Summary
Background
Multiple endocrine neoplasia type 2B is a rare syndrome caused mainly by Met918Thr germline RET mutation, and characterised by medullary thyroid carcinoma, phaeochromocytoma, and extra-endocrine features. Data are scarce on the natural history of multiple endocrine neoplasia type 2B. We aimed to advance understanding of the phenotype and natural history of multiple endocrine neoplasia type 2B, to increase awareness and improve detection.
Methods
This study was a retrospective, multicentre, international study in patients carrying the Met918Thr RET variant with no age restrictions. The study was done with registry data from 48 centres globally. Data from patients followed-up from 1970 to 2016 were retrieved from May 1, 2016, to May 31, 2018. Our primary objectives were to determine overall survival, and medullary thyroid carcinoma-specific survival based on whether the patient had undergone early thyroidectomy before the age of 1 year. We also assessed remission of medullary thyroid carcinoma, incidence and treatment of phaeochromocytoma, and the penetrance of extra-endocrine features.
Findings
345 patients were included, of whom 338 (98%) had a thyroidectomy. 71 patients (21%) of the total cohort died at a median age of 25 years (range <1–59). Thyroidectomy was done before the age of 1 year in 20 patients, which led to long-term remission (ie, undetectable calcitonin level) in 15 (83%) of 18 individuals (2 patients died of causes unrelated to medullary thyroid carcinoma). Medullary thyroid carcinoma-specific survival curves did not show any significant difference between patients who had thyroidectomy before or after 1 year (comparison of survival curves by log-rank test: p=0·2; hazard ratio 0⋅35; 95% CI 0.07–1.74). However, there was a significant difference in remission status between patients who underwent thyroidectomy before and after the age of 1 year (p<0·0001). There was a significant difference in remission status between patients who underwent thyroidectomy before and after the age of 1 year (p<0·0001). In the other 318 patients who underwent thyroidectomy after 1 year of age, biochemical and structural remission was obtained in 47 (15%) of 318 individuals. Bilateral phaeochromocytoma was diagnosed in 156 (50%) of 313 patients by 28 years of age. Adrenal-sparing surgery was done in 31 patients: three (10%) of 31 patients had long-term recurrence, while normal adrenal function was obtained in 16 (62%) patients. All patients with available data (n=287) had at least one extra-endocrine feature, including 106 (56%) of 190 patients showing marfanoid body habitus, mucosal neuromas, and gastrointestinal signs.
Interpretation
Thyroidectomy done at no later than 1 year of age is associated with a high probability of cure. The reality is that the majority of children with the syndrome will be diagnosed after this recommended age. Adrenal-sparing surgery is feasible in multiple endocrine neoplasia type 2B and affords a good chance for normal adrenal function. To improve the prognosis of such patients, it is imperative that every health-care provider be aware of the extra-endocrine signs and the natural history of this rare syndrome. The implications of this research include increasing awareness of the extra-endocrine symptoms and also recommendations for thyroidectomy before the age of 1 year.
Funding
None.
Introduction
Multiple endocrine neoplasia type 2B is a rare genetic syndrome (prevalence, 0⋅9–1⋅6 per million individuals) caused by germline mutations in the proto-oncogene RET.1,2 The syndrome is most commonly caused by the Met918Thr RET mutation (>95% cases) followed by the Ala883Phe RET mutation (<5% cases);3,4 very rarely, the disease results from tandem RET mutations.5
Precise data for the phenotypic characteristics and natural history of patients carrying the most common Met918Thr mutation remain scarce. Multiple endocrine neoplasia type 2B can include medullary thyroid carcinoma, phaeochromocytoma, and extra-endocrine features. Prognosis is hampered by the syndrome’s main clinical feature, medullary thyroid carcinoma. Multiple endocrine neoplasia type 2B that is associated with medullary thyroid carcinoma is observed worldwide, often clinically aggressive, and of early onset.6 It is preceded by a C-cell hyperplasia and can evolve quickly to lymph node invasion and systemic disease through vascular invasion.6,7
The largest study to date of 75 patients concluded that the prognosis has improved over the past 20 years because of earlier management of medullary thyroid carcinoma.8 Three previous studies, based on 18–44 patients, gave insight into the natural history of the disease and became the basis for the guidelines on the management of the disease.9–12 These studies showed that the prognosis of medullary thyroid carcinoma in multiple endocrine neoplasia type 2B depends on the stage at diagnosis and that only early diagnosis and intervention provided a chance for cure. Brauckhoff and co-workers11 emphasised the need for awareness of the extra-endocrine signs, given the fact that medullary thyroid carcinoma would be only palpable on clinical examination far later than the age recommended of younger than 1 year for thyroidectomy,11,13 and the majority of multiple endocrine neoplasia type 2B cases arise because of de novo RET mutations.10
Therefore, all health-care providers treating children should be aware of the various extra-endocrine clinical signs4,14,15 that should raise concern for multiple endocrine neoplasia type 2B in the differential diagnosis. This earlier identification would help patients benefit from early thyroidectomy to avoid metastatic disease.12 The second principal feature of multiple endocrine neoplasia type 2B is phaeochromocytoma, but few data about this feature of the disease are available. The two largest series reported 26 and 15 patients with multiple endocrine neoplasia type 2B and phaeochromocytoma.8,16 The penetrance of phaeochromocytoma is lower than that of medullary thyroid carcinoma.16
Given the sparsity of published data, and taking advantage of a large international tertiary referral centre network, we sought to provide more insight into the natural history of multiple endocrine neoplasia type 2B. Our main aim was to improve understanding of the phenotype and natural history of the condition, on the basis of current guidelines, which in turn would lead to improved awareness of this complex syndrome and the extra-endocrine signs to allow for optimal detection and essential and functional prognosis of these predominantly young patients.
Methods
Study design and participants
This international study was initiated on April 1, 2016. Experts from centres all over the world (appendix) founded a consortium to include all their available retrospective data in a registry to analyse in detail the diagnosis, management, and outcomes of patients with multiple endocrine neoplasia type 2B.
Patients included in the database had to be diagnosed as carriers of a germline pathogenic Met918Thr RET mutation. There were no age restrictions and no other inclusion criteria were used. Additionally, first-degree relatives with histologically proven medullary thyroid carcinoma and patients presenting with the association of medullary thyroid carcinoma, phaeochromocytoma, and extra-endocrine signs suggestive of multiple endocrine neoplasia type 2B (at a time when genetic testing was not available) were included. Some of these patients had been previously reported with incomplete data. For such patients, updated and exhaustive data were re-extracted from their updated medical records (appendix).2,9,17–19 Patients who were carriers of a germline pathogenic RET Ala883Phe mutation were not included because of a recent report on the natural history of such patients.20 This work conforms to the Declaration of Helsinki, Good Clinical Practice guidelines, and was approved by the appropriate local institutional review boards or ethics committees. All patients gave signed informed consent for genetic DNA analysis. For the retrospective analysis of existing data sets from routine patient care, the majority of the centres did not require additional specific institutional review board approval. Some centres had ethics committee approval or institutional review board authorisation for the use of anonymised data without additional patient consent (Belgium, Canada, China, Czech Republic, Denmark, France, Hungary, Japan, Netherlands, and Poland). Some centres had an additional consent form signed by the patients (Argentina, Chile, Spain, and UK). National Institutes of Health patients included in this study were enrolled in a National Cancer Institute, Pediatric Oncology Branch medullary thyroid cancer natural history study (NCT01660984).
Procedures
We included retrospective, routinely collected data about medullary thyroid carcinoma, phaeochromocytoma, extra-endocrine features with no age restrictions, and schooling and professional attainment (in patients older than 10 years). The following data were collected regarding medullary thyroid carcinoma: age at diagnosis and first surgery, sex, type of surgery, pTNM classification, calcitonin level before and after surgery, treatments received, and final outcome (disease status and final vital status, including cause of death). The following data were collected regarding phaeochromocytoma: age at diagnosis and type of surgery (adrenal-sparing vs total adrenalectomy), pathological diagnosis, recurrence, postsurgical adrenal insufficiency, and final outcome. Data from patients followed up from Jan 1, 1970 to April 1, 2016 were retrieved from May 1, 2016 to May 31, 2018. The principal investigator (FC) collected the data, which were provided by each centre. A potential confounder was the risk of incomplete data in the medical files of each patient. To minimise the effect of this unavoidable confounder, for extra-endocrine features, the presence or absence of a specific sign was recorded only when it was specifically noted as present or absent in the medical records.
Outcomes
Our primary objective was to analyse the overall survival and medullary thyroid carcinoma-specific survival in patients with multiple endocrine neoplasia type 2B based on whether they had undergone early thyroidectomy at the age of 1 year or less, as recommended by international guidelines.12 Our secondary objectives were specifically to better characterise the natural history of medullary thyroid carcinoma and phaeochromocytoma and to establish the penetrance of extra-endocrine features. For medullary thyroid carcinoma, final disease status was defined as follows: remission by normal calcitonin and carcinoembryonic antigen levels and no residual disease on imaging; biochemical persistence by increased calcitonin or carcinoembryonic antigen, or both, with no residual disease on imaging; biochemical and cervical persistence by increased calcitonin or carcinoembryonic antigen, or both, with pathological cervical lymph nodes; biochemical and systemic persistence by increased calcitonin or carcinoembryonic antigen, or both, with systemic metastases on imaging. Follow-up imaging modalities were selected by each investigator. For phaeochromocytoma, the size of the tumour was defined by the pathological analysis when available, or initial imaging in other cases. Phaeochromocytoma was determined to be malignant by the presence of metastases to lymph nodes or other tissues (eg, lungs, bones, or liver). We also compared disease-free survival after adrenal-sparing surgery or adrenalectomy in patients with phaeochromocytoma, and examined adrenal function recovery after adrenal-sparing surgery. Data for extra-adrenal tumours (eg, paraganglioma) were also collected. Finally, data were also gathered about the extra-endocrine features of multiple endocrine neoplasia type 2B, and any other diseases the patients might have presented with during their follow-up. The presence or absence of a specific extra-endocrine feature was noted only when it was specifically reported in the data file of the patients. Suggestions of a particular feature were not counted unless the file explicitly said it was present.
Statistical analysis
We summarised continuous variables as mean and SD or median (range), or both. We did statistical comparisons of quantitative data with Student’s t test or ANOVA. For statistical comparisons of dichotomous data, we used the χ2 test with Yates’ correction. We established overall survival and medullary cancer-specific survival and compared patients with thyroidectomy before or after 1 year of age with log-rank analysis. We calculated age-dependent penetrance estimates of phaeochromocytoma with the Kaplan-Meier method. All statistical tests were two-sided, and p values of less than 0·05 were considered statistically significant. All analyses were done with Prism (version 6) for MacOs X.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
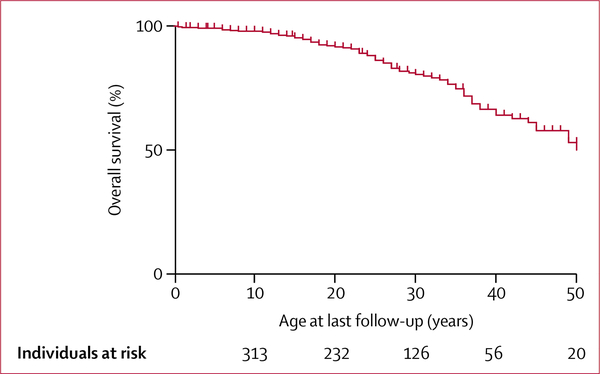
48 centres were part of the consortium. 20 patients were excluded because of incomplete data. 345 patients (184 women, 161 men) were included. Among them, 276 (84%) of 330 with available data on familial status were de novo cases (non-familial). 115 patients had been previously reported in six different studies, with incomplete data (appendix p 4). At a median age of 25 years (range <1–66, IQR 17) at last follow-up, 71 (21%) patients had died. Median age at death was 25 years (range <1–59). 209 (92%) of 227 patients were alive by age 20, 37 (65%) of 57 by age 40, and 5 (42%) of 12 by age 60. Overall survival is shown in figure 1.
Figure 1:
Overall survival based on age at last follow-up (Kaplan-Meier estimates)
338 (98%) of 345 patients had a thyroidectomy. Seven (2%) patients did not have a thyroidectomy: six patients because of metastatic and progressive disease at the time of diagnosis (median age 27 years; range 21–32, IQR 6), and one because of fatal complications of intestinal ganglioneuromatosis at the age of 6 months. At a median age at thyroidectomy of 14 years (range 3 months–47 years, IQR 17), medullary thyroid carcinoma was found in 327 (97%) of 338 patients. It was limited to the thyroid in only 53 (17%) of 312 patients for whom lymph node dissection was done. 43 (14%) of 338 patients died due to metastatic medullary thyroid carcinoma (with metastasis in one or more sites: liver [28], lungs [26], bone [17] and/or brain [8]) at a median age of 25 years (range 6–59, IQR 16; table 1).
Table 1:
Multiple endocrine neoplasia type 2B-related medullary thyroid carcinoma with thyroid surgery
| All patients operated on (n=338) | Patients operated on at 1 year old or younger (n=20) | Patients operated on at older than 1 year old (n=318) | p values | |
|---|---|---|---|---|
| Age at thyroidectomy (IQR) | 14 years (17) | 9 months (6) | 14 years (18·5) | <0·0001 |
| Calcitonin (fold upper limit of normal; IQR) | 180·3 (1145·1) | 6·2 (11·4) | 182·0 (1082·2) | <0·0001 |
| Pathology | <0·0001 | |||
| Normal | 2 (0·5%) | 2 (10%) | 0 | |
| C-cell hyperplasia without medullary thyroid carcinoma | 9 (2·6%) | 3 (15%) | 6 (2%) | |
| Medullary thyroid carcinoma | 327 (97%) | 15 (83%) | 312 (98%) | |
| Medullary thyroid carcinoma size (mm, IQR) | 21 (27) | 4 (3) | 23 (24) | <0·0001 |
| Medullary thyroid carcinoma size <10 mm | 51 (15%) | 14 (70%) | 37 (12%) | |
| Systematic lymph node dissection | 241/312 (77%) | 14/19 (74%) | 227/293 (77%) | 0·24 |
| Normal calcitonin after surgery | 57/223 (26%) | 15/20 (83%) | 43/206 (21%) | <0·0001 |
| Age at last follow-up, years (IQR) | 25 (17) | 6 (11) | 26 (17) | <0·0001 |
| Final outcome | <0·0001 | |||
| Remission | 62 (18%) | 15/18* (83%) | 47 (15%) | |
| Biochemical persistence | 58 (17%) | 0 | 58 (18%) | |
| Biochemical and cervical persistence | 47 (14%) | 2 (10%) | 45 (14%) | |
| Biochemical and systemic persistence | 105 (31%) | 1 (5%) | 104 (33%) | |
| Deaths | 66 (20%) | 2 (10%) | 64 (20%) | |
| Medullary thyroid carcinoma | 43 (65%) | 0 | 43 (67%) | |
| Phaeochromocytoma | 2 (3%) | 0 | 2 (3%) | |
| Gastrointestinal complications of multiple endocrine neoplasia type 2B | 2 (3%) | 1 (50%) | 1 (2%) | |
| Unknown reasons | 19 (44%) | 1 (50%) | 18 (28%) | |
Data are n (%), n/N (%), or median (IQR) unless specified. p values compare thyroidectomy before and after 1 year. Remission was defined by normal calcitonin and carcinoembryonic antigen levels and no residual disease on imaging; biochemical persistence by increased calcitonin or carcinoembryonic antigen, or both, with no residual disease on imaging; biochemical and cervical persistence by increased calcitonin or carcinoembryonic antigen, or both, with pathological cervical lymph nodes; biochemical and systemic persistence by increased calcitonin or carcinoembryonic antigen, or both, with systemic metastases on imaging.
2 patients died of causes unrelated to medullary thyroid carcinoma.
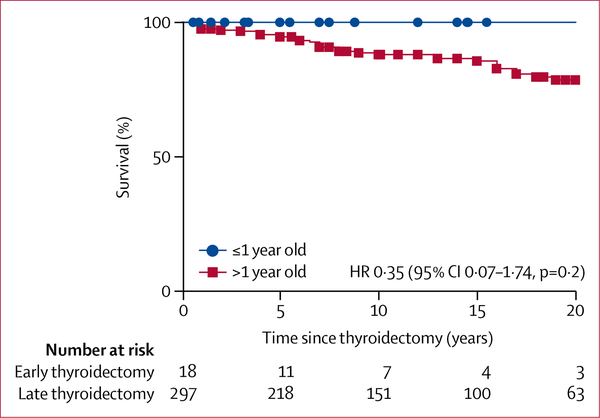
Medullary thyroid carcinoma-specific survival curves did not show any significant difference between patients who had early or delayed thyroidectomy (figure 2; comparison of survival curves by log-rank test: p=0⋅2; hazard ratio 0⋅35; 95% CI 0⋅07–1⋅74). By contrast, there was a significant difference in remission status between patients operated before and after the age of 1 year (p<0·0001; table 1).
Figure 2:
Kaplan-Meier estimates of medullary thyroid carcinoma-specific survival in patients who underwent early (≤1 year old) versus late (>1 year old) thyroidectomy
HR=hazard ratio.
As shown in the appendix, 20 patients had a thyroidectomy at no later than 1 year of age (median 9 months, range 3–12, IQR 0·5) as identified based on their medical records. During follow-up, two patients had died (neither from medullary thyroid carcinoma): one at the age of 6 months because of complications of intestinal ganglioneuromatosis and the other at the age of 3 years with no cause of death specified. At last follow-up, at a median of 5·2 years after thyroidectomy (range 3 months–32·0 years, IQR 10·6), 15 (83%) of 18 surviving patients who underwent thyroidectomy at the age of 1 year or less were in biochemical and structural remission, while three (17%) of 18 individuals had persistent biochemical and structural disease.
In the group of 318 patients who had a thyroidectomy after 1 year of age, 64 (20%) patients were deceased, including 43 due to medullary thyroid carcinoma, at a median age of 25 years (range 8–59, IQR 18·5). At last follow-up, at a median of 13 years after thyroidectomy (range 1⋅7–25·0, IQR 13·9), 47 (15%) patients were in biochemical and structural remission, while 207 (65%) had persistent disease, including 104 (33%) who progressed to systemic metastases requiring chemotherapy (n=8), oral targeted therapy (n=34), or liver-directed radiofrequency ablation (n=2; data not shown). Data were not available for the remaining 59 patients. T and N status at diagnosis is shown in the appendix. Of note, nine (56%) of 16 patients operated between the ages of 1 year and 4 years had persistent disease at last follow-up (data not shown).
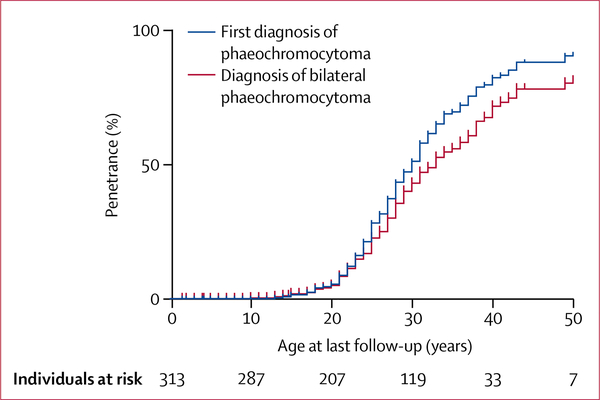
Clinical data regarding phaeochromocytoma were available for 313 patients. A diagnosis of at least one phaeochromocytoma was made in 153 (49%) of 313 patients at a median age of 24 years (range 13–52, IQR 9; table 2). 146 (95%) patients had undergone surgery as treatment for phaeochromocytoma at last follow-up. Bilateral synchronous phaeochromocytoma was the initial diagnosis in 79 (52%) patients. The median size of the larger phaeochromocytoma was 30 mm in diameter (range 6–175, IQR 31) whereas the contralateral one was 19 mm (range 3–75, IQR 17). Among the 74 remaining patients (48%) with a first diagnosis of unilateral phaeochromocytoma, 32 patients developed a metachronous contralateral phaeochromocytoma at a median of 4 years (range 2–16, IQR 3·2) after the first phaeochromocytoma diagnosis (table 2). Penetrance of unilateral phaeochromocytoma was 50% by the age of 25 years, whereas penetrance of bilateral phaeochromocytoma was 50% by the age of 28 years (figure 3).
Table 2:
Phaeochromocytoma
| Number of patients | |
|---|---|
| Patients with phaeochromocytoma | 153/313 (49%) |
| Median age at first diagnosis of phaeochromocytoma (years [IQR]) | 24 (9) |
| Symptomatic at first diagnosis | 71/127 (56%) |
| Diagnosis | |
| Before medullary thyroid carcinoma | 8/137 (6%; delay of 3 years, IQR 2) |
| At the same time as medullary thyroid carcinoma | 35/137 (26%) |
| After medullary thyroid carcinoma | 94/137 (69%; delay of 8 years, IQR 8) |
| Unilateral phaeochromocytoma at last follow-up | 42/153 (27%) |
| Bilateral phaeochromocytoma at last follow-up | 111/153 (73%) |
| Synchronous bilateral phaeochromocytoma | 79/153 (52%) |
| Asynchronous bilateral phaeochromocytoma | 32/153 (21%) |
| Patients with adrenal surgery | 146/153 (95%) |
| Unilateral | 51 (35%) |
| Bilateral | 95 (65%) |
| Patients with adrenalectomy | 115/153 (79%) |
| Recurrence | 11 (10%) |
| Adrenal insufficiency | 69* (100%) |
| Patients with at least one adrenal-sparing surgery | 31/153 (20%) |
| Right adrenal | 13 (42%) |
| Left adrenal | 11 (36%) |
| Both sides | 7 (23%) |
| Recurrence | 3 (10%) |
| Adrenal insufficiency | 10† (38%) |
| Metastatic phaeochromocytoma | 4/153 (3%) |
| Median age at last follow-up (years [IQR]) | 25 (8) |
| Death due to phaeochromocytoma | 2/153 (<1%) |
Data are n (%) or n/N (%) unless specified.
69 patients underwent bilateral adrenalectomy.
A total of 26 patients underwent bilateral adrenal surgery and at least one adrenal-sparing surgery.
Figure 3:
Kaplan-Meier estimates of unilateral and bilateral phaeochromocytoma penetrance
Comparing adrenal sparing and total adrenalectomy in patients with multiple endocrine neoplasia type 2B and pheochromocytoma, 31 (20%) of 153 patients had at least one adrenal-sparing surgery. In all patients who underwent surgery, 14 (10%) of 146 patients developed recurrence at a median age of 34 years (range 24–54, IQR 10·8), including three patients who had undergone adrenal-sparing surgery and 11 who had an adrenalectomy (p=0⋅56; data not shown). For the three patients, recurrence had been diagnosed at 5 years in one individual and 6 years in the other two, after the organ-sparing surgery. Among the 26 patients with bilateral adrenal surgery and at least one adrenal-sparing surgery, normal adrenocortical function was observed in 16 (62%) patients (vs 0% in the adrenalectomy group; p<0·0001; table 2). Four patients (3%) had metastatic phaeochromocytoma at last follow-up, as evidenced by metanephrines hypersecretion, at a median age of 41 years (range 35–45, IQR 5). Two patients had bilateral synchronous phaeochromocytoma at 27 and 40 years old, and two patients had metachronous phaeochromocytoma at the age of 19 and 23 years for the first patient, and 25 and 29 years for the second patient.
The prevalence of extra-endocrine features is reported in table 3. All patients with available data (n=287) had at least one extra-endocrine feature. The association of marfanoid body habitus, ganglioneuromatosis, and gastrointestinal signs was reported in 106 (56%) of 190 patients for whom the presence or absence of these features was clearly noted in their medical records. Surgical intervention for extra-endocrine features was necessary in 28 patients (19 gastrointestinal surgeries including ten cases for symptomatic megacolon and nine with achalasia requiring oesophageal dilatation; five hip surgeries; and four bladder, prostate, or ureteral surgeries). Additional extra-endocrine features, probably not linked to multiple endocrine neoplasia type 2B, included seizures in four patients (without brain metastases), micrognathia in four cases, and congenital cataract in two cases (data not shown).
Table 3:
Extra-endocrine features
| Number (%) | |
|---|---|
| Ganglioneuromatosis | 258/266 (97%) |
| Tongue | 165/266 (62%) |
| Lips | 142/266 (53%) |
| Eyelid or conjunctival | 51/266 (19%) |
| Marfanoid habitus | 193/266 (73%) |
| Pseudo Hirschsprung’s disease or severe constipation | 168/257 (65%) |
| Achalasia or gastroparesis | 9/257 (4%) |
| Pes cavus | 63/166 (38%) |
| Pectus excavatum | 45/172 (26%) |
| Motor or muscle weakness (hypotonia) | 51/190 (27%) |
| Scoliosis | 18/190 (9%) |
| Corneal hypertrophy | 82/182 (45%) |
| Alacrima | 66/167 (40%) |
| Kidney anomalies (eg, kidney atrophy, kidney cysts, hydronephrosis, and ureteral atonia) | 25/189 (13%) |
Data are n (%) of N. Note that the penetrance was based on the presence or absence of each feature specifically noted in the medical files of each patient. When no mention of the feature was noted, the information was considered as not available.
School and professional data from patients aged more than 10 years were available for 129 patients (median age 31 years, range 10–66, IQR 17): 32 (25%) patients were unable to work or attend school because of disability at last follow-up (data not shown).
Discussion
This international, retrospective study provides a detailed description of the natural history and outcomes of Met918Thr RET multiple endocrine neoplasia type 2B. It emphasises the need for early thyroidectomy to improve survival, and the possibility of adrenal-sparing surgery to improve the functional outcome of patients with Met918Thr RET multiple endocrine neoplasia type 2B. It also highlights the need for an optimal extra-endocrine management of these patients, because a quarter of them could be unable to work or attend school because of endocrine and extra-endocrine related disability, a point that has not been raised previously, to our knowledge, in studies mainly focusing on the outcome of medullary thyroid carcinoma. These results should thus modify patient care and help establish future guidelines.
The importance of early diagnosis is shown by the medullary thyroid carcinoma outcome of patients who underwent thyroidectomy. Although only 15% of patients were cured when thyroidectomy was done after the age of 1 year, 83% were cured when thyroidectomy was done before or at the age of 1 year (p<0·0001; table 1). This finding shows the need for early thyroidectomy—ie, before the age of 1 year. Although there had been suggestions to postpone thyroidectomy to the age of 4 years,10 our study calls for caution as nine (56%) of our 16 patients operated between the ages of 1 year and 4 years had persistent disease at last follow-up (data not shown). Although our study did not collect data for surgical complications, the high rate of cure of medullary thyroid carcinoma with early thyroidectomy could outweigh the associated risks, such as laryngeal nerve injury or hypoparathyroidism, even though these can substantially affect an infant’s life. To minimise risks, surgeries in patients with multiple endocrine neoplasia type 2B should only be done by experienced, high-volume thyroid cancer surgeons. However, the possibility of early thyroidectomy is hampered by the difficulty in ma king an ea rly di agnosis.11,13 In deed, the diagnosis of multiple endocrine neoplasia type 2B remains a challenge because of the high proportion of de novo mutations and the absence of endocrine symptoms at an early age. In a series of 44 patients, Brauckhoff and colleagues10 reported that the chance of remission was higher in patients who had been diagnosed based on recognition of extra-endocrine features of multiple endocrine neoplasia type 2B (ie, by a non-endocrinologist). Although marfanoid body habitus and mucosal neuromas typically appear after the age of 1 year, features such as constipation, pseudo-obstruction, or feeding difficulties are early signs that should lead to further evaluation for the syndrome. Gastrointestinal signs were present in two-thirds of our patients and Brauckhoff and co-workers11 reported tear production abnormalities (alacrima) as an additional early sign of multiple endocrine neoplasia type 2B. Proper education of paediatricians, ophthalmologists, dentists, gastroenterologists, orthopaedic surgeons, and general practitioners is thus crucial, as they represent the front line for early diagnosis. Notably, we also found a surprisingly large number of patients (n=47, 15%) who underwent thyroidectomy after the age of 1 year who were in biochemical and structural remission at last follow-up. These results also emphasise the heterogeneity and phenotypic variability of multiple endocrine neoplasia type 2B, as all but one of these 47 patients required only a single delayed surgery to achieve remission.
In this study, the penetrance of phaeochromocytoma was high: 50% of all patients developed a phaeochromocytoma and 50% of these were bilateral by 28 years of age. As a comparison, we had previously reported that 20% of patients with the RET 634 codon mutation and 5% with RET exon 10 mutations had bilateral phaeochromocytoma by 28 years of age.18 Makri and colleagues19 reported in a study of eight patients with multiple endocrine neoplasia type 2B-associated phaeochromocytoma a 10-year-old patient with an asymptomatic phaeochromocytoma. This identification of phaeochromocytoma in a 10-year-old is in line with international guidelines, which recommend a first biological screening at 11 years of age in Met918Thr multiple endocrine neoplasia type 2B. Overall, considering synchronous or metachronous appearance, phaeochromocytoma was bilateral in most of our patients (73%). This observation highlights the need to consider adrenal-sparing surgery in these patients, who would otherwise be at risk for life-long adrenal insufficiency. As 61% of patients who underwent adrenal-sparing surgery had normal adrenocortical function, the results seem similar to those reported for multiple endocrine neoplasia type 2A.18 The rate of malignant phaeochromocytoma was similar to reported numbers for other RET mutations,18 and two patients of 66 died of phaeochromocytoma or consequences of catecholamine hypersecretion.
Our study has inherent limitations due to its retrospective nature. However, multiple endocrine neoplasia type 2B is a very rare syndrome and the most suitable approach to improve its understanding was to retrospectively analyse the data from a large number of centres. This study design results in incomplete data (especially for the earlier signs that allowed diagnosis, the age at which these signs were first seen by the physician, or the presence or absence of extra-endocrine features in patients diagnosed during adulthood; some precise pathological characteristics such as TNM status for phaeochromocytoma or extent of lymph node resection), subjective data extraction, and heterogeneous follow-up. We collected the data of only 20 patients with thyroidectomy before the age of 1 year. This low number, associated with a short follow-up, probably explains why we did not find any significant difference between patients operated before and after the age of 1 year, but there was a significant difference in remission status. This finding should not modify the aggressive early management of such patients, as shown in table 1.
In summary, the different clinical courses and outcomes of medullary thyroid carcinoma and phaeochromocytoma, in addition to the varying penetrance of extra-endocrine features, make multiple endocrine neoplasia type 2B a complex syndrome characterised by wide phenotypic variability despite being a defined single gene disorder. Our data underscore the importance of pursuing thyroidectomy before 1 year of age, which requires an early non-endocrine diagnosis; highlight the value of doing adrenal-sparing surgery in patients with phaeochromocytoma when technically feasible; and emphasise the need for a multidisciplinary approach lifelong to the treatment of both the endocrine and extra-endocrine features, which should be systematically reported in medical files whether present or not. Additionally, our data highlight that a large proportion of these young patients become disabled. One of our future aims will be to show whether the natural history of patients with multiple endocrine neoplasia type 2B can be modified by novel drugs or techniques. Therefore, our study improves understanding regarding the long-term outcomes of multiple endocrine neoplasia type 2B, but more work remains to be done to obtain a global understanding of this rare syndrome.
Supplementary Material
Research in context.
Evidence before this study
Multiple endocrine neoplasia type 2B is a rare genetic syndrome that arises from an activating mutation (primarily the Met918Thr variant) in the rearranged during transfection (RET) proto-oncogene. Given the rarity of the syndrome, published data are scarce and are primarily limited to a few referral centres. Medullary thyroid cancer is the predominant finding in multiple endocrine neoplasia type 2B, developing within the first year of life and leading to early death due to metastatic medullary thyroid carcinoma in most cases. It is currently recommended that thyroidectomy be done before the age of 1 year to prevent the early metastatic spread of medullary thyroid carcinoma, yet this recommendation is not evidence-based and is rarely done because most multiple endocrine neoplasia type 2B cases arise de novo and the diagnosis is not made until long after the onset of metastatic and incurable medullary thyroid carcinoma. The second major component of multiple endocrine neoplasia type 2B is phaeochromocytoma, for which the first-line treatment is adrenalectomy. Historically, the treatment has been total adrenalectomy, although surgical advances have led to the broader use of adrenal-sparing procedures, which have not been studied in multiple endocrine neoplasia type 2B specifically. Finally, multiple endocrine neoplasia type 2B is also associated with extra-endocrine features, such as alacrima and symptoms of gastrointestinal dysmotility, which can present during infancy, thus allowing the astute clinician who recognises this association to make the correct diagnosis early enough to prevent the onset of metastatic medullary thyroid carcinoma through early thyroidectomy.
Added value of this study
345 patients from 48 centres across the world were included in the largest study on multiple endocrine neoplasia type 2B ever published, to our knowledge. We report for the first time an extensive description of the phenotype and natural history of multiple endocrine neoplasia type 2B. This study emphasises the benefits of early thyroidectomy before the age of 1 year, as it led to a long-term cure in 15 (83%) of 18 patients who were operated on. Surprisingly, at a median age of 25 years, 47 (15%) patients operated on later than 1 year were still cured, while 64 (20%) of our patients had died, highlighting the phenotypic heterogeneity of the syndrome. All patients had at least one extra-endocrine feature, and more than half of them had the classic association of marfanoid body habitus, gastrointestinal signs, and mucosal neuromas—clinical signs that should warn health providers of a potential diagnosis of multiple endocrine neoplasia type 2B. Half of the patients had bilateral phaeochromocytomas by the age of 28 years and, for the first time, the benefits of adrenal-sparing surgery are reported, which resulted in normal adrenocortical function in 62% of patients and was associated with a low risk of recurrence. Finally, we report for the first time, to our knowledge, the social consequences of the syndrome, as a quarter of our patients were disabled due to its clinical features.
Implications of all the available evidence
Multiple endocrine neoplasia type 2B is an orphan disease associated with a substantially altered quality of life and poor survival primarily due to metastatic medullary thyroid carcinoma. Means to improve the prognosis of patients with multiple endocrine neoplasia type 2B include making the diagnosis earlier and better understanding the natural history and management of disease features. The evidence has clearly shown that the clinical presentation of the syndrome begins at birth, and improved education of all health providers about the extra-endocrine signs should lead to earlier diagnosis and improved outcomes through earlier thyroidectomy. Improving the functional prognosis can also be achieved through appropriate management of phaeochromocytoma via adrenal-sparing surgery, which should lead to less adrenal insufficiency and the need to take life-long adrenal replacement therapy. Future studies should focus on the factors explaining the phenotypic variations in terms of disease aggressiveness.
Acknowledgments
We thank our patients and all the clinicians looking after them. FC would like to thank Lucie Hergott-Faure for the analysis of preliminary data. JM and CG would like to thank the Danish Thyroid Cancer Group (DATHYRCA) and the Danish MEN2 group. SD received a national grant (AZV 16–32665A). JK and BJ were supported by the Polish National Center of Research and Development MILESTONE Project (grant number STRATEGMED2/267398/4/NCBR/2015). This research was supported in part by the National Cancer Institute Center for Cancer Research Intramural Research Program, but specific funding was not assigned for this study.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Frederic Castinetti, Aix-Marseille Université, Institut National de la Santé et de la Recherche Médicale, Marseille Medical Genetics, Marseille, France; Assistance Publique-Hôpitaux de Marseille, Department of Endocrinology, Hôpital de la Conception, Centre de Référence des Maladies Rares de l’hypophyse, Marseille, France.
Steven G Waguespack, Department of Endocrine Neoplasia and Hormonal Disorders, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Andreas Machens, Department of General, Visceral and Vascular Surgery, Martin Luther University Halle-Wittenberg, Halle (Saale), Germany.
Shinya Uchino, Noguchi Thyroid Clinic and Hospital Foundation, Beppu, Japan.
Kornelia Hasse-Lazar, Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie Institute, Oncology Center, Gliwice Branch, Gliwice, Poland.
Gabriella Sanso, Centro de Investigaciones Endocrinológicas, “Dr César Bergadá”, Hospital de Niños Ricardo Gutiérrez, Buenos Aires, Argentina.
Tobias Else, Division of Metabolism, Endocrinology, & Diabetes, Department of Internal Medicine, University of Michigan Health System, Ann Arbor, MI, USA.
Sarka Dvorakova, Department of Molecular Endocrinology, Institute of Endocrinology, Prague, Czech Republic.
Xiao Ping Qi, Departments of Oncologic and Urologic Surgery, The 117th People’s Liberation Army Hospital, People’s Liberation Army Hangzhou Clinical College, Anhui Medical University, Hangzhou, China.
Rossella Elisei, Department of Endocrinology, University Hospital, Pisa, Italy.
Ana Luisa Maia, Thyroid Section, Endocrinology Division, Hospital de Clίnicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
John Glod, Pediatric Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Delmar Muniz Lourenço, Jr, Endocrine Genetics Unit, Endocrinology Division, Hospital das Clίnicas, University of São Paulo School of Medicine, São Paulo, Brazil; Endocrine Oncology Division, Institute of Cancer of the State of São Paulo, Faculty of Medicine of the University of São Paulo, São Paulo, Brazil.
Nuria Valdes, Department of Endocrinology and Nutrition, Hospital Universitario Central de Asturias, Oviedo, Spain; Unit of Endocrinology, Nutrition, Diabetes and Obesity, Institute of Sanitary Research of Asturias, Oviedo, Spain.
Jes Mathiesen, Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Odense University Hospital, Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Nelson Wohllk, Endocrine Section, Universidad de Chile, Hospital del Salvador, Santiago de Chile, Santiago, Chile.
Tushar R Bandgar, Department of Endocrinology, Seth G S Medical College, King Edward Memorial Hospital, Parel, Mumbai, India.
Delphine Drui, L’Institut du thorax, Department of Endocrinology, Centre Hospitalier Universitaire Nantes, Nantes, France.
Marta Korbonits, Department of Endocrinology, St Bartholomew’s Hospital, London, UK; London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Maralyn R Druce, Department of Endocrinology, St Bartholomew’s Hospital, London, UK; London School of Medicine and Dentistry, Queen Mary University of London, London, UK.
Caroline Brain, Division of Endocrine Surgery, University College Hospital and Great Ormond Street Hospital, London, United Kingdom.
Tom Kurzawinski, Division of Endocrine Surgery, University College Hospital and Great Ormond Street Hospital, London, United Kingdom.
Atila Patocs, Hungarian Academy of Sciences and Semmelweis University, HSA-SE “Lendület” Hereditary Endocrine Tumour Research Group, Budapest, Hungary.
Maria Joao Bugalho, Serviço de Endocrinologia, Diabetes e Metabolismo, Centro Hospitalar Universitário Lisboa Norte-Hospital Santa Maria, Lisboa, Portugal; Centro Académico de Medicina de Lisboa, Universidade Lisboa, Lisboa, Portugal.
Andre Lacroix, Endocrine Division, Department of Medicine, Centre Hospitalier de l’Universite de Montréal, Montreal, QC, Canada.
Philippe Caron, Centre Hospitalier Universitaire de Toulouse, Hôpital Larrey, Service d’Endocrinologie, Maladies métaboliques, Nutrition, Toulouse, France.
Patricia Fainstein-Day, Endocrine and Nuclear Medicine Unit, Hospital Italiano de Buenos Aires, Buenos Aires, Argentina.
Francoise Borson Chazot, Hospices Civils de Lyon, Fédération d’Endocrinologie, Université Claude Bernard Lyon 1, Lyon, France.
Marc Klein, Department of Endocrinology, University Hospital, Nancy, France.
Thera P Links, Department of Endocrinology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands.
Claudio Letizia, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Rome, Italy.
Laura Fugazzola, Division of Endocrine and Metabolic Diseases, Istituto di Ricovero e Cura a Carattere Scientifico, Istituto Auxologico Italiano, University of Milan, Milan, Italy ‘; Department of Pathophysiology and Transplantation, University of Milan, Milan, Italy.
Olivier Chabre, Centre Hospitalier Universitaire de Grenoble, Hôpital Albert Michallon, Service d’Endocrinologie-Diabétologie-Nutrition, Grenoble, France.
Letizia Canu, Department of Experimental and Clinical Biomedical Sciences, Endocrinology Unit, University of Florence, Florence, Italy.
Regis Cohen, Endocrinologie et Métabolismes, Centre Hospitalier de Saint Denis, Saint-Denis, France.
Antoine Tabarin, Centre Hospitalier Universitaire de Bordeaux, Hôpital du Haut Lévêque, Service d’Endocrinologie-Diabétologie et Maladies Métaboliques, Pessac, France.
Anita Spehar Uroic, Department of Pediatrics, University Hospital Centre Zagreb, Zagreb, Croatia.
Dominique Maiter, Service d’Endocrinologie et Nutrition, Cliniques Universitaires Saint-Luc, Universite Catholique de Louvain, Brussels, Belgium.
Sandrine Laboureau, Department of Endocrinology, Diabetes and Nutrition, Reference Centre of Rare Thyroid Disease, Hospital of Angers, Angers, France.
Caterina Mian, Operative Unit of the Endocrinology, Department of Medicine, University of Padua, Padua, Italy.
Mariola Peczkowska, Department of Hypertension, Institute of Cardiology, Warsaw, Poland.
Frederic Sebag, Department of Endocrine Surgery, La Conception Hospital, Marseille, France.
Thierry Brue, Aix-Marseille Université, Institut National de la Santé et de la Recherche Médicale, Marseille Medical Genetics, Marseille, France; Assistance Publique-Hôpitaux de Marseille, Department of Endocrinology, Hôpital de la Conception, Centre de Référence des Maladies Rares de l’hypophyse, Marseille, France; Department of Endocrine Neoplasia and Hormonal Disorders.
Delphine Mirebeau-Prunier, Département de Biochimie et Génétique, Centre Hospitalier Universitaire d’Angers, Angers, France.
Laurence Leclerc, Centre Hospitalier Régional Universitaire de Lille, Hopital Huriez, Service d’Endocrinologie, Lille, France.
Birke Bausch, Section for Preventive Medicine, Department of Nephrology and General Medicine, Freiburg, Germany.
Amandine Berdelou, Endocrine Oncology, Institut Gustave Roussy Ecole Doctorale de Cancerologie, Villejuif, France.
Akihiro Sukurai, Department of Medical Genetics and Genomics, Sapporo Medical University School of Medicine, Chuo-ku, Sapporo, Hokkaido, Japan.
Petr Vlcek, Department of Nuclear Medicine and Endocrinology, Second Faculty of Medicine, Charles University, Prague, Czech Republic; Motol University Hospital, Prague, Czech Republic.
Jolanta Krajewska, Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie Institute, Oncology Center, Gliwice Branch, Gliwice, Poland.
Marta Barontini, Centro de Investigaciones Endocrinológicas, “Dr César Bergadá”, Hospital de Niños Ricardo Gutiérrez, Buenos Aires, Argentina.
Carla Vaz Ferreira Vargas, Thyroid Section, Endocrinology Division, Hospital de Clίnicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Laura Valerio, Department of Endocrinology, University Hospital, Pisa, Italy.
Lucieli Ceolin, Thyroid Section, Endocrinology Division, Hospital de Clίnicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
Srivandana Akshintala, Department of Pediatrics, New York University Langone Medical Center, New York, NY, USA.
Ana Hoff, Endocrine Genetics Unit, Endocrinology Division, Hospital das Clίnicas, University of São Paulo School of Medicine, São Paulo, Brazil; Endocrine Oncology Division, Institute of Cancer of the State of São Paulo, Faculty of Medicine of the University of São Paulo, São Paulo, Brazil.
Christian Godballe, Department of Otorhinolaryngology, Head and Neck Surgery and Audiology, Odense University Hospital, Odense, Denmark; Department of Clinical Research, University of Southern Denmark, Odense, Denmark.
Barbara Jarzab, Department of Nuclear Medicine and Endocrine Oncology, Maria Sklodowska-Curie Institute, Oncology Center, Gliwice Branch, Gliwice, Poland.
Camilo Jimenez, Department of Endocrine Neoplasia and Hormonal Disorders, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Charis Eng, Genomic Medicine Institute, Lerner Research Institute and Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA.
Tsuneo Imai, Department of Breast and Endocrine Surgery, National Hospital Organization Higashinagoya National Hospital, Nagoya, Japan.
Martin Schlumberger, Endocrine Oncology, Institut Gustave Roussy Ecole Doctorale de Cancerologie, Villejuif, France.
Elizabeth Grubbs, Department of Surgical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Henning Dralle, Section of Endocrine Surgery, Department of General, Visceral, and Transplantation Surgery, University Hospital Essen, Essen, Germany.
Hartmut P Neumann, Section for Preventive Medicine, Department of Nephrology and General Medicine, Freiburg, Germany.
Eric Baudin, Endocrine Oncology, Institut Gustave Roussy Ecole Doctorale de Cancerologie, Villejuif, France.
References
- 1.Carlson KM, Dou S, Chi D, et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA 1994; 91: 1579–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Incidence and prevalence of multiple endocrine neoplasia 2B in Denmark: a nationwide study. Endocr Relat Cancer 2017; published online April 24. DOI: 10.1530/ERC-17-0122. [DOI] [PubMed] [Google Scholar]
- 3.Eng C, Smith DP, Mulligan LM, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet 1994; 3: 237–41. [DOI] [PubMed] [Google Scholar]
- 4.Marquard J, Eng C. Multiple endocrine neoplasia type 2. In: Adam MP, Ardinger HH, Pagon RA, et al. , eds. GeneReviews. Seattle: University of Washington, 1993. [PubMed] [Google Scholar]
- 5.Castinetti F, Moley J, Mulligan L, Waguespack SG. A comprehensive review on MEN2B. Endocr Relat Cancer 2018; 25: T29–39. [DOI] [PubMed] [Google Scholar]
- 6.Waguespack SG, Rich TA, Perrier ND, Jimenez C, Cote GJ. Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol 2011; 7: 596–607. [DOI] [PubMed] [Google Scholar]
- 7.O’Riordain DS, O’Brien T, Weaver AL, et al. Medullary thyroid carcinoma in multiple endocrine neoplasia types 2A and 2B. Surgery 1994; 116: 1017–23. [PubMed] [Google Scholar]
- 8.Raue F, Dralle H, Machens A, Bruckner T, Frank-Raue K. Long-term survivorship in multiple endocrine neoplasia type 2b diagnosed before and in the new millennium. J Clin Endocrinol Metab 2018; 103: 235–43. [DOI] [PubMed] [Google Scholar]
- 9.Leboulleux S, Travagli JP, Caillou B, et al. Medullary thyroid carcinoma as part of a multiple endocrine neoplasia type 2B syndrome: influence of the stage on the clinical course. Cancer 2002; 94: 44–50. [DOI] [PubMed] [Google Scholar]
- 10.Brauckhoff M, Machens A, Lorenz K, Bjoro T, Varhaug JE, Dralle H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg 2014; 259: 800–06. [DOI] [PubMed] [Google Scholar]
- 11.Brauckhoff M, Machens A, Hess S, et al. Premonitory symptoms preceding metastatic medullary thyroid cancer in MEN 2B: an exploratory analysis. Surgery 2008; 144: 1044–50. [DOI] [PubMed] [Google Scholar]
- 12.Wells SA Jr, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 2015; 25: 567–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wray CJ, Rich TA, Waguespack SG, Lee JE, Perrier ND, Evans DB. Failure to recognize multiple endocrine neoplasia 2B: more common than we think? Ann Surg Oncol 2008; 15: 293–301. [DOI] [PubMed] [Google Scholar]
- 14.Eng C, Clayton D, Schuffenecker I, et al. The relationship between specific RET proto-oncogene mutations and disease phenotype in multiple endocrine neoplasia type 2. International RET mutation consortium analysis. JAMA 1996; 276: 1575–79. [PubMed] [Google Scholar]
- 15.O’Riordain DS, O’Brien T, Crotty TB, Gharib H, Grant CS, van Heerden JA. Multiple endocrine neoplasia type 2B: more than an endocrine disorder. Surgery 1995; 118: 936–42. [DOI] [PubMed] [Google Scholar]
- 16.Thosani S, Ayala-Ramirez M, Palmer L, et al. The characterization of pheochromocytoma and its impact on overall survival in multiple endocrine neoplasia type 2. J Clin Endocrinol Metab 2013; 98: E1813–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castinetti F, Qi XP, Walz MK, et al. Outcomes of adrenal-sparing surgery or total adrenalectomy in phaeochromocytoma associated with multiple endocrine neoplasia type 2: an international retrospective population-based study. Lancet Oncol 2014; 15: 648–55. [DOI] [PubMed] [Google Scholar]
- 18.Makri A, Akshintala S, Derse-Anthony C, et al. Pheochromocytoma in children and adolescents with multiple endocrine neoplasia type 2B. J Clin Endocrinol Metab 2019; 104: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai T, Uchino S, Okamoto T, et al. High penetrance of pheochromocytoma in multiple endocrine neoplasia 2 caused by germ line RET codon 634 mutation in Japanese patients. Eur J Endocrinol 2013; 168: 683–87. [DOI] [PubMed] [Google Scholar]
- 20.Mathiesen JS, Habra MA, Bassett JH, et al. Risk profile of the RET A883F germline mutation: an international collaborative study. J Clin Endocrinol Metab 2017; 102: 2069–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.