Abstract
Most legume plants can associate with diazotrophic soil bacteria called rhizobia, resulting in new root organs called nodules that enable N2 fixation. Nodulation is an energy-consuming process, and nodule number is tightly regulated by independent systemic signaling pathways controlled by CLE/SUNN and CEP/CRA2. Moreover, nitrate inhibits legume nodulation via local and systemic regulatory pathways. In Medicago truncatula, NLP1 plays important roles in nitrate-induced inhibition of nodulation, but the relationship between systemic and local pathways in mediating nodulation inhibition by nitrate is poorly understood. In this study, we found that nitrate induces CLE35 expression in an NLP1-dependent manner and that NLP1 binds directly to the CLE35 promoter to activate its expression. Grafting experiments revealed that the systemic control of nodule number involves negative regulation by SUNN and positive regulation by CRA2 in the shoot, and that NLP1's control of the inhibition of rhizobial infection, nodule development, and nitrogenase activity in response to nitrate is determined by the root. Unexpectedly, grafting experiments showed that loss of CRA2 in the root increases nodule number at inhibitory nitrate levels, probably because of CEP1/2 upregulation in the cra2 mutants, suggesting that CRA2 exerts active negative feedback regulation in the root.
Key words: CRA2, CEP, CLE, nitrate inhibition of nodulation, NLP1, SUNN
Root nodule formation is suppressed locally and systemically by nitrate. This study reports that NLP1 directly controls CLE35 in the root to systemically regulate nodulation in response to nitrate.
Introduction
Nodulation, the symbiotic association of N-fixing bacteria and legumes, imposes a large energetic burden on the host plant. Consequently, legumes have evolved an elaborate process to balance nitrogen demand against energy expenditure by regulating the number of nodules via a long-distance signaling pathway (Caetano-Anolles and Gresshoff, 1991; Voisin et al., 2003; Reid et al., 2011). This mechanism of root–shoot–root long-distance feedback regulation is known as autoregulation of nodulation (AON). The existence of AON long-distance signaling has been demonstrated both in split-root inoculation experiments and in grafting experiments using hypernodulation mutants (Delves et al., 1986; Olsson et al., 1989; Kouchi et al., 2010). The CLAVATA1 (CLV1)-like leucine-rich repeat receptor-like kinase plays a central role in the AON pathway. The CLV1-like gene has different names in different legume species: NARK (Nodule Autoregulation Receptor Kinase) in Glycine max and Phaseolus vulgaris, HAR1 (Hypernodulation Aberrant Root formation 1) in Lotus japonicus, SUNN (Super Numeric Nodules) in Medicago truncatula, and SYM29 in Pisum sativum. Previous studies have shown that mutations in these genes result in hypernodulation, and reciprocal grafting experiments have confirmed that the hypernodulation phenotype is determined by the shoot genotype (Krusell et al., 2002; Nishimura et al., 2002; Searle et al., 2003; Schnabel et al., 2005; Ferguson et al., 2014). The root–shoot signals are the CLE peptides encoded by MtCLE12/13 (Mortier et al., 2010), LjCLE-RS1/2 (Okamoto et al., 2013), and GmRIC1/2 (Lim et al., 2011; Reid et al., 2011). Further studies found that the symbiotic-specific transcription factor NIN (Nodule Inception) can directly bind to the promoter of LjCLE-RS1/2 or MtCLE13 and activate its expression (Soyano et al., 2014; Laffont et al., 2020). The LjCLE-RS2 peptide is arabinosylated and then translocated to the shoot through the xylem, where it directly binds to LjHAR1 to suppress nodulation (Okamoto et al., 2013). In M. truncatula, nodule-expressed MtCLE12 and MtCLE13 are essential for AON. Genetic and chemical synthesis experiments have shown that RDN1 (Root-Determined Nodulation 1), a hydroxyproline-O-arabinosyl transferase, arabinosylates MtCLE12 but not MtCLE13 to enable perception by SUNN (Schnabel et al., 2011; Kassaw et al., 2017; Imin et al., 2018). Subsequently, miR2111 functions as a positive regulator to control nodulation through post-transcriptional regulation of the symbiosis suppressor Too Much Love (TML) in roots (Tsikou et al., 2018; Gautrat et al., 2020; Okuma et al., 2020; Zhang et al., 2021).
In addition to the classical AON negative regulation pathway, another systemic pathway was identified in M. truncatula that positively regulates nodulation and negatively regulates lateral root formation via another leucine-rich repeat receptor-like kinase, CRA2 (Compact Root Architecture 2). Grafting experiments revealed that CRA2 control of lateral root initiation and nodulation is determined by its expression in the root and shoot, respectively (Huault et al., 2014). The sunn cra2 double mutant has an intermediate nodule-number phenotype, suggesting that the CRA2 and SUNN systemic pathways act independently to regulate nodulation from the shoot (Laffont et al., 2019). Recent studies revealed that CRA2 regulates auxin biosynthesis and ethylene signaling to coordinate root growth and symbiotic nodulation (Zhu et al., 2020), and miR2111 acts as a systemic effector at the intersection of the SUNN negative and CRA2 positive pathways, allowing the dynamic fine-tuning of nodulation (Gautrat et al., 2020). The closest homolog of CRA2 in Arabidopsis is CEPR1 (CEP Receptor 1)/XIP1(Xylem Intermixed in Phloem 1), which, along with CEPR2, can bind directly to AtCEP1 peptides to regulate nitrate signaling (Tabata et al., 2014). The CEP (C-terminally encoded peptide) signaling peptides are produced mostly under low N conditions (Imin et al., 2013), and MtCEP7, a specific rhizobium-induced CEP, is directly regulated by NIN (Laffont et al., 2020). CEP is expressed in the stele of lateral roots and then loaded into xylem vessels, where the hydroxylated CEP is transported to the shoots (Ohkubo et al., 2017). Previous studies have shown that in M. truncatula, exogenous application of MtCEP1 peptides negatively regulates lateral root formation but positively regulates nodulation in a CRA2-dependent manner (Imin et al., 2013; Mohd-Radzman et al., 2016). These studies indicate that CEPs are likely to be CRA2 ligands in M. truncatula, although there is no direct evidence that MtCRA2 can directly bind to MtCEPs.
In addition to the positive and negative systemic mechanisms that regulate nodulation in response to existing infections, nitrate also suppresses nodulation (Streeter and Wong, 1988). MtNLP1 (NIN-like protein 1) was identified as a key regulator involved in nitrate-induced suppression of nodulation in M. truncatula (Lin et al., 2018; Nishida et al., 2018). We found that nitrate triggers the translocation of MtNLP1 from the cytosol to the nucleus, where it interacts with the symbiotic transcription factor NIN, suppressing NIN's activation of target genes (Lin et al., 2018). In L. japonicus, a different NLP family member, LjNRSYM1 (LjNLP4), was shown to play a similar role in nitrate-mediated inhibition of nodulation (Nishida et al., 2018). Both MtNLP1 and LjNRSYM1 mediate multiple aspects of nodulation, including rhizobial infection, nodule number, nodule development, and nitrogen-fixation activity (Lin et al., 2018; Nishida et al., 2018). LjCLE-RS2 expression is induced by rhizobia and nitrate, and LjNRSYM1 accumulates in the nucleus in response to nitrate, where it directly regulates LjCLE-RS2 expression (Nishida et al., 2018). GmNIC1 was strongly induced by nitrate but not by rhizobial inoculation and functioned locally to inhibit nodulation (Reid et al., 2011). Very recent work has shown that CLE34 and CLE35 are induced by nitrate in M. truncatula. CLE34 was identified as a pseudogene that lacks a functional CLE domain, and CLE35 systemically suppresses nodulation in an AON- and NLP1-dependent manner (Lebedeva et al., 2020; Mens et al., 2021; Moreau et al., 2021). These studies suggest that CLE35 may mediate nitrate-induced inhibition of nodulation in M. truncatula. However, whether CLE35 is directly activated by NLP1 has not yet been determined.
In this study, we show that NLP1 can directly bind to the CLE35 promoter and activate its expression. Genetic analyses and grafting experiments show that CRA2 acts positively and SUNN acts negatively in the shoot to control nodule number in response to nitrate in M. truncatula, whereas loss of CRA2 in the root inhibits nodulation in the presence of nitrate through negative feedback regulation.
Results
Nitrate-induced CLE35 expression is dependent on NLP1
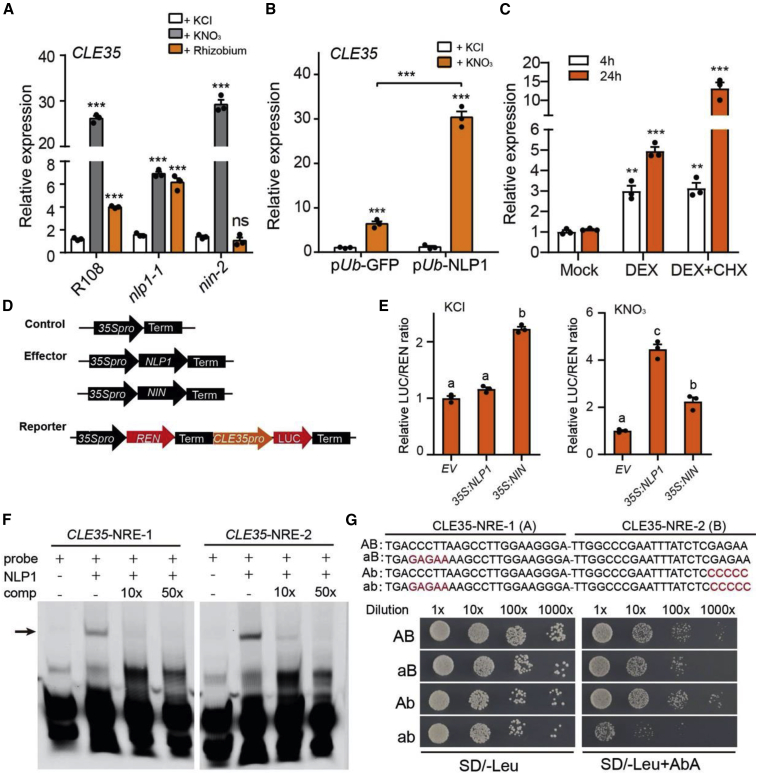
CLE35 is induced by nitrate, and CLE35 regulates nodule numbers in response to both rhizobia and nitrate (Lebedeva et al., 2020; Mens et al., 2021). To investigate how CLE35 is activated, we tested whether the induction of CLE35 by nitrate or rhizobia was dependent on the transcription factor NLP1 or NIN in M. truncatula. qRT-PCR analyses revealed that nitrate induction of CLE35 expression was strongly impaired in nlp1 roots but was unaffected in nin-2 roots. By contrast, rhizobial induction of CLE35 expression was not affected in nlp1-1 roots but was abolished in nin-2 roots (Figure 1A and Supplemental Figure 1A).
Figure 1.
NLP1 directly binds and activates CLE35 expression.
(A) Relative expression of CLE35 in wild-type (R108), nlp1-1, and nin-2 roots in response to nitrate treatment (10 mM, 3 days) or rhizobial inoculation (3 dpi).
(B) Relative expression of CLE35 in EV control (pUb-GFP) and NLP1-overexpressing (pUb-NLP1) hairy roots in response to nitrate (10 mM, 3 days).
(C) Relative expression of CLE35 in pUb-NLP1-GR roots. Roots were incubated in liquid FP medium supplemented with mock (DMSO), dexamethasone (DEX), or DEX + cycloheximide (CHX) for 4 or 24 h. Asterisks indicate significant differences (two-tailed t-test, ∗∗P < 0.01, ∗∗∗P < 0.001).
(D and E) NLP1 and NIN activate CLE35 expression in Nicotiana benthamiana leaves. qRT-PCR was used to measure the gene expression levels in treated roots; expression levels were normalized relative to the value of the ubiquitin reference gene. Data from one representative experiment of three biological replicates are shown. (D) Schematic diagrams of the CLE35 promoter-driven dual-luciferase (LUC) reporter gene and effector genes (control, NLP1, and NIN). (E) The luciferase activity of co-expressed effector gene and CLE35 promoter in N. benthamiana in the absence (KCl) or presence (KNO3) of nitrate. Luciferase activity was normalized to REN activity, and fold changes are relative to the co-expression with EV. Different letters in (E) indicate significant differences from the respective controls based on ANOVA with a post hoc Tukey's test (P < 0.05). Error bars represent mean ± SEM.
(F) Gel-shift assays of NLP1 binding to the promoter of CLE35. The protein–DNA complexes were separated by electrophoresis, and the fluorescence-labeled DNA was detected by fluorimetry. Unlabeled DNA fragments were added as competitors (comp). Arrow indicates the retardation of migration of the NLP1 bound to the probes.
(G) Yeast one-hybrid assay of NLP1 binding to the two NRE regions in the CLE35 promoter. Binding assays were performed for NLP1 against the synthetic NRE sequence (AB) or the mutated NRE sequence (aB, Ab, and ab, in red). The medium lacking leucine (−Leu) was supplemented with 250 ng/ml aureobasidin A (AbA). Yeast cells were diluted in a 10× dilution series.
To further test whether NLP1 can induce the expression of CLE35, we measured CLE35 transcript levels in M. truncatula hairy roots overexpressing NLP1 (pUb-NLP1) after KCl or KNO3 (10 mM) treatment. CLE35 expression was induced by nitrate, and its transcript levels were much higher in hairy roots expressing pUb-NLP1 than in those transformed with the empty vector (EV; pUb-GFP) (Figure 1B and Supplemental Figure 1B). These results suggested that nitrate induction of CLE35 expression is NLP1 dependent. CLE35 is also induced by rhizobia, and NIN is required for this induction.
NLP1 directly activates the expression of CLE35
To determine whether NLP1 can directly activate CLE35 expression, a construct in which NLP1 was fused to the glucocorticoid receptor (NLP1-GR) and driven by the Lotus ubiquitin promoter was created and then used to generate M. truncatula hairy roots. The expression of CLE35 was induced by treatment with dexamethasone (DEX) alone or together with cycloheximide (CHX) (Figure 1C). This result suggests that NLP1 might directly target CLE35 to activate its expression. We then used a dual-luciferase reporter assay to analyze whether NLP1 or NIN affected CLE35 transcriptional activity in N. benthamiana leaves. We co-expressed pCLE35-LUC with p35S-NLP1, p35S-NIN, or EV in N. benthamiana leaves. Luciferase activity analysis revealed that NIN could activate pCLE35:LUC either with or without nitrate; however, NLP1 could activate pCLE35:LUC only in the presence of nitrate (Figure 1D and 1E, Supplemental Figure 1C).
In Arabidopsis thaliana and L. japonicus, NLPs bind to the nitrate-responsive cis element (NRE) of a number of nitrate-inducible genes (Konishi and Yanagisawa, 2013; Soyano et al., 2015; Nishida et al., 2018). Promoter sequence analyses revealed two putative NRE sequences in the CLE35 promoter region (Supplemental Figure 2). The binding of NLP1 to the CLE35 promoter region was examined by electrophoretic mobility-shift assay and yeast one-hybrid assay. Specifically shifted bands were detected when CLE35-NRE-1 and CLE35-NRE-2 probes were incubated with NLP1 recombinant protein, and unlabeled DNA probes (comp in Figure 1F) competed for this binding (Figure 1F). This notion was further tested by a yeast one-hybrid assay. The results showed that NLP1 can bind to either of the NRE motifs and that mutation of the NRE motifs reduced this binding activity (Figure 1G). Based on these results, we conclude that NLP1 binds to the NRE motifs in the CLE35 promoter to activate its expression in response to nitrate.
Shoot SUNN is required for NLP1-mediated nitrate-induced inhibition of nodule numbers
Nitrate-induced inhibition of nodulation is known to be regulated by local and systemic pathways, and the shoot SUNN-mediated AON pathway is involved in the systemic regulation of nodulation (Jeudy et al., 2010). To further evaluate the role of SUNN in NLP1-mediated nitrate-induced inhibition of nodulation, a homozygous M. truncatula (R108) Tnt1 insertion mutant (designated sunn-6) was identified from line NF1526 (Pislariu et al., 2012), and found to have an insertion in the first exon, 400 bp from the start codon (Supplemental Figure 3A). Nodulation was evaluated at 3 weeks post-inoculation (wpi) with Sm1021. Nodule number was significantly decreased in the presence of nitrate in the wild-type R108 (Supplemental Figure 3B–3C), but only a very high concentration of nitrate (20 mM KNO3) led to significant suppression of nodule number in sunn-6 (Supplemental Figure 3D–3E). On the other hand, nodule size was dramatically decreased by a moderate concentration of nitrate (5 mM KNO3) in both wild type and sunn-6 (Supplemental Figure 3B–3E). Thus, sunn had an increased ability to form nodules under high nitrate, but its sensitivity to nitrate inhibition of nodule development was normal.
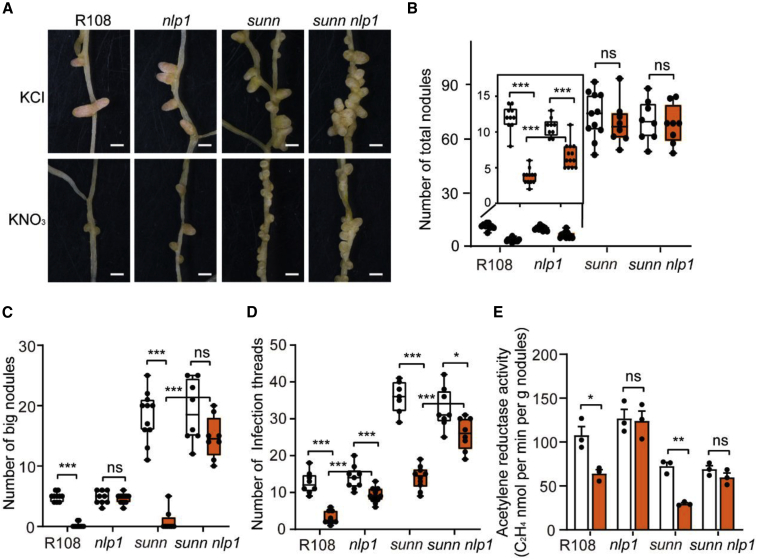
A sunn nlp1 double mutant was generated, and its nodulation phenotype was analyzed in response to nitrate. The total nodule number did not differ significantly between sunn and sunn nlp1 with or without nitrate (Figure 2A and 2B). However, in the presence of nitrate, the number of large nodules (≥1 mm) was reduced in wild type and sunn but not in nlp1 and sunn nlp1 (Figure 2A and 2C). There were similar numbers of infection threads (ITs) in sunn and sunn nlp1 in the absence of nitrate; however, IT numbers were slightly reduced in sunn nlp1 but were still significantly higher than in sunn in the presence of nitrate at 7 days post-inoculation (dpi) (Figure 2D). The nodules’ N-fixation ability was then determined by an acetylene reduction assay (ARA), and the results showed that nitrate treatment clearly decreased N-fixation activity in wild type (R108) and sunn-6, but not in nlp1 or sunn nlp1 (Figure 2E). Together, these results show that although nodule numbers were similar in sunn and sunn nlp1, sunn nlp1 developed more large nodules and ITs and had a higher acetylene reduction rate than sunn in response to nitrate.
Figure 2.
Effects of nitrate on nodulation in the sunn nlp1 double mutant.
(A–D) Nodule phenotype (A), number of total nodules (B), number of big nodules (≥1 mm) (C), and number of infection threads (D) in wild type (R108), nlp1-1, sunn-6, and sunn nlp1. Plants were grown in a vermiculite and perlite (1:1) mixture and irrigated with 10 mM KCl or KNO3. Total nodules and large nodules were counted at 3 wpi, and the infection threads were scored at 7 dpi.
(E) Acetylene reduction assay (nM min−1 g nodule−1) of nodules formed on wild-type and nlp1-1, sunn-6, and sunn nlp1 mutant plants (n = 3). Scale bars in (A), 1 mm; error bars show ±SEM. Data points from one representative experiment of three independent experiments are plotted as dots. Asterisks in (B–E) indicate significant differences (two-tailed t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, not significant).
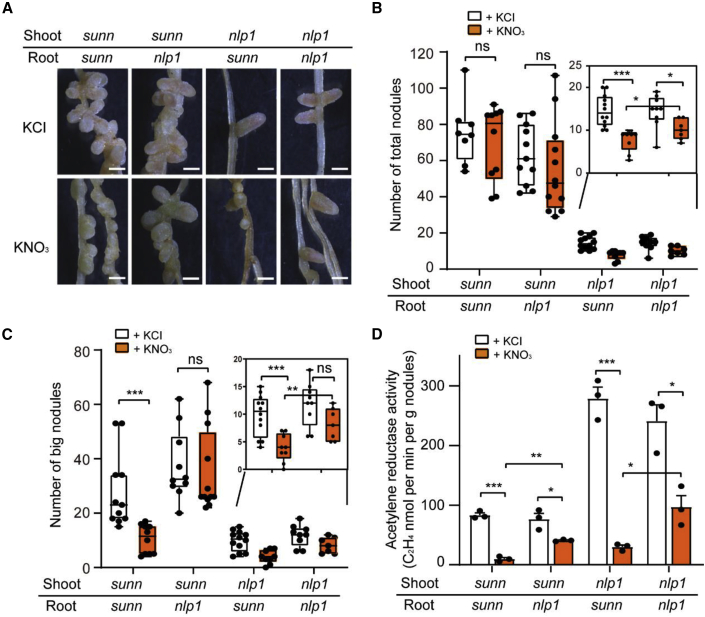
We next conducted grafting experiments between sunn-6 and wild type (R108) or nlp1-1 to analyze nodule numbers. In the absence of nitrate, the sunn-6 scion grafted onto wild-type or nlp1 rootstock produced significantly more nodules than did the wild-type or nlp1 scion grafted onto sunn-6 rootstock (Figure 3A and 3B and Supplemental Figure 3F and 3G). In the presence of nitrate, the sunn-6 scion-grafted plants (sunn/R108 and sunn/nlp1) were hypernodulated, and the total nodule number was similar to that of sunn self-grafted plants (Figure 3A and 3B and Supplemental Figure 3F and 3G). However, the number of big nodules (≥1 mm) was significantly higher in sunn/nlp1 than in sunn/R108 or sunn self-grafted plants in response to nitrate (Figure 3A and 3C and Supplemental Figure 3F and 3H). Moreover, nitrogenase activity was decreased to a greater extent in plants with sunn rootstocks than in those with nlp1 rootstocks (Figure 3D).
Figure 3.
Nodulation phenotypes of sunn-6 and nlp1-1 grafted plants.
(A–C) The grafted plants were grown in nitrogen-starved substrate and irrigated with 10 mM KCl or KNO3. Total nodules and large nodules were counted at 3 wpi. Nodule phenotypes (A), total nodule number (B), and number of big nodules (≥1 mm) (C) in grafting combinations of sunn-6 and nlp1-1.
(D) Acetylene reduction assay (nM min−1 g nodule−1) of nodules from different grafting combinations of sunn-6 and nlp1-1 grown in nitrogen-free substrate and irrigated with 10 mM KCl or KNO3 (n = 3). Scale bar in (A), 1 mm; error bars show ±SEM. Data points from one representative experiment of two independent experiments are plotted as dots. Asterisks indicate significant differences (two-tailed t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, not significant).
Together, these results demonstrate that NLP1 control of nitrate-induced inhibition of nodule number is dependent on SUNN activity in the shoot, whereas NLP acts independently of SUNN in the root to control nitrate inhibition of rhizobial infection, nodule nitrogenase activity, and nodule development.
Loss of CRA2 in the shoot versus the root has opposing effects on nodulation under high nitrate
CRA2 is a positive systemic regulator of nodulation in M. truncatula, and cra2 mutants form few pink nodules under nitrogen-limited conditions (Huault et al., 2014). To determine whether CRA2 affects nitrate-induced inhibition of nodulation, we analyzed nodulation of the cra2-2 and cra2-16 mutants in response to nitrate. cra2-16 is a new allele of CRA2 that contains a Tnt1 insertion (NF9553) 1363 bp from the start codon (Supplemental Figure 4A and 4B). This mutant showed nodulation and lateral root phenotypes similar to those of other cra2 mutants (Huault et al., 2014; Laffont et al., 2019; Zhu et al., 2020). As shown in Supplemental Figure 4, cra2 mutants showed reduced nodule numbers, similar to wild type, after nitrate treatment. However, nodule size was more sensitive to nitrate in cra2 than in wild type, and cra2 formed only small nodules under 5 mM KNO3 treatment (Supplemental Figure 4C–4H).
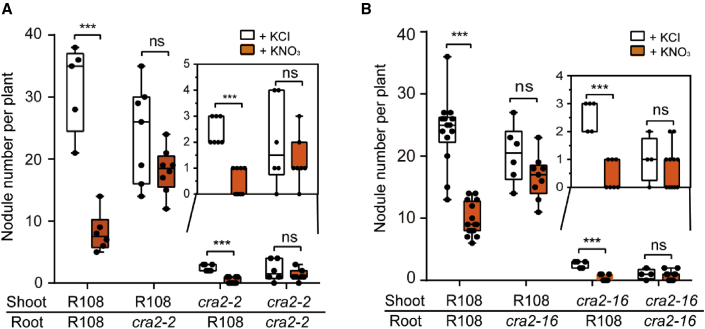
To further analyze the function of CRA2 in nitrate-induced inhibition of nodulation, we conducted reciprocal grafting experiments between wild type (R108) and cra2 mutants. In the absence of nitrate, plants with a wild-type scion (R108/cra2-2 and R108/cra2-16) formed markedly more nodules than did those with a cra2 scion (cra2-2/R108 and cra2-16/R108) and cra2 self-grafted plants (Figure 4). In the presence of nitrate, significantly fewer nodules formed on wild-type (R108/R018) and cra2 scion-grafted plants (cra2-2/R108 and cra2-16/R108) (Figure 4), suggesting that shoot CRA2 positively controls nodule numbers in response to nitrate. Surprisingly, we found that cra2 rootstock-grafted plants (R108/cra2-2 and R108/cra2-16) were almost unaffected in terms of nodule numbers and produced more nodules than plants on R108 rootstock in the presence of nitrate (Figure 4). This result suggests that root cra2 nodulation is insensitive to nitrate and that root CRA2 negatively controls nodulation in response to nitrate. Taken together, these results show that shoot and root CRA2 play distinct roles in the regulation of nodulation, dependent on nitrate availability.
Figure 4.
Shoot and root CRA2 function differently to regulate nodule number in response to nitrate.
Nodule number in different grafting combinations of wild type (R108) and cra2-2(A) or cra2-16(B). The grafted plants were grown in nitrogen-starved substrate and irrigated with 10 mM KCl or KNO3. Nodule numbers were counted at 3 wpi. Data points from one representative experiment of three independent experiments are plotted as dots. Asterisks indicate significant differences (two-tailed t-test, ∗∗∗P < 0.001; ns, not significant).
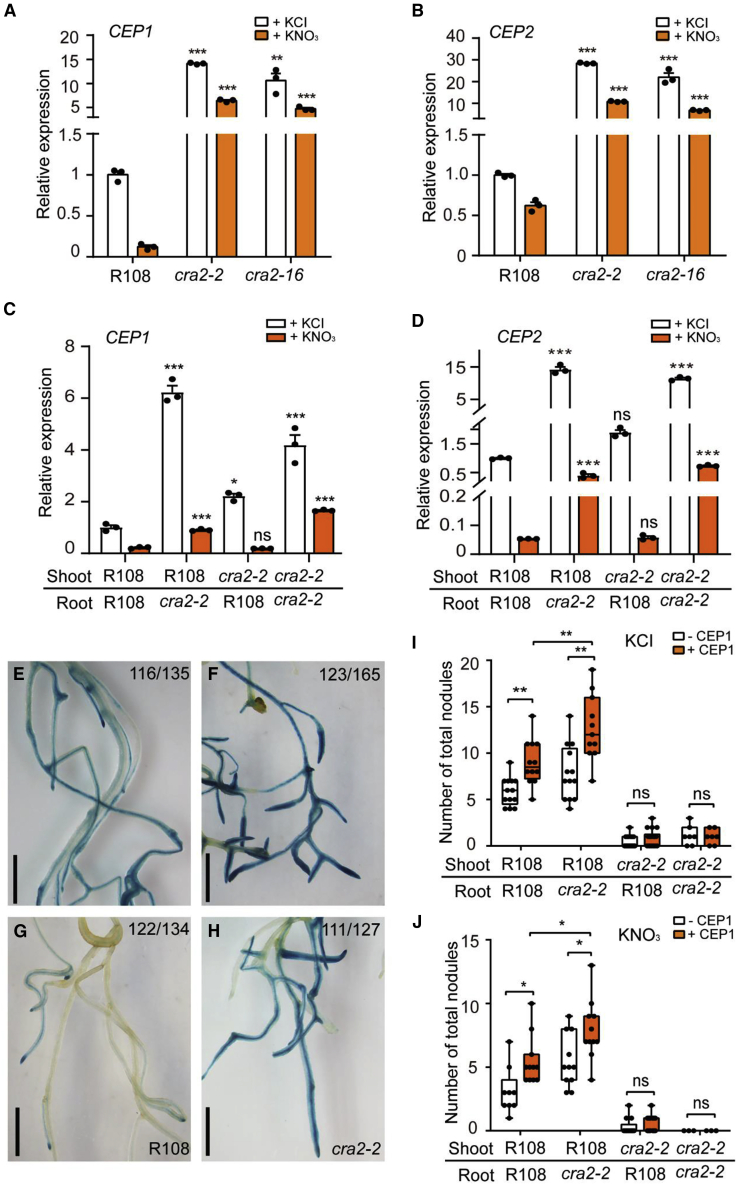
Negative feedback by root CRA2 regulates CEP1 and CEP2 expression
The insensitivity of R108/cra2 root nodulation to nitrate inhibition suggests that the CEP/CRA2 pathway may be constitutively activated independent of nitrogen availability in this grafting combination. To test this idea, we first conducted qRT-PCR analyses to determine the transcript levels of CEP1 and CEP2 in cra2 roots with or without nitrate. Consistent with the results of a previous study (Imin et al., 2013), CEP1 and CEP2 transcript levels were reduced in wild-type roots in the presence of nitrate (Figure 5A and 5B). However, the transcript levels of CEP1 and CEP2 were significantly higher in the roots of cra2 than in wild-type roots, either with or without nitrate (Figure 5A and 5B). To verify this, we examined CEP1 and CEP2 transcript levels in the roots using grafting. The results showed that the expression of CEP1 and CEP2 was higher in cra2 rootstocks (R108/cra2 and cra2/cra2) than in wild type or cra2/R108, with or without nitrate (Figure 5C and 5D).
Figure 5.
Root CRA2 negative feedback regulation of CEP1/2 expression.
(A and B) Relative expression of CEP1(A) and CEP2(B) in the roots of wild type (R108) and cra2 mutants in response to nitrate.
(C and D) Relative expression of CEP1(C) and CEP2(D) in different grafting combinations of wild type and cra2-2 roots in response to nitrate. qRT-PCR was used to measure the gene expression levels in treated roots, normalized relative to KCl-treated roots. Error bars represent the mean ± SEM. Statistical data were derived from one representative experiment of three biological replicates (two-tailed Student's t-test, ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001; ns, not significant).
(E–H) Bright-field images of wild-type (R108) (E and G) or cra2-2(F and H) roots expressing pCEP1:GUS. The transgenic hairy roots were watered with 10 mM KCl (E and F) or KNO3(G and H), then harvested at 5 days and histochemically stained. The numbers in the upper right corners indicate the number of GUS-stained roots (blue) versus total stained roots. Scale bars, 2.5 mm.
(I and J) Effects of synthetic CEP1 peptide application on nodulation in wild-type (R108)- and cra2-grafted plants. Grafted plants were grown on BNM medium plates with 5 mM KCl (I) or KNO3(J) plus or minus 10−6 M CEP1 peptide, and the nodule number was scored at 3 wpi. Error bar shows ±SEM. Data points from one representative experiment of three independent experiments are plotted as dots (two-tailed Student's t-test, ∗P < 0.05, ∗∗P < 0.01; ns, not significant).
To further validate this hypothesis, we expressed pCEP1-GUS in wild-type (R108) and cra2-2 hairy roots. The results of GUS staining analysis revealed that nitrate strongly suppressed CEP1 expression in wild-type hairy roots (Figure 5E and 5G). GUS expression was stronger in cra2 roots than in wild type roots under both conditions and was only slightly reduced by nitrate (Figure 5F and 5H). These results suggested that root CRA2 is involved in the negative feedback regulation of CEP expression. Thus, the overactivation of CEP genes in the roots of the R108/cra2 graft combination promotes nodulation through CRA2 in the shoot, even under inhibitory nitrate conditions.
Synthetic CEP1 peptide increases nodule number through CRA2 in the shoot
To test whether CEPs can indeed promote nodulation under nitrate inhibitory conditions, we synthesized the M. truncatula CEP1 peptide and verified its bioactivity to restrict the number of lateral roots and promote circumferential cell proliferation sites (Mohd-Radzman et al., 2015) (Supplemental Figure 5). Then, we applied the synthetic CEP1 peptide to grafted plants between R108 and cra2-2. The grafted plants were grown on plates with or without nitrate (10 mM KCl or KNO3) and inoculated with Sm1021, and their nodule number was scored at 3 wpi. After application of the synthetic CEP1 peptide, nodule number was enhanced in wild-type scion (R108/R108 and R108/cra2)-grafted plants but not in cra2 scion (cra2/R108 and cra2/cra2)-grafted plants in either the presence or absence of nitrate (Figure 5I and 5J). However, the grafted plants with cra2 rootstocks (R108/cra2) produced more nodules than wild-type grafts (R108/R108) after treatment with CEP1 peptides, with or without nitrate (Figure 5I and 5J). Together, these results show that CEP1 can promote nodulation even at inhibitory nitrate levels and that this requires CRA2 activity in the shoot. This result is consistent with the previous hypothesis that loss of the negative feedback exerted by CRA2 on CEP1 and CEP2 expression in the R108/cra2 grafts may promote nodulation under nitrate inhibitory conditions through CRA2 receptor activity in shoots.
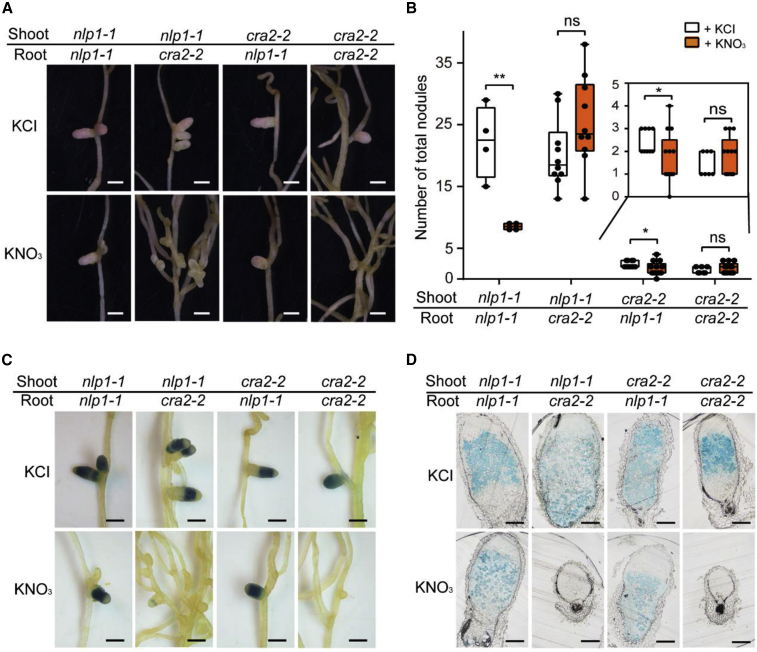
NLP1 coordinates CRA2 signaling in the shoot to mediate nitrate inhibition of nodulation
Because CRA2 and NLP1 are tightly linked in the M. truncatula genome, the cra2 nlp1 double mutant would be very difficult to obtain. To investigate whether CRA2 activity in the shoot is required for NLP1's role in nitrate-induced inhibition of nodulation, we performed a reciprocal grafting experiment between cra2-2 and nlp1-1. The cra2 scion-grafted plants produced very few nodules, which made it difficult to analyze nitrogenase activity. We therefore inoculated these plants with Sm1021 carrying pNifH:GUS and used GUS-staining analyses to detect NifH expression in the nodules as a qualitative indicator of nodule nitrogen-fixation activity. As expected, the cra2 scion (cra2/nlp1 and cra2/cra2)-grafted plants produced very few nodules compared with the nlp1 scion-grafted plants at 3 wpi with Sm1021 in the absence of nitrate (Figure 6A and 6B). In the presence of nitrate, nlp1 scions grafted to cra2 rootstock (nlp1/cra2) produced more nodules than nlp1 (Figure 6A and 6B); this is consistent with the R108/cra2 grafting results. Moreover, compared with cra2 rootstock-grafted plants, the nlp1 rootstock (nlp1/nlp1 and cra2/nlp1)-grafted plants showed a slightly decreased nodule number (Figure 6B), but their nodules were larger (Figure 6A) and showed detectable NifH expression in the presence of nitrate (Figure 6C). Examination of histochemically stained nodule sections by light microscopy revealed that nodules on nlp1 rootstock were large and contained bacteria, whereas those on cra2 rootstock were small and contained no bacteria in the presence of nitrate (Figure 6D). Together, these results show that loss of shoot CRA2 positively affects nodule numbers, whereas loss of root CRA2 has the opposite effect, and root NLP1 independently controls nodule N fixation in response to nitrate.
Figure 6.
Shoot CRA2 coordinates root NLP1 to mediate nitrate-induced inhibition of nodulation.
(A and B) Nodule phenotypes (A) and total nodule number (B) in different grafting combinations of nlp1-1 and cra2-2 irrigated with 5 mM KCl or KNO3. Nodule number was scored at 3 wpi.
(C and D) Nodules with pNifH:GUS staining (C) and sections of nodules (D) in grafting combinations of nlp1-1 and cra2-2. Scale bar, 1 mm (A and C) and 250 μm (D). Error bar shows ±SEM. Data points in (B) from a representative experiment of three independent experiments are plotted as dots (two-tailed Student's t-test, ∗P < 0.05, ∗∗P < 0.01; ns, not significant).
Discussion
The legume–rhizobia symbiotic interaction involves long-distance signaling pathways between the root and the shoot to mediate root nodule formation. A series of studies revealed that two independent systemic signaling pathways regulate nodulation in the shoot: the CLE/SUNN negative regulation pathway and the CEP/CRA2 positive regulation pathway (Bhuvaneswari et al., 1981; Kosslak and Bohlool, 1984; Caetano-Anolles and Gresshoff, 1991; Huault et al., 2014; Laffont et al., 2019; Lin et al., 2020). High nitrate levels inhibit nodulation, and most AON-related mutants in different legume species are nitrate-tolerant nodulators (Carroll et al., 1985; Krusell et al., 2002; Searle et al., 2003; Oka-Kira et al., 2005; Schnabel et al., 2005; Magori et al., 2009; Elise Schnabel et al., 2010). The NIN-like proteins MtNLP1 and LjNRSYM1 were shown to act locally to control nitrate suppression of nodulation in M. truncatula and L. japonicus, respectively (Lin et al., 2018; Nishida et al., 2018). In L. japonicus, the nitrate-induced inhibition of nodulation mediated by LjNRSYM1 and the AON systemic regulation of nodulation mediated by HAR1 are linked by the rhizobia- and nitrate-induced CLE peptide LjCLE-RS2 (Nishida et al., 2018). In M. truncatula, CLE12 and CLE13 are induced by rhizobia but not by nitrate (Mens et al., 2021). Thus, MtCLE12 and MtCLE13 might not be involved in nitrate-induced inhibition of nodulation by the AON pathway. In this study, we found that rhizobia-induced CLE35 expression was dependent on NIN, and nitrate-induced expression of CLE35 was largely but not totally blocked in nlp1 mutants. This might be because other NLPs, such as NLP4, act redundantly with NLP1 in nitrate inhibition of nodulation and may also be required for the full induction of CLE35 expression by nitrate. Our work demonstrates that NLP1 can directly bind to the CLE35 promoter and activate its expression in N. benthamiana and M. truncatula. This finding is supported by very recent work showing that MtCLE34 and MtCLE35 are induced by nitrate and that MtCLE35 inhibits nodulation in a SUNN- and RDN1-dependent manner (Lebedeva et al., 2020; Mens et al., 2021; Moreau et al., 2021). Thus, the regulation of CLE35 by NLP1 constitutes an important link between nitrate-induced inhibition of nodulation and the systemic AON pathway. miR2111 acts as a common signal downstream of SUNN and CRA2 by post-transcriptionally regulating the symbiosis suppressor TML1/2 in M. truncatula roots (Gautrat et al., 2020). Mature miR2111 is repressed by rhizobia or nitrate in L. japonicus and M. truncatula (Tsikou et al., 2018; Gautrat et al., 2020). However, a recent study found that CLE35 overexpression represses miR2111 independent of nitrate, and ectopic expression of miR2111 partially bypasses nitrate inhibition of nodulation (Moreau et al., 2021). These findings together suggested that an NLP1–CLE35–SUNN–TML pathway is involved in nitrate regulation of nodulation, but the signals from shoot to root in nitrate systemic regulation of nodulation were still unknown.
Operating in a fashion complementary to AON, the CRA2 signaling pathway positively regulates nodulation from the shoot (Huault et al., 2014). The ortholog of Medicago CRA2 in Arabidopsis is CEPR1/XIP1, which functions as a CEP receptor (Huault et al., 2014; Tabata et al., 2014). We found that, like SUNN, CRA2 acts in the shoot to control nodule number, but it is not involved in the suppression of N-fixation activity in response to nitrate. CRA2 is expressed in the shoots, roots, and nodules (Huault et al., 2014). CEP1 peptide, the presumed CRA2 ligand and a positive regulator of nodulation, is expressed in roots and nodules and is suppressed by nitrate (Imin et al., 2013). Unexpectedly, R108/cra2-grafted plants showed less sensitivity to nitrate in terms of nodule numbers. Our results indicate that enhanced nodulation in CRA2/cra2 plants results from loss of CRA2-dependent negative feedback regulation of CEP1 expression, resulting in CEP1 overproduction, which leads to increased activation of CRA2 in the shoot. Such ligand overproduction has been documented in various hormone receptor mutants; for example, loss of multiple cytokinin receptors increases cytokinin content as a consequence of feedback (Riefler et al., 2006). This hypothesis was supported by the increased expression of CEP1 and CEP2 in cra2 roots compared with wild-type roots with or without nitrate. However, in the absence of nitrate, the number of nodules did not differ significantly between R108/cra2 and wild type, even though CEP transcript levels were higher in cra2 roots than in wild-type roots. This may be due to the enhanced lateral root system of cra2 or to other unknown roles of CRA2 in the regulation of nodulation.
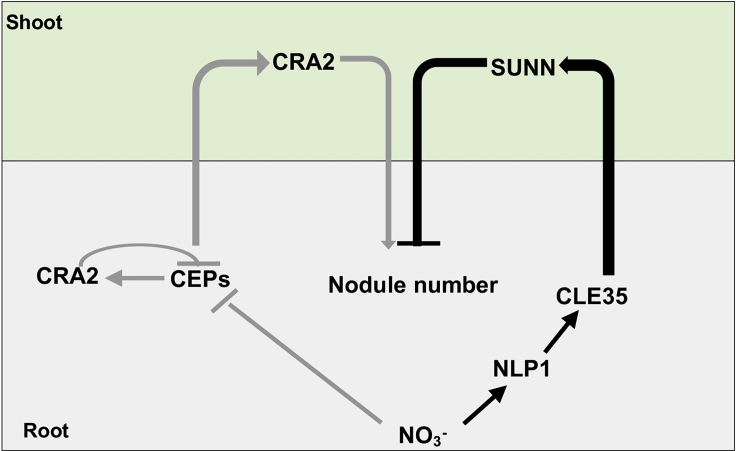
In this study, we show that systemic signaling through SUNN and CRA2 is limited to the control of nodule number, whereas NLP1 controls rhizobial infection, nodule development, and nitrogenase activity in response to nitrate. This system allows the plant to set upper limits on nodule number through the balanced action of CLE/SUNN and CEP/CRA2 signaling, while enabling the dynamic control of rhizobial infection and N fixation based on nitrate availability in the root. Our side-by-side analysis of these three regulatory pathways and their interactions extends our knowledge of nitrate regulation of nodulation in M. truncatula and demonstrates the conservation of these pathways in L. japonicus, suggesting their likely conservation across all papilionoid legumes. On the basis of our results, we have proposed a model for nitrate-mediated inhibition of nodulation via systemic and local regulatory pathways (Figure 7). In the presence of nitrate, the nitrate signal triggers NLP1 to shuttle to the nucleus, where NLP1 activates CLE35, whose product is then transported to the shoot to activate SUNN and further restrict nodule number. Nitrate also inhibits the expression of CEPs, so that shoot CRA2 is not activated to positively regulate root nodule formation. Meanwhile, negative feedback regulation by CRA2 in the root is involved in the suppression of CEP expression by nitrate.
Figure 7.
Proposed model for nitrate-induced inhibition of nodulation in M. truncatula.
In the presence of nitrate, activation of NLP1 increases the expression of CLE35, and the encoded peptides systemically inhibit nodule initiation through shoot SUNN (indicated in black). Nitrate also suppresses CEP expression, and the encoded CEPs systemically positively regulate nodule numbers through shoot CRA2 (indicated in gray). In addition, loss of CRA2 in the root results in increased CEP expression via loss of negative feedback inhibition, thereby promoting nodule initiation.
Materials and methods
Biological materials and growth conditions
M. truncatula ecotype R108 was used as the wild type in this study, and all the mutants used were isolated from that background. The cra2-2 mutant was described by Huault et al. (2014), and the cra2-16 and sunn-6 mutants were isolated from the collection of Tnt1 retrotransposon-tagged mutants in M. truncatula. The new allele cra2-16 and the sunn-6 mutants were isolated by PCR genotyping. The insertion sites were identified by sequencing the PCR-amplification products. The relevant primers are shown in Supplemental Table 1. Sinorhizobium meliloti strain 1021 (Sm1021) harboring hemA:lacZ or Sm1021 carrying a pnifH:GUS plasmid were used in inoculation assays. Agrobacterium rhizogenes strain AR1193 was used for hairy-root transformation of M. truncatula, and Agrobacterium tumefaciens EHA105 was used for expression in Nicotiana benthamiana. The sunn nlp1 double mutant was generated by crossing the sunn-6 and nlp1-1 mutants and screening F2 plants.
Plant growth conditions, hairy-root transformation, rhizobial inoculation, and phenotype observation
M. truncatula seeds were scarified with sandpaper, then surface sterilized in 10% sodium hypochlorite for 3 min and rinsed five times with sterilized water. The sterilized seeds were imbibed in water and then transferred to 0.8% water agar plates that were kept at 4°C for 3 days and then placed in the dark at 22°C overnight. The seedlings were transferred to a mixture of perlite and vermiculite (1:1) and grown in a growth chamber under a 16 h/8 h light/dark photoperiod.
For grafting experiments, 7-day-old shoots were cut at the hypocotyl and grafted onto reciprocal roots using small plastic tubes. After 10–14 days, the successfully grafted plants were transferred to a vermiculite and perlite (1:1) mixture and irrigated every other day with water containing KCl or KNO3 at the indicated concentrations.
The seedlings, grafted plants, or hairy-root transgenic plants were inoculated with rhizobia 5–7 days after transfer to the vermiculite and perlite (1:1) mixture. To analyze infection events, the plants were harvested at 7 dpi with Sm1021/LacZ, and the roots were stained with 5-bromo-4-chloro-3-indolyl-β-D-galactosidase. The ITs were observed and scored under a microscope, and 10–12 plants of each genotype were analyzed. The nodule number was scored at 3 wpi.
Nitrogen-fixation activity
M. truncatula seedlings were transferred into a vermiculite and perlite (1:1) mixture and inoculated with Sm1021 5–7 days later. At 3 wpi, the plants were treated with 10 mM KNO3 or KCl for another 3 days. The plant roots were cut and immediately placed into 10-ml glass vials (three plants per vial). The vials were sealed with a rubber septum, and 0.5 ml acetylene was injected into each vial. The vials were then incubated for 30 min at 28°C. Gas samples (0.2 ml) were extracted from the glass vials using a syringe, and ethylene was measured by gas chromatography (GC9310, Shanghai Chromatographic Instruments Co., Ltd., Shanghai, China) as an indicator of nitrogenase activity. Nodules were detached from the roots and weighed. The ARA was calculated as nanomoles of ethylene produced per minute per unit fresh weight of nodule (Hardy et al., 1968).
Gene expression analysis
To analyze nitrate-induced gene expression, M. truncatula wild-type (R108), cra2, nin-2, or nlp1 seedlings were grown on BNM or FP plates supplemented with 50 nM aminoethoxyvinylglycine (Sigma-Aldrich) for 3 days, then transferred to plates with BNM or FP containing 10 mM KCl or KNO3 for another 3 days. To analyze rhizobium-induced gene expression, R108, nin-2, or nlp1 seedlings were grown on BNM or FP agar plates for 3 days, then inoculated with Sm1021 (OD600 ≈ 0.05) and grown for another 3 days. Total RNA was extracted from M. truncatula roots using the TRIpure isolation reagent (Aidlab, Beijing, China) according to the manufacturer's instructions. RNA was quantified using a NanoDrop 2000 instrument (Thermo Fisher, Waltham, MA, USA) and then reverse transcribed into cDNA using the TransScript One-Step gDNA Removal and cDNA Synthesis SuperMix (TransGen, Beijing, China). Gene transcript levels were analyzed by real-time PCR using TB Green Premix Ex Taq (Takara, Dalian, China) with the StepOnePlus PCR system (Thermo Fisher, Waltham, MA, USA). Relative transcript levels were normalized against that of the reference gene (MtUBQ10). Statistical significance based on three biological replicates was calculated using the 2−ΔΔCt method. The primers used for qRT-PCR are listed in Supplemental Table 1.
Promoter:GUS analysis
The CEP1 promoter (about 2 kb upstream of the translation start codon) was amplified by PCR using M. truncatula leaf genomic DNA as the template. The relevant primers are shown in Supplemental Table 1. The PCR products were cloned into pDONR207 and then recombined into pKGWFS7 to generate pCEP1:GUS. The sequences were confirmed by DNA sequencing. The constructs were transferred into A. rhizogenes AR1193 by electroporation. Then the pCEP1:GUS was transformed into M. truncatula R108 or cra2-2 seedlings to generate composite plants with transgenic roots. The transformed chimeric plants were transferred into pots with vermiculite and perlite (1:1) and watered every other day with 10 mM KCl or KNO3. The roots were harvested for GUS staining 5 days after transfer and then incubated in GUS staining solution for 4–6 h at 37°C.
Activation of CLE35 expression in N. benthamiana leaves or M. truncatula hairy roots by NLP1
To assess the effects of NLP1 on CLE35 expression in M. truncatula roots, pUb-GFP or pUb-NLP1 was expressed in M. truncatula R108 hairy roots. The transformed plants were transferred into pots with vermiculite and perlite (1:1) and watered with 10 mM KCl or KNO3. The roots were harvested 7 days after transfer (n = 3–5 plants for each biological replicate), and CLE35 transcript levels were analyzed using qRT-PCR.
For DEX induction of gene expression in M. truncatula roots, the full-length NLP1 and GR sequences were separately amplified by PCR and then inserted into the pUb-GW-GFP vector using XbaI/KpnI and KpnI/AscI restriction sites. The constructs were introduced into A. rhizogenes AR1193 by electroporation and then transformed into M. truncatula wild-type R108 to generate transgenic hairy roots. After 3 weeks, pUb-NLP1-GR transgenic roots were preincubated with 10 mM CHX or DMSO for 30 min before the addition of DEX (10 μM) or CHX + DEX (10 μM CHX, 10 μM DEX) for 4 h or 24 h. The transgenic roots were harvested to measure CLE35 gene expression. A GFP reporter gene included in this construct was used to identify transgenic roots using a fluorescence stereomicroscope (SMZ1500, Nikon).
For the dual-luciferase reporter assay in N. benthamiana leaves, the NRE-1 sequence (186 bp) and the 2-kb promoter region upstream of the CLE35 start codon were amplified by PCR using the primers shown in Supplemental Table 1. The PCR products were separately inserted into the pGreenII-0800 vector using KpnI and HindIII/SpeI restriction sites to generate the pCLE35-LUC reporter construct. The effector construct was generated by inserting the NLP1 CDS into the pRI101 vector containing the 35S promoter via KpnI/EcoRI restriction sites. These constructs were transferred into A. tumefaciens GV3101 and then introduced into N. benthamiana leaves by infiltration. After 2 days of watering with either 20 mM KCl or 20 mM KNO3, the LUC/REN ratio was measured by the dual-luciferase reporter assay system according to the manufacturer's protocols (Promega). For the transient LUC assay, LUC images were viewed with a charge-coupled device camera (Tanon, China) after spraying tobacco leaves with 1 mM luciferin.
Electrophoretic mobility-shift assays
NLP1 (NLP1 C-, residues 571–909) was expressed in E. coli Rosetta (Lin et al., 2018). The CLE35 promoter regions were synthesized, and probes were labeled with CY5. After incubation, the reaction mixture was electrophoresed on a 6% native polyacrylamide gel, and the labeled DNA was detected with a Starion FLA-9000 scanner (Fujifilm).
Yeast one-hybrid assay
The bait sequences were amplified by PCR using synthesized NRE sequences as a template and then cloned into the pAbAi vector and integrated into the genome of the Y1HGold yeast strain. The NLP1 full-length coding sequences were amplified, cloned into the pGADT7-Rec prey vector, and fused with the bait yeast strain according to the protocol of the Matchmaker Gold Yeast One-Hybrid Library screening system (Clontech). The primers used are listed in Supplemental Table 1. The yeast strains were selected on medium lacking leucine with 250 ng/ml of aureobasidin A antibiotic.
CEP1 peptide synthesis and treatment of roots
HyP4- and HyP11-modified CEP1 peptides (Mohd-Radzman et al., 2015) were synthesized by GL Biochem Pty Ltd. (Shanghai, China). For the CEP1 peptide treatment of roots, grafted plants were transferred to 100-mm square plates with 40 ml BNM agar medium containing 10 mM KNO3 or KCl, with or without 1 μM CEP1 peptide. The grafted plants were inoculated with Sm1021 3–5 days after transfer, and the nodule number was scored at 3 wpi.
Statistical analyses
Statistically significant differences were detected by ANOVA or the Kruskal–Wallis non-parametric test using IBM SPSS Statistics version 25 or a two-tailed Student's t-test (Microsoft Office Excel 2016), as indicated in the figure legends. Vertical box plots were generated using GraphPad Prism 6.0 software. For each boxplot, the center line in the box shows the median; the box limits indicate the upper and lower quartiles; and the whiskers represent the maximum and minimum values.
Funding
This work was supported by grants from The National Key Research & Development Program of China (2016YFA0500500 and 2016YFD0100702) and the NSFC (31670242) to F.X.
Author contributions
Z.L., J.L., and F.X. designed the research; Z.L., J.L., and M.F. conducted the experiments and analyzed the data; Y.Z. identified the cra2-16 mutant; X.L. performed some grafting experiments; and F.X. and Z.L. wrote the paper. All authors read and approved the final article.
Acknowledgments
We thank Florian Frugier (IPS2, CNRS, INRA, France) for providing cra2-2 seeds and Jeremy Murray (CEMPS, China) for providing sunn-6 seeds and S. meliloti Sm1021 harboring pNifH:GUS and for helpful editing of the manuscript. We thank Brett Ferguson (University of Queensland, Australia) for discussion about nitrate-induced CLEs in M. truncatula and Michael A. Djordjevic (Australian National University, Australia) for the suggestion to create synthetic CEP1 peptides. The authors declare no competing interest.
Published: March 27, 2021
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Supplemental information is available at Plant Communications Online.
Supplemental information
References
- Bhuvaneswari T.V., Bhagwat A.A., Bauer W.D. Transient susceptibility of root cells in four common legumes to nodulation by rhizobia. Plant Physiol. 1981;68:1144–1149. doi: 10.1104/pp.68.5.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano-Anolles G., Gresshoff P.M. Efficiency of nodule initiation and autoregulatory responses in a supernodulating soybean mutant. Appl. Environ. Microbiol. 1991;57:2205–2210. doi: 10.1128/aem.57.8.2205-2210.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll B.J., McNeil D.L., Gresshoff P.M. Isolation and properties of soybean [Glycine max (L.) Merr.] mutants that nodulate in the presence of high nitrate concentrations. Proc. Natl. Acad. Sci. U S A. 1985;82:4162–4166. doi: 10.1073/pnas.82.12.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves A.C., Mathews A., Day D.A., Carter A.S., Carroll B.J., Gresshoff P.M. Regulation of the soybean-Rhizobium nodule symbiosis by shoot and root factors. Plant Physiol. 1986;82:588–590. doi: 10.1104/pp.82.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elise Schnabel A.M., Smith L., Kassaw T., Long S., Frugoli J. The lss supernodulation mutant of Medicago truncatula reduces expression of the SUNN gene. Plant Physiol. 2010;154:1390–1402. doi: 10.1104/pp.110.164889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B.J., Li D., Hastwell A.H., Reid D.E., Li Y., Jackson S.A., Gresshoff P.M. The soybean (Glycine max) nodulation-suppressive CLE peptide, GmRIC1, functions interspecifically in common white bean (Phaseolus vulgaris), but not in a supernodulating line mutated in the receptor PvNARK. Plant Biotechnol. J. 2014;12:1085–1097. doi: 10.1111/pbi.12216. [DOI] [PubMed] [Google Scholar]
- Gautrat P., Laffont C., Frugier F. Compact Root Architecture 2 promotes root competence for nodulation through the miR2111 systemic effector. Curr. Biol. 2020;30:1339–1345.e3. doi: 10.1016/j.cub.2020.01.084. [DOI] [PubMed] [Google Scholar]
- Hardy R.W., Holsten R.D., Jackson E.K., Burns R.C. The acetylene-ethylene assay for N2 fixation: laboratory and field evaluation. Plant Physiol. 1968;43:1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huault E., Laffont C., Wen J., Mysore K.S., Ratet P., Duc G., Frugier F. Local and systemic regulation of plant root system architecture and symbiotic nodulation by a receptor-like kinase. Plos Genet. 2014;10:e1004891. doi: 10.1371/journal.pgen.1004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imin N., Mohd-Radzman N.A., Ogilvie H.A., Djordjevic M.A. The peptide-encoding CEP1 gene modulates lateral root and nodule numbers in Medicago truncatula. J. Exp. Bot. 2013;64:5395–5409. doi: 10.1093/jxb/ert369. [DOI] [PubMed] [Google Scholar]
- Imin N., Patel N., Corcilius L., Payne R.J., Djordjevic M.A. CLE peptide tri-arabinosylation and peptide domain sequence composition are essential for SUNN-dependent autoregulation of nodulation in Medicago truncatula. New Phytol. 2018;218:73–80. doi: 10.1111/nph.15019. [DOI] [PubMed] [Google Scholar]
- Jeudy C., Ruffel S., Freixes S., Tillard P., Santoni A.L., Morel S., Journet E.P., Duc G., Gojon A., Lepetit M. Adaptation of Medicago truncatula to nitrogen limitation is modulated via local and systemic nodule developmental responses. New Phytol. 2010;185:817–828. doi: 10.1111/j.1469-8137.2009.03103.x. [DOI] [PubMed] [Google Scholar]
- Kassaw T., Nowak S., Schnabel E., Frugoli J. ROOT DETERMINED NODULATION1 is required for M. truncatula CLE12, but not CLE13, peptide signaling through the SUNN receptor kinase. Plant Physiol. 2017;174:2445–2456. doi: 10.1104/pp.17.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Yanagisawa S. Arabidopsis NIN-like transcription factors have a central role in nitrate signalling. Nat. Commun. 2013;4:1617. doi: 10.1038/ncomms2621. [DOI] [PubMed] [Google Scholar]
- Kosslak R.M., Bohlool B.B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984;75:125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H., Imaizumi-Anraku H., Hayashi M., Hakoyama T., Nakagawa T., Umehara Y., Suganuma N., Kawaguchi M. How many peas in a pod? Legume genes responsible for mutualistic symbioses underground. Plant Cell Physiol. 2010;51:1381–1397. doi: 10.1093/pcp/pcq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusell L., Madsen L.H., Sato S., Aubert G., Genua A., Szczyglowski K., Duc G., Kaneko T., Tabata S., de Bruijn F. Shoot control of root development and nodulation is mediated by a receptor-like kinase. Nature. 2002;420:422–426. doi: 10.1038/nature01207. [DOI] [PubMed] [Google Scholar]
- Laffont C., Huault E., Gautrat P., Endre G., Kalo P., Bourion V., Duc G., Frugier F. Independent regulation of symbiotic nodulation by the SUNN negative and CRA2 positive systemic pathways. Plant Physiol. 2019;180:559–570. doi: 10.1104/pp.18.01588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffont C., Ivanovici A., Gautrat P., Brault M., Djordjevic M.A., Frugier F. The NIN transcription factor coordinates CEP and CLE signaling peptides that regulate nodulation antagonistically. Nat. Commun. 2020;11:3167. doi: 10.1038/s41467-020-16968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva M., Azarakhsh M., Yashenkova Y., Lutova L. Nitrate-induced CLE peptide systemically inhibits nodulation in Medicago truncatula. Plants (Basel) 2020;9(11):1456. doi: 10.3390/plants9111456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C.W., Lee Y.W., Hwang C.H. Soybean nodule-enhanced CLE peptides in roots act as signals in GmNARK-mediated nodulation suppression. Plant Cell Physiol. 2011;52:1613–1627. doi: 10.1093/pcp/pcr091. [DOI] [PubMed] [Google Scholar]
- Lin J., Frank M., Reid D. No home without hormones: how plant hormones control legume nodule organogenesis. Plant Commun. 2020;1:100104. doi: 10.1016/j.xplc.2020.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.S., Li X., Luo Z., Mysore K.S., Wen J., Xie F. NIN interacts with NLPs to mediate nitrate inhibition of nodulation in Medicago truncatula. Nat. Plants. 2018;4:942–952. doi: 10.1038/s41477-018-0261-3. [DOI] [PubMed] [Google Scholar]
- Magori S.O.-K.E., Shibata S., Umehara Y., Kouchi H., Hase Y., Tanaka A., Sato S., Tabata S., Kawaguchi M. TOO MUCH LOVE, a root regulator associated with the long-distance control of nodulation in Lotus japonicus. Mol. Plant Microbe Interact. 2009;22:259–268. doi: 10.1094/MPMI-22-3-0259. [DOI] [PubMed] [Google Scholar]
- Mens C., Hastwell A.H., Su H., Gresshoff P.M., Mathesius U., Ferguson B.J. Characterisation of Medicago truncatula CLE34 and CLE35 in nitrate and rhizobia regulation of nodulation. New Phytol. 2021;229:2525–2534. doi: 10.1111/nph.17010. [DOI] [PubMed] [Google Scholar]
- Mohd-Radzman N.A., Binos S., Truong T.T., Imin N., Mariani M., Djordjevic M.A. Novel MtCEP1 peptides produced in vivo differentially regulate root development in Medicago truncatula. J. Exp. Bot. 2015;66:5289–5300. doi: 10.1093/jxb/erv008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd-Radzman N.A., Laffont C., Ivanovici A., Patel N., Reid D., Stougaard J., Frugier F., Imin N., Djordjevic M.A. Different pathways act downstream of the CEP peptide receptor CRA2 to regulate lateral root and nodule development. Plant Physiol. 2016;171:2536–2548. doi: 10.1104/pp.16.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C., Gautrat P., Frugier F. Nitrate-induced CLE35 signaling peptides inhibit nodulation through the SUNN receptor and miR2111 repression. Plant Physiol. 2021;00:1–13. doi: 10.1093/plphys/kiaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortier V., Den Herder G., Whitford R., Van de Velde W., Rombauts S., D'Haeseleer K., Holsters M., Goormachtig S. CLE peptides control Medicago truncatula nodulation locally and systemically. Plant Physiol. 2010;153:222–237. doi: 10.1104/pp.110.153718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida H., Tanaka S., Handa Y., Ito M., Sakamoto Y., Matsunaga S., Betsuyaku S., Miura K., Soyano T., Kawaguchi M. A NIN-LIKE PROTEIN mediates nitrate-induced control of root nodule symbiosis in Lotus japonicus. Nat. Commun. 2018;9:499. doi: 10.1038/s41467-018-02831-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura R., Hayashi M., Wu G.J., Kouchi H., Imaizumi-Anraku H., Murakami Y., Kawasaki S., Akao S., Ohmori M., Nagasawa M. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- Ohkubo Y., Tanaka M., Tabata R., Ogawa-Ohnishi M., Matsubayashi Y. Shoot-to-root mobile polypeptides involved in systemic regulation of nitrogen acquisition. Nat. Plants. 2017;3:17029. doi: 10.1038/nplants.2017.29. [DOI] [PubMed] [Google Scholar]
- Oka-Kira E., Tateno K., Miura K., Haga T., Hayashi M., Harada K., Sato S., Tabata S., Shikazono N., Tanaka A. Klavier (klv), a novel hypernodulation mutant of Lotus japonicus affected in vascular tissue organization and floral induction. Plant J. 2005;44:505–515. doi: 10.1111/j.1365-313X.2005.02543.x. [DOI] [PubMed] [Google Scholar]
- Okamoto S., Shinohara H., Mori T., Matsubayashi Y., Kawaguchi M. Root-derived CLE glycopeptides control nodulation by direct binding to HAR1 receptor kinase. Nat. Commun. 2013;4:2191. doi: 10.1038/ncomms3191. [DOI] [PubMed] [Google Scholar]
- Okuma N., Soyano T., Suzaki T., Kawaguchi M. MIR2111-5 locus and shoot-accumulated mature miR2111 systemically enhance nodulation depending on HAR1 in Lotus japonicus. Nat. Commun. 2020;11:5192. doi: 10.1038/s41467-020-19037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J.E., Nakao P., Bohlool B.B., Gresshoff P.M. Lack of systemic suppression of nodulation in split root systems of supernodulating soybean (Glycine max [L.] Merr.) mutants. Plant Physiol. 1989;90:1347–1352. doi: 10.1104/pp.90.4.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pislariu C.I., Murray J.D., Wen J., Cosson V., Muni R.R.D., Wang M., Benedito V.A., Andriankaja A., Cheng X., Jerez I.T. A Medicago truncatula tobacco retrotransposon insertion mutant collection with defects in nodule development and symbiotic nitrogen fixation. Plant Physiol. 2012;159:1686–1699. doi: 10.1104/pp.112.197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid D.E., Ferguson B.J., Gresshoff P.M. Inoculation- and nitrate-induced CLE peptides of soybean control NARK-dependent nodule formation. Mol. Plant Microbe Interact. 2011;24:606–618. doi: 10.1094/MPMI-09-10-0207. [DOI] [PubMed] [Google Scholar]
- Reid D.E., Ferguson B.J., Hayashi S., Lin Y.H., Gresshoff P.M. Molecular mechanisms controlling legume autoregulation of nodulation. Ann. Bot. 2011;108:789–795. doi: 10.1093/aob/mcr205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riefler M., Novak O., Strnad M., Schmülling T. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell. 2006;18:40–54. doi: 10.1105/tpc.105.037796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel E., Journet E.P., de Carvalho-Niebel F., Duc G., Frugoli J. The Medicago truncatula SUNN gene encodes a CLV1-like leucine-rich repeat receptor kinase that regulates nodule number and root length. Plant Mol. Biol. 2005;58:809–822. doi: 10.1007/s11103-005-8102-y. [DOI] [PubMed] [Google Scholar]
- Schnabel E.L., Kassaw T.K., Smith L.S., Marsh J.F., Oldroyd G.E., Long S.R., Frugoli J.A. The ROOT DETERMINED NODULATION1 gene regulates nodule number in roots of Medicago truncatula and defines a highly conserved, uncharacterized plant gene family. Plant Physiol. 2011;157:328–340. doi: 10.1104/pp.111.178756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I.R., Men A.E., Laniya T.S., Buzas D.M., Iturbe-Ormaetxe I., Carroll B.J., Gresshoff P.M. Long-distance signaling in nodulation directed by a CLAVATA1-like receptor kinase. Science. 2003;299:109–112. doi: 10.1126/science.1077937. [DOI] [PubMed] [Google Scholar]
- Soyano T., Hirakawa H., Sato S., Hayashi M., Kawaguchi M. Nodule Inception creates a long-distance negative feedback loop involved in homeostatic regulation of nodule organ production. Proc. Natl. Acad. Sci. U S A. 2014;111:14607–14612. doi: 10.1073/pnas.1412716111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soyano T., Shimoda Y., Hayashi M. NODULE INCEPTION antagonistically regulates gene expression with nitrate in Lotus japonicus. Plant Cell Physiol. 2015;56:368–376. doi: 10.1093/pcp/pcu168. [DOI] [PubMed] [Google Scholar]
- Streeter J., Wong P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988;7:1–23. [Google Scholar]
- Tabata R., Sumida K., Yoshii T., Ohyama K., Shinohara H., Matsubayashi Y. Perception of root-derived peptides by shoot LRR-RKs mediates systemic N-demand signaling. Science. 2014;346:343–346. doi: 10.1126/science.1257800. [DOI] [PubMed] [Google Scholar]
- Tsikou D., Yan Z., Holt D.B., Abel N.B., Reid D.E., Madsen L.H., Bhasin H., Sexauer M., Stougaard J., Markmann K. Systemic control of legume susceptibility to rhizobial infection by a mobile microRNA. Science. 2018;362:233–236. doi: 10.1126/science.aat6907. [DOI] [PubMed] [Google Scholar]
- Voisin A.S.S.C., Jeudy C., Warembourg F.R. Symbiotic N2 fixation in relation to C economy of Pisum sativum L. as a function of plant phenology. J. Exp. Bot. 2003;54:2733–2744. doi: 10.1093/jxb/erg290. [DOI] [PubMed] [Google Scholar]
- Zhang M., Su H., Gresshoff P.M., Ferguson B.J. Shoot-derived miR2111 controls legume root and nodule development. Plant Cell Environ. 2021 doi: 10.1111/pce.13992. [DOI] [PubMed] [Google Scholar]
- Zhu F., Deng J., Chen H., Liu P., Zheng L., Ye Q., Li R., Brault M., Wen J., Frugier F. A CEP peptide receptor-like kinase regulates auxin biosynthesis and ethylene signaling to coordinate root growth and symbiotic nodulation in Medicago truncatula. Plant Cell. 2020;32:2855–2877. doi: 10.1105/tpc.20.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.