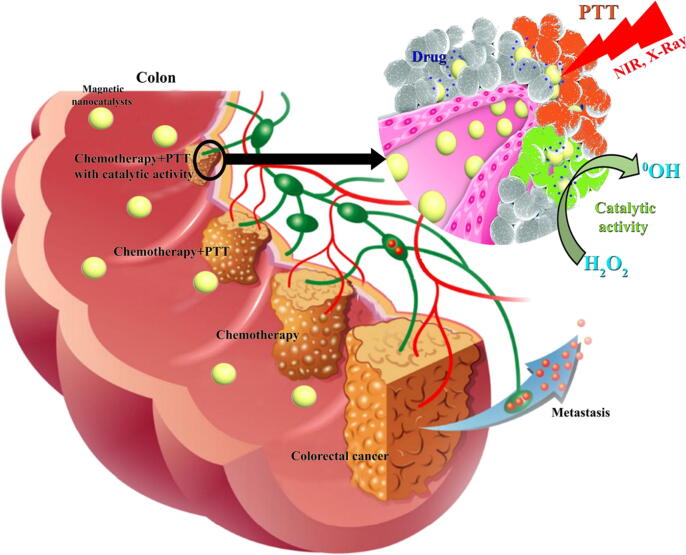
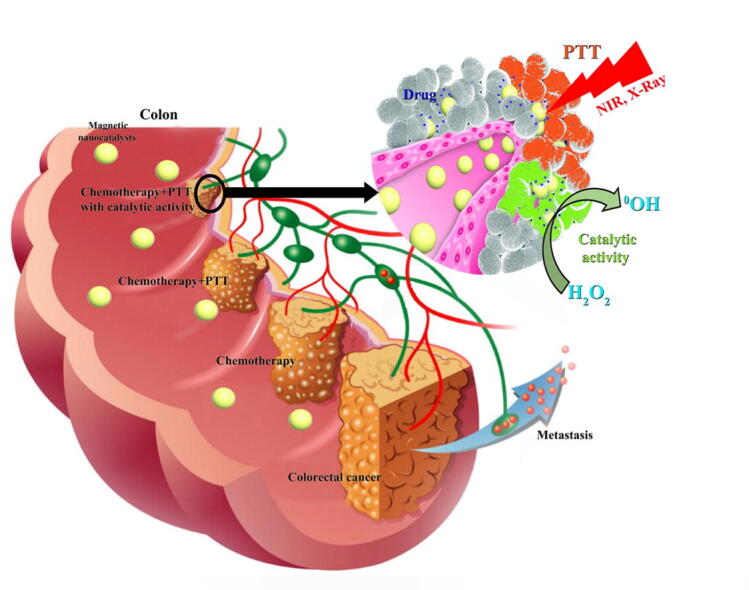
Graphical abstract
Keywords: Magnetic nanocatalysts, Heterocyclic compounds, Anticancer drugs, Fenton reaction, Cancer therapy
Abstract
Background
Heterocyclic compounds have always been used as a core portion in the development of anticancer drugs. However, there is a pressing need for developing inexpensive and simple alternatives to high-cost and complex chemical agents-based catalysts for large-scale production of heterocyclic compounds. Also, development of some smart platforms for cancer treatment based on nanoparticles (NPs) which facilitate Fenton reaction have been widely explored by different scientists. Magnetic NPs not only can serve as catalysts in the synthesis of heterocyclic compounds with potential anticancer properties, but also are widely used as smart agents in targeting cancer cells and inducing Fenton reactions.
Aim of Review
Therefore, in this review we aim to present an updated summary of the reports related to the main clinical or basic application and research progress of magnetic NPs in cancer as well as their application in the synthesis of heterocyclic compounds as potential anticancer drugs. Afterwards, specific tumor microenvironment (TME)-responsive magnetic nanocatalysts for cancer treatment through triggering Fenton-like reactions were surveyed. Finally, some ignored factors in the design of magnetic nanocatalysts- triggered Fenton-like reaction, challenges and future perspective of magnetic nanocatalysts-assisted synthesis of heterocyclic compounds and selective cancer therapy were discussed.
Key Scientific Concepts of Review:
This review may pave the way for well-organized translation of magnetic nanocatalysts in cancer therapy from the bench to the bedside.
Introduction
Catalysts are widely used in the production of chemicals [1], [2] and pharmaceutical ingredients [3], [4]. A large number of products require the application of catalysts; where, it has been estimated that a large portion of manufactured products of industrialized countries' GDP is dependent on catalysts [5]. Today, researchers have a big effort to incorporate nanomaterials into catalysts as a successful and promising strategy in the development of nano-based platforms. Because of their great significance of nanocatalysts in different areas of nanotechnology such as nanochemistry, nanopharmaceuticals, and nanomedicine, the number of articles and books published about their application is continuously increasing. Nanomaterials show distinctive catalytic characteristics and are widely used in the preparation of nanocatalysts [6], [7]. Indeed, many of the properties of materials such as electrical, optical, and magnetic behaviors change at nanoscale dimension [8], [9]. These novel characteristics are derived from the following three properties: small size less than 10 nm, potential high surface (S)/volume (V) ratio, and an increase in the number of surface atoms. In fact, these three factors are the most important reasons for the emergence of catalytic properties in nanomaterials. Basically, when particles become small (nanoscale), because of their high curvature, they have many atoms on their surface establishing weak bonds with the lattice atoms [10]. Therefore, these particles have high surface energy and are highly active, so-called surface atoms are physically unstable and chemically active and are prone to many chemical reactions [11], [12]. Since the electronic structure of the NPs depends on the dimension of the particle, their capability to interact with other compounds also depends on their dimension [13], [14]. For example, one group of materials that behave differently in bulk and nanoscale forms is magnetic particles. Magnetic particles have a limited catalytic activity and are considered as inactive metals in the bulk state, but at the nanoscale, they exhibit profound catalytic activity and are commonly used as intermediate metals in the development of nanocatalysts [15], [16].
The catalyst design at the NP scale is heavily based on the principles of size [17] and mass transfer [18]. Recent advances have shown that the amelioration of the catalytic activities of catalysts can be achieved through nanoscale structural modification [19], [20]. Indeed, due to the high cost and scarcity of the most catalytically active metal group, there has been a great interest in the use of these materials with low concentration and optimized catalytic activity. At the nanoscale, the properties of materials are dictated by the arrangement of atoms [21]. Table 1 summarizes the factors affecting the catalytic properties of nanomaterials.
Table 1.
Factors influencing the catalytic properties of nanomaterials.
| Factors | Explanation | Ref. |
|---|---|---|
| NP size | In most cases, the catalytic activity of NPs is inversely related to the size of NPs | [22] |
| NP shape | As the spatial distribution of the NPs increases, the number of surface atoms becomes more available which result in the enhancement of the catalytic activity | [22] |
| NP distribution | NPs with many edges and corners, such as tetrahedral, octahedral, and cubic, as the ratio of surface to volume increases more than other NPs, these nanomaterials show more catalytic activities. | [23] |
| NP supports | The solid substrate can be used for immobilization of NPs to prevent the NPs from accumulating and their so-called agglomeration and increase their catalytic activity | [24] |
| Reaction conditions | When NPs react under microwave conditions, the catalytic activity and selectivity over normal conditions (reflux) are greatly improved. | [25] |
Different kinds of magnetic nanocatalysts
Despite the variation in the classification of magnetic nanocatalysts due to different physicochemical properties of NPs, the method of fabrication, surface modification, crystal structure, and composition of magnetic nanocatalysts derived from iron (Fe), gold (Au), palladium (Pd), and platinum (Pt) can be organized based on their oxidase-, peroxidase-, superoxide dismutase-, and catalase-like activities (Table 2).
Table 2.
Summary of magnetic nanocatalysts based on their intrinsic activity.
| NPs | Oxidase | Peroxidase | Superoxide dismutase | Catalase |
|---|---|---|---|---|
| Fe | FeNPs | Fe3O4, Fe2O3 | FePO4 | Fe3O4, Fe2O3 |
| Au | AuNPs, Au@Pt | AuNPs, Au@Pt | AuNPs, | AuNPs, |
| Mn | MnO2, Mn3O4 | MnO2, Mn3O4, MnFe2O4 | MnO2, Mn3O4 | MnO2, Mn3O4 |
| Ni | NiPd | NiPd | NiPd | |
| Ag | AgNPs | AgNPs | AgNPs | AgNPs |
| Pt | PtNP, PtCo | PtNP, PtNPs/GO | PtNP | PtNP |
| Pd | PdNP | PdNP | PdNP | PdNP |
| Co | CoFe2O4 | Co3O4, CoFe2O4 | Co3O4 |
However, the most commonly used magnetic nanomaterial is iron oxide (IO), whose most important catalytic activities in medical activities are peroxidase-, superoxide dismutase-, and catalase-like activities (Table 3). Peroxidase is the most well-known enzymatic activity mimicked by IO nanocatalysis. For peroxidase activity, magnetic nanocatalysts similar to the Horseradish peroxidase (HRP) require an optimum temperature of 37 to 40 °C with a pH of 3 to 6.5. Moreover, in order to increase the peroxidase activity of NPs, the presence of the optimum concentration of hydrogen peroxide (H2O2) is important.
Table 3.
Metallic nanocatalysts and thier parameters in medical platforms.
| Material | Size (nm) | Shape | Activity | Ref. |
|---|---|---|---|---|
| Fe | ||||
| Fe3O4 | 13 | Polyhedral | Peroxidase | [26] |
| Fe3O4 | – | Spherical | Catalase | [27] |
| GO-Fe3O4 | 6–8 | NPs | Peroxidase | [28] |
| Fe3O4@Pt | 5–10 | Spherical | Peroxidase | [29] |
| Fe3O4@Cu@Cu2O | 20–40 | Spherical | Peroxidase | [30] |
| GO-Fe2O3 | – | NPs | Catalase | [31] |
| PB-Fe2O3 | 30 | NPs | Peroxidase | [32] |
| Pd@γ-Fe2O3 | 14–25 | Polyhedral | Peroxidase | [33] |
| Au | ||||
| Au2O3 | 50 | NPs | Peroxidase | [34] |
| AuNPs | 15–34 | Spherical | Multi | [35] |
| Au@PVP NPs | 1–3 | NPs | Oxidase | [36] |
| Pt | ||||
| PtNPs | 3.8 | NPs | Oxidase | [37] |
| PtNPs | 1.2 | Nanoplate | Oxidase | [38] |
| PtNPs | 40 | Multi-octahedra | Oxidase | [39] |
| Pd | ||||
| AgPd-GO | 7–10 | NPs | Peroxidase | [40] |
| Pd@MIL-101 | 1.4–1.8 | NPs | Hydrolysis | [41] |
| PdNPs | 1.5 | NPs | Peroxidase | [42] |
| Rhodium (Rh) | ||||
| Rh-SiO2 NPs | 5–15 | Tetrahedral | Oxidase | [43] |
| RhNPs | 1–2 | NPs | Peroxidase | [44] |
| Rudium (Ru) | ||||
| RuNPs | 1.1 | NPs | Peroxidase | [45] |
Function of magnetic nanocatalysts
Magnetic NPs as catalysts have different capabilities in medical fields such as diagnosis [46], imaging [47], drug delivery [48], drug discovery [49], and cancer therapy [50] due to the inherent enzymatic activity. Since catalytic activities in chemistry are highly surface dependent, the use of metallic NPs is of particular importance due to their highly active surface [51]. Although the reaction kinetics of magnetic nanocatalyst-based catalysts are slightly lower than those of native enzymes [52], resistance to environmental changes such as heat and acidity, easy separation, excellent reusability, and cost effective have made them potential candidates in different applications [15], [53]. On the other hand, the occurrence of dual behavior of magnetic nanocatalysts or the bridge between homogeneous and heterogeneous catalysis by nanocatalysts [54], as well as the provision of a platform to induce a certain reaction like photooxidation have encouraged scientietis to use magnetic nanocatalysts [55]. Hence, a large number of magnetic nanocatalysts have been produced in the industry to catalysis the synthesis of different chemicals or drugs. Magnetic nanocatalysts in the form of homogeneous and heterogeneous catalysts have been used in biomedical activities [56], [57], among which IONPs have the highest application due to their very low toxicity [58]. Furthermore, magnetic nanocatalysts are highly considered in therapeutic platforms, especially in cancer therapy, anti-inflammatory activities, antibacterial, tissue engineering, and immunotherapy. The integration of the homogeneous and heterogeneous catalysts in biomedical fields enabled by medical nanocatalysts through light and magnetic waves has provided a potential system for development of therapeutic platforms.
Main clinical or basic application and research progress of magnetic NPs in cancer
Before magnetic NPs were considered as catalysts, they were used in the fields of chemotherapy [59], imaging [60], gene therapy [61], photothermal therapy (PTT) [62], magnetic hyperthermia [63], radiation therapy [64], and photodynamic therapy (PDT) [65]. The drug delivery by magnetic NPs has become a popular way to transfer drugs to the tumor site due to controllable physicochemical properties. For example, the loading and delivery of paclitaxel [66], doxorubicin [62], [63], 5-fluorouracil [67], and even small molecules or proteins such as lactoferrin [68] or Bcl-2 shRNA [69] to tumors has been successfully conducted. Furthermore, evaluation of vital organs around the target tissue has shown that magnetic NPs have triggered a minor effect on the pathological changes and systemic toxicity [62], [63], [70], [71], [72]. It has been shown that the use of magnetic NPs enhances the therapeutic activity against tumors by inducing more damage to DNA, while NPs activated by radiotherapy did not show such toxicity on the cancer cells [73]. Moreover, magnetic NPs significantly sensitize the tumor cells to radiation therapy [74] by inducing a hypoxic condition for the generation of active oxygen.
Furthermore, it was found that the use of magnetic NPs, in addition to improving cancer immunotherapy through facilitating the antibody penetration into the tumor, increases the quality of imaging of target tissue by MRI [75]. Furthermore, different types of magnetic NPs have shown anti-cancer activities. In this regard, Zanganeh et al. [76] showed that ferromoxytol-functionalized IONPs can trigger potential anticancer activity against lung, liver and breast cancers.
Thermal therapy enabled by magnetic NPs for treatment of cancer has received much attention due to their high efficiency and very low toxicity in critical tissues [50]. The most important methods of tumor ablation therapies through increase in free radical species enabled by magnetic NPs include magnetic hyperthermia, PTT [62], [63], [77], and PDT [78]. Despite the methods mentioned in the treatment of cancer with magnetic NPs, today the combination of these methods with the catalytic activity of magnetic NPs is done in order to make the primary treatment easier or more effective. For example, Nie et al. [79] showed that CuS-Fe@polymer nanocatalysts highly improved chemodynamic therapy through PTT and peroxidase-like activity in tumor site. Similarly, Zhao et al. [80] by developing ROS-activatable liposomes@oxaliplatin@Fe3O4 nanocatalysts designed a platform for synergistic photo/chemodynamic therapies.
Magnetic nanocatalysts in the synthesis of anticancer drugs
NPs can be used as promising platforms to accelerate the process of converting organic compounds to one another [81]. These include organic compounds widely used in chemical and pharmaceutical industries [82]. As heterocyclic compounds with anticancer properties widely used in the pharmaceutical industry, an easy, efficient and environmentally friendly method for synthesizing these organic compounds has been introduced by using nanocatalysts [83], [84].
Nanocatalysts in fabrication of heterocyclic compounds as novel anticancer drugs
Heterocyclic compounds are known to act as promising anticancer drugs [85]; where, they have been reported as the key structural components of several anticancer agents present on the market, recently. Indeed, of the potential anticancer drugs approved by the FDA between 2010 and 2019, about 40% had heterocyclic compounds within their formulations. The importance of this plan is in the feasible and inexpensive synthesis of these compounds using nanocatalysts and the investigation of their structural characteristics and performance through computational studies. Expensive homogeneous catalysts, multistage and complex manufacturing processes are commonly used in drug production processes; however, the application of nanocatalyst in the development of anticancer drugs is highly efficient and has the ability to be reused in the drug manufacturing process [86]. The nanocatalysts decrease production costs, increase biocompatibility via the use of non-toxic metals and non-volatile solvents, and limit the production of by-products [87]. Thereby, nanocatalyst can not only be extracted from the inexpensive sources, but they also are resistant to air and moisture and exhibit potential catalytic performance in chemical reactions [88]. Also, heterocyclic compounds in the presence of nanocatalysts can be synthesized via different routes [86], [89].
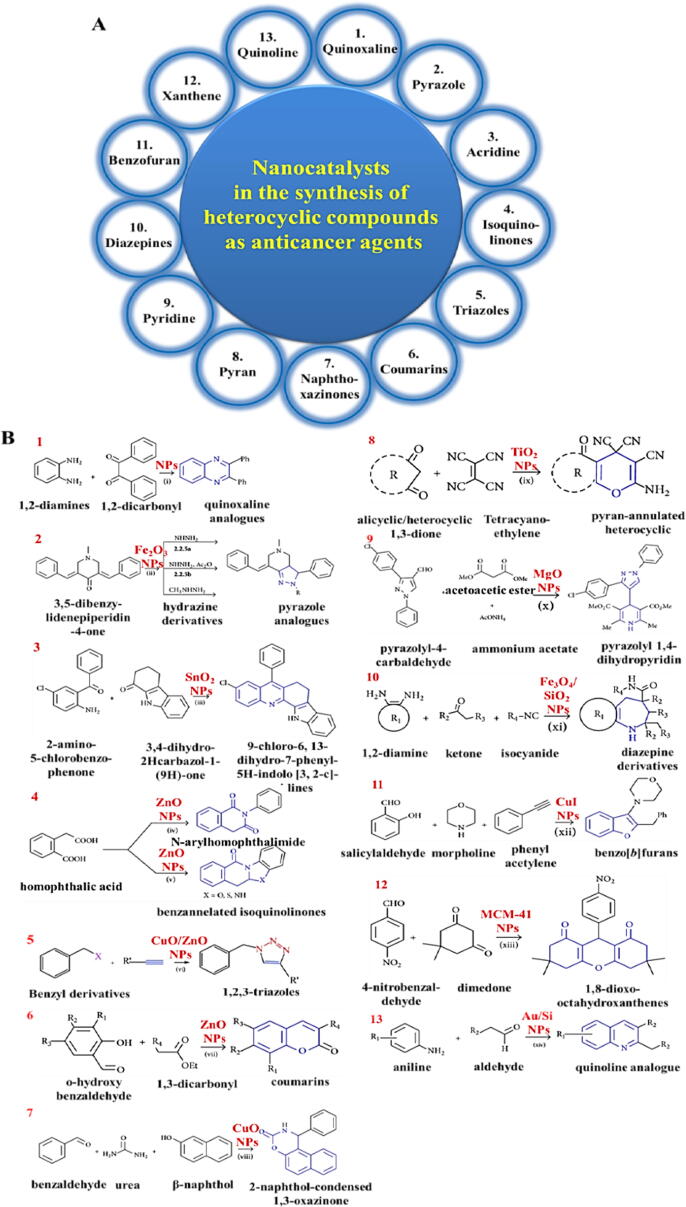
Quinoxaline, Pyrazole, Acridine, Isoquinolinone, Triazoles, Coumarins, Naphthoxazinones, Pyran, Pyridine, Diazepines, Benzofuran, Xanthene, and Quinoline are the most common heterocyclic compounds used as anticancer agents (Fig. 1A) [90].
Fig. 1.
(A (Schematic illustration of using nanocatalysts in the synthesis of different heterocyclic compounds. (B(The reactions of synthesis of heterocyclic compounds. Adapted with permission from Ref. [90].
These compounds can be synthesized in a variety of mechanisms by using different nanocatalysts (Fig. 1B). One of the performances of synthesized pharmaceutical compounds can be highlighted as following: estrogen receptors (ER) and aromatase enzymes are two important factors in cancer therapy, more precisely in breast cancer; hormone therapy can inhibit estrogen production by blocking aromatase or using ligands to block estrogen receptors and stopping estrogen activity and cell growth [85]. The other mechanisms of anticancer properties of heterocyclic compounds are tabulated in Table 4.
Table 4.
Mechanism of different heterocyclic compounds in cancer therapy.
| Heterocyclic compounds | Drugs approved by FDA | Type of cancer | Mechanism of anticancer | Ref. |
|---|---|---|---|---|
| Nitrogen-based heterocycles | Vincristine and vinblastine | Acute lymphoblastic leukemia, Hodgkin’s and non-Hodgkin’s lymphoma, and testicular cancer | Inhibition of cell signaling, cell cycle arrest, inhibition of tumor vascularization and DNA repair, induction of oxidative stress, tubulin depolymerization | [91] |
| Oxygen-based heterocycles | Cabazitaxel and eribulin | Prostate and metastatic breast cancer | Depolymerisation of microtubule, inhibition of mitosis | [92] |
| Sulfur-based heterocycles | Dabrafenib | Melanoma, lung cancer | Inhibition of tyrosine kinase | [93] |
The molecules synthesized in the presence of nanocatalysts show similar size, shape, polarity, and cytotoxic effects against cancer cells as compared to classical compounds. Potentially, the interaction of these compounds with the estrogen receptor have been further investigated through molecular docking calculations and density-functional theory (DFT) studies and yielded favorable results. Therefore, in the presence of the nanocatalyst, the anticancer drug can be prepared in a simple and inexpensive way [85]. Also, the synthesis of heterocyclic compounds by using nanocatalysts shows several advantages such as short reaction time, high throughput, and solvent-free medium [84]. A range of nanocatalysts such as magnesium oxide [94], SBA‐15 [86], [95], cobalt [96], palladium [97], [98], copper [99], graphene oxide [100], [101], and carbon nanotubes [102] have been also used as promising and efficient agents for synthesis of heterocyclic compounds with potential anticancer effects [103], [104], [105].
Magnetic nanocatalysts: Homogeneous or heterogeneous catalysis in drug synthesis
Also, the main strategy of catalysis science and technology is to increase the catalytic function and selectivity as well as recovery of the nanocatalysts. Indeed, the recovery and reutilization of nanocatalysts seem to be crucial parameters owing to strict biological and economical demand for sustainability [106], [107], [108]. Homogeneous catalysts show several positive points that they are well-structured on a molecular basis and easily dissolvable in the reaction milieu [54]. Therefore, these catalysts are extremely approachable to the substrates and normally demonstrate profound catalytic function, even under harsh environments. However, their separation from the reaction medium to circumvent the interactions with product needs exorbitant and repetitive extraction processes. Moreover, the inorganic nanocatalysts are usually composed of an expensive noble metal. Hence, in spite of their innate superior points, homogeneous catalysts are rarely applied in pharmacologically or medically applicable systems. On the other hand, there are frequently catalytically functional sets with several activities and specificities in the material of a heterogeneous catalyst, which are not suitable to be addressed in the molecular approach. Therefore, purification and reutilization of homogeneous catalysts is a principal concern in the sustainable and wide-ranging fabrication of organic compounds [54]. The catalyst can be purified through dissolving products and nanocatalysts in separate non-miscible solutions, which result in the purification of nanocatalysts by uncomplicated phase separation. However, the solubility of reactants in the reaction milieu and the mass transfer should be considered in the purification process [54]. Also, the activity of the purified catalyst may be partially inhibited by increasing their tendency to agglomeration during purification processes [109]. Nanocatalysts can be easily solubilized in a liquid solution to form a stable nanosuspension; however, during conventional purification process, they can be aggregated into particles with diameters of more than 100 nm. Therefore, to overcome this circumstance, ultracentrifugation is normally applied as the only way to purify the nanocatalyst. However, this approach needs high-priced and high technology facilities to achieve the potential results. Thus, magnetic nanocatalysts, which can be readily purified from the reaction medium by magnetic utilization, can be introduced as promising nanocatalysts in the pharmacological industry.
Therefore, catalytic capabilities of magnetic NPs consisting of Fe2O3 and an organic component are further investigated to accelerate the introduced production process. Also, the magnetic nanocatalysts provide a higher activity than their bulk counterparts due to its high S/V ratio, which greatly increases the efficiency of chemical synthesis process. In addition, having magnetic properties enables them to be separated from the manufactured product upon completion of the reaction. Therefore, the final product purification process will be performed with greater ease and speed [110]. For example, magnetic nickel-Fe2O4 nanocatalysts were employed as effective and reusable nanocatalyst for fabrication of acetylferrocene chalcones as potential anticancer candidates against colon cancer (HCT116), breast cancer (MCF7), and liver cancer (HEPG2) [111]. Therefore, it may be concluded that different species of magnetic NPs can be utilized as recoverable nanocatalysts in the synthesis of heterocyclic drugs both in solvent-free and aqueous media [112], [113], [114], [115].
Magnetic nanocatalysts-facilitated Fenton reaction for cancer therapy
As current cancer therapeutic strategies may stimulate some adverse effects against surrounding normal tissues and/or trigger unwanted tumor metastasis, to improve the anti-tumor therapeutic efficacy, targeting tumor microenvironment (TME) has been used for cancer therapy. Indeed, in TME, the metabolism, intermediates and pH are considerably different from those in non-cancer cells. Therefore, TME-responsive nanocatalysts for cancer therapy have been recently evaluated in vitro and in vivo.
In fact, it may be suggested that if the intrinsic properties of the TME in the presence of nanocatalysts could activate the Fenton reaction, the potential cancer therapy can be achieved with minimized side effects against off-targets.
Metal NPs like IONPs have been shown to provide multiple enzyme-like functions in a pH-dependent fashion. For example, these NPs could mimic a catalase-like activity to convert H2O2 into safe H2O and O2 at pH 7.5. More fascinating, they could mimic a peroxidase-like activity to transform H2O2 into highly hydroxyl radicals (OH•) at acidic pH. Therefore, IONPs are believed to serve as promising nanozymes in augmentation of the anti-tumor therapeutic efficacy of OH•, because such a site-selective production of the highly toxic radicals could trigger the induction of apoptosis in acidic cancer cells and leave the normal cells undamaged. Indeed, magnetic nanocatalysts could potentially stimulate the cancer cells-specified Fenton reaction to produce plentiful toxic OH• in the acidic TME.
According to Fenton reaction that was first introduced in 1894 by H. J. H. Fenton, H2O2 can be converted to toxic OH• in the presence of ferrous (Fe2+/ Fe2+) ions [116]. This catalytic reaction has been widely investigated in the various areas for instance elimination of organic pollutants from water through decomposition of contaminants into harmless materials like water, inorganic salts and so on [116], [117].
Fenton reaction in the cancer cells termed ferroptosis, which depends on Fe and reactive oxygen species (ROS) [118]. Indeed, in cancer cells the H2O2 molecule acts as a reactant to trigger the ferroptosis and the Fe2+ is the catalytic agent. It is well-documented that acidic condition is desirable for Fenton reaction; therefore, the acidic pH of TME can be considered as an effective characteristic to trigger the Fenton reaction [119]. Fig. 2(A), schematically illustrates the Fenton reactions in cancer cells.
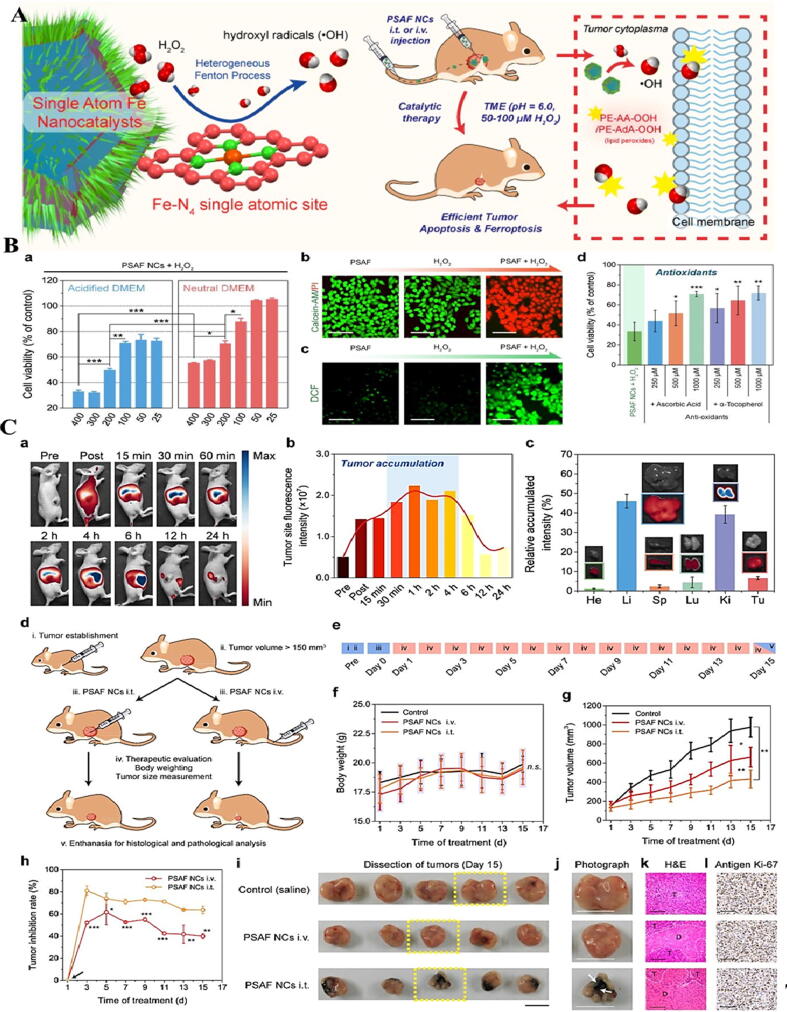
Fig. 2.
(A(Schematic representation of PEGylated single-atom Fe (PSAF) NCs in TTE. (B(Cell viability assays. Viability assays of tumor cell (4 T1) in the presence of PSAF NCs as well as H2O2 at 2 different pHs (a), Calcein-AM/PI cell staining and (b) and DCFH-DA cell- staining (c), viability percentage of the cells pre-incubated with antioxidants (d). (C) In vivo biodistribution and anti-tumor activity of PSAF NCs. In vivo biodistribution of NCs (a, b, c), in vivo treatment timescale of PSAF NCs (d, e), body weight (f) and tumor volume (g). tumor inhibition rates (h), Digital photos (i-j), and histological and staining microscopic images (k, l) [124]. Reprinted with permission from Ref. [124].
A lot of researchers have focused on the design and development of nanostructures, which are able to induce ferroptosis reactions in different cancers [80], [120]. Based on the literature review, Fe-based nanostructures are appropriate candidates in specific accumulation at tumor region through passive as well as active targeting mechanisms. Indeed, these nano-based platforms are degraded in endocytic organelles of cancer cells in the form of Fe2+ or Fe3+ to initiate the ferroptosis process [121], [122]. In general, this catalytic process leads to imbalance between production and destruction of ROS that in turn can stimulate the severe oxidative stress-induced apoptosis. In fact, induction of intratumoral Fenton reaction can result in the disproportionation of H2O2. Therefore, development of the efficient ferroptosis-based nanocatalysts that possess transformation capacity of endogenous H2O2 to OH• is an outstanding strategy for cancer therapy [123].
Besides, regarding to the stronger oxidation capacity of OH• compared to singlet oxygen, the generation of these species are highly demanded for Fenton reaction. On the other hand, shelf life of OH• is very short (9–10 s), which can trigger only a few oxidative reactions such as DNA damage, lipid oxidation, and protein oxidation, whereas their diffusion into remote sites is difficult [125]. Considering the aforementioned explanations, Fenton-based nanocatalysts which serve as tumor-selective nano-based platforms would be preferred for cancer therapy.
In general, it has been reported that free radicals could trigger apoptosis induction in malignant cancer cells and subsequent magnificent tumor suppression (Fig. 2A-C) [124].
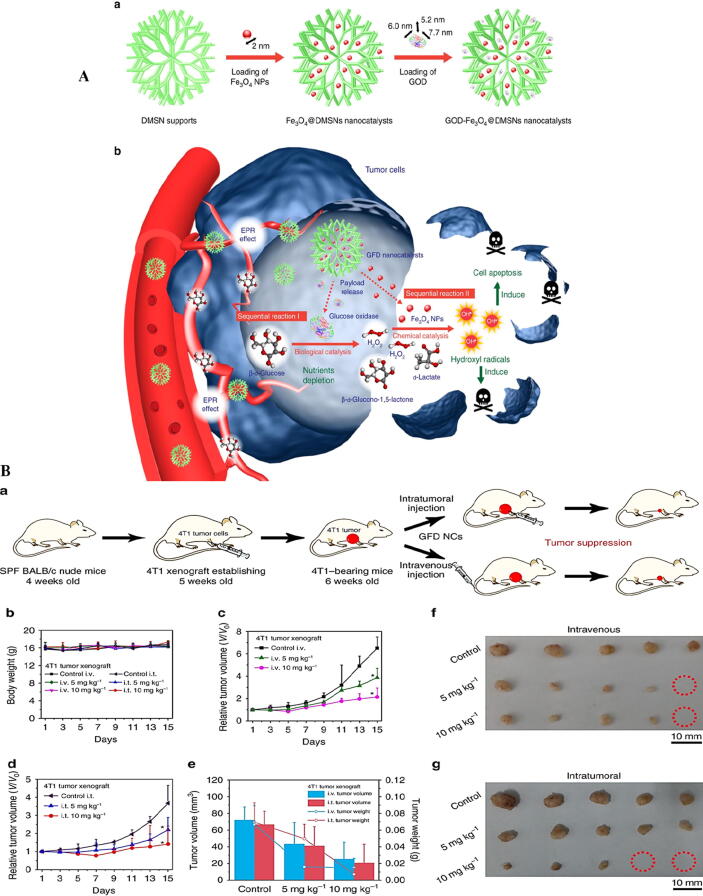
Nevertheless, the intracellular level of H2O2 in cancer cells is not high enough for nanocatalysts to produce a large amount of OH• to ameliorate Fenton chemical reaction for nanocatalytic tumor therapy. Therefore, a satisfactory approach to increase the intratumoral level of H2O2 was developed by Huo et al. [126]. Indeed, they reported that glucose oxidase (GOD) should be combined with nanocatalyst to increase the level of H2O2. Afterwards, IONPs integrated into the dendritic mesoporous silica NPs (DMSNs) to increase their dispersion and the ability to convert H2O2 to OH• with high efficacy, which could further stimulate the anticancer activity (Fig. 3A, B).
Fig. 3.
(A) Schematic representation. Fabrication (a) and catalytic-therapeutic activities of GFD NCs (b). (B) anticancer activity of fabricated GFD NCs. Schematic presentation of tumor xenograft induction and NCs administration routes, and therapeutic results (a). The body weights after treating with NCs intravenously (i.v.) and intratumorally (i.t.) (b), The relative tumor volumes mice treated with NCs via i.v. (c) and i.p. modes (d). The tumor volumes and weights of tumors (e), image of tumors after i.v. (f) and i.p. treatments (g) [126]. Reprinted with permission from Ref. [126].
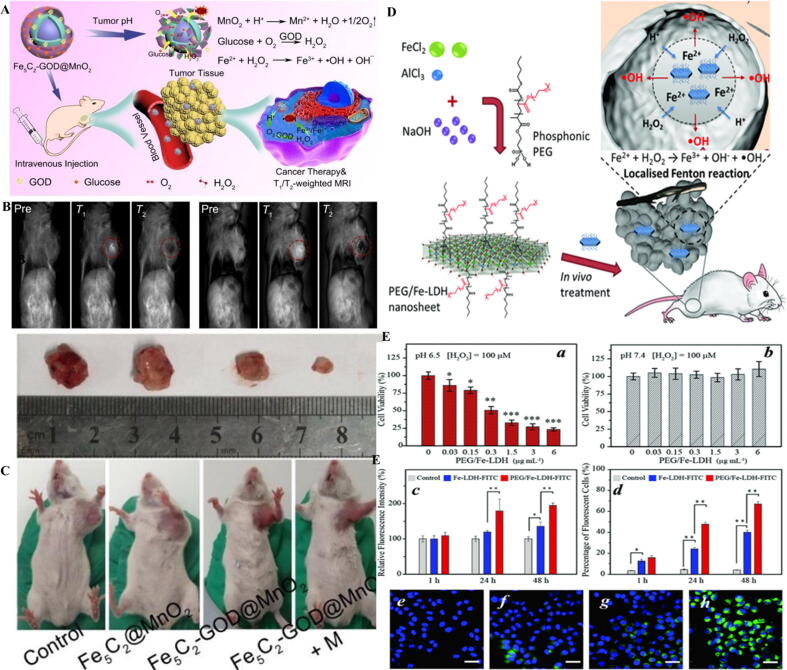
The strategy was proposed by Huo et al. [126] can be hampered by unwanted distribution of nanocatalyst in normal tissues and inactivation of GOD or leaking before reaching the carrier to the targeted site in vivo. Therefore, a gatekeeper cover can be designed as a shield on the surface of the carrier in a pH-sensitive manner to release GOD at the tumor site. Also, GOD needs oxygen to catalyze the conversion of glucose to H2O2 and carrier should provide a large amount of oxygen. The study done by Feng et al. [127], which reported the fabrication of a potential nanocatalyst equipped with a gate keeper and a source of oxygen, was performed to overcome these limitations. They constructed smart, magnetic targeted, and TME-responsive nanocatalysts that can cause oxidative stress-mediated apoptosis in tumor cells. Indeed, application of magnetic field (MF) can result in the targeted accumulation of magnetic nanocatalyst [127]. The magnetic core of IO carbide (Fe5C2)-GOD and the manganese dioxide (MnO2) nanoshell as a smart “gatekeeper” mask GOD from unwanted leakage until entering cancer cells. The Fe5C2-GOD@MnO2 nanocatalysts did not show any activity in off-targets, whereas in cancer cells, TME triggered conversion of MnO2 shell into Mn2+ and O2 along with leaking GOD (Fig. 4A). Mn2+ could be used as a magnetic resonance imaging (MRI) contrast material and O2 in the presence of GOD could be converted into H2O2 which may speed up the following Fenton reaction catalyzed by the Fe5C2 core (Fig. 4B, C).
Fig. 4.
(A) Design and preparation of Fe5C2-GOD@MnO2 nanocatalysts. (B) In vivo T1/T2 MR images. Tumor bearing mice with i.v. injected Fe5C2-GOD@MnO2 either alone (a) or presence (b) of MF. (C) Digital images of the dissected tumors after 14 days treatment [127]. (D) Schematic illustration of TME of 2D PEG/Fe‐LDH nanosheets. (E) In vitro assays. Cell viability assays at different pH of 6.5 (a) and 7.4 (b), relative fluorescence intensity (c), and percentage of fluorescence intensity (d) based on cellular internalization, images of DCFH‐DA and DAPI staining incubated with PEG/Fe‐LDH, (e), H2O2 (f), PEG/Fe‐LDHs and H2O2 at pH 7.4 (g) and pH 6.5 (h) [76]. Reprinted with permission from Refs.[76], [127].
Another approach to increase the amount of OH• is to use 2D nanocatalyst. In this case, 2D hydroxide nanosheets can be integrated with magnetic nanocomposite to yield abundant OH• in the acidic state of TME. For example, Cao et al. [128] developed a conjugated Fe2+‐containing double layers of hydroxide (LDH) nanosheet through a simple and useful strategy with profound catalytic function to disproportionate H2O2 in cancer cells, and subsequently produce a high amount of OH• at mildly acidic milieu (Fig. 4D). Also, nanocomposites can be functionalized with PEG to achieve more biocompatibility and increase the level of internalization into the cells (Fig. 4E) [128].
Ignored factors in design of Fenton-based nanocatalysts
In agreement with reported studies, Fenton nanocatalysts could effectively trigger the production of highly toxic OH• to suppress the tumor growth in acidic TME. This phenomenon does not occur in normal tissue under neutral conditions, representing the low adverse effect of these nanoplatforms against off-targets. Nevertheless, it should be considered that cancer therapy based on Fenton nanocatalysts is a newborn approach and still remains some crucial issues to introduce this strategy to the clinical translations [129]. In the following, we will represent several important ignored factors in relationship with design of Fenton nanocatalysts for cancer therapy.
Intrinsic instability over a long period of time is one of the major problems, which can be correlated with the high tendency of nanocatalysts to form aggregated species with aim of their reduced surface energy. Another critical issue for these nanocatalysts is ease of their oxidation in air, which limits their storage for a long period of time. Reduction in interfacial area is consequence of aggregation of these magnetic nanostructures that subsequently lead to the loss of their magnetism as well as dispersibility properties [130].
To date, various strategies are applied to synthesize the magnetic nanocatalysts with high catalytic activity to provide more catalytic-active sites through the reduction of particle size or the fabrication of amorphous nanostructures [123], [131]. In spite of the presence of various routes for fabrication of nanocatalysts, their upscale production is extremely controversial.
Since, the catalytic properties of different nanocatalysts do not follow a similar mechanism and investigation of these mechanisms is not fully understood in the reported studies, the optimization of their catalytic efficacy is another critical issue that is overlooked in the most of performed studies. Therefore, catalytic mechanisms of nanocatalysts for cancer therapy should be carefully assessed in the future research.
The next issue to consider in the following research is feasibility of characterization of catalytic reaction in vivo. Due to the complications of and complex intracellular circumstances as well as lack of acceptable procedures at current time to characterize the catalytic mechanism in vivo, constructing a standard protocol for carefully characterization of catalytic reactions at in vivo microenvironment is more essential to conduct the future investigations in this field.
The surface engineering of nanocatalysts is a key prerequisite for their potential tumor accumulation and subsequently for achievement to excellent therapeutic efficacy. It is well documented that nanocatalysts could circumvent the reticuloendothelial system uptake via a suitable surface modification, leading the long-term blood circulation of designed structure [122]. Based on the performed literature review, the surface-engineering methodologies for design of targeted Fenton nanocatalysts are much less have been investigated [122], [132]. Therefore, efficient strategies should be established for surface modifications of Fenton nanocatalysts to improve the nanocatalytic-therapeutic efficacy in the following studies.
The next critical parameter should be noted for design of effective Fenton nanocatalysts is their biocompatibility and biosafety, guaranteeing their clinical translations in future. Although, several reports have demonstrated good biocompatibility of some Fenton nanocatalysts like Fe-based nanostructures and also their composites [133], [134]. their cytotoxicity and adverse biological effects in long periods have not been well-explored. Moreover, most Fenton nanocatalysts with high stability possess low biodegradation rates. Hence, it is expecting to optimize the biodegradation rate of these nanocatalysts and also their elimination from blood circulation for their clinical translations.
Generally, further investigations must be conducted to resolve the aforementioned challenges of Fenton nanocatalysts for cancer therapy in clinical trials.
Challenges and future prospects
Regarding the challenges of nanocatalyst in cancer therapy it should be noted that the intracellular level of H2O2 in cancer cells should be increased to produce a large amount of OH• to stimulate promising catalytic performance of nanocatalysts. Therefore, some strategies like combination of magnetic nanocatalysts with different sources of H2O2 production should be developed. In the meantime, the approving biodegradability and biocompatibility of magnetic nanocatalysts should be considered to guarantee their potential safety in vivo.
Magnetic NPs with different physicochemical properties synthesized in a number of routes can be used as effective catalysts for organic reactions to develop heterocyclic compounds as potential anticancer drugs. Furthermore, their magnetic characteristics enable the simple and effective separation of the nanocatalyst using a magnetic MF to be reutilized up to several times without any remarkable changes in the initial catalytic function. These advantages can be carried out both in aqueous and non– aqueous environments. Additionally, the activity of fabricated compounds can be tested against a wide range of cancers. Furthermore, magnetic NPs can be integrated in the form of nanocomposite to develop the superparamagnetic properties and magnetization even at ambient temperature. The fabricated magnetic nanocomposite could demonstrate potential catalytic activity as a novel heterogeneous magnetic agent for the development of some heterocyclic compounds with profound anticancer activities.
Additionally, there are a number of approaches to selectively activate anticancer agents in the tumor site by means of nanocatalyst to reduce the relevant adverse effects. For instance, in the case of magnetic nanocatalysts, external MFs are utilized to target the magnetic nanocatalyst in the selected tissue, which can then catalytically result in activation of Fenton reactions.
Also based on the specific properties of TME, a new inception of PTT in combination with magnetic nanocatalyst could be a promising approach in augmentation of Fenton chemical reaction for nanocatalytic tumor therapy (Fig. 5). These systems change the performance of nanocatalytic Fenton reaction for production of OH• and enhancing the tumor mortality at a subsequent time. Indeed, GOD for generation of large amounts of H2O2 and Fe3O4 ‐modified with photothermal inducer‐based nanocatalyst can be fabricated to attain diagnostic imaging‐guided and photothermal‐improved nanocatalytic tumor therapy. Interestingly, the high photothermal‐turning efficacy of the inducer increases the defined tumor temperature to dramatically speed up and ameliorate the nanocatalytic activity, which potentially results in outstanding synergistic anticancer activities with minimal adverse impacts.
Fig. 5.
PTT in combination with magnetic nanocatalyst could be used as a potential platform in augmentation of Fenton chemical reaction for tumor therapy.
Conclusion
The wide application of magnetic nanocatalysts derives from the fact that these agents have provided very encouraging outcomes in the synthesis of heterocyclic compounds as potential anticancer drugs as well as selective cancer treatments in several preclinical studies. In fact, magnetic nanocatalysts either alone or in conjugation with other NPs/molecules can be used as multiple promising nanocatalysts in synthesis of heterocyclic compounds based on magnetic NPs-catalyzed reactions, targeted drug delivery and facilitated Fenton reaction for cancer therapy. Nevertheless, only limited attempts have been done to translate the preclinical achievements of these smart agents to the clinics. Indeed, several challenges such as rapid metabolism, limited bioavailability and biodegradability should be taken into account in advance in order to bring magnetic nanocatalysts from the bench to the bedside. Pharmaceutical companies should try to overcome these drawbacks by reformulating nanocatalysts such as conjugation with other NPs or natural compounds.
Key Scientific Concepts of review
This review may pave the way for well-organized translation of magnetic nanocatalysts in cancer therapy from the bench to the bedside.
Declaration of Competing Interest
The authors have none to declare.
Acknowledgement
The authors gratefully acknowledge the China Postdoctoral Science Foundation research grant No. 2020 M672291; Henan Medical Science and Technology Research Youth Project Co-Sponsored by the Province and Ministry in China No: SB201902020; Top Talent Fund of the Second Affiliated Hospital of Zhengzhou University, No. 2020BJRCA03; Henan Middle-Aged Youth Health Technology Innovation Talent Project No. YXKC2020059.
Biographies

Dr. Suliman Khan has obtained PhD degree from Chinese Academy of Sciences. He is working at the second affiliated hospital of Zhengzhou University as postdoctoral teacher. He has recently received two research grants from Chinese Postdoctoral Sciences foundation and the Second Affiliated hospital of Zhengzhou University. He has published more 60 papers in SCI journals including Scientific Data, Clinical Microbiology and Infection, Journal of Advanced Research, andJournal of Clinical Microbiology.

Dr. Majid Sharifi has completed his scientific activities in the field of Animal Nutrition in the universities of Guilan (B.Sc.), Tehran (M.Sc.) and then Tabriz (Ph.D.) in 2003, 2006 and 2017. He re-educated in nanomedicine (M.Sc.) and graduated in 2019 due to his great interest in using nanomaterials for treating diseases and pathological disorders. He has implemented several projects in the fields of nano-biosensing, phototherapy therapy, and drug delivery, and has published various articles in prestigious journals such as Biosensors and Bioelectronics, Controlled release, Advanced Research, Talanta, Scientific Reports, and Nanomedicine.

Dr. Anwarul Hasan is an Associate Professor in the Department of Mechanical and Industrial Engineering, and Biomedical Research Center at Qatar University. Earlier he worked as an Assistant Professor in the Department of Biomedical Engineering and Mechanical Engineering at American University of Beirut, Lebanon. He was also a visiting Assistant Professor during 2014 to 2017 and an NSERC Postdoctoral Fellow during 2012-2013 at the Harvard-MIT Division of Health Sciences and Technology at the Harvard Medical School and Massachusetts Institute of Technology in Boston, USA. Dr Hasan obtained his PhD from University of Alberta, Canada in 2010 and worked in industry in Canada during 2010-2011. Dr Hasan has more than 200 peer reviewed publications including over 150 journal articles, and more than 50 conference proceeding papers as well as two edited books on “Tissue Engineering for Artificial Organs”. He is a winner of more than sixteen national and international awards. In the latest ranking of world’s top 2% highly cited scientists’ list by Stanford University researchers published in October 2020, Dr Hasan has been ranked 320 out of 50331 top biomedical researchers in the world. Dr Hasan’s current research interests involve Biomaterials, Tissue Engineering, 3D Bioprinting and Organs on chips platforms and microneedle arrays for Diabetic wound healing, cancer biochips, Covid-19 diagnostics, and cardiovascular tissue engineering.

Dr. Farnoosh Attar has obtained Ph.D. and M.Sc. in Biochemistry from the Institute of Biochemistry and Biophysics (IBB) of Tehran University between 2002-2010. Since 2012, she is Assistant Professor at the Department of Food Toxicology, Research Center of Food Technology and Agricultural Products, Standard Research Institute (SRI), Karaj, Iran. Besides developing standards and standardization based on scientific and technical research to providing assurance of food quality and safety, Dr. Attar has collaborated with other scientists to publish more than 30 papers in the fields of nanozymes, nanoparticles-proteins interaction, and cancer.

Dr. Zehra Edis has obtained BSc., MSc. and PhD. from the University of Cologne, Germany. She conducted her PhD. under the Scholarship of the University of Cologne. She worked as scientific employee at Akzo Nobel Chemicals and research Intern in Bayer AG, Leverkusen, Germany. She joined Ajman University and Sharjah University, UAE as lecturer. Since February 2014 she is Assistant Professor in Ajman University, UAE. She has published more than 31 papers and received from the Deanship of Graduate Studies and Research (DGSR), Ajman University, 6 research grants as Principal Investigator.

Dr. Qian Bai has obtained her MD and PhD degrees from Zhengzhou University, China. She is currently working as deputy director of research and consultant at department of pain management in the second affiliated hospital of Zhengzhou University, She has received four funding grants from the Henan Medical Science and Technology Research Youth Project Co-Sponsored by the Province and Ministry in China, Top Talent Fund of the Second Affiliated Hospital of Zhengzhou University, Henan Middle-aged Youth Health Technology Innovation Talent Project, and National Natural Science Foundation of China Youth Project. She has published more than 15 papers in high impact factor SCI journals including “Brain”, “Molecular Neurobiology” and “Pain”.

Dr. Hossein Derakhshankhah is an Assistant Professor at the Pharmaceutical Biomaterials Department /Pharmaceutical Science Reserach Center (PSRC) of the Kermanshah University of Medical Sciences (KUMS). He obtained his Ph.D. in 2017 from Tehran University of Medical Sciences (TUMS), under supervision of Professor MortezaMahmoudi, on earning deep understanding of “hidden” factors in neurodegenerative disease with focus on Alzheimer disease. His current research area is in development/design of Nano/Bio materials for treatment of Neurodegenerative Diseases (Alzheimer, Parkinson, etc.) in collaboration with Prof. Mahmoudi’s laboratory. Also, Dr. Derakhshankhah is currently Chief Executive Officer (CEO) of Zistmavad Pharmed knowledge-based company.

Dr. Mojtaba Falahati completed his Bachelor Curriculum in the field of Biology at Ferdowsi University in 2004 (Mashhad, Iran). Due to his high interests in Biophysical mechanisms related to diseases and pathological disorders, he performed his master thesis in Biophysics in the area of nerve membrane with focusing on medicinal polymers for the treatment of spinal cord injury at the Institute of Biochemistry and Biophysics (IBB) between 2005-2007 (Tehran, Iran). Dr. Mojtaba Falahati received his PhD in Biophysics from IBB (Tehran, Iran) in 2011. During his PhD thesis he had a visit from Bremen, Jacob, Göttingen, and Tübingen Universities in Germany. His main research area during the PhD was the immobilization of enzyme into the nanoporous materials and uncovering the factors influencing the activity and stability of enzymes after interaction with nanoparticles. Since 2012, Dr. Falahati is an assistant professor at the Department of Nanotechnology, Faculty of Advanced Science and Technology, Tehran Medical Sciences, Azad University, Tehran, Iran. Dr. Falahati has recently received several national and international funding for the investigating the interaction of nanomaterials with proteins and cells, development of nanozymes, nanomaterials-mediated drug delivery for cancer therapy, and tissue engineering.
Footnotes
Peer review under responsibility of Cairo University.
Contributor Information
Qian Bai, Email: baiqian@zzu.edu.cn.
Mojtaba Falahati, Email: Mojtaba.falahati@alumni.ut.ac.ir.
References
- 1.Dhakshinamoorthy A., Opanasenko M., Čejka J., Garcia H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal Sci Technol. 2013;3:2509–2540. [Google Scholar]
- 2.Aljammal N., Jabbour C., Thybaut J.W., Demeestere K., Verpoort F., Heynderickx P.M. Metal-organic frameworks as catalysts for sugar conversion into platform chemicals: State-of-the-art and prospects. Coord Chem Rev. 2019;401 [Google Scholar]
- 3.Simonetti M., Cannas D.M., Just-Baringo X., Vitorica-Yrezabal I.J., Larrosa I. Cyclometallated ruthenium catalyst enables late-stage directed arylation of pharmaceuticals. Nat Chem. 2018;10:724. doi: 10.1038/s41557-018-0062-3. [DOI] [PubMed] [Google Scholar]
- 4.Li B., Wendlandt A.E., Stahl S.S. Replacement of Stoichiometric DDQ with a Low Potential o-Quinone Catalyst Enabling Aerobic Dehydrogenation of Tertiary Indolines in Pharmaceutical Intermediates. Org Lett. 2019;21:1176–1181. doi: 10.1021/acs.orglett.9b00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armor J.N. A history of industrial catalysis. Catal Today. 2011;163:3–9. [Google Scholar]
- 6.Wang N., Sun Q., Yu J. Ultrasmall Metal Nanoparticles Confined within Crystalline Nanoporous Materials: A Fascinating Class of Nanocatalysts. Adv Mater. 2019;31:1803966. doi: 10.1002/adma.201803966. [DOI] [PubMed] [Google Scholar]
- 7.Bodaghifard M.A. Bis sulfamic acid functionalized magnetic nanoparticles as a retrievable nanocatalyst for the green synthesis of polyhydroquinolines and tetrahydrobenzopyrans. J Nanostruct. 2019;9:29–40. [Google Scholar]
- 8.Alves H., Costa A., Correa M., Bohn F., Della Pace R., Acchar W. Structural, magnetic and electric properties of ZrO2 tapes decorated with magnetic nanoparticles. Ceram Int. 2019;45:14500–14504. [Google Scholar]
- 9.Lertcumfu N, Sayed FN, Shirodkar SN, Radhakrishnana S, Mishra A, Rujijanagul G, et al. Structure‐Dependent Electrical and Magnetic Properties of Iron Oxide Composites. Physica Status solidi (a); 2019, 1801004.
- 10.Zhang J. Lattice mismatch induced curved configurations of hybrid boron nitride–carbon nanotubes. Physica E. 2016;84:372–377. [Google Scholar]
- 11.Lopes P.P., Strmcnik D., Tripkovic D., Connell J.G., Stamenkovic V., Markovic N.M. Relationships between atomic level surface structure and stability/activity of platinum surface atoms in aqueous environments. ACS Catal. 2016;6:2536–2544. [Google Scholar]
- 12.Liu P., Guan P., Hirata A., Zhang L., Chen L., Wen Y. Visualizing under-coordinated surface atoms on 3D nanoporous gold catalysts. Adv Mater. 2016;28:1753–1759. doi: 10.1002/adma.201504032. [DOI] [PubMed] [Google Scholar]
- 13.Yang F., Deng D., Pan X., Fu Q., Bao X. Understanding nano effects in catalysis. Natl Sci Rev. 2015;2:183–201. [Google Scholar]
- 14.Tanatar B, Moldoveanu V, Dragomir R, Stanciu S. Interaction and Size Effects in Open Nano‐Electromechanical Systems. Phys Status Solidi (b); 2019, 1800443.
- 15.Dalpozzo R. Magnetic nanoparticle supports for asymmetric catalysts. Green Chem. 2015;17:3671–3686. [Google Scholar]
- 16.Chen M.-N., Mo L.-P., Cui Z.-S., Zhang Z.-H. Magnetic nanocatalysts: synthesis and application in multicomponent reactions. Curr Opin Green Sustainable Chem. 2019;15:27–37. [Google Scholar]
- 17.Yen H., Seo Y., Kaliaguine S., Kleitz F. Role of metal–support interactions, particle size, and metal–metal synergy in CuNi nanocatalysts for H2 generation. ACS Catal. 2015;5:5505–5511. [Google Scholar]
- 18.Lee J., Yoo J.M., Ye Y., Mun Y., Lee S., Kim O.H. Development of Highly Stable and Mass Transfer-Enhanced Cathode Catalysts: Support-Free Electrospun Intermetallic FePt Nanotubes for Polymer Electrolyte Membrane Fuel Cells. Adv Energy Mater. 2015;5:1402093. [Google Scholar]
- 19.He L., Weniger F., Neumann H., Beller M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: catalysis beyond electrochemistry. Angew Chem Int Ed. 2016;55:12582–12594. doi: 10.1002/anie.201603198. [DOI] [PubMed] [Google Scholar]
- 20.Trovarelli A., Llorca J. Ceria catalysts at nanoscale: how do crystal shapes shape catalysis? ACS Catal. 2017;7:4716–4735. [Google Scholar]
- 21.Gabrys P.A., Zornberg L.Z., Macfarlane R.J. Programmable Atom Equivalents: Atomic Crystallization as a Framework for Synthesizing Nanoparticle Superlattices. Small. 2019;1805424 doi: 10.1002/smll.201805424. [DOI] [PubMed] [Google Scholar]
- 22.Cao S., Tao F.F., Tang Y., Li Y., Yu J. Size-and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem Soc Rev. 2016;45:4747–4765. doi: 10.1039/c6cs00094k. [DOI] [PubMed] [Google Scholar]
- 23.Xu Q., Song Y., Li Y., Liu Z. Nanocatalysis for Organic Chemistry. Curr Org Chem. 2016;20:2013–2021. [Google Scholar]
- 24.Kumar S., Varma R., Zboril R., Gawande M. Support Morphology-dependent Activity of Nanocatalysts, Nanoparticle Design and Characterization for Catalytic Applications in Sustainable. Chemistry. 2019;38:84. [Google Scholar]
- 25.Quiroz J., Barbosa E.C., Araujo T.P., Fiorio J.L., Wang Y.-C., Zou Y.-C. Controlling reaction selectivity over hybrid plasmonic nanocatalysts. Nano Lett. 2018;18:7289–7297. doi: 10.1021/acs.nanolett.8b03499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vallabani N.S., Karakoti A.S., Singh S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: one step detection of blood glucose at physiological pH. Colloids Surf, B. 2017;153:52–60. doi: 10.1016/j.colsurfb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Fan K., Wang H., Xi J., Liu Q., Meng X., Duan D. Optimization of Fe 3 O 4 nanozyme activity via single amino acid modification mimicking an enzyme active site. Chem Commun. 2017;53:424–427. doi: 10.1039/c6cc08542c. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y.-L., Zhang H.-G., Rahman Z.U., Su L., Chen X.-J., Hu J. Graphene oxide–Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale. 2012;4:3969–3976. doi: 10.1039/c2nr12109c. [DOI] [PubMed] [Google Scholar]
- 29.Ma M., Xie J., Zhang Y., Chen Z., Gu N. Fe3O4@ Pt nanoparticles with enhanced peroxidase-like catalytic activity. Mater Lett. 2013;105:36–39. [Google Scholar]
- 30.Wang Z., Chen M., Shu J., Li Y. One-step solvothermal synthesis of Fe3O4@ Cu@ Cu2O nanocomposite as magnetically recyclable mimetic peroxidase. J Alloy Compd. 2016;682:432–440. [Google Scholar]
- 31.Song L., Huang C., Zhang W., Ma M., Chen Z., Gu N. Graphene oxide-based Fe2O3 hybrid enzyme mimetic with enhanced peroxidase and catalase-like activities. Colloids Surf, A. 2016;506:747–755. [Google Scholar]
- 32.Dutta A.K., Maji S.K., Biswas P., Adhikary B. New peroxidase-substrate 3, 5-di-tert-butylcatechol for colorimetric determination of blood glucose in presence of Prussian Blue-modified iron oxide nanoparticles. Sens Actuators, B. 2013;177:676–683. [Google Scholar]
- 33.Kluenker M., Nawaz Tahir M., Ragg R., Korschelt K., Simon P., Gorelik T.E. Pd@ Fe2O3 superparticles with enhanced peroxidase activity by solution phase epitaxial growth. Chem Mater. 2017;29:1134–1146. [Google Scholar]
- 34.Merga G., Saucedo N., Cass L.C., Puthussery J., Meisel D. “Naked” gold nanoparticles: synthesis, characterization, catalytic hydrogen evolution, and SERS. J Phys Chem C. 2010;114:14811–14818. [Google Scholar]
- 35.da Silva A.G., Rodrigues T.S., Macedo A., da Silva R.T., Camargo P.H. An undergraduate level experiment on the synthesis of Au nanoparticles and their size-dependent optical and catalytic properties. Quim Nova. 2014;37:1716–1720. [Google Scholar]
- 36.Tsunoyama H., Ichikuni N., Sakurai H., Tsukuda T. Effect of electronic structures of Au clusters stabilized by poly (N-vinyl-2-pyrrolidone) on aerobic oxidation catalysis. J Am Chem Soc. 2009;131:7086–7093. doi: 10.1021/ja810045y. [DOI] [PubMed] [Google Scholar]
- 37.Shin C., Park T.E., Park C., Kwon S.J. Observation of single Pt nanoparticle collisions: enhanced electrocatalytic activity on a Pd ultramicroelectrode. ChemPhysChem. 2016;17:1637–1641. doi: 10.1002/cphc.201600032. [DOI] [PubMed] [Google Scholar]
- 38.Matos J., Ono L.K., Behafarid F., Croy J.R., Mostafa S., DeLaRiva A.T. In situ coarsening study of inverse micelle-prepared Pt nanoparticles supported on γ-Al2O3: pretreatment and environmental effects. PCCP. 2012;14:11457–11467. doi: 10.1039/c2cp41339f. [DOI] [PubMed] [Google Scholar]
- 39.Lim B., Lu X., Jiang M., Camargo P.H.C., Cho E.C., Lee E.P. Facile Synthesis of Highly Faceted Multioctahedral Pt Nanocrystals through Controlled Overgrowth. Nano Lett. 2008;8:4043–4047. doi: 10.1021/nl802959b. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y., Zhu Q.-L., Tsumori N., Xu Q. Immobilizing Highly Catalytically Active Noble Metal Nanoparticles on Reduced Graphene Oxide: A Non-Noble Metal Sacrificial Approach. J Am Chem Soc. 2015;137:106–109. doi: 10.1021/ja511511q. [DOI] [PubMed] [Google Scholar]
- 41.Dai H., Su J., Hu K., Luo W., Cheng G. Pd nanoparticles supported on MIL-101 as high-performance catalysts for catalytic hydrolysis of ammonia borane. Int J Hydrogen Energy. 2014;39:4947–4953. [Google Scholar]
- 42.Zhu Q.-L., Tsumori N., Xu Q. Immobilizing Extremely Catalytically Active Palladium Nanoparticles to Carbon Nanospheres: A Weakly-Capping Growth Approach. J Am Chem Soc. 2015;137:11743–11748. doi: 10.1021/jacs.5b06707. [DOI] [PubMed] [Google Scholar]
- 43.Moock D., Wiesenfeldt M.P., Freitag M., Muratsugu S., Ikemoto S., Knitsch R. Mechanistic Understanding of the Heterogeneous, Rhodium-Cyclic (Alkyl)(Amino)Carbene-Catalyzed (Fluoro-)Arene Hydrogenation. ACS Catal. 2020;10:6309–6317. doi: 10.1021/acscatal.0c01074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim M., Wei M.M., Deydier E., Manoury E., Poli R., Lecante P. Rhodium nanoparticles stabilized by ferrocenyl-phosphine ligands: synthesis and catalytic styrene hydrogenation. Dalton Trans. 2019;48:6777–6786. doi: 10.1039/c9dt01006h. [DOI] [PubMed] [Google Scholar]
- 45.Touge T., Kuwana M., Komatsuki Y., Tanaka S., Nara H., Matsumura K. Development of Asymmetric Transfer Hydrogenation with a Bifunctional Oxo-Tethered Ruthenium Catalyst in Flow for the Synthesis of a Ceramide (d-erythro-CER[NDS]) Org Process Res Dev. 2019;23:452–461. [Google Scholar]
- 46.Xie L., Jin W., Chen H., Zhang Q. Superparamagnetic iron oxide nanoparticles for cancer diagnosis and therapy. J Biomed Nanotechnol. 2019;15:215–416. doi: 10.1166/jbn.2019.2678. [DOI] [PubMed] [Google Scholar]
- 47.Chen L., Zhong H., Qi X., Shao H., Xu K. Modified core–shell magnetic mesoporous zirconia nanoparticles formed through a facile “outside-to-inside” way for CT/MRI dual-modal imaging and magnetic targeting cancer chemotherapy. RSC Adv. 2019;9:13220–13233. doi: 10.1039/c9ra01063g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roohi R., Emdad H., Jafarpur K. A comprehensive study and optimization of magnetic nanoparticle drug delivery to cancerous tissues via external magnetic field. J Test Eval. 2019;47:681–703. [Google Scholar]
- 49.Zanoni M., Pignatta S., Arienti C., Bonafè M., Tesei A. Anticancer drug discovery using multicellular tumor spheroid models. Expert Opin Drug Discov. 2019;14:289–301. doi: 10.1080/17460441.2019.1570129. [DOI] [PubMed] [Google Scholar]
- 50.Raouf I., Khalid S., Khan A., Lee J., Kim H.S., Kim M.-H. A review on numerical modeling for magnetic nanoparticle hyperthermia: Progress and challenges. J Therm Biol. 2020;102644 doi: 10.1016/j.jtherbio.2020.102644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xie J., Liu G., Eden H.S., Ai H., Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc Chem Res. 2011;44:883–892. doi: 10.1021/ar200044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raita M., Arnthong J., Champreda V., Laosiripojana N. Modification of magnetic nanoparticle lipase designs for biodiesel production from palm oil. Fuel Process Technol. 2015;134:189–197. [Google Scholar]
- 53.Ghasemi H., Aghabarari B., Alizadeh M., Khanlarkhani A., Abu-Zahra N. High efficiency decolorization of wastewater by Fenton catalyst: Magnetic iron-copper hybrid oxides. J Water Process Eng. 2020;37 [Google Scholar]
- 54.Shylesh S., Schünemann V., Thiel W.R. Magnetically separable nanocatalysts: bridges between homogeneous and heterogeneous catalysis. Angew Chem Int Ed. 2010;49:3428–3459. doi: 10.1002/anie.200905684. [DOI] [PubMed] [Google Scholar]
- 55.Garrido I., Pastor-Belda M., Campillo N., Viñas P., Yañez M.J., Vela N. Photooxidation of insecticide residues by ZnO and TiO2 coated magnetic nanoparticles under natural sunlight. J Photochem Photobiol, A. 2019;372:245–253. [Google Scholar]
- 56.Shi F., Tse M.K., Pohl M.M., Brückner A., Zhang S., Beller M. Tuning catalytic activity between homogeneous and heterogeneous catalysis: improved activity and selectivity of free nano-Fe2O3 in selective oxidations. Angew Chem Int Ed. 2007;46:8866–8868. doi: 10.1002/anie.200703418. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Q., Yang X., Guan J. Applications of Magnetic Nanomaterials in Heterogeneous Catalysis. ACS Appl Nano Mater. 2019;2:4681–4697. [Google Scholar]
- 58.Voinov M.A., Pagán J.O.S., Morrison E., Smirnova T.I., Smirnov A.I. Surface-mediated production of hydroxyl radicals as a mechanism of iron oxide nanoparticle biotoxicity. J Am Chem Soc. 2011;133:35–41. doi: 10.1021/ja104683w. [DOI] [PubMed] [Google Scholar]
- 59.Xie L., Jin W., Zuo X., Ji S., Nan W., Chen H. Construction of small-sized superparamagnetic Janus nanoparticles and their application in cancer combined chemotherapy and magnetic hyperthermia. Biomater Sci. 2020;8:1431–1441. doi: 10.1039/c9bm01880h. [DOI] [PubMed] [Google Scholar]
- 60.Cai Z., Wu C., Yang L., Wang D., Ai H. Assembly-Controlled Magnetic Nanoparticle Clusters as MRI Contrast Agents. ACS Biomater Sci Eng. 2020;6:2533–2542. doi: 10.1021/acsbiomaterials.9b01198. [DOI] [PubMed] [Google Scholar]
- 61.Du M., Chen Y., Tu J., Liufu C., Yu J., Yuan Z. Ultrasound Responsive Magnetic Mesoporous Silica Nanoparticle-Loaded Microbubbles for Efficient Gene Delivery. ACS Biomater Sci Eng. 2020;6:2904–2912. doi: 10.1021/acsbiomaterials.0c00014. [DOI] [PubMed] [Google Scholar]
- 62.Sharifi M., Jafari S., Hasan A., Paray B.A., Gong G., Zheng Y. Antimetastatic Activity of Lactoferrin-Coated Mesoporous Maghemite Nanoparticles in Breast Cancer Enabled by Combination Therapy. ACS Biomater Sci Eng. 2020;6:3574–3584. doi: 10.1021/acsbiomaterials.0c00086. [DOI] [PubMed] [Google Scholar]
- 63.Sharifi M., Hasan A., Nanakali N.M.Q., Salihi A., Qadir F.A., Muhammad H.A. Combined chemo-magnetic field-photothermal breast cancer therapy based on porous magnetite nanospheres. Sci Rep. 2020;10:5925. doi: 10.1038/s41598-020-62429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farahani S., Alam N.R., Haghgoo S., Shirazi A., Geraily G., Gorji E. The effect of bismuth nanoparticles in kilovoltage and megavoltage radiation therapy using magnetic resonance imaging polymer gel dosimetry. Radiat Phys Chem. 2020;170 [Google Scholar]
- 65.Zhang P., Wu G., Zhao C., Zhou L., Wang X., Wei S. Magnetic stomatocyte-like nanomotor as photosensitizer carrier for photodynamic therapy based cancer treatment. Colloids Surf, B. 2020;194 doi: 10.1016/j.colsurfb.2020.111204. [DOI] [PubMed] [Google Scholar]
- 66.Cai D., Liu L., Han C., Ma X., Qian J., Zhou J. Cancer cell membrane-coated mesoporous silica loaded with superparamagnetic ferroferric oxide and Paclitaxel for the combination of Chemo/Magnetocaloric therapy on MDA-MB-231 cells. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-51029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dabaghi M., Quaas R., Hilger I. The Treatment of Heterotopic Human Colon Xenograft Tumors in Mice with 5-Fluorouracil Attached to Magnetic Nanoparticles in Combination with Magnetic Hyperthermia Is More Efficient than Either Therapy Alone. Cancers. 2020;12:2562. doi: 10.3390/cancers12092562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharifi M., Rezayat S.M., Akhtari K., Hasan A., Falahati M. Fabrication and evaluation of anti-cancer efficacy of lactoferrin-coated maghemite and magnetite nanoparticles. J Biomol Struct Dyn. 2020;38:2945–2954. doi: 10.1080/07391102.2019.1650114. [DOI] [PubMed] [Google Scholar]
- 69.Rajendrakumar S.K., Venu A., Revuri V., George Thomas R., Thirunavukkarasu G.K., Zhang J. Hyaluronan-Stabilized Redox-Sensitive Nanoassembly for Chemo-Gene Therapy and Dual T1/T2 MR Imaging in Drug-Resistant Breast Cancer Cells. Mol Pharm. 2019;16:2226–2234. doi: 10.1021/acs.molpharmaceut.9b00189. [DOI] [PubMed] [Google Scholar]
- 70.Tadic M., Trpkov D., Kopanja L., Vojnovic S., Panjan M. Hydrothermal synthesis of hematite (α-Fe2O3) nanoparticle forms: Synthesis conditions, structure, particle shape analysis, cytotoxicity and magnetic properties. J Alloy Compd. 2019;792:599–609. [Google Scholar]
- 71.Sharifi M., Hosseinali S.H., Saboury A.A., Szegezdi E., Falahati M. Involvement of planned cell death of necroptosis in cancer treatment by nanomaterials: Recent advances and future perspectives. J Control Release. 2019;299:121–137. doi: 10.1016/j.jconrel.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 72.Sharifi M., Hosseinali S.H., Yousefvand P., Salihi A., Shekha M.S., Aziz F.M. Gold nanozyme: Biosensing and therapeutic activities. Mater Sci Eng, C. 2020;108 doi: 10.1016/j.msec.2019.110422. [DOI] [PubMed] [Google Scholar]
- 73.Duval K.E., Vernice N.A., Wagner R.J., Fiering S.N., Petryk J.D., Lowry G.J. Immunogenetic effects of low dose (CEM43 30) magnetic nanoparticle hyperthermia and radiation in melanoma cells. Int J Hyperth. 2019;36:37–46. doi: 10.1080/02656736.2019.1627433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang C., Yan L., Gu Z., Zhao Y. Strategies based on metal-based nanoparticles for hypoxic-tumor radiotherapy. Chem Sci. 2019;10:6932–6943. doi: 10.1039/c9sc02107h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y., Ran Y., Wang Z., Cheng J., Cao Y., Yang C. Magnetic-responsive and targeted cancer nanotheranostics by PA/MR bimodal imaging-guided photothermally triggered immunotherapy. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119370. [DOI] [PubMed] [Google Scholar]
- 76.Zanganeh S., Hutter G., Spitler R., Lenkov O., Mahmoudi M., Shaw A. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song G., Kenney M., Chen Y.-S., Zheng X., Deng Y., Chen Z. Carbon-coated FeCo nanoparticles as sensitive magnetic-particle-imaging tracers with photothermal and magnetothermal properties. Nat Biomed Eng. 2020;4:325–334. doi: 10.1038/s41551-019-0506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu J., Xiao T., Zhang J., Che H., Shi Y., Shi X. Surface-Charge-Switchable Nanoclusters for Magnetic Resonance Imaging-Guided and Glutathione Depletion-Enhanced Photodynamic Therapy. ACS Nano. 2020;14:11225–11237. doi: 10.1021/acsnano.0c03080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nie X., Xia L., Wang H.-L., Chen G., Wu B., Zeng T.-Y. Photothermal therapy nanomaterials boosting transformation of Fe (III) into Fe (II) in tumor cells for highly improving chemodynamic therapy. ACS Appl Mater Interfaces. 2019;11:31735–31742. doi: 10.1021/acsami.9b11291. [DOI] [PubMed] [Google Scholar]
- 80.Zhao Z., Wang W., Li C., Zhang Y., Yu T., Wu R. Reactive oxygen species–activatable liposomes regulating hypoxic tumor microenvironment for synergistic photo/chemodynamic therapies. Adv Funct Mater. 2019;29:1905013. [Google Scholar]
- 81.Wang MR, Deng L, Liu GC, Wen L, Wang JG, Huang KB, et al. Porous Organic Polymer-Derived Nanopalladium Catalysts for Chemoselective Synthesis of Antitumor Benzofuro [2, 3-b] pyrazine from 2-Bromophenol and Isonitriles. Organ Lett; 2019. [DOI] [PubMed]
- 82.Aremu O.S., Singh P., Singh M., Mocktar C., Koorbanally N.A. Synthesis of chloro, fluoro, and nitro derivatives of 7-amino-5-aryl-6-cyano-5H-pyrano pyrimidin-2, 4-diones using organic catalysts and their antimicrobial and anticancer activities. J Heterocycl Chem. 2019 [Google Scholar]
- 83.Zhang X., Li G., Wu D., Li X., Hu N., Chen J. Recent progress in the design fabrication of metal-organic frameworks-based nanozymes and their applications to sensing and cancer therapy. Biosens Bioelectron. 2019 doi: 10.1016/j.bios.2019.04.061. [DOI] [PubMed] [Google Scholar]
- 84.Maleki A., Paydar R. Graphene oxide–chitosan bionanocomposite: a highly efficient nanocatalyst for the one-pot three-component synthesis of trisubstituted imidazoles under solvent-free conditions. RSC Adv. 2015;5:33177–33184. [Google Scholar]
- 85.Hajipour A.R., Khorsandi Z., Mortazavi M., Farrokhpour H. Green, efficient and large-scale synthesis of benzimidazoles, benzoxazoles and benzothiazoles derivatives using ligand-free cobalt-nanoparticles: as potential anti-estrogen breast cancer agents, and study of their interactions with estrogen receptor by molecular docking. RSC Adv. 2015;5:107822–107828. [Google Scholar]
- 86.Bardajee G.R., Mohammadi M., Kakavand N. Copper (II)–diaminosarcophagine-functionalized SBA-15: a heterogeneous nanocatalyst for the synthesis of benzimidazole, benzoxazole and benzothiazole derivatives under solvent-free conditions. Appl Organomet Chem. 2016;30:51–58. [Google Scholar]
- 87.Kaur G., Devi P., Thakur S., Kumar A., Chandel R., Banerjee B. Magnetically Separable Transition Metal Ferrites: Versatile Heterogeneous Nano-Catalysts for the Synthesis of Diverse Bioactive Heterocycles. ChemistrySelect. 2019;4:2181–2199. [Google Scholar]
- 88.Mayoral E.P., Soriano E., Calvino-Casilda V., Rojas-Cervantes M., Martín-Aranda R. Silica-based nanocatalysts in the CC and C-heteroatom bond forming cascade reactions for the synthesis of biologically active heterocyclic scaffolds. Catal Today. 2017;285:65–88. [Google Scholar]
- 89.Godino-Ojer M., López-Peinado A.J., Maldonado-Hódar F.J., Bailón-García E., Pérez-Mayoral E. Cobalt oxide–carbon nanocatalysts with highly enhanced catalytic performance for the green synthesis of nitrogen heterocycles through the Friedländer condensation. Dalton Trans. 2019;48:5637–5648. doi: 10.1039/c8dt04403a. [DOI] [PubMed] [Google Scholar]
- 90.Hemalatha K., Madhumitha G., Kajbafvala A., Anupama N., Sompalle R., Roopan S.M. Function of nanocatalyst in chemistry of organic compounds revolution: an overview. J Nanomater. 2013;2013:4. [Google Scholar]
- 91.Rai V., Tandon P.K., Khatoon S. Effect of chromium on antioxidant potential of Catharanthus roseus varieties and production of their anticancer alkaloids: vincristine and vinblastine. Biomed Res Int. 2014;2014 doi: 10.1155/2014/934182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azarenko O., Smiyun G., Mah J., Wilson L., Jordan M.A. Antiproliferative mechanism of action of the novel taxane cabazitaxel as compared with the parent compound docetaxel in MCF7 breast cancer cells. Mol Cancer Ther. 2014;13:2092–2103. doi: 10.1158/1535-7163.MCT-14-0265. [DOI] [PubMed] [Google Scholar]
- 93.Planchard D., Kim T.M., Mazieres J., Quoix E., Riely G., Barlesi F. Dabrafenib in patients with BRAFV600E-positive advanced non-small-cell lung cancer: a single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:642–650. doi: 10.1016/S1470-2045(16)00077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Naeimi H., Rashid Z., Zarnani A.-H., Ghahremanzadeh R. Nanocrystalline magnesium oxide: an efficient promoter and heterogeneous nano catalyst for the one-pot synthesis of pyrazolotriazoles in green medium. J Nanopart Res. 2014;16:2416. [Google Scholar]
- 95.Khalafy J., Arlan F.M., Chalanchi S.S. One-pot, Three-component Synthesis of a New Series of 2-Amino-4-aroyl-5-oxo-5, 6-dihydro-2H-pyrano [3, 2-c] quinoline-3-carbonitrile in the Presence of SBA-15 as a Nanocatalyst. J Heterocycl Chem. 2018;55:149–153. [Google Scholar]
- 96.Losada E., Rajabi F., Feiz A., Luque R. An Efficient and Reusable Cobalt Nanocatalyst for the Synthesis of Bis (indolyl) methanes under Solvent-Free Conditions. Curr Org Synth. 2016;13:888–892. [Google Scholar]
- 97.Ganapathy D., Sekar G. Efficient synthesis of polysubstituted olefins using stable palladium nanocatalyst: applications in synthesis of tamoxifen and isocombretastatin A4. Org Lett. 2014;16:3856–3859. doi: 10.1021/ol5017367. [DOI] [PubMed] [Google Scholar]
- 98.Cornelio B., Laronze-Cochard M., Ceruso M., Ferraroni M., Rance G.A., Carta F. 4-Arylbenzenesulfonamides as human carbonic anhydrase Inhibitors (hCAIs): Synthesis by Pd nanocatalyst-mediated Suzuki-Miyaura reaction, enzyme inhibition, and X-ray crystallographic studies. J Med Chem. 2016;59:721–732. doi: 10.1021/acs.jmedchem.5b01771. [DOI] [PubMed] [Google Scholar]
- 99.Das D. Multicomponent Reactions in Organic Synthesis Using Copper-Based Nanocatalysts. ChemistrySelect. 2016;1:1959–1980. [Google Scholar]
- 100.Behravesh S., Fareghi-Alamdari R., Badri R. Sulfonated reduced graphene oxide (RGO-SO3H): As an efficient nanocatalyst for one-pot synthesis of 2-amino-3-cyano-7-hydroxy-4H-chromenes derivatives in water. Polycyclic Aromat Compd. 2018;38:51–65. [Google Scholar]
- 101.Bahadorikhalili S., Ma'mani L., Mahdavi H., Shafiee A. Copper supported β-cyclodextrin functionalized PEGylated mesoporous silica nanoparticle-graphene oxide hybrid: An efficient and recyclable nano-catalyst for straightforward synthesis of 2-arylbenzimidazoles and 1, 2, 3-triazoles. Micropor Mesopor Mater. 2018;262:207–216. [Google Scholar]
- 102.Sharghi H., Aboonajmi J., Mozaffari M., Doroodmand M.M., Aberi M. Application and developing of iron-doped multi-walled carbon nanotubes (Fe/MWCNTs) as an efficient and reusable heterogeneous nanocatalyst in the synthesis of heterocyclic compounds. Appl Organomet Chem. 2018;32 [Google Scholar]
- 103.Martins P., Jesus J., Santos S., Raposo L.R., Roma-Rodrigues C., Baptista P.V. Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules. 2015;20:16852–16891. doi: 10.3390/molecules200916852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Curado N., Giménez N., Miachin K., Aliaga-Lavrijsen M., Cornejo M.A., Jarzecki A.A. Preparation of Titanocene-Gold Compounds Based on Highly Active Gold (I)-N-Heterocyclic Carbene Anticancer Agents: Preliminary in vitro Studies in Renal and Prostate Cancer Cell Lines. ChemMedChem. 2019 doi: 10.1002/cmdc.201800796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fayed E.A., Eissa S.I., Bayoumi A.H., Gohar N.A., Mehany A.B., Ammar Y.A. Design, synthesis, cytotoxicity and molecular modeling studies of some novel fluorinated pyrazole-based heterocycles as anticancer and apoptosis-inducing agents. Mol Diversity. 2019;23:165–181. doi: 10.1007/s11030-018-9865-9. [DOI] [PubMed] [Google Scholar]
- 106.Mousavi S.R., Sereshti H., Rashidi Nodeh H., Foroumadi A. A novel and reusable magnetic nanocatalyst developed based on graphene oxide incorporated strontium nanoparticles for the facial synthesis of β-enamino ketones under solvent-free conditions. Appl Organomet Chem. 2019;33 [Google Scholar]
- 107.Kalantari F., Ramazani A., Heravi M.R. Recent Advances in the Applications of Hybrid Magnetic Nanomaterials as Magnetically Retrievable Nanocatalysts. Curr Org Chem. 2019;23:136–163. [Google Scholar]
- 108.Zhang D., Zhou C., Sun Z., Wu L.-Z., Tung C.-H., Zhang T. Magnetically recyclable nanocatalysts (MRNCs): a versatile integration of high catalytic activity and facile recovery. Nanoscale. 2012;4:6244–6255. doi: 10.1039/c2nr31929b. [DOI] [PubMed] [Google Scholar]
- 109.Zhang N., Xu Y.-J. Aggregation-and leaching-resistant, reusable, and multifunctional Pd@ CeO2 as a robust nanocatalyst achieved by a hollow core–shell strategy. Chem Mater. 2013;25:1979–1988. [Google Scholar]
- 110.Javidi J., Esmaeilpour M. Fe3O4@ SiO2–imid–PMAn magnetic porous nanosphere as recyclable catalyst for the green synthesis of quinoxaline derivatives at room temperature and study of their antifungal activities. Mater Res Bull. 2016;73:409–422. [Google Scholar]
- 111.Abu-Dief A.M., Nassar I.F., Elsayed W.H. Magnetic NiFe2O4 nanoparticles: efficient, heterogeneous and reusable catalyst for synthesis of acetylferrocene chalcones and their anti-tumour activity. Appl Organomet Chem. 2016;30:917–923. [Google Scholar]
- 112.Nasr-Esfahani M., Rafiee Z., Montazerozohori M., Kashi H. A highly efficient magnetic solid acid nanocatalyst for the synthesis of new bulky heterocyclic compounds. RSC Adv. 2016;6:47298–47313. [Google Scholar]
- 113.Taghavi F., Gholizadeh M., Saljooghi A.S., Ramezani M. Cu (ii) immobilized on Fe 3 O 4@ APTMS-DFX nanoparticles: an efficient catalyst for the synthesis of 5-substituted 1 H-tetrazoles with cytotoxic activity. MedChemComm. 2017;8:1953–1964. doi: 10.1039/c7md00302a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kazemi M., Ghobadi M., Mirzaie A. Cobalt ferrite nanoparticles (CoFe2O4 MNPs) as catalyst and support: magnetically recoverable nanocatalysts in organic synthesis. Nanotechnol Rev. 2018;7:43–68. [Google Scholar]
- 115.Ghavidel H., Mirza B., Soleimani-Amiri S., Novel A. Efficient, and Recoverable Basic Fe3O4@ C Nano-Catalyst for Green Synthesis of 4 H-Chromenes in Water via One-Pot Three Component Reactions. Polycycl Aromat Compd. 2019:1–22. [Google Scholar]
- 116.Fenton H. LXXIII.—Oxidation of tartaric acid in presence of iron. J Chem Soc Trans. 1894;65:899–910. [Google Scholar]
- 117.Yusuf A.A., Fazal-ur-Rehman M. Photo-oxidative degradation of methyl orange in aqueous medium by Photo-Fenton reaction. Sci Technol. 2018;4:90–96. [Google Scholar]
- 118.Ma S., Dielschneider R.F., Henson E.S., Xiao W., Choquette T.R., Blankstein A.R. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0182921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Southworth B.A., Voelker B.M. Hydroxyl radical production via the photo-Fenton reaction in the presence of fulvic acid. Environ Sci Technol. 2003;37:1130–1136. doi: 10.1021/es020757l. [DOI] [PubMed] [Google Scholar]
- 120.Shen Z., Song J., Yung B.C., Zhou Z., Wu A., Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin H., Chen Y., Shi J. Nanoparticle-triggered in situ catalytic chemical reactions for tumour-specific therapy. Chem Soc Rev. 2018;47:1938–1958. doi: 10.1039/c7cs00471k. [DOI] [PubMed] [Google Scholar]
- 122.Dai C., Wang C., Hu R., Lin H., Liu Z., Yu L. Photonic/magnetic hyperthermia-synergistic nanocatalytic cancer therapy enabled by zero-valence iron nanocatalysts. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.119374. [DOI] [PubMed] [Google Scholar]
- 123.Zhang C., Bu W., Ni D., Zhang S., Li Q., Yao Z. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew Chem Int Ed. 2016;55:2101–2106. doi: 10.1002/anie.201510031. [DOI] [PubMed] [Google Scholar]
- 124.Huo M., Wang L., Wang Y., Chen Y., Shi J. Nanocatalytic Tumor Therapy by Single-Atom Catalysts. ACS Nano. 2019;13:2643–2653. doi: 10.1021/acsnano.9b00457. [DOI] [PubMed] [Google Scholar]
- 125.Chiarugi P., Cirri P. Redox regulation of protein tyrosine phosphatases during receptor tyrosine kinase signal transduction. Trends Biochem Sci. 2003;28:509–514. doi: 10.1016/S0968-0004(03)00174-9. [DOI] [PubMed] [Google Scholar]
- 126.Huo M., Wang L., Chen Y., Shi J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Feng L., Xie R., Wang C., Gai S., He F., Yang D. Magnetic targeting, tumor microenvironment-responsive intelligent nanocatalysts for enhanced tumor ablation. ACS Nano. 2018;12:11000–11012. doi: 10.1021/acsnano.8b05042. [DOI] [PubMed] [Google Scholar]
- 128.Cao Z., Zhang L., Liang K., Cheong S., Boyer C., Gooding J.J. Biodegradable 2D Fe–Al Hydroxide for Nanocatalytic Tumor-Dynamic Therapy with Tumor Specificity. AdV. Sci. 2018;5:1801155. doi: 10.1002/advs.201801155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qian X., Zhang J., Gu Z., Chen Y. Nanocatalysts-augmented Fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials. 2019 doi: 10.1016/j.biomaterials.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 130.Diz P., Pernas P., El Maatougui A., Tubio C.R., Azuaje J., Sotelo E. Sol–gel entrapped Cu in a silica matrix: An efficient heterogeneous nanocatalyst for Huisgen and Ullmann intramolecular coupling reactions. Appl Catal A. 2015;502:86–95. [Google Scholar]
- 131.Nadejde C., Neamtu M., Hodoroaba V.-D., Schneider R., Paul A., Ababei G. Green Fenton-like magnetic nanocatalysts: Synthesis, characterization and catalytic application. Appl Catal B. 2015;176:667–677. [Google Scholar]
- 132.Hu R., Fang Y., Huo M., Yao H., Wang C., Chen Y. Ultrasmall Cu2-xS nanodots as photothermal-enhanced Fenton nanocatalysts for synergistic tumor therapy at NIR-II biowindow. Biomaterials. 2019;206:101–114. doi: 10.1016/j.biomaterials.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 133.Wang L., Huo M., Chen Y., Shi J. Iron-engineered mesoporous silica nanocatalyst with biodegradable and catalytic framework for tumor-specific therapy. Biomaterials. 2018;163:1–13. doi: 10.1016/j.biomaterials.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 134.Cao Z., Li B., Sun L., Li L., Xu Z.P., Gu Z. 2D Layered Double Hydroxide Nanoparticles: Recent Progress toward Preclinical/Clinical Nanomedicine. Methods. 2019:1900343. [Google Scholar]