cardiovasc Comput Tomogr 2018;12:223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nemec U, Heidinger B, Sokas C, et al. Diagnosing sarcopenia on thoracic computed tomography: quantitative assessment of skeletal muscle mass in patients undergoing transcatheter aortic valve replacement. Acad Radiol 2017;24:1154–61. [DOI] [PubMed] [Google Scholar]
- 36.Damluji AA, Forman DE. van Diepen S, Alexander KP, Page RL 2nd, Hummel SL, Menon V, Katz JN, Albert NM, Afilalo J, Cohen MG, American Heart Association Council on Clinical C, Council on C and Stroke N. Older adults in the cardiac intensive care unit: factoring geriatric syndromes in the management, prognosis, and process of care: a scientific statement from the American Heart Association. Circulation 2020;141:e6–e32. [DOI] [PubMed] [Google Scholar]
- 37.Woo T, Yu S, Visvanathan R. Systematic literature review on the relationship between biomarkers of sarcopenia and quality of life in older people. J Frailty Aging 2016;5:88–99. [DOI] [PubMed] [Google Scholar]
- 38.Kim M, Kim J, Won CW. Association between involuntary weight loss with low muscle mass and health-related quality of life in community-dwelling older adults: nationwide surveys (KNHANES 2008–2011). Exp Gerontol 2018;106:39–45. [DOI] [PubMed] [Google Scholar]
- 39.Yoo SZ, No MH, Heo JW, et al. Role of exercise in age-related sarcopenia. J Exerc Rehabil 2018;14:551–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart PD, Buck DJ. The effect of resistance training on health-related quality of life in older adults: systematic review and meta-analysis. Health Promot Perspect 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soto GE, Jones P, Weintraub WS, et al. Prognostic value of health status in patients with heart failure after acute myocardial infarction. Circulation 2004;110:546–51. [DOI] [PubMed] [Google Scholar]
- 42.Flynn KE, Lin L, Moe GW, et al. Relationships between changes in patient-reported health status and functional capacity in outpatients with heart failure. Am Heart J 2012;163:88–94. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prado CM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr 2014;38:940–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery 2011;150:505–14. [DOI] [PubMed] [Google Scholar]
- 46.Carli F, Chora D, Awasthi R, et al. Preoperative pulse and thermal radiofrequency facilitates prehabilitation and subsequent rehabilitation of a patient scheduled for total knee arthroplasty. Can J Anaesth 2015;62:1355–6. [DOI] [PubMed] [Google Scholar]
- 47.Soud M, Alahdab F, Ho G, et al. Usefulness of skeletal muscle area detected by computed tomography to predict mortality in patients undergoing transcatheter aortic valve replacement: a meta-analysis study. Int J Cardiovasc Imaging 2019;35:1141–7. [DOI] [PubMed] [Google Scholar]