Graphical abstract
Keywords: Recurrent implantation failure, Endometrial receptivity, PIBF1, IL6, p-STAT3, Endometrial decidualization
Highlights
-
•
PIBF1 levels peaked in the mid-secretory phase of endometrium.
-
•
PIBF1 expression decreased in the mid-secretory endometrium of RIF patients.
-
•
PIBF1 regulated HESC proliferation and decidualization via IL6/p-STAT3 signaling.
-
•
The IL6/p-STAT3, Ki-67, prolactin, and IGFBP1 levels were lower in RIF patients.
-
•
Low PIBF1 expression may account for poor endometrial receptivity in RIF patients.
Abstract
Introduction
Recurrent implantation failure (RIF) is a challenging problem of assisted reproductive technology that arises mainly due to inadequate endometrial receptivity and its pathogenesis is still unclear.
Objectives
In this study, we conducted the first investigation of the effect of decreased PIBF1 expression in mid-secretory phase on endometrial receptivity in patients with RIF.
Methods
Microarray assay, reverse transcriptase-quantitative polymerase chain reaction, western blot, and in-vitro experiments were conducted.
Results
The results showed that progesterone-induced blocking factor 1 (PIBF1) expression was highest in the mid-secretory endometrium in control subjects, but was significantly lower in RIF patients. In Ishikawa and human endometrial stromal cells (HESCs), rather than human endometrial epithelial cells, PIBF1 knockdown significantly downregulated cell proliferation and the levels of interleukin 6 (IL6) and phosphorylated signal transducer and activator of transcription-3 (p-STAT3). Besides, in HESCs, the levels of IL6, p-STAT3, prolactin and insulin-like growth factor binding-protein-1 (IGFBP1) decreased after PIBF1 knockdown during in-vitro decidualization. All these cellular changes could be notably restored by PIBF1 or IL6 overexpression. Consistent with our findings with PIBF1, the levels of IL6, p-STAT3, ki-67, prolactin, and IGFBP1 in the mid-secretory endometrium were notably lower in patients with RIF compared with controls.
Conclusion
In summary, in the mid-secretory phase, decreased expression of PIBF1, IL6, and p-STAT3 inhibited HESC proliferation and decidualization, which is of theoretical and clinical importance for future research and clinical-treatment strategies.
Introduction
Recurrent implantation failure (RIF), known as a failure to achieve clinical pregnancy after having undergone more than three embryo transfer cycles with the transfer of a total at least four good-quality embryos (score ≥ 7 or grade of 3BB or better) [1], is a difficult clinical issue in the area of in-vitro fertilization and embryo transfer (IVF-ET) [2]. Approximately 10% of sterile women receiving IVF-ET undergo RIF, which is associated with a substantial emotional and economic burden in these women [2]. To date, effective treatment for RIF has only rarely been achieved [3]. Endometrial receptivity and embryo quality were reported to be the main factors influencing the efficacy of embryo implantation [4], and inadequate endometrial receptivity was the main cause of RIF [5], [6].
Decidualization is vital for the acquisition of endometrial receptivity during the window phase. Proper proliferation of endometrial stromal cells is necessary for decidualization, and attenuating proliferation inhibited decidualization and, hence, led to pregnancy failure [7]. For example, decreased expression of homeobox A10 (HOXA10), a known marker of endometrial receptivity, was related to the inhibition of stromal cell proliferation during decidualization and HOXA10-mutant mice had reduced fertility [8], [9]. Notch 1 has been reported to regulate cell proliferation and promote successful decidualization, and depletion of the Notch1 gene in mice led to fewer offspring [10], [11].
Progesterone-induced blocking factor 1 (PIBF1), first found in progesterone-treated lymphocytes from fertile women [12], has a great effect on cell proliferation. For example, increased PIBF1 expression promoted tumor cell proliferation [13], and PIBF1 depletion caused mitotic arrest [14]. Some researchers have found that the lower PIBF1 expression was associated with abortion, preterm delivery and eclampsia [15], [16], [17]. It was also reported that PIBF1 could induce the decidual transformation with mouse endometrial stromal cells [18]. However, the underlying mechanisms remain unclear. Previously, mid-secretory endometrial specimens were collected from patients who successfully achieved clinical pregnancy after the first embryo transfer and from patients with RIF for microarray analysis [1], and we found that PIBF1 was significantly downregulated in the patients with RIF. These findings raised the possibility that decreased PIBF1 expression was responsible for RIF. In this study, we conducted the first investigation of the effect of decreased PIBF1 expression in mid-secretory phase on endometrial receptivity in patients with RIF.
Material and methods
Study population
Ethical approval was obtained from the Institutional Ethics Committee of Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University (2012–57), and all participants recruited signed informed consent forms.
In this study, patients with a history of no pregnancies after at least 3 embryo transfers (including a total of ≥ 4 good-quality embryos) were recruited in the RIF group. Patients with tubal obstruction or unexplained infertility who achieved a clinical pregnancy after the first embryo transfer were assigned to the control group. All patients in the control group underwent cleavage-stage embryo transfer. Blastocyst was transferred in patients after being diagnosed with RIF. The inclusion and exclusion criteria for all patients were as follows: the patients were 25–35 years old with regular menstrual cycles and normal endocrine profiles. Patients that used any intrauterine device or contraceptive drug within the last 6 months, or with any of the following conditions were excluded from the study: intrauterine pathology, hydrosalpinx, salpingitis, polycystic ovary syndrome, endometriosis, adenomyosis, chromosome abnormalities, or autoimmune disease.
Previously [1], we performed comprehensive mRNA profiling on mid-secretory endometrial samples from three controls and three individuals with RIF. The patient details are displayed in Supplementary Table 1, and their microarray data were analyzed in this study. Between January 2016 and January 2020, endometrium samples were collected from 58 control subjects. The samples were divided into the following 6 phases of the menstrual cycle using the criteria defined by Noyes et al. [19] and the chronological dating described in Obstetrics and Gynecology (9th ed, People's Medical Publishing House): early proliferative (days 5–7 of the cycle, n = 8), mid-proliferative (days 8–10 of the cycle, n = 8), late proliferative (days 11–14, n = 20), early secretory (days 15–19, n = 8), mid-secretory (LH + 7 [20], days 20–23, n = 18), and late secretory (days 24–28, n = 8). Specimens were obtained from all 20 RIF patients on day LH + 7 of one natural cycle after at least 3 failed cycles. All samples were collected using pipe-suction curettage (LILYCLEANER, Shanghai, China). The endometria of 20 patients with RIF and 18 control subjects on day LH + 7 were collected to analyze the expression of PIBF1 and PIBF1 signaling relevant molecules (Table 3). Endometrium samples from 48 control subjects in all 6 phases of the menstrual cycle were collected for PIBF1-expression analysis (Table 4). What else, for isolation of primary endometrial cells, endometrium from RIF patients (mid-secretory phase, n = 11) and woman with history of pregnancy who were acted as controls (late-proliferative phase, n = 15; mid-secretory phase, n = 15) were collected.
Table 3.
Demographic characteristics of control subject and patients with RIF in mid-secretory phase recruited in this study.
| Variable | Control (n = 18) | RIF (n = 20) | P |
|---|---|---|---|
| Age (y) | 30.56 ± 3.45 | 30.50 ± 3.25 | 0.960 |
| BMI (kg/m2) | 22.88 ± 3.52 | 21.63 ± 2.34 | 0.215 |
| Basal FSH level (mIU/mL) | 6.08 ± 1.35 | 6.85 ± 1.87 | 0.164 |
| Basal LH level (mIU/mL) | 4.19 ± 2.29 | 3.56 ± 2.16 | 0.946 |
| Basal E2 level (pg/mL) | 37.99 ± 13.56 | 40.50 ± 18.99 | 0.646 |
| P4 level on the day of ET (ng/mL) * | 13.27 ± 6.72 | 16.61 ± 5.20 | 0.055 |
| P4 level on the day of endometrial biopsy (ng/mL) | 15.40 ± 4.17 | 17.21 ± 3.14 | 0.137 |
| Endometrial thickness on the day of embryo transfer (mm) | 8.67 ± 0.39 | 8.47 ± 0.38 | 0.125 |
| Median embryo-transfer number (range) | 1 (1, 1) | 4 (3, 10) | < 0.001a |
| Number of embryos per transfer (%) | 0.909b | ||
| 1 | 6/18 (33.3%) | 31/97 (32.0%) | |
| 2 | 12/18 (66.7%) | 66/97 (68.0%) | |
| Median score of transferred day-3 embryos (range) | 8 (7, 9) | 8 (7, 10) | 0.564a |
| Score of transferred blastocysts (%) | – | ||
| 3BB | (/, /) | 11/58 (19.0%) | |
| 4AB | (/, /) | 25/58 (41.3%) | |
| 4BB | (/, /) | 22/58 (37.9%) |
The data are presented as the mean ± SD and were analyzed by Student’s t-test, unless otherwise stated. *P4 levels on the day of ET > 40 ng/ml in the control (5 for the fresh embryo-transfer cycle and 2 for the frozen embryo-transfer cycle) and RIF (5 for the fresh embryo-transfer cycle and 8 for the frozen embryo-transfer cycle) groups were excluded. BMI, body–mass index; LH, luteinizing hormone; FSH, follicle-stimulating hormone; E2, estradiol; P4, progesterone. a, Mann–Whitney test; b, Fisher’s exact test
Table 4.
Demographic characteristics of the recruited control subjects during the indicated phases of the menstrual cycle.
| Variable | EP (n = 8) | MP (n = 8) | LP (n = 8) | ES (n = 8) | MS (n = 8) * | LS (n = 8) | P |
|---|---|---|---|---|---|---|---|
| Age (y) | 29.00 ± 2.73 | 27.25 ± 3.11 | 28.38 ± 4.27 | 28.75 ± 2.66 | 29.63 ± 4.07 | 26.50 ± 3.96 | 0.508 |
| BMI (kg/m2) | 22.65 ± 1.48 | 21.03 ± 1.44 | 21.58 ± 2.02 | 20.98 ± 1.34 | 22.85 ± 2.26 | 21.17 ± 1.57 | 0.119 |
| Basal FSH level (mIU/mL) | 6.40 ± 1.19 | 6.87 ± 1.31 | 6.02 ± 1.16 | 6.50 ± 1.04 | 5.60 ± 1.40 | 6.52 ± 1.24 | 0.403 |
| Basal LH level (mIU/mL) | 4.17 ± 2.06 | 4.96 ± 1.62 | 3.93 ± 1.48 | 4.69 ± 2.32 | 3.46 ± 1.58 | 4.30 ± 1.65 | 0.630 |
| Basal E2 level (pg/mL) | 37.25 ± 9.82 | 38.25 ± 6.54 | 34.38 ± 8.16 | 35.63 ± 6.99 | 35.63 ± 11.29 | 41.75 ± 4.83 | 0.547 |
| Endometrial thickness on ET day (mm) | 8.39 ± 0.36 | 8.51 ± 0.37 | 8.50 ± 0.46 | 8.54 ± 0.32 | 8.65 ± 0.48 | 8.83 ± 0.18 | 0.286 |
| Number of embryos per transfer, (%) | 0.788a | ||||||
| 1 | 3/8 (37.5%) | 2/8 (25.0%) | 4/8 (50.0%) | 2/8 (25.0%) | 1/8 (12.5%) | 2/8 (25.0%) | |
| 2 | 5/8 (50.0%) | 6/8 (75.0%) | 4/8 (50.0%) | 6/8 (75.0%) | 7/8 (87.5%) | 6/8 (75.0%) | |
| Median score of transferred day-3 embryos (range) | 8 (7, 9) | 8 (7, 9) | 7 (7, 9) | 8 (7, 9) | 8 (7, 8) | 8 (7, 10) | 0.512b |
The data are presented as the mean ± SD and were analyzed by ANOVA, unless otherwise stated. BMI, body–mass index; FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; EP: early proliferative phase; MP: mid-proliferative phase; LP: late proliferative phase; ES: early secretory phase; MS: mid-secretory phase; LS: late secretory phase. * The information for this group overlaps with that in the control group shown in Table 3. a, Fisher’s exact test; b, Kruskal–Wallis test
Microarray assay
The mRNA expression profiles of three control and three individuals with RIF were analyzed applying the Affymetrix GeneChip® PrimeView™ Human Gene Expression Array (Thermo Fisher Scientific, Meridian, USA). Data from the Gene Expression Omnibus (GEO) database (series GSE103465) were analyzed. We also analyzed data from another 2 datasets from the GEO database (GSE58144 and GSE111974), which include data from 96 control subjects and 67 patients with RIF. P-value between two groups was automatically generated with the limma algorithm in the R package, which uses a linear-regression model and Bayes testing for differentially expressed gene (DEG) screening.
Complementary DNA (cDNA) preparation and quantitative real-time polymerase chain reaction (RT-qPCR) analysis
RNA extraction from endometrial biopsy tissues or cultured cells was performed according to published instruction (9767; Takara, Beijing, China). cDNA was synthesized using the reverse-transcription kit (RR036A, Takara, Beijing, China). The cDNA was frozen at –20 °C until use.
qPCR was performed as we described previously [21], and mRNA expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The 2–ΔΔCt method was applied to calculate the relative mRNA expression levels. The primer pairs used in this study are shown in Table 1.
Table 1.
Primers used for RT-qPCR.
| Primer | Sequence (5ʹ-3ʹ) |
|---|---|
| PIBF1-F | TTAGTGTTCGCTGTGCTCAT |
| PIBF1-R | AATGGTCTCTGGATGCTACATAT |
| IL6-F | AGAACAGATTTGAGAGTAGTGAGGAAC |
| IL6-R | GGCATTTGTGGTTGGGTCAGG |
| MKI67-F | GTGGAAGTTCTGCCTACGGA |
| MKI67-R | TAGTGCCCAATTTCTCAGGC |
| PRL-F | AGGAGCAAGCCCAACAGATG |
| PRL-R | TACTTCCGTGACCAGATGATACAG |
| IGFBP1-F | CCCAGAGAGCACGGAGATAAC |
| IGFBP1-R | GGTGACATGGAGAGCCTTCG |
| GAPDH-F | TGACTTCAACAGCGACACCCA |
| GAPDH-R | CACCCTGTTGCTGTAGCCAAA |
Western blot immunoassay
Protein was isolated from samples using RIPA buffer (89900, Thermo, Meridian, USA) with 10% protease inhibitor (5892970001, Roche, Mannheim, Germany). 60 µg of protein was separated by 10% SDS-PAGE and transferred onto 0.22-μm polyvinylidene difluoride membranes at 90 mV for 90 min. After blocking, the membranes were incubated with primary antibodies (Table 2) for 12 h at 4 °C. The membranes were rinsed with Tris-buffered saline and 0.1% Tween-20 for 30 min and incubated with secondary antibodies for 1 h at room temperature. The membranes were washed again before analysis with Immobilon Western Chemiluminescent HRP Substrate (WBKLS0100, Merck, Darmstadt, Germany). After enhanced chemiluminescence analysis of phosphorylated signal transducer and activator of transcription-3 (p-STAT3), the membrane was then stripped with stripping buffer (Solarbio, Beijing, China) and re-probed with an anti-STAT3 antibody.
Table 2.
Antibodies used in this research.
| Antibody | Dilution ratio | Application | Product details |
|---|---|---|---|
| Anti-PIBF1 rabbit polyclonal antibody | 1:1000 | Western blotting | ab183107, Abcam, CA, USA |
| Anti-IL6 mouse monoclonal antibody | 1:1000 | Western blotting | ab9324, Abcam, CA, USA |
| Anti-Phospho-STAT3 (Tyr705) rabbitmonoclonal antibody | 1:1000 | Western blotting | #9145, Cell Signaling, MA, USA |
| Anti-STAT3 mouse monoclonal antibody | 1:1000 | Western blotting | #9139, Cell Signaling, MA, USA |
| Anti-HOXA10 goat polyclonal antibody | 1:1000 | Western blotting | ab191470, Abcam, CA, USA |
| Anti-PRL rabbit monoclonal antibody | 1:1000 | Western blotting | ab188229, Abcam, CA, USA |
| Anti-IGFBP1 rabbit monoclonal antibody | 1:1000 | Western blotting | ab180948, Abcam, CA, USA |
| Anti-GAPDH rabbit monoclonal antibody | 1:1000 | Western blotting | #5174, Cell Signaling, MA, USA |
| HRP-linked anti-rabbit IgG antibody | 1:5000 | Western blotting | #7074, Cell Signaling, MA, USA |
| HRP-linked anti-mouse IgG antibody | 1:5000 | Western blotting | #7076, Cell Signaling, MA, USA |
| HRP-linked anti-goat IgG antibody | 1:1000 | Western blotting | A0266, Beyotime, Shanghai, China |
| Anti-PIBF1 rabbit polyclonal antibody | 1:100 | Immunohistochemical staining | ab183107, Abcam, CA, USA |
| Anti-Ki67 rabbit polyclonal antibody | 1:200 | Immunohistochemical staining | ab16667, Abcam, CA, USA |
| Anti-Vimentin rabbit monoclonal antibody | 1:500 | Immunocytochemical staining | ab92547, Abcam, CA, USA |
| Anti-Cytokeratin 7 rabbit monoclonal antibody | 1:100 | Immunocytochemical staining | ab181598, Abcam, CA, USA |
| Goat polyclonal Secondary Antibody to Rabbit IgG - H&L (Alexa Fluor® 488) | 1:500 | Immunocytochemical staining | ab150077, Abcam, CA, USA |
| Alexa Fluor® 594 GAR | 1:100 | Immunocytochemical staining | R37117, Invitrogen, OR, USA |
Immunohistochemical staining
Tissues were fixed with 4% paraformaldehyde (BL539A, Biosharp, Shanghai, China) and embedded in paraffin. Next, each section (thickness, 5-μm) was deparaffinized, rehydrated, subjected to antigen retrieval, and submerged in 3% hydrogen peroxide (10 min). Then, the slides were flushed with Tris-buffered saline and incubated with 5% normal goat serum (room temperature, 30 min). Thereafter, the slides were incubated with anti-PIBF1 (1:100), anti-Ki-67 (1:200), or normal rabbit IgG antibodies at 4 °C (12 h). Subsequently, they were incubated with secondary antibodies, stained with diaminobenzidine, and counterstained with hematoxylin before visualizing the slides using the Nikon Eclipse 80i microscope.
The intensity of PIBF1 and Ki-67 staining was graded by calculating the immunohistochemical (IHC) score [21]:
IHC score = Σ (% of immunostained cells × intensity of staining).
The staining intensity of PIBF1 and Ki-67 was graded from 0 to 3 (corresponding to negative, weak, moderate, and strong staining, respectively). A 600 × 600 pixel area within each slide used for scoring was randomly chosen under 400 × magnification. Two pathologists performed the scoring independently, and the IHC scores of each section were used for analysis.
Culturing human endometrial stromal cells (HESCs), human endometrial epithelial cells (HEECs), and Ishikawa cells
Primary cells were separated from endometrial samples. First, the tissues were minced and digested for 1 h with 0.1% collagenase (17100017, Gibco, NY, USA) in a 37 °C shaker. Each mixture was then passed through a 100-µm sieve and then a 40-µm sieve. Converge and centrifuged the flushing and the reverse-flushing percolate from the 40-µm sieve to collect primary HESCs and HEECs, respectively. HESCs represent spindle-shaped fibroblast-like morphology while HEECs were polygonal cells and the purity was > 95%. Cultured HESCs and HEECs were identified by immunocytochemistry after staining with stromal cell marker vimentin (Table 2) or epithelial marker cytokeratin (Table 2) as described previously (Fig. S1) [21], [22].
The cells were incubated in medium in the following 2 weeks containing DMEM/F12, 1% penicillin/streptomycin (15140122, Gibco, NY, USA), and 10% fetal bovine serum (10091141, Gibco, NY, USA) at 37 °C (5% CO2).
Lentiviral silencing and plasmid overexpression
Short-hairpin RNA (shRNA) against human PIBF1 and negative shRNA were produced by Hanbio company (Shanghai, China), and PIBF1 and interleukin 6 (IL6) overexpression and mock plasmids were produced by GeneCopoeia (Guangzhou, China). The negative shRNA (5′-TTCTCCGAACGTGTCACGTAA-3′) or PIBF1-shRNA (5′-TCGTGAGGAATTGGCAGCAATGAAA-3′) were transduced into cells (concentration, 2 × 107 transducing units/mL) using polybrene (10 μg/ml). The cells were selected with puromycin (1 μg/ml) and then cultured with the puromycin reagent (0.5 μg/ml). The plasmids bought from GeneCopoeia were transfected into cells with transfection reagent (06366236001, Roche, Mannheim, Germany), following the manufacturer’s instructions.
Proliferation assay
Cell proliferation was assayed in Ishikawa cells and HESCs with the Cell Counting Kit (CCK-8; CK-04, Dojindo Molecular Technologies, Inc., Japan). The cells were plated in 96-well plates (1,000 cells/well), and CCK-8 solution (10 µl/well) was added on the second day after plating. The optical density (OD) was measured using a Multiskan GO instrument at 450 nm.
The CCK-8 has rarely been used to examine the proliferation of HEECs because the cell mass is difficult to manipulate, which readily causes errors. Instead, cell proliferation is widely measured using the well-established marker, MKI67 [23], [24]. Therefore, we analyzed MKI67 expression by qPCR to explore HEEC proliferation. Each experiment was repeated at least three times.
In-vitro decidualization assay
HESCs isolated from late proliferative-phase endometrium samples were isolated for analysis. After 4 days of lentivirus transduction and 3 days of plasmid transfection, the cells were then cultured in serum-free medium with β-estradiol (10 nM, E2758, Sigma, MO, USA), progesterone (1 μM, V900699, Sigma), and 8-Br-cAMP (1 mM, ab141448, Abcam, CA, USA) for 48 h. Total RNA was extracted and two markers of decidualization (prolactin, [PRL] and insulin-like growth factor-binding protein 1 [IGFBP1]) were analyzed with RT-qPCR and western blot. The primers and antibodies used are shown in Table 1 and Table 2, respectively. Each experiment was repeated at least three times.
Enzyme-linked immunosorbent assay (ELISA) analysis
IL6 secretion was measured using ELISA kits (ml058097, Mlbio, Shanghai, China) with 50 μl culture medium, following the manufacturer’s instructions. The standard curves were plotted using recombinant human IL6. The OD was measured using a Multiskan GO instrument at 450 nm. The IL6 levels for each sample were normalized to the amount of the total protein in the cultured cells.
Statistical analysis
For continuous variables, the two-tailed Student’s t-test was applied to calculate differences between two groups. Differences across more than two groups were analyzed by analysis of variance (ANOVA) and the Bonferroni test for data with established normality and homogeneity of variance. Otherwise, a nonparametric test was applied to analyze the data. For analyzing categorical variables, the Fisher's exact test was used. All data processing was managed using SPSS software (version 23.0, IBM, Inc.), and statistical significance was set at P < 0.05. All data related to the figures are displayed in Supplementary Table 2. The western blot images of in-vitro cell experiments in triplicates and the western blot images for endometrial tissues of all patients are provided in Supplementary Fig. S7-S14.
Results
Patient characteristics
Except for the number of embryo transfers, there was no significant difference in the characteristics between the control and RIF patients, or among patients during different phases of the menstrual cycle (Table 3, Table 4).
Microarray results
According to the results of our previous microarray assay [1], 2519 DEGs displaying > 2-fold changes (adjusted P-value < 0.05) were identified in the RIF group. Among these genes, PIBF1 expression was lower in the RIF group (fold change = -2.15, P < 0.05, Fig. 1a). In addition, the gene-expression profiles of GSE58144 and GSE111974 showed that PIBF1 expression was lower in the RIF group (fold change = -1.06 and −1.21 respectively, P < 0.01, Fig. S2). In our microarray assay, Pearson correlation tests were carried out between PIBF1 and all genes detected. The top 300 genes with the highest correlations were screened and passed through the STRING database to construct a protein − protein interaction network. STAT3 was identified as a hub gene with a rather high node degree (Fig. 1b).
Fig. 1.
Microarray results for PIBF1 expression. (a) Microarray results for PIBF1 expression in the three control individuals and three RIF patients. (b) Protein − protein-interaction network of the 300 genes that were most significantly correlated with PIBF1 expression. The red and blue nodes indicate upregulated and downregulated genes in the RIF group. The node size corresponds to the number of edges linked to the node, including the in and out links. The green arrow points to STAT3. *P < 0.05.
Characterization of PIBF1 expression in endometrial tissues and in HESCs after progesterone treatment
In the endometria of control subjects, the mRNA and protein levels of PIBF1 were low during the proliferative phases, increased in the early secretory phase, and peaked in the mid-secretory phase during the menstrual cycle (Fig. 2a–2c). Immunohistochemical staining revealed that PIBF1 was localized in the epithelium and stroma of the endometrial tissues (Fig. 2d). In the stroma, the staining intensity was lower in the RIF group compared to the control group (Fig. 2e, P < 0.01). In the epithelium, although the staining intensity showed a tendency towards decreased levels in the RIF group, no statistical difference was found (Fig. 2e). The mRNA and protein levels of PIBF1 in mid-secretory endometrium (Fig. 2f–2 h, P < 0.01) and HESCs (Fig. 2i-2 k) were notably lower in the RIF group compared those in the control group. HOXA10 protein expression was significantly lower in the RIF group (Fig. 2g–2 h, P < 0.01). In addition, PIBF1 expression in HESCs was induced by progesterone in a dose-dependent manner (Fig. S3a-b), and the levels of PIBF1, IL6, PRL, IGFBP1, p-STAT3 and STAT3 could be induced by progesterone or by the cocktail of estrogen, progesterone and cAMP (Fig. S3c-d).
Fig. 2.
Expression of PIBF1 and HOXA10 in endometrial tissues and HESCs. The mRNA (a) and protein (b and c) expression levels of PIBF1 in endometrial tissues from control subjects in different menstrual phases (n = 8 per group). (d) Representative immunohistochemical-staining images showing PIBF1 expression in endometrium tissue from control and RIF patients, as well as negative-control (no staining) and positive-control (decidua tissue) slides. Negative, week, moderate, and strong staining are indicated with gray, black, green, and red arrows, respectively. Scale bar = 50 μm. (e) Quantification of PIBF1 expression in stromal and epithelial tissue from the control (n = 18) and RIF (n = 20) groups. mRNA-expression level (f) of PIBF1 and protein-expression levels (g and h) of HOXA10 and PIBF1 in the controls (n = 18) and RIF (n = 20) patients. The mRNA (i) and protein (j and k) expression levels of PIBF1 in endometrial stromal cells from control and RIF patients (n = 3 per group). EP, early proliferative phase; MP, mid-secretory phase; LP, late proliferative phase; ES, early secretory phase; MS, mid-secretory phase; LS, late secretory phase. GAPDH was detected as the loading control. Data were expressed as the median (range) except for panel (h, i and k), which were expressed as mean ± SD. *P < 0.05, **P < 0.01.
Changes following knockdown of PIBF1 in Ishikawa cells, HESCs, and HEECs
Fig. 3a–3 h revealed that the mRNA and protein levels of IL6, the protein levels of phosphorylated STAT3, and cell proliferation decreased significantly in Ishikawa cells and HESCs following PIBF1 knockdown. However, in HEECs, the mRNA and protein levels of IL6 (Fig. 3i–3 k) and the mRNA-expression level of MKI67 (Fig. 3l), a marker of proliferation, were not significantly changed after PIBF1 knockdown. Our ELISA results confirmed that, after PIBF1 knockdown, IL6 protein expression was considerably more decreased in HESCs (P < 0.01) than in HEECs (P > 0.05) (Fig. S4). In addition, measuring the in-vitro decidualization activity revealed that the mRNA and protein levels of PRL and IGFBP1 (markers of decidualization) and the protein levels of IL6 and p-STAT3 decreased following PIBF1 knockdown in HESCs (Fig. 3m–3o).
Fig. 3.
Changes in IL6 expression, STAT3 phosphorylation, cell proliferation, and stromal cell decidualization following PIBF1 knockdown. The mRNA-expression levels of PIBF1 and IL6 after PIBF1 knockdown in Ishikawa cells (a), HESCs (e), and HEECs (i). The protein levels of PIBF1, IL6, phosphorylated STAT3 (Y705), and STAT3 after PIBF1 knockdown in Ishikawa cells (b and c) and HESCs (f and g). Protein-expression levels of PIBF1 and IL6 after PIBF1 knockdown in HEECs (j and k). Cell proliferation after PIBF1 knockdown in Ishikawa cells (d) and HESCs (h), as determined by CCK-8 assays, and in HEECs (l), as determined based on MKI67 expression, a marker of proliferation. The mRNA-expression levels (m) of PRL and IGFBP1 (decidualization markers) and the protein levels (n and o) of IL6, PRL, IGFBP1, and phosphorylated STAT3 in stromal cells after PIBF1 knockdown during in vitro decidualization. NC, negative control; PIBF1-KD, PIBF1-knockdown group. GAPDH and STAT3 were detected as loading controls. All data are presented as the mean ± SD. The detail of *P < 0.05, **P < 0.01.
Changes following PIBF1 overexpression in PIBF1 shRNA-transduced Ishikawa cells and in HESCs and HEECs
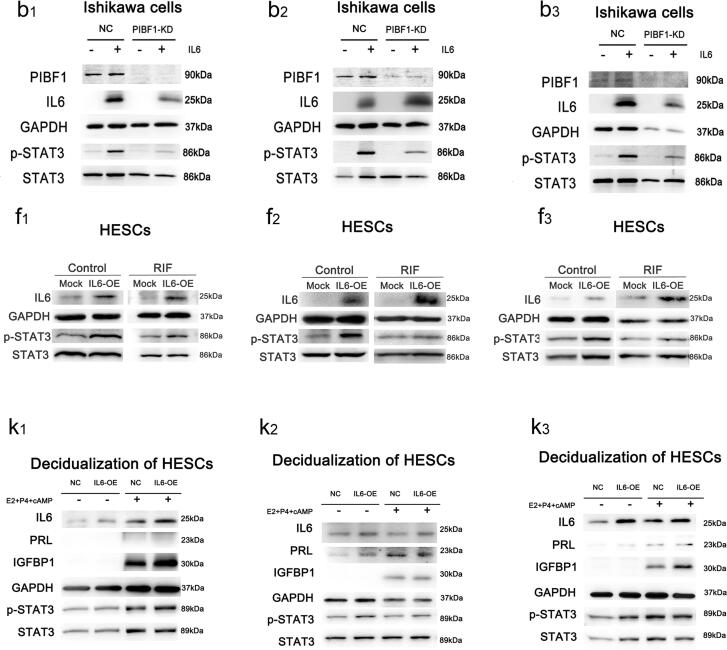
Fig. 4a–4 h shows that in Ishikawa cells transduced with negative-control or PIBF1 shRNA, as well as in HESCs isolated from control subjects and patients with RIF, IL6 and p-STAT3 production and cell proliferation increased notably following PIBF1 overexpression. The elevated levels of p-STAT3 and increased cell proliferation with PIBF-1 overexpression could be suppressed by inhibition of IL-6 (Fig. S5). However, in HEECs, the mRNA and protein levels of IL6 (Fig. 4i–4 k) and mRNA levels of MKI67 (Fig. 4l) did not significantly change after PIBF1 overexpression. Furthermore, the in vitro-decidualization activity assay revealed that the mRNA and protein levels of PRL and IGFBP1, as well as the protein levels of IL6 and p-STAT3, clearly increased in HESCs following PIBF1 overexpression (Fig. 4m–4o).
Fig. 4.
Changes in IL6 expression, STAT3 phosphorylation, cell proliferation, and stromal cell decidualization following PIBF1 overexpression. The mRNA-expression levels of PIBF1 and IL6 after PIBF1 overexpression in Ishikawa cells transduced with negative-control or PIBF1 shRNA (a) and in HESCs (e) and HEECs (i). Protein levels of PIBF1, IL6, phosphorylated STAT3 (Y705), and STAT3 in Ishikawa cells transduced with negative-control or PIBF1 shRNA (b and c) and in HESCs isolated from control or RIF patients (f and g). Protein-expression levels of PIBF1 and IL6 after PIBF1 overexpression in HEECs (j and k). Cell proliferation after PIBF1 overexpression in Ishikawa cells transduced with negative-control or PIBF1 shRNA (d) and in HESCs (h), as determined by the CCK-8 assay, and in HEECs (l), as determined by measuring the mRNA-expression level of MKI67, a marker of proliferation. The mRNA-expression levels (m) of PRL and IGFBP1 (decidualization markers) and the protein levels (n and o) of IL6, PRL, IGFBP1, and phosphorylated STAT3 (Y705) after PIBF1 overexpression during in vitro decidualization. HESCs were isolated from control subjects or patients with RIF as indicated (f and g). NC, negative control; PIBF1-OE, PIBF1-overexpression group. GAPDH and STAT3 were detected as loading controls. All data are presented as the mean ± SD. *P < 0.05, **P < 0.01.
Changes following IL6 overexpression in PIBF1 shRNA-transduced Ishikawa cells and in HESCs and HEECs
Using Ishikawa cells transduced with negative-control and PIBF1 shRNA, as well as HESCs isolated from control subjects and RIF patients, we found that p-STAT3 production and cell proliferation increased notably following IL6 overexpression (Fig. 5a–5 h). In Ishikawa cells and HESCs, dose-dependent induction of STAT3 phosphorylation was demonstrated following IL6 overexpression, but this effect was blocked when treated with an IL6-neutralizing antibody (Fig. S6). In HEECs, however, the mRNA-expression level of MKI67 did not significantly change following IL6 overexpression (Fig. 5i). In addition, assaying the in vitro decidualization activity revealed that the mRNA and protein levels of PRL and IGFBP1, and the protein levels of p-STAT3 increased in HESCs following IL6 overexpression (Fig. 5j–5 l).
Fig. 5.
Changes in IL6 expression, STAT3 phosphorylation, cell proliferation, and stromal cell decidualization following IL6 overexpression. IL6 mRNA expression after IL6 overexpression in Ishikawa cells transduced with negative-control or PIBF1 shRNA (a) and in HESCs (e) and HEECs (i). Protein levels of IL6, phosphorylated STAT3 (Y705), and STAT3 in Ishikawa cells transduced with negative-control or PIBF1 shRNA (b and c) and in HESCs isolated from control subjects or patients with RIF (f and g). Cell proliferation after IL6 overexpression in Ishikawa cells transduced with negative-control or PIBF1 shRNA (d) and in HESCs (h), as determined by the CCK-8 assay, and in HEECs (i), as determined by measuring the mRNA-expression level of MKI67, a marker of cell proliferation. The-mRNA expression levels (j) of PRL and IGFBP1 (decidualization markers) and protein levels (k and l) of PRL, IGFBP1, and phosphorylated STAT3 after IL6 overexpression during in vitro decidualization. HESCs were isolated from control subjects and patients with RIF as indicated (f and g). NC, negative control; PIBF1-KD: PIBF1-knockdown group; IL6-OE: IL6-overexpression group. GAPDH and STAT3 were detected as loading controls. All data are presented as the mean ± SD. *P < 0.05, **P < 0.01.
In vivo expression of IL6, p-STAT3, PRL, IGFBP1, and Ki-67 in endometrial tissues
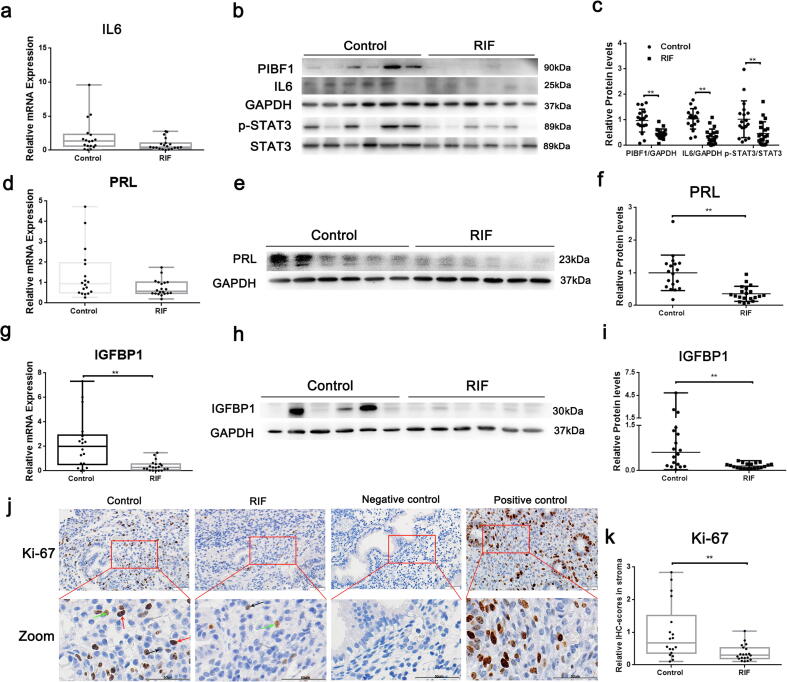
In the three microarray datasets above mentioned, IL6, PRL, and IGFBP1 were downregulated in the RIF group, except for the IL6 in GSE111974 and the PRL in GSE103465, which were slightly upregulated without significance (Fig. S2). Subsequent validation revealed that the mRNA and protein levels of IL6, PRL, and IGFBP1 were remarkably lower in the RIF group (Fig. 6a–6i). What else, the p-STAT3 level was lower in the RIF group (Fig. 6b–6c, P < 0.01) and the staining intensity of Ki-67 was lower in the stroma of the RIF group (Fig. 6j–6 k, P < 0.01).
Fig. 6.
Expression of PIBF1, IL6, p-STAT3 (Y705), PRL, IGFBP1, and Ki-67 in endometrial tissues. The mRNA levels of IL6 (a), PRL (d), and IGFBP1 (g) in the control (n = 18) and RIF groups (n = 20) are shown. The protein levels of PIBF1/IL6/p-STAT3 (b and c), PRL (e and f), and IGFBP1 (h and i) in the controls (n = 18) and RIF patients (n = 20) are shown. (j) Representative images of Ki-67 expression determined via immunohistochemical analysis in the endometrium from control subjects and patients with RIF, as well as negative-control (no staining) and positive-control (human proliferative endometrium) slides. Negative, week, moderate, and strong staining are indicated with gray, black, green, and red arrows, respectively. Scale bar = 50 μm. (k) Quantification of Ki-67 expression in stroma in the controls (n = 18) and RIF patients (n = 20). GAPDH and STAT3 were detected as loading controls. Data were expressed as mean ± SD in panels c and f, and others were expressed as the median (range). *P < 0.05, **P < 0.01.
Discussion
Implantation failure is a problematic clinical issue in reproductive medicine that is mainly related to inadequate endometrial receptivity, and no clear explanatory mechanisms have been purported [5], [6]. In this study, we found that decreased PIBF1/IL6/p-STAT3 expression during the mid-secretory phase inhibited human endometrial stromal cell proliferation and decidualization, which may be one of the causes of implantation failure.
During the menstrual cycle, PIBF1 expression in the endometrium of the controls was relatively low in the proliferative phase, elevated in the early secretory phase, and peaked in the mid-secretory phase, suggesting that high PIBF1 expression during the implantation window helps the acquisition of endometrial receptivity. The microarray analysis results and further validation confirmed that PIBF1 expression decreased significantly in the RIF group during the mid-secretory phase, which was consistent with the expression of HOXA10, an accepted marker of endometrial receptivity [25]. These results implied that decreased expression of PIBF1 during the window phase may be one of the prime causes underlying the inadequate endometrial receptivity.
Previously, it was reported that PIBF1 could bind to the promoter of IL6 to enhance its expression [26] and that IL6 stimulated STAT3 phosphorylation to promote cell proliferation in many types of cancer cells [27], [28], [29]. Furthermore, prior research demonstrated that IL6 could induce the proliferation of stromal cells from patients with endometriosis by activating STAT3 [30]. In this study, our microarray analysis revealed that STAT3 belonged to the hub of PIBF1-related genes, and the IL6 and p-STAT3 levels decreased markedly in the RIF group, which corresponded with the PIBF1-expression changes in individual patients. Following PIBF1 knockdown, the proliferation and levels of IL6 and phosphorylated STAT3 in Ishikawa cells and HESCs decreased significantly, which could be reversed by overexpressing PIBF1 or IL6. What else, the increased cell proliferation and elevated levels of p-STAT3 following PIBF-1 overexpression could be suppressed by inhibition of IL-6. However, the changes in HEECs showed no statistical significance. Ishikawa cells are surrogates for human endometrial cells that are usually used to study the proliferative function of endometrial cells [31], [32], which were derived from endometrial adenocarcinoma with higher proliferative and invasive characteristics than that primary HEECs. In addition, previous research showed that the gene-expression profile differed between Ishikawa cells and HEECs [33], which may account for the different reactions to PIBF1.
Proper proliferation of endometrial stromal cells is necessary for decidualization, and both IL6 and STAT3 phosphorylation play important roles in the decidualization process [34], [35]. We further found that, in HESCs, the levels of IGFBP1 and PRL (decidualization markers) and levels of IL6 and p-STAT3 notably decreased following PIBF1 knockdown, but increased after PIBF1 or IL6 overexpression during the in-vitro decidualization. In vivo expression levels revealed that, consistent with the PIBF1/IL6/p-STAT3 levels, the expression levels of Ki-67, IGFBP1 and PRL decreased notably in the RIF group. These results indicate that in the mid-secretory endometrium, decreased PIBF1 can inhibit the proliferation and decidualization of HESCs by inhibiting the IL6/p-STAT3 pathway. Furthermore, immunohistochemistry revealed that PIBF1 expression was significantly lower in the stroma than in epithelium of the endometrial tissues in RIF patients, which suggested that PIBF1 may act a pivotal part in HESCs in terms of the establishment of endometrial receptivity.
During the menstrual cycle, PIBF1 expression in the endometrium is consistent with the levels of progesterone, and our in-vitro experiment showed that PIBF1 expression increased in dose-dependent manner after progesterone treatment in HESCs. Further study proved that both progesterone and the cocktail of decidualization containing progesterone could induce the expression levels of PIBF1/IL6/p-STAT3 in HESCs, suggesting the effect of progesterone on the induction of this pathway. However, levels of PIBF1/IL6/p-STAT3 decreased significantly while the serum progesterone level on the day of biopsy was not reduced in the RIF group. These results implied that the decreased expression of PIBF1 in RIF patients during mid-secretory phase was affected by other factors besides progesterone. For example, some groups reported that PIBF1 expression was also affected by the IL-33 concentration, as well as the number of B cells and mitochondrial DNA copies [15], [36], [37]. The reason(s) underlying PIBF1 downregulation in patients with RIF should be further investigated.
Conclusion
In summary, we found that PIBF1 expression changed dynamically during the menstrual cycle and peaked in mid-secretory phase. During the same period, in the endometrium of RIF group, the levels of PIBF1, IL6, and p-STAT3 decreased significantly, and the proliferation and decidualization of HESCs were notably inhibited. Our results suggested that lower PIBF1 expression inhibited the proliferation and decidualization of HESCs by decreasing the levels of IL6 and p-STAT3, which could be one of the main causes of poor endometrial receptivity in RIF patients. However, further study on the mechanism of decreased PIBF1 levels in the mid-secretory endometrium and the mechanism by which PIBF1 may regulate endometrial receptivity should be conducted with animal models.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was funded by Shanghai Jiao Tong University Medicine-Engineering Fund (No. YG2017ZD11 and YG2017MS57), by the National Natural Science Foundation of China (No. 81873857, 81771656, 81501249 and 81701513), and by the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (No. 20181803).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2020.09.002.
Contributor Information
Qiang Liu, Email: qliu0122@shsmu.edu.cn.
Bufang Xu, Email: bufangxu@163.com.
Aijun Zhang, Email: zhaj1268@163.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.

Supplementary figure 3.
Supplementary figure 4.
Supplementary figure 5.
Supplementary figure 6.
Supplementary figure 7.
Supplementary figure 8.
Supplementary figure 9.
Supplementary figure 10.
Supplementary figure 11.
Supplementary figure 12.
Supplementary figure 13.
Supplementary figure 14.
References
- 1.Guo F., Si C., Zhou M., Wang J., Zhang D., Leung P.C.K. Decreased PECAM1-mediated TGF-beta1 expression in the mid-secretory endometrium in women with recurrent implantation failure. Hum Reprod. 2018;33:832–843. doi: 10.1093/humrep/dey022. [DOI] [PubMed] [Google Scholar]
- 2.Coughlan C., Ledger W., Wang Q., Liu F., Demirol A., Gurgan T. Recurrent implantation failure: definition and management. Reprod Biomed Online. 2014;28:14–38. doi: 10.1016/j.rbmo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Steiner N., Shrem G., Tannus S., Dahan S.Y., Balayla J., Volodarsky-Perel A. Effect of GnRH agonist and letrozole treatment in women with recurrent implantation failure. Fertil Steril. 2019;112:98–104. doi: 10.1016/j.fertnstert.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Norwitz E.R., Schust D.J., Fisher S.J. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–1408. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 5.Munro S.K., Farquhar C.M., Mitchell M.D., Ponnampalam A.P. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod. 2010;16:297–310. doi: 10.1093/molehr/gaq010. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian-Leon P., Garrido N., Remohi J., Pellicer A., Diaz-Gimeno P. Asynchronous and pathological windows of implantation: two causes of recurrent implantation failure. Hum Reprod. 2018;33:626–635. doi: 10.1093/humrep/dey023. [DOI] [PubMed] [Google Scholar]
- 7.Fullerton P.T., Monsivais D., Kommagani R., Matzuk M.M. Follistatin is critical for mouse uterine receptivity and decidualization. Proc Natl Acad Sci U S A. 2017;114:4772–4781. doi: 10.1073/pnas.1620903114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sroga J.M., Gao F., Ma X., Das S.K. Overexpression of cyclin D3 improves decidualization defects in Hoxa-10(-/-) mice. Endocrinology. 2012;153:5575–5586. doi: 10.1210/en.2012-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson G.V., Lim H., Paria B.C., Satokata I., Dey S.K., Maas R.L. Mechanisms of reduced fertility in Hoxa-10 mutant mice: uterine homeosis and loss of maternal Hoxa-10 expression. Development. 1996;122:2687–2696. doi: 10.1242/dev.122.9.2687. [DOI] [PubMed] [Google Scholar]
- 10.Afshar Y., Jeong J.W., Roqueiro D., DeMayo F., Lydon J., Radtke F. Notch1 mediates uterine stromal differentiation and is critical for complete decidualization in the mouse. FASEB J. 2012;26:282–294. doi: 10.1096/fj.11-184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afshar Y., Miele L., Fazleabas A.T. Notch1 is regulated by chorionic gonadotropin and progesterone in endometrial stromal cells and modulates decidualization in primates. Endocrinology. 2012;153:2884–2896. doi: 10.1210/en.2011-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szekeres-Bartho J., Kilar F., Falkay G., Csernus V., Torok A., Pacsa A.S. The mechanism of the inhibitory effect of progesterone on lymphocyte cytotoxicity: I. Progesterone-treated lymphocytes release a substance inhibiting cytotoxicity and prostaglandin synthesis. Am J Reprod Immunol Microbiol. 1985;9:15–18. doi: 10.1111/j.1600-0897.1985.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Arenas A., Valadez-Cosmes P., Jimenez-Arellano C., Lopez-Sanchez M., Camacho-Arroyo I. Progesterone-induced blocking factor is hormonally regulated in human astrocytoma cells, and increases their growth through the IL-4R/JAK1/STAT6 pathway. J Steroid Biochem Mol Biol. 2014;144:463–470. doi: 10.1016/j.jsbmb.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Kim K., Rhee K. The pericentriolar satellite protein CEP90 is crucial for integrity of the mitotic spindle pole. J Cell Sci. 2011;124:338–347. doi: 10.1242/jcs.078329. [DOI] [PubMed] [Google Scholar]
- 15.Huang B., Faucette A.N., Pawlitz M.D., Pei B., Goyert J.W., Zhou J.Z. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat Med. 2017;23:128–135. doi: 10.1038/nm.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polgar B., Nagy E., Miko E., Varga P., Szekeres-Bartho J. Urinary progesterone-induced blocking factor concentration is related to pregnancy outcome. Biol Reprod. 2004;71:1699–1705. doi: 10.1095/biolreprod.104.030437. [DOI] [PubMed] [Google Scholar]
- 17.Wu S., Zhang H., Tian J., Liu L., Dong Y., Mao T. Expression of kisspeptin/GPR54 and PIBF/PR in the first trimester trophoblast and decidua of women with recurrent spontaneous abortion. Pathol Res Pract. 2014;210:47–54. doi: 10.1016/j.prp.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Mulac-Jericevic B., Sucurovic S., Gulic T., Szekeres-Bartho J. The involvement of the progesterone receptor in PIBF and Gal-1 expression in the mouse endometrium. 2019;81(e13104) doi: 10.1111/aji.13104. [DOI] [PubMed] [Google Scholar]
- 19.Noyes R.W., Hertig A.T., Rock J. Reprint of: Dating the Endometrial Biopsy. Fertil Steril. 2019;112:93–115. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 20.Xu B., Sun X., Li L., Wu L., Zhang A., Feng Y. Pinopodes, leukemia inhibitory factor, integrin-β3, and mucin-1 expression in the peri-implantation endometrium of women with unexplained recurrent pregnancy loss. Fertil Steril. 2012;98:389–395. doi: 10.1016/j.fertnstert.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 21.Xu B., Zhou M., Wang J., Zhang D., Guo F., Si C. Increased AIF-1-mediated TNF-alpha expression during implantation phase in IVF cycles with GnRH antagonist protocol. Hum Reprod. 2018;33:1270–1280. doi: 10.1093/humrep/dey119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou X., Xu B., Zhang D., Jiang X., Chang H.-M., Leung P.C.K. Loss of CDYL Results in Suppression of CTNNB1 and Decreased Endometrial Receptivity. Fron Cell Dev Biol. 2020;8:105. doi: 10.3389/fcell.2020.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghazal S., McKinnon B., Zhou J., Mueller M., Men Y., Yang L. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med. 2015;7:996–1003. doi: 10.15252/emmm.201505245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niklaus A.L., Aubuchon M., Zapantis G., Li P., Qian H., Isaac B. Assessment of the proliferative status of epithelial cell types in the endometrium of young and menopausal transition women. Hum Reprod. 2007;22:1778–1788. doi: 10.1093/humrep/dem032. [DOI] [PubMed] [Google Scholar]
- 25.Fogle R.H., Li A., Paulson R.J. Modulation of HOXA10 and other markers of endometrial receptivity by age and human chorionic gonadotropin in an endometrial explant model. Fertil Steril. 2010;93:1255–1259. doi: 10.1016/j.fertnstert.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Halasz M., Polgar B., Berta G., Czimbalek L., Szekeres-Bartho J. Progesterone-induced blocking factor differentially regulates trophoblast and tumor invasion by altering matrix metalloproteinase activity. Cell Mol Life Sci. 2013;70:4617–4630. doi: 10.1007/s00018-013-1404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tu B., Du L., Fan Q.M., Tang Z., Tang T.T. STAT3 activation by IL-6 from mesenchymal stem cells promotes the proliferation and metastasis of osteosarcoma. Cancer Lett. 2012;325:80–88. doi: 10.1016/j.canlet.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lau W.W., Ng J.K., Lee M.M., Chan A.S., Wong Y.H. Interleukin-6 autocrine signaling mediates melatonin MT(1/2) receptor-induced STAT3 Tyr(705) phosphorylation. J Pineal Res. 2012;52:477–489. doi: 10.1111/j.1600-079X.2011.00965.x. [DOI] [PubMed] [Google Scholar]
- 30.Hsiao K.-Y., Chang N., Tsai J.-L., Lin S.-C., Tsai S.-J., Wu M.-H. Hypoxia-inhibited DUSP2 expression promotes IL-6/STAT3 signaling in endometriosis. Am J Reprod Immunol. 2017;78 doi: 10.1111/aji.12690. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh T.-F., Tseng C.-J., Tang J.-B., Chen Y.-H. A proline rich acidic protein PRAP identified from uterine luminal fluid of estrous mice is able to enhance the estrogen responsiveness of Ishikawa cells. J Cell Biochem. 2011;112:3122–3128. doi: 10.1002/jcb.23238. [DOI] [PubMed] [Google Scholar]
- 32.Yuan M., Hu M., Lou Y., Wang Q., Mao L., Zhan Q. Environmentally relevant levels of bisphenol A affect uterine decidualization and embryo implantation through the estrogen receptor/serum and glucocorticoid-regulated kinase 1/epithelial sodium ion channel α-subunit pathway in a mouse model. Fertil Steril. 2018;109:735–744. doi: 10.1016/j.fertnstert.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Bui T.D., Zhang L., Rees M.C., Bicknell R., Harris A.L. Expression and hormone regulation of Wnt 2, 3, 4, 5a, 7a, 7b and 10b in normal human endometrium and endometrial carcinoma. Br J Cancer. 1997;75:1131–1136. doi: 10.1038/bjc.1997.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoumakis E., Margioris A.N., Stournaras C., Dermitzaki E., Angelakis E., Makrigiannakis A. Corticotrophin-releasing hormone (CRH) interacts with inflammatory prostaglandins and interleukins and affects the decidualization of human endometrial stroma. Mol Hum Reprod. 2000;6:344–351. doi: 10.1093/molehr/6.4.344. [DOI] [PubMed] [Google Scholar]
- 35.Teng C.B., Diao H.L., Ma X.H., Xu L.B., Yang Z.M. Differential expression and activation of Stat3 during mouse embryo implantation and decidualization. Mol Reprod Dev. 2004;69:1–10. doi: 10.1002/mrd.20149. [DOI] [PubMed] [Google Scholar]
- 36.Ferrarelli L.K. B cells prevent preterm labor. Sci Signal. 2017;10:eaam7592. doi: 10.1126/scisignal.aam7592. [DOI] [PubMed] [Google Scholar]
- 37.Delsite R., Kachhap S., Anbazhagan R., Gabrielson E., Singh K.K. Nuclear genes involved in mitochondria-to-nucleus communication in breast cancer cells. Mol Cancer. 2002;1:6. doi: 10.1186/1476-4598-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.