Key Points
Question
Among older adults undergoing coronary revascularization, is the type of procedure (coronary artery bypass grafting [CABG] or percutaneous coronary intervention [PCI]) associated with differences in memory decline over time?
Findings
In this retrospective cohort study of 1680 US older adults, the change in the rate of memory decline from pre- to postprocedure was not significantly different for those undergoing CABG vs PCI.
Meaning
There was no significant difference in the change in the rate of memory decline after CABG compared with PCI in a cohort of older adults.
Abstract
Importance
It is uncertain whether coronary artery bypass grafting (CABG) is associated with cognitive decline in older adults compared with a nonsurgical method of coronary revascularization (percutaneous coronary intervention [PCI]).
Objective
To compare the change in the rate of memory decline after CABG vs PCI.
Design, Setting, and Participants
Retrospective cohort study of community-dwelling participants in the Health and Retirement Study, who underwent CABG or PCI between 1998 and 2015 at age 65 years or older. Data were modeled for up to 5 years preceding and 10 years following revascularization or until death, drop out, or the 2016-2017 interview wave. The date of final follow-up was November 2017.
Exposures
CABG (including on and off pump) or PCI, ascertained from Medicare fee-for-service billing records.
Main Outcomes and Measures
The primary outcome was a summary measure of cognitive test scores and proxy cognition reports that were performed biennially in the Health and Retirement Study, referred to as memory score, normalized as a z score (ie, mean of 0, SD of 1 in a reference population of adults aged ≥72 years). Memory score was analyzed using multivariable linear mixed-effects models, with a prespecified subgroup analysis of on-pump and off-pump CABG. The minimum clinically important difference was a change of 1 SD of the population-level rate of memory decline (0.048 memory units/y).
Results
Of 1680 participants (mean age at procedure, 75 years; 41% female), 665 underwent CABG (168 off pump) and 1015 underwent PCI. In the PCI group, the mean rate of memory decline was 0.064 memory units/y (95% CI, 0.052 to 0.078) before the procedure and 0.060 memory units/y (95% CI, 0.048 to 0.071) after the procedure (within-group change, 0.004 memory units/y [95% CI, −0.010 to 0.018]). In the CABG group, the mean rate of memory decline was 0.049 memory units/y (95% CI, 0.033 to 0.065) before the procedure and 0.059 memory units/y (95% CI, 0.047 to 0.072) after the procedure (within-group change, −0.011 memory units/y [95% CI, −0.029 to 0.008]). The between-group difference-in-differences estimate for memory decline for PCI vs CABG was 0.015 memory units/y (95% CI, −0.008 to 0.038; P = .21). There was statistically significant increase in the rate of memory decline after off-pump CABG compared with after PCI (difference-in-differences: mean increase in the rate of decline of 0.046 memory units/y [95% CI, 0.008 to 0.084] after off-pump CABG), but not after on-pump CABG compared with PCI (difference-in-differences: mean slowing of decline of 0.003 memory units/y [95% CI, −0.024 to 0.031] after on-pump CABG).
Conclusions and Relevance
Among older adults undergoing coronary revascularization with CABG or PCI, the type of revascularization procedure was not significantly associated with differences in the change of rate of memory decline.
This cohort study compares the change in the rate of memory decline after coronary artery bypass grafting (CABG) vs percutaneous coronary intervention (PCI) in older adults.
Introduction
Postoperative neurocognitive disorder (NCD), formerly called postoperative cognitive decline, is a concern for older adults after coronary artery bypass grafting (CABG). In 2001, an observational study showed that 5 years after CABG, 42% of patients had demonstrable cognitive decline, considerably greater than in a comparison cohort of Medicare beneficiaries.1 Partly motivated by the hypothesis that exposure to a cardiopulmonary bypass pump during CABG causes adverse neurologic outcomes,2,3 off-pump CABG was developed in the late 1990s.4,5 Although randomized trials of on- vs off-pump CABG have shown no short- (1 year)5,6,7 or intermediate- (5 years)8 term cognitive benefit of off-pump CABG, these trials were small and had restrictive enrollment criteria. To date, there is no conclusive evidence on whether CABG in older adults causes a meaningful deterioration from patients’ presurgical cognitive trajectory and whether off-pump CABG avoids such adverse cognitive effects.
Percutaneous coronary intervention (PCI) is an alternative to CABG for coronary revascularization. PCI avoids most medical exposures hypothesized to contribute to postoperative cognitive decline after CABG, including the cardiopulmonary bypass pump, general anesthesia, surgical trauma, invasive mechanical ventilation, intensive care unit exposure, and postoperative delirium. Patients who undergo PCI have coronary artery disease severe enough to merit revascularization and thus offer the natural comparison group for patients who undergo CABG when evaluating the effects of CABG on long-term cognitive decline.
The primary objective of this study was to compare change in the rate of memory decline before vs after CABG vs PCI for older adults enrolled in the population-representative Health and Retirement Study (HRS). It was hypothesized that CABG would be associated with greater increase in the rate of memory decline than PCI.
Methods
Study Design and Ethical Approval
The HRS is a prospective longitudinal survey of US adults that has been collecting economic, health, family, and lifestyle data on a population-representative sample of community-dwelling participants since 1992.9 HRS participants undergo detailed interviews (either in person or by telephone) every 2 years from cohort entry at the age of 50 years or older until death or dropout, with a focus on the experience of aging for older adults. Ethical approval for data analysis was obtained from the University of California, San Francisco, Committee on Human Research, which waived the requirement for patient consent. Participants in the HRS gave verbal informed consent for participation in the study, and data collection practices are approved by the institutional review board at the University of Michigan, which administers the HRS.
The goal of this study was to model changes in memory trajectory before vs after CABG vs PCI by establishing a longitudinal preprocedure rate of memory decline and comparing it with postprocedure rate of memory change using cognitive test results collected for a cohort of US older adults. The secondary outcome was a calculated probability of dementia. In an a priori subgroup analysis, we also modeled CABG recipients who underwent on-pump (traditional) vs off-pump (without cardiopulmonary bypass) surgery, on the hypothesis that avoiding cardiopulmonary bypass may be associated with improved cognitive outcome.4,5 In addition, we performed a post hoc analysis applying adapted definitions for NCD (formerly named postoperative cognitive decline/dysfunction) to the cohort to facilitate comparison with other studies of cognition following CABG.
Study Participants
We analyzed HRS participants aged 65 years or older who enrolled in the HRS from 1992 through 2010, agreed to release their Medicare inpatient billing records (approximately 80% of the cohort),9 and received fee-for-service care between 1998 and 2015. Participants were excluded if they did not participate in an HRS assessment, or did not complete the HRS cognitive assessment (either directly or by proxy), within 3 years prior to the cardiac procedure, or if they were assigned zero sampling weight (ie, were noncommunity dwelling) at the interview prior to the cardiac procedure. Participants were followed up until death, dropout, or the 2016 interview wave; the last follow-up interview in the analytic sample occurred in November 2017 (the interview “wave” spans a 2-year time frame).
Ascertainment of Cardiac Procedure
CABG and PCI events were identified by International Classification of Diseases, Ninth Revision procedure code (CABG: 36.1 × and 36.03; PCI: 00.66, 36.04, 36.06, and 36.07) in Medicare inpatient billing files between 1998 and 2015. Participants who underwent both CABG and PCI during the study period during separate hospitalizations were assigned to the earliest procedure. If there were billing codes for both procedures during a single hospitalization, the participant was assigned to the CABG group. Off-pump vs on-pump CABG was ascertained with the International Classification of Diseases, Ninth Revision code 39.61 (extracorporeal circulation auxiliary to open heart surgery).10 Participants who subsequently underwent any CABG were recorded but not censored because subsequent CABG was hypothesized to be a potential mediator of cognitive change.
Outcomes
The primary outcome was a summary measure of cognitive tests in the HRS, called memory score, which was developed by calibrating core HRS cognition questionnaire items against the Aging, Demographics, and Memory Study sample of HRS participants who underwent detailed in-person neuropsychologic batteries.11 The memory score uses both proxy cognition reports and/or direct participant responses to the immediate and delayed word list recall, a strategy to reduce attrition bias from participants who cannot be directly assessed but are known to be impaired.12 It has also been previously applied for modeling longitudinal memory change in the HRS.13,14,15,16,17
Memory score was normalized as a z score (ie, mean of 0, SD of 1) in the population of HRS participants 72 years of age and older in the 1995 wave.12 In the study population described here, 95% of memory score measurements fell between −0.93 and 1.50. The minimum clinically important difference in memory score has not been formally established, but was defined as a change of 1 SD of the population (in this case, the standard deviation for the rate of cognitive decline in the study population, or 0.048 memory units/y), which has been suggested as a reasonable threshold for clinical significance in cognition research.18
The primary comparison was the difference-in-differences estimate of the rate of memory score decline from the preprocedure period (ie, 3 years prior to the procedure until the time of the procedure) vs the postprocedure period (ie, 6 months after the procedure to 5 years after the procedure), comparing CABG vs PCI.
The secondary outcome was probability of dementia12,19 derived from the memory score, the Telephone Interview for Cognitive Status, and serial-7 subtractions. We modeled memory score and dementia probability from 5 years prior to 10 years after the procedure to reduce the influence of participants who contributed an unusually long period of pre- or postprocedure data.
We performed a post hoc analysis to align with objective testing criteria for the outcomes of presumptive mild and major NCD in the cohort. The actual change in memory score from pre- to postprocedure was adjusted by the expected cognitive change derived from a group of age- and sex-matched older adults without reported cardiac disease (eMethods 7 in the Supplement). We used the standard deviation of the preprocedure memory score for CABG/PCI recipients to identify participants whose adjusted postprocedure memory score was more than 2 SDs below their expected score (major NCD) or 1 to 2 SDs below expectation (mild NCD).20
Adjustment Variables
Adjustment variables were drawn from the closest HRS interview within 3 years prior to the procedure. Where possible, cleaned and processed variables prepared by the RAND Center for the Study of Aging were used (eMethods 1 in the Supplement). Models were adjusted for sex; education; age at procedure; marital status; financial assets above the median; body mass index (calculated as weight in kilograms divided by height in meters squared); current smoking; moderate/severe pain; presence of difficulty in any activity of daily living; depressive symptoms; and patient-reported diagnoses of hypertension, diabetes, lung disease, stroke, and heart disease. We also adjusted models for race and ethnicity, which were participant reported according to investigator-defined categories. Categories used in this analysis were non-Hispanic White, non-Hispanic Black, Hispanic ethnicity, and other, a category that included masked classifications (Asian, Pacific Islander, American Indian, and Alaska Native) to preserve participant confidentiality. Race and ethnicity were included in modeling to serve as a proxy for a complex set of social risk factors, which were hypothesized to influence both receipt of PCI/CABG and late-life cognitive test performance.
Specific variables were a priori selected for incorporation into propensity scores, in addition to variables listed above. These included hypothesized factors associated with CABG vs PCI21: heart failure, angina, lung disease, active nonskin malignancy, urban/rural residence, a frailty score,22,23 and participant-reported new myocardial infarction at the postprocedure interview (a surrogate for urgent revascularization). Missing data were addressed using multiple imputation with chained equations for variables missing values in more than 0.5% of participants (eMethods 2 in the Supplement).
Statistical Analysis
To assess balance on measured group characteristics, we calculated the absolute weighted difference between the groups and its 95% CI. The primary analysis was a linear mixed-effects model for memory score over time defined with respect to exact date of the procedure. The mixed model included flexible specifications of time to accommodate the potentially complex trajectories of cognitive change. We included fixed intercepts and slopes for the independent variables in the model, random intercepts for a derived survey cluster variable to account for the correlation of respondents within clusters created by the HRS complex sampling design, random intercepts and slopes for each respondent to account for the correlation among repeated observations on the same individual, and participant sampling weights. We considered that model flexibility could induce spurious findings, so models were developed with qualitative comparison with a nonprocedural control group of age- and sex-matched older adults (eMethods 3 and eFigures 1 and 2 in the Supplement). The final model used restricted cubic splines with 4 knots (for figures) and piecewise linear splines (for ease of interpretation when reporting numerical results) with knots at −3 years, 0 years (ie, time of procedure), 0.5 years, and 5 years after confirming conclusions were similar between linear and cubic splines. See eMethods 4 in the Supplement for an alternative model incorporating a discontinuity at time = 0, which added complexity without materially affecting the findings (also eFigure 3 and eTables 1 and 2 in the Supplement).
We applied the same modeling strategy to the logit of dementia probability, which was then back transformed to probability of dementia,24 and in an a priori subgroup analysis comparing on- vs off-pump CABG recipients. We used the same modeling strategy for memory score after applying inverse probability of treatment weights (eMethods 6 in the Supplement). We also conducted a sensitivity analysis to evaluate selective survival or dropout bias with a joint model for longitudinal and survival data (eMethods 5 and eTable 3 in the Supplement).
Statistical analysis was conducted in Stata version 16.0 using the svy suite of commands (StataCorp LLC), SAS/STAT version 15.1 (SAS Institute), and R version 3.6.3 (R Core Team, 2020). The a priori significance threshold was P < .05, and all P values were 2-sided. Because of the potential for type I error due to multiple comparisons, findings for secondary analyses should be interpreted as exploratory.
Results
Participants and Group Characteristics
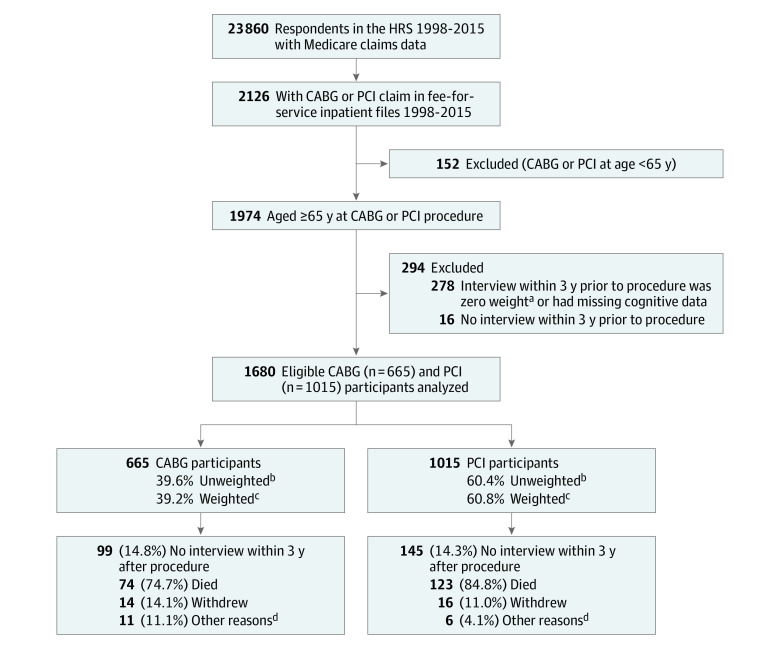
Among 665 respondents who underwent CABG, 497 had on-pump procedures and 168 had off-pump procedures; 1015 participants underwent PCI (Figure 1). Values for at least 1 variable were missing for 12.9% of respondents; after multiple imputation, 13 participants (2.0%) in the CABG group and 14 participants (1.4%) in the PCI group had missing data, which were not imputed, and may not be included in some models. The mean number of interviews per participant, and other measures of lead-in and follow-up, were not significantly different between the CABG and PCI groups (Table). The first postprocedure interview occurred at a mean (SD) of 1.12 (0.78) years after the procedure in the CABG group and 1.06 (0.75) years in the PCI group. After the index procedure, 98.9% of the CABG group and 93.7% of the PCI group remained free of subsequent CABG or other open-heart surgery during study follow-up.
Figure 1. Cohort Creation and Follow-up.
Detailed reasons for lack of participation in postprocedure trajectory modeling are included for completeness; all 1680 participants were used in modeling of the preprocedure trajectory. CABG indicates coronary artery bypass grafting; HRS, Health and Retirement Study; and PCI, percutaneous coronary intervention.
aZero weight was assigned to participants who were not community dwelling (ie, lived in a nursing home or similar care facility) at the time of the preprocedure interview.
bUnweighted values represent each participant contributing equally to the study sample.
cWeighted values apply population-level sampling weights, as assigned by the HRS’s complex sampling schema, so that the study population is representative of the US population of older adults.
dOther reasons for nonparticipation in an interview within 3 years after procedure: CABG (n = 11): 10 had no interview within 3 years, but resumed participation at a subsequent HRS interview, and 1 participated in the postprocedure interview but did not participate in cognitive testing. PCI (n = 6): 6 had no interview within 3 years, but resumed participation at a subsequent HRS interview.
Table. Description of the Underlying Population-Weighted Cohort and the Cohort Following Inverse Probability of Treatment Weighting for CABG vs PCIa.
| Cohort after population weighting | Inverse probability-weighted cohort | |||||
|---|---|---|---|---|---|---|
| No. (%) | Difference, % (95% CI) | % | Difference, % (95% CI) | |||
| CABG | PCI | CABG | PCI | |||
| Sample No. | 665 | 1015 | 665 | 1015 | ||
| Sample No. (complete case)b | 652 | 1001 | NA | 649 | 997 | NA |
| Demographics | ||||||
| Age at procedure, mean (SD), y | 74.8 (6.6) | 75.0 (7.2) | −0.2 (−1.0 to 0.5) | 74.8 (5.3) | 74.7 (6.9) | 0.1 (−0.7 to 1.0) |
| Sex | ||||||
| Male | 446 (65.8) | 556 (54.4) | 11.4 (6.2 to 16.7) | 58.7 | 58.3 | 0.4 (−6.1 to 7.0) |
| Female | 219 (34.2) | 459 (45.6) | 41.3 | 41.7 | ||
| Race/ethnicity | ||||||
| Non-Hispanic | ||||||
| White | 572 (89.4) | 829 (85.5) | 3.9 (−0.4 to 8.3) | 86.5 | 86.8 | −0.3 (−5.9 to 5.4) |
| Black | 41 (3.9) | 98 (6.2) | −2.3 (−4.3 to 0.3) | 5.8 | 5.5 | 0.2 (−3.1 to 3.6) |
| Hispanic | 45 (5.5) | 61 (5.7) | −0.2 (−3.5 to 3.1) | 5.8 | 5.7 | 0.1 (−3.4 to 3.6) |
| Otherc | 7 (1.2) | 27 (2.7) | −1.5 (−3.1 to 0.1) | 1.9 | 1.9 | −0.1 (−2.4 to 2.2) |
| Education | ||||||
| <High school/GED | 220 (30.8) | 329 (30.3) | 0.5 (−5.5 to 6.4) | 28.4 | 28.3 | 0.2 (−7.2 to 7.6) |
| High school | 200 (30.8) | 343 (34.3) | −3.5 (−8.5 to 1.5) | 32.8 | 32.7 | 0.1 (−6.5 to 6.7) |
| Some college | 113 (18.5) | 192 (19.8) | −1.3 (5.6 to 3.1) | 20.1 | 20.9 | −0.8 (5.7 to 4.2) |
| >College | 132 (19.9) | 151 (15.5) | 4.3 (0 to 8.7) | 18.7 | 18.2 | 0.5 (−5.2 to 6.2) |
| Wealth greater than cohort median | 298 (56.5) | 507 (49.7) | 6.9 (1.2 to 12.5) | 46.1 | 46.3 | 0.2 (−6.2 to 6.7) |
| Married/partnered | 479 (69.4) | 647 (59.3) | 10.1 (4.7 to 15.5) | 63.6 | 63.0 | 0.6 (−6.3 to 7.4) |
| Health covariates | ||||||
| BMI | ||||||
| <18.5 | 4 (0.8) | 9 (0.9) | −0.1 (−1.0 to 0.8) | 0.8 | 0.8 | 0.04 (−1.3 to 1.4) |
| 18.5-24.9 | 187 (27.3) | 277 (26.9) | 0.4 (−4.8 to 5.6) | 27.2 | 26.5 | 0.8 (−4.6 to 6.1) |
| 25-29.9 | 297 (47.0) | 452 (45.5) | 1.5 (−4.4 to 7.4) | 46.4 | 46.8 | −0.4 (−6.4 to 5.6) |
| ≥30 | 172 (24.9) | 272 (26.7) | −1.8 (−6.8 to 3.2) | 25.6 | 26.0 | −0.4 (−5.9 to 5.1) |
| Currently smoking | 89 (13.0) | 137 (15.7) | −2.7 (−5.6 to 0.2) | 12.4 | 12.9 | −0.6 (−5.4 to 4.3) |
| Hypertension | 457 (68.3) | 676 (66.1) | 2.2 (−2.9 to 7.4) | 64.2 | 64.7 | −0.5 (−6.9 to 5.9) |
| Prior diagnosis of heart problems | 300 (45.7) | 476 (46.5) | −0.8 (−7.1 to 5.6) | 44.1 | 43.7 | 0.3 (−7.1 to 7.9) |
| Diabetes | 211 (31.4) | 314 (30.6) | 0.8 (−4.1 to 5.7) | 28.2 | 28.6 | −0.5 (−7.0 to 6.1) |
| Moderate-severe paind | 169 (24.3) | 299 (30.0) | −5.7 (−11.0 to −0.5) | 27.0 | 27.5 | −0.5 (−6.8 to 5.7) |
| Depressive symptomse | 129 (21.0) | 243 (24.9) | −3.9 (−8.8 to 1.0) | 20.7 | 21.1 | −0.3 (−5.6 to 4.9) |
| Prior stroke | 88 (13.3) | 146 (13.8) | −0.6 (−4.0 to 2.9) | 13.2 | 13.4 | −0.2 (−4.6 to 4.2) |
| Lung disease | 54 (7.8) | 135 (14.8) | −7.0 (−10.7 to −3.2) | 8.4 | 10.4 | −2.0 (−5.4 to 1.4) |
| Difficulty with at least 1 activity of daily living | 126 (19.1) | 196 (19.2) | −0.1 (−5.1 to 4.9) | 16.9 | 17.5 | −0.6 (−6.4 to 5.2) |
| Interview data, mean (SD) | ||||||
| No. of interviews | 5.0 (1.9) | 5.0 (1.9) | 0 (−0.2 to 0.2) | 5.4 (1.3) | 5.5 (1.7) | −0.1 (−0.2 to 0.1) |
| No. of preprocedure interviews | 2.2 (0.7) | 2.2 (0.7) | −0.01 (−0.08 to 0.07) | 2.3 (0.5) | 2.3 (0.7) | −0.01 (−0.08 to 0.06) |
| No. of postprocedure interviews | 2.8 (1.8) | 2.8 (1.9) | −0.01 (−0.19 to 0.17) | 3.2 (1.3) | 3.2 (1.6) | −0.06 (−0.25 to 0.14) |
| Mean person-years of preprocedure data | 2.4 (1.4) | 2.3 (1.4) | −0.02 (−0.07 to 0.02) | 2.4 (1.2) | 2.4 (1.5) | 0.003 (−0.05 to 0.06) |
| Mean person-years of postprocedure data | 3.9 (2.7) | 3.9 (2.7) | 0.07 (−0.18 to 0.11) | 3.9 (2.4) | 4.0 (2.9) | −0.06 (−0.22 to 0.11) |
| Preprocedure memory score, mean (SD) | ||||||
| Preprocedure memory score, memory unitsf | 0.84 (0.49) | 0.82 (0.54) | 0.01 (−0.04 to 0.07) | 0.90 (0.35) | 0.89 (0.46) | 0.01 (−0.04 to 0.07) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CABG, coronary artery bypass grafting; GED, General Educational Development test; NA, not applicable; PCI, percutaneous coronary intervention.
The actual number of participants prior to weighting, and proportion of the sample after application of population-level survey weights, are reported in the cells.
Complete case No. includes those who were completed via multiple imputation, and best reflects the No. included in the final analytic models. The inverse probability of treatment weighted models required more variables (ie, those used in the CABG vs PCI propensity score, as well as the other main adjustment variables), hence the complete case No. is lower.
Other race is a composite of Asian, Pacific Islander, American Indian, and Alaska Native, which are masked as a single category to protect participant confidentiality.
Moderate-severe pain was determined at the preprocedure interview (as were all health covariates listed here) and was ascertained as a 2-part question: “Are you often troubled with pain?” and “Is the pain mild, moderate, or severe?” Participants who reported they were often troubled by moderate or severe pain met the definition for this health covariate.
Depressive symptoms were considered a score of 3 or greater on the revised 8-item Center for Epidemiologic Studies Depression scale (range, 0-8).
Memory score is a summary measure that uses direct response to immediate and delayed word recall or proxy cognition assessments for those who do not or cannot directly participate in cognitive assessments. The score is roughly analogous to a population cognitive z score and is normally distributed. A total of 95% of the study population memory score values were between −0.93 and 1.5, to offer an idea of range. Higher memory scores indicate better composite memory function.
Participants who underwent CABG were more likely than participants who underwent PCI to be male, married or partnered, and have financial assets above the median (Table). The mean memory score at the preprocedure interview was not significantly different between the CABG and PCI groups (Table). After inverse probability of treatment weighting, the groups were well-matched on measured participant characteristics (Table).
Primary Analysis
In the PCI group, the mean rate of covariate-adjusted memory decline was 0.064 memory units/y (95% CI, 0.052 to 0.078) before the procedure and 0.060 memory units/y (95% CI, 0.048 to 0.071) after the procedure (within-group change, 0.004 memory units/y [95% CI, −0.010 to 0.018]). In the CABG group, the mean adjusted rate of memory decline was 0.049 memory units/y (95% CI, 0.033 to 0.065) before the procedure and 0.059 memory units/y (95% CI, 0.047 to 0.072) after the procedure (within-group change, −0.011 memory units/y [95% CI, −0.029 to 0.008]). The between-group difference-in-differences estimate for memory decline for PCI vs CABG was 0.015 memory units/y (95% CI, −0.008 to 0.038; P = .21). After revascularization, the rate of memory score decline in the CABG group vs PCI group was not significantly different (difference-in-differences, CABG-PCI, 0.0002 memory units/y [95% CI, −0.018 to 0.018]; P = .98) (Figure 2A). Results of additional models for memory score incorporating a discontinuity at time = 0 (eFigure 3 and eTables 1 and 2) and a joint model for longitudinal and survival outcomes (eTable 3) are available in the Supplement.
Figure 2. Adjusted Linear Mixed-Effects Models for Memory Score and Dementia Probability for CABG vs PCI Recipients.
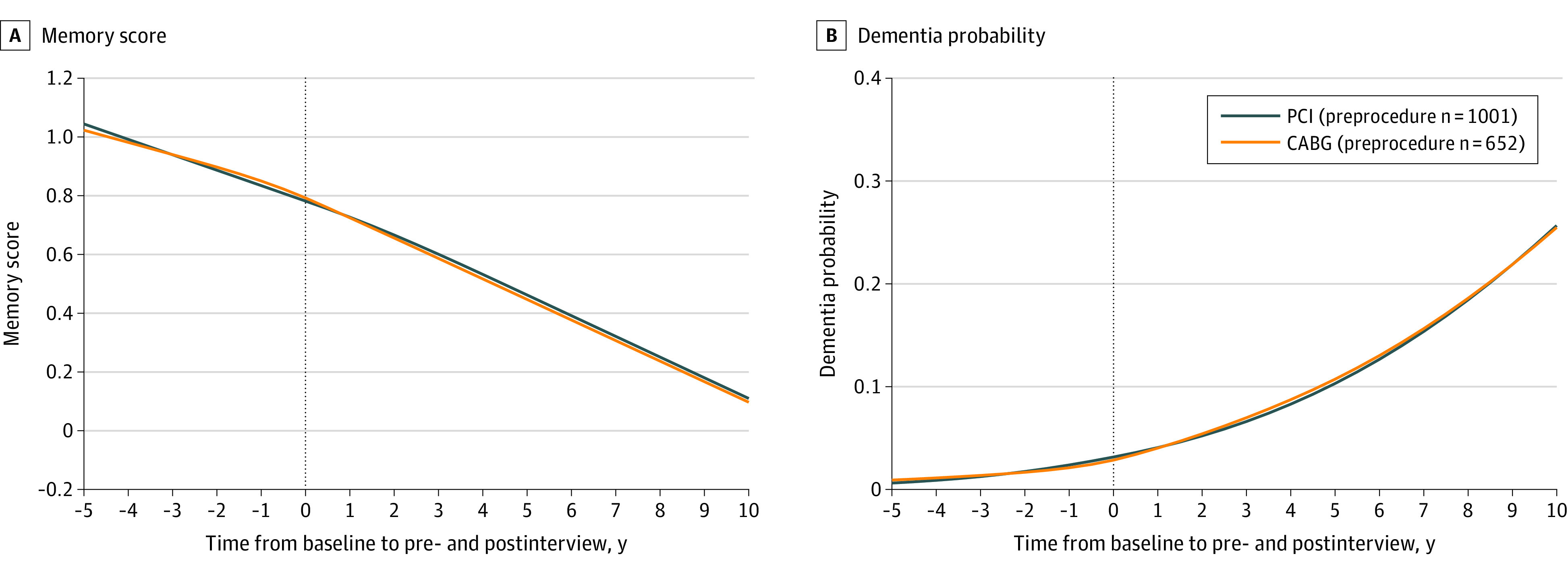
Models in A and B included 652 coronary artery bypass grafting (CABG) recipients and 1001 percutaneous coronary intervention (PCI) recipients at the preprocedure interview. Memory score is a summary measure of word recall and proxy cognition assessments roughly analogous to a population cognitive z score. Higher memory scores indicate better composite memory function. Dementia probability was derived from the memory score, the Telephone Interview for Cognitive Status, and serial-7 subtractions, and it ranges from 0 to 1.
Secondary Analyses
Long-term dementia probability trajectory was not significantly different between the CABG and PCI groups (Figure 2B). Predicted probability of dementia 5 years after revascularization, at population mean characteristics, was 10.5% (95% CI, 8.4% to 13.0%) in the CABG group and 9.6% (95% CI, 7.9% to 11.5%) in the PCI group (difference, 0.9% [95% CI, −2.0% to 3.8%]). Analysis of the memory score using inverse probability weighting to compare CABG and PCI cognitive trajectories yielded substantially similar results to the primary analysis (eFigure 4 in the Supplement).
Prespecified Subgroup Analysis for On- and Off-Pump CABG
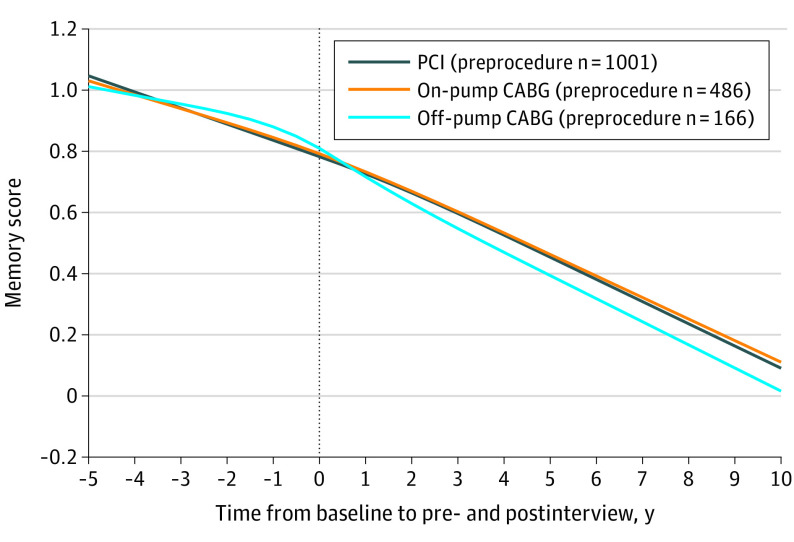
For the on-pump CABG group, the mean rate of covariate-adjusted memory decline was 0.055 memory units/y (95% CI, 0.036 to 0.074) before the procedure and 0.054 memory units/y (95% CI, 0.038 to 0.071) after the procedure (within-group change, 0.001 memory units/y [95% CI, −0.022 to 0.024] slower decline after the procedure). For the off-pump CABG group, the mean adjusted rate of memory decline was 0.032 memory units/y (95% CI, 0.001 to 0.063) before the procedure and 0.074 memory units/y (95% CI, 0.052 to 0.096) after the procedure (within-group change, 0.042 memory units/y [95% CI, 0.006 to 0.078] increase in the rate of decline after off-pump CABG).
The between-group difference-in-differences estimate for on-pump CABG vs PCI was a mean slowing of decline of 0.003 memory units/y (95% CI, −0.024 to 0.031) after on-pump CABG compared with PCI. The between-group difference-in-differences estimate for off-pump CABG vs PCI was a mean increase in the rate of decline of 0.046 memory units/y (95% CI, 0.008 to 0.084) after off-pump CABG compared with PCI. This rate increase approached, and the confidence intervals include, 1 SD of the population rate of memory decline,18 which was 0.048 memory units/y (Figure 3). Direct comparison of on-pump vs off-pump CABG yielded a between-group difference-in-difference estimate of 0.043–memory units/y (95% CI, −0.003 to 0.088) faster decline in the off-pump CABG group, a difference that was not statistically significant.
Figure 3. Adjusted Memory Score Trajectory Model for PCI, On-Pump CABG, and Off-Pump CABG Recipients.
The model included 1001 percutaneous coronary intervention (PCI) recipients, 486 on-pump coronary artery bypass grafting (CABG) recipients, and 166 off-pump CABG recipients at the preprocedure interview. Memory score is a summary measure of word recall and proxy cognition assessments roughly analogous to a population cognitive z score (see footnote f in the Table for details). Higher memory scores indicate better composite memory function.
Post Hoc Analysis for Mild and Major Postoperative NCD
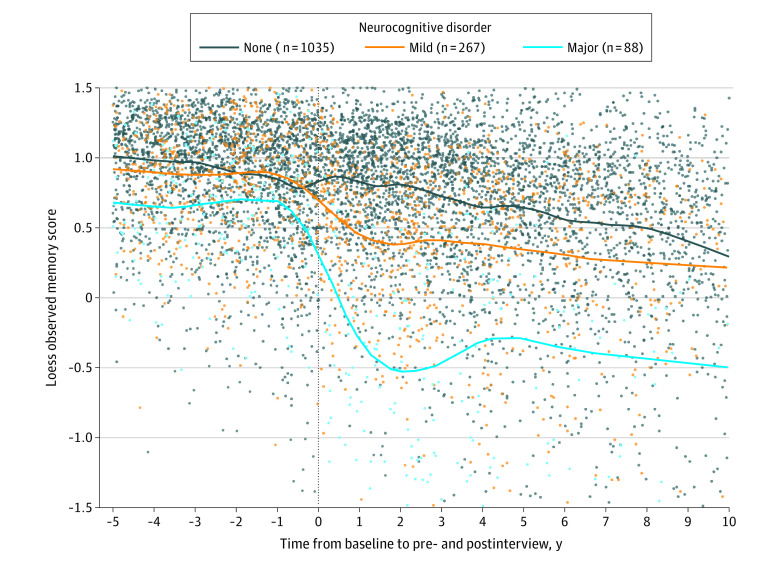
Although postprocedure memory trajectories were not significantly different for participants who underwent CABG vs PCI, some participants met an adapted definition, applied post hoc, for the objective criteria of major or mild NCD after the procedure (Figure 4; eFigure 5 in the Supplement). Both major (6.4% of CABG group, 4.8% of PCI group; absolute difference, 1.5% [95% CI, −1.1% to 4.2%]) and mild (20% of CABG group and 16% of PCI group; absolute difference, 3.6% [95% CI, −0.3% to 7.5%]) NCD were not significantly different for participants who underwent CABG compared with PCI. However, older adults who underwent CABG were significantly more likely to meet any NCD definition (CABG: 26% any NCD; PCI: 21% any NCD; absolute difference, 5.1% [95% CI, 0.6% to 9.6%]; P = .03).
Figure 4. Loess Smoother Graphs Fitting Observed Memory Score Measurements for Participants Who Met Adapted Neurocognitive Disorder Definitions.
Neurocognitive disorder determinations are made on the basis of the first postprocedure memory score measurement, excluding those within 6 months of procedure, and included adapted definitions for mild neurocognitive disorder (orange; n = 267), major neurocognitive disorder (light blue; n = 88), and no neurocognitive disorder (dark blue; n = 1035). Graphs are nonparametric, unadjusted, and do not use survey weights.
Discussion
In this nationally representative cohort of 1680 US older adults, 2 methods of coronary revascularization—1 surgical and 1 minimally invasive—were not associated with statistically significant differential change in 5-year rate of memory decline. The absence of a significant difference between groups persisted after balancing the cohort using inverse probability of treatment weighting. That the rate of late-life cognitive change was not significantly different after CABG compared with PCI suggests that the modality of revascularization is not a strong determinant of subsequent cognitive aging. CABG was not associated with significantly different modeled probability of late-life dementia compared with PCI, closing an important gap posed by studies demonstrating elevated dementia risk in patients who undergo CABG when compared with population norms (ie, without controlling for severe cardiac disease).25,26
However, an a priori subgroup analysis of off-pump CABG vs on-pump CABG demonstrated statistically significant increase in the rate of cognitive decline following off-pump CABG, but not on-pump CABG, compared with PCI. The difference in memory trajectory after off-pump CABG was qualitatively sustained until the end of the modeling timeframe. Incomplete revascularization and late cardiovascular events are more common with off-pump CABG than on-pump CABG.27,28 Although the OCTOPUS trial demonstrated no significant difference in 5-year cognitive outcomes between on- and off-pump CABG recipients, this study enrolled only 281 patients who underwent CABG at a mean age of 61 years, and confidence limits for the effect size were wide.8 Other off-vs-on-pump CABG randomized trials did not study 5-year cognitive outcomes, so observational data provide the only available source for measurement of long-term potential differential cognitive change, particularly for older adults.
Consistent with prior evidence, neither on-pump CABG nor PCI were associated with a statistically significant change in population-level long-term cognitive trajectory compared with preprocedure trajectory. The absence of comparison with a preprocedure trajectory has historically been a limitation of longitudinal studies of cognition after surgery,29 with notable exceptions also leveraging epidemiologic data.23,30 The absence of a statistically significant change in cognitive trajectory after revascularization empirically confirms the conclusions of Selnes et al,31 who suggested that cognitive decline for older adults undergoing CABG is driven by population-level cognitive aging and the cognitive effects of severe cardiac disease. This conclusion is further supported by comparison of older adults who underwent on- vs off-pump CABG: off-pump CABG may be a less durable method of revascularization.27,28 Further, in the present study, it was associated with worse long-term cognitive outcome than PCI, offering the hypothesis that the more durable and complete revascularization achieved by on-pump CABG may have beneficial cognitive implications. However, a post hoc analysis showed that, within both the CABG and PCI groups, there were individuals who met an adapted definition for mild or major NCD,20 implying an adverse cognitive outcome. This emphasizes that, while population-level metrics are important, they can obscure meaningful individual-level differences that include long-term adverse cognitive outcomes32 regardless of modality of revascularization.
Limitations
This study has several limitations. First, these models capture population means, thus, population-level trajectories include some individuals who decline and some who improve. This was highlighted in a post hoc analysis that demonstrated variability in postprocedure cognitive trajectory regardless of modality of revascularization.
Second, dementia probability and adapted NCD determinations were modeled based on brief neuropsychologic assessments incorporated in the HRS, not clinical diagnoses, so they have uncertain clinical relevance at the individual level. Further, because NCD stratification could occur on the basis of interviews more than 1 year after the procedure, the alignment with postoperative NCD (a diagnosis that must be made <1 year after surgery) is imperfect.
Third, because treatment modality was ascertained from Medicare billing records, only participants receiving fee-for-service care were modeled. Medicare Advantage recipients tend to be younger, are more likely to be female and of a racial/ethnic minority group, and may be less likely to receive invasive cardiac procedures.33,34 However, it is unlikely that the association between CABG and late-life cognition would be different, after covariate adjustment, for Medicare Advantage vs fee-for-service recipients. These findings should not be extended to non–Medicare-eligible adults (eg, <65 years).
Fourth, some potentially relevant confounding variables (eg, coronary anatomy prompting revascularization; surgeon-specific variables) were unavailable in this secondary data analysis, and no comparable nonprocedural control group exists for inferential purposes. However, data sources that include both detailed clinical data and long-term (eg, 5-year) presurgical cognitive trajectory are not available; retrospective cohort data therefore provide an important complement to what is already known about this topic.
Fifth, participants in this study underwent PCI or CABG between 1998 and 2015; as surgical and interventional techniques evolve over time, it is unknown whether these findings apply to contemporary procedures. Sixth, the memory score that was modeled is heavily weighted toward memory function and may not be sensitive to change in other cognitive modalities (eg, executive function; processing speed).
Conclusions
Among older adults undergoing coronary revascularization with CABG or PCI, the type of revascularization procedure was not significantly associated with differences in the change of rate of memory decline.
eMethods 1. Adjustment Variables
eMethods 2. Missing Data
eMethods 3. Nonprocedural qualitative comparison group
eFigure 1. Flow Diagram for the Heart Healthy and Heart Disease Control Subjects
eFigure 2. Primary Analysis, Including Nonprocedural Controls
eMethods 4. Alternative models incorporating a discontinuity at time=0
eTable 1. Intercept Change at Time=0 for Model Depicted in eFigure 3
eTable 2. Results of Primary Analysis in Models With and Without a Discontinuity
eFigure 3. Primary Analysis, Including a Discontinuity at Time=0
eMethods 5. Joint model for longitudinal and survival data
eTable 3. Mixed-Effects Model Compared With Joint Model Coefficients
eMethods 6. Propensity-Weighted Analysis
eFigure 4. Inverse Probability of Treatment-Weighted Trajectories
eMethods 7. Neurocognitive Disorder Stratification
eFigure 5. Point Clouds for NCD Groups
eReferences
References
- 1.Newman MF, Kirchner JL, Phillips-Bute B, et al. ; Neurological Outcome Research Group and the Cardiothoracic Anesthesiology Research Endeavors Investigators . Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395-402. doi: 10.1056/NEJM200102083440601 [DOI] [PubMed] [Google Scholar]
- 2.Branthwaite MA. Neurological damage related to open-heart surgery: a clinical survey. Thorax. 1972;27(6):748-753. doi: 10.1136/thx.27.6.748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sotaniemi KA. Brain damage and neurological outcome after open-heart surgery. J Neurol Neurosurg Psychiatry. 1980;43(2):127-135. doi: 10.1136/jnnp.43.2.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diegeler A, Hirsch R, Schneider F, et al. Neuromonitoring and neurocognitive outcome in off-pump versus conventional coronary bypass operation. Ann Thorac Surg. 2000;69(4):1162-1166. doi: 10.1016/S0003-4975(99)01574-X [DOI] [PubMed] [Google Scholar]
- 5.Van Dijk D, Jansen EW, Hijman R, et al. ; Octopus Study Group . Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287(11):1405-1412. doi: 10.1001/jama.287.11.1405 [DOI] [PubMed] [Google Scholar]
- 6.Lamy A, Devereaux PJ, Prabhakaran D, et al. ; CORONARY Investigators . Effects of off-pump and on-pump coronary-artery bypass grafting at 1 year. N Engl J Med. 2013;368(13):1179-1188. doi: 10.1056/NEJMoa1301228 [DOI] [PubMed] [Google Scholar]
- 7.Shroyer AL, Grover FL, Hattler B, et al. ; Veterans Affairs Randomized On/Off Bypass (ROOBY) Study Group . On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med. 2009;361(19):1827-1837. doi: 10.1056/NEJMoa0902905 [DOI] [PubMed] [Google Scholar]
- 8.van Dijk D, Spoor M, Hijman R, et al. ; Octopus Study Group . Cognitive and cardiac outcomes 5 years after off-pump vs on-pump coronary artery bypass graft surgery. JAMA. 2007;297(7):701-708. doi: 10.1001/jama.297.7.701 [DOI] [PubMed] [Google Scholar]
- 9.Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort profile: the Health and Retirement Study (HRS). Int J Epidemiol. 2014;43(2):576-585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benedetto U, Angelini GD, Caputo M, et al. Off- vs on-pump coronary artery bypass graft surgery on hospital outcomes in 134,117 octogenarians. J Thorac Dis. 2017;9(12):5085-5092. doi: 10.21037/jtd.2017.11.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langa KM, Plassman BL, Wallace RB, et al. The Aging, Demographics, and Memory Study: study design and methods. Neuroepidemiology. 2005;25(4):181-191. doi: 10.1159/000087448 [DOI] [PubMed] [Google Scholar]
- 12.Wu Q, Tchetgen EJ, Osypuk TL, White K, Mujahid M, Maria Glymour M. Combining direct and proxy assessments to reduce attrition bias in a longitudinal study. Alzheimer Dis Assoc Disord. 2013;27(3):207-212. doi: 10.1097/WAD.0b013e31826cfe90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marden JR, Mayeda ER, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. High hemoglobin A1c and diabetes predict memory decline in the Health and Retirement Study. Alzheimer Dis Assoc Disord. 2017;31(1):48-54. doi: 10.1097/WAD.0000000000000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Q, Mejía-Guevara I, Rist PM, Walter S, Capistrant BD, Glymour MM. Changes in memory before and after stroke differ by age and sex, but not by race. Cerebrovasc Dis. 2014;37(4):235-243. doi: 10.1159/000357557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marden JR, Mayeda ER, Walter S, et al. Using an Alzheimer disease polygenic risk score to predict memory decline in Black and White Americans over 14 years of follow-up. Alzheimer Dis Assoc Disord. 2016;30(3):195-202. doi: 10.1097/WAD.0000000000000137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vable AM, Eng CW, Mayeda ER, et al. Mother’s education and late-life disparities in memory and dementia risk among US military veterans and non-veterans. J Epidemiol Community Health. 2018;72(12):1162-1167. doi: 10.1136/jech-2018-210771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitlock EL, Diaz-Ramirez LG, Glymour MM, Boscardin WJ, Covinsky KE, Smith AK. Association between persistent pain and memory decline and dementia in a longitudinal cohort of elders. JAMA Intern Med. 2017;177(8):1146-1153. doi: 10.1001/jamainternmed.2017.1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh-Manoux A, Kivimäki M. The importance of cognitive aging for understanding dementia. Age (Dordr). 2010;32(4):509-512. doi: 10.1007/s11357-010-9147-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianattasio KZ, Ciarleglio A, Power MC. Development of Algorithmic dementia ascertainment for racial/ethnic disparities research in the US Health and Retirement Study. Epidemiology. 2020;31(1):126-133. doi: 10.1097/EDE.0000000000001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Evered L, Silbert B, Knopman DS, et al. ; Nomenclature Consensus Working Group . Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121(5):1005-1012. doi: 10.1016/j.bja.2017.11.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med. 2012;366(16):1467-1476. doi: 10.1056/NEJMoa1110717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cigolle CT, Ofstedal MB, Tian Z, Blaum CS. Comparing models of frailty: the Health and Retirement Study. J Am Geriatr Soc. 2009;57(5):830-839. doi: 10.1111/j.1532-5415.2009.02225.x [DOI] [PubMed] [Google Scholar]
- 23.Whitlock EL, Diaz-Ramirez LG, Smith AK, Boscardin WJ, Avidan MS, Glymour MM. Cognitive change after cardiac surgery versus cardiac catheterization: a population-based study. Ann Thorac Surg. 2019;107(4):1119-1125. doi: 10.1016/j.athoracsur.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Package ‘logitnorm’: Functions for the Logitnormal Distribution. Version 0.8.372018. The R Project.
- 25.Kuźma E, Airdrie J, Littlejohns TJ, et al. Coronary artery bypass graft surgery and dementia risk in the Cardiovascular Health Study. Alzheimer Dis Assoc Disord. 2017;31(2):120-127. doi: 10.1097/WAD.0000000000000191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evered LA, Silbert BS, Scott DA, Maruff P, Ames D. Prevalence of dementia 7.5 years after coronary artery bypass graft surgery. Anesthesiology. 2016;125(1):62-71. doi: 10.1097/ALN.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 27.Diegeler A, Börgermann J, Kappert U, et al. Five-year outcome after off-pump or on-pump coronary artery bypass grafting in elderly patients. Circulation. 2019;139(16):1865-1871. doi: 10.1161/CIRCULATIONAHA.118.035857 [DOI] [PubMed] [Google Scholar]
- 28.Shroyer AL, Hattler B, Wagner TH, et al. ; Veterans Affairs ROOBY-FS Group . Five-year outcomes after on-pump and off-pump coronary-artery bypass. N Engl J Med. 2017;377(7):623-632. doi: 10.1056/NEJMoa1614341 [DOI] [PubMed] [Google Scholar]
- 29.Nadelson MR, Sanders RD, Avidan MS. Perioperative cognitive trajectory in adults. Br J Anaesth. 2014;112(3):440-451. doi: 10.1093/bja/aet420 [DOI] [PubMed] [Google Scholar]
- 30.Krause BM, Sabia S, Manning HJ, Singh-Manoux A, Sanders RD. Association between major surgical admissions and the cognitive trajectory: 19 year follow-up of Whitehall II cohort study. BMJ. 2019;366:l4466. doi: 10.1136/bmj.l4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selnes OA, Grega MA, Bailey MM, et al. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann Thorac Surg. 2009;88(2):445-454. doi: 10.1016/j.athoracsur.2009.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179-1185. doi: 10.1213/ANE.0b013e318215217e [DOI] [PubMed] [Google Scholar]
- 33.Figueroa JF, Blumenthal DM, Feyman Y, et al. Differences in management of coronary artery disease in patients with Medicare advantage vs traditional fee-for-service Medicare among cardiology practices. JAMA Cardiol. 2019;4(3):265-271. doi: 10.1001/jamacardio.2019.0007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matlock DD, Groeneveld PW, Sidney S, et al. Geographic variation in cardiovascular procedure use among Medicare fee-for-service vs Medicare Advantage beneficiaries. JAMA. 2013;310(2):155-162. doi: 10.1001/jama.2013.7837 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Adjustment Variables
eMethods 2. Missing Data
eMethods 3. Nonprocedural qualitative comparison group
eFigure 1. Flow Diagram for the Heart Healthy and Heart Disease Control Subjects
eFigure 2. Primary Analysis, Including Nonprocedural Controls
eMethods 4. Alternative models incorporating a discontinuity at time=0
eTable 1. Intercept Change at Time=0 for Model Depicted in eFigure 3
eTable 2. Results of Primary Analysis in Models With and Without a Discontinuity
eFigure 3. Primary Analysis, Including a Discontinuity at Time=0
eMethods 5. Joint model for longitudinal and survival data
eTable 3. Mixed-Effects Model Compared With Joint Model Coefficients
eMethods 6. Propensity-Weighted Analysis
eFigure 4. Inverse Probability of Treatment-Weighted Trajectories
eMethods 7. Neurocognitive Disorder Stratification
eFigure 5. Point Clouds for NCD Groups
eReferences