Abstract
OBJECTIVE
Depression is common in people with diabetes, but data from developing countries are scarce. We evaluated the prevalence and risk factors for depressive symptoms in patients with diabetes using data from the International Diabetes Management Practices Study (IDMPS).
RESEARCH DESIGN AND METHODS
IDMPS is an ongoing multinational, cross-sectional study investigating quality of care in patients with diabetes in real-world settings. Data from wave 5 (2011), including 21 countries, were analyzed using the 9-item Patient Health Questionnaire (PHQ-9) to evaluate depressive symptoms. Logistic regression analyses were conducted to identify risk factors of depressive symptoms.
RESULTS
Of 9,865 patients eligible for analysis, 2,280 had type 1 and 7,585 had type 2 diabetes (treatment: oral glucose-lowering drugs [OGLD] only, n = 4,729; OGLDs plus insulin, n = 1,892; insulin only, n = 964). Depressive symptoms (PHQ-9 score ≥5) were reported in 30.7% of those with type 1 diabetes. In patients with type 2 diabetes, the respective figures were 29.0% for OGLDs-only, 36.6% for OGLDs-plus-insulin, and 46.7% for insulin-only subgroups. Moderate depressive symptoms (PHQ-9 score 10–19) were observed in 8–16% of patients with type 1 or type 2 diabetes. Female sex, complications, and low socioeconomic status were independently associated with depressive symptoms. In type 1 diabetes and in the type 2 diabetes OGLDs-only group, depression was associated with poor glycemic control.
CONCLUSIONS
Depressive symptoms are common in patients with diabetes from developing countries, calling for routine screening, especially in high-risk groups, to reduce the double burden of diabetes and depression and their negative interaction.
Introduction
Patients with type 1 and type 2 diabetes both have a high prevalence of depressive symptoms, with ∼30% having depressive symptoms and 11% having major depression (1). A systematic review of studies predominantly from the U.S. and Europe reported the prevalence of depression was threefold higher in patients with type 1 diabetes and nearly twofold higher in patients with type 2 diabetes compared with the general population (2). A U.S. study also showed that poor glycemic control, female sex, and low levels of general education were independent risk factors for major depressive symptoms (3).
There is emerging evidence regarding the bidirectional association between depression and diabetes. In some studies, depression was associated with an increased risk of type 2 diabetes, whereas others showed that diabetes was a risk factor for depression (4,5). While the causal nature of this association remains uncertain, depression in people with diabetes may lead to poor treatment adherence, suboptimal glycemic control, increased risks of microvascular and macrovascular complications, and increased health care costs (6–9). Studies from the U.S. show that depression in people with diabetes is also associated with an increased risk of premature death, especially among older adults (10,11). For these reasons, the American Diabetes Association and the International Diabetes Federation recommend screening for depressive symptoms in people with diabetes at the initial visit (12,13). American Diabetes Association guidelines suggest screening should be performed at periodic intervals and when there is a change in disease, treatment, or life circumstance (13).
Most data documenting the diabetes–depression link are from high-income countries, yet almost 80% of people with diabetes reside in developing countries (14). Our aim was to address this knowledge gap by using data from the International Diabetes Management Practices Study (IDMPS) to estimate the frequency of depressive symptoms and associated risk factors in people with type 1 and type 2 diabetes living in developing countries. The latter is an ongoing worldwide observational study program that describes patient profiles, management, and patterns of care over time in developing countries (15). Data for IDMPS have been collected in separate successive waves since 2005 and have provided new information on health care resource use and glycemic control and their associations with self-monitoring of blood glucose and patient education in people with diabetes living in developing countries (15–18). The present analysis explores the prevalence and associated risk factors of depressive symptoms in people with type 1 and type 2 diabetes by using data from the fifth wave of IDMPS, performed in 2011.
Research Design and Methods
Study Design and Procedures
The IDMPS is an ongoing international, multicenter, observational study that has collected data from adult patients with type 1 or type 2 diabetes in distinct waves since 2005 (15). Each wave consists of a 2-week cross-sectional survey period when data on patient profiles and routine care practices were collected. The fifth wave of IDMPS was conducted in 2011 by 879 physicians in 21 countries:
Africa: Algeria, Cameroon, Egypt, Morocco, Senegal, and Tunisia; Eurasia: Georgia, Kazakhstan, Russia, Ukraine, and Uzbekistan; Latin America: Argentina, Colombia, and Venezuela; Middle East: Jordan, Lebanon, Kingdom of Saudi Arabia, and United Arab Emirates; and South Asia: India, Pakistan; and Turkey.
Patients’ demographic and socioeconomic characteristics, medical history, previous and current treatments, glycemic control, cardiovascular risk factors, diabetes-related complications, diabetes-related education, and mode of follow-up were collected in case report forms.
In addition, patients were asked to complete the 9-item Patient Health Questionnaire (PHQ-9) to evaluate depressive symptoms (19). The PHQ-9 is a brief screening questionnaire used for screening patients for depressive symptoms. Each of the nine criteria for depression of the DSM-IV are scored as “0” (not at all) to “3” (nearly every day). PHQ-9 scores of 5–9, 10–14, 15–19, and ≥20 are suggestive of mild, moderate, moderately severe, and severe depression, respectively (19). A PHQ-9 score of <5 represents no depression (19). The study was conducted in accordance with the relevant ethical standards and was approved by appropriate regulatory and ethics committees in all participating countries and investigational centers.
Sanofi researchers and staff coordinated and monitored the study in each participating country, assisting the local coordinators and investigators in collecting data.
Statistical Analysis
Physicians with experience of prescribing insulin therapy were selected randomly after stratification based on specialty. The number of participating physicians recruited was based on the country-specific estimated patient sample size required. Each physician recruited the first 10 patients with type 2 diabetes and first 5 patients with type 1 diabetes who presented during routine clinical practice in a 2-week period. The patient sample size of IDMPS wave 5 was determined on a country basis, based on the primary objective of IDMPS, which was to assess the therapeutic management of patients with type 2 diabetes over time. In addition to the primary objective, each wave of IDMPS has a particular focus. In wave 5, we aimed to analyze the pattern of depressive symptoms in patients with type 1 and type 2 diabetes, and the results are reported here.
SAS 9.2 software (SAS Institute, Cary, NC) was used for statistical analysis. Descriptive analyses were performed in patients with type 1 and type 2 diabetes. The latter group was further analyzed according to treatment-type subgroups: oral glucose-lowering drugs (OGLDs) only, OGLDs plus insulin, and insulin only. In wave 5 of IDMPS, use of glucagon-like 1 receptor agonists was captured; however, only very few patients (1.3%) used this class of drug. As such, when considering the type 2 diabetes therapy subgroups, these patients were included in the OGLD-only, OGLD-plus-insulin, or insulin-only therapy subgroups.
All data are expressed as mean ± SD or median (interquartile range), as appropriate. Depression was defined as a PHQ-9 score of ≥5, with subcategories defined as follows: mild, PHQ-9 score of 5–9; moderate, PHQ-9 score of 10–19; and severe, PHQ-9 score of 20–27. Univariate logistic regression analyses were conducted to identify risk factors for depression. Potential risk factors included ethnicity, age, sex, living area, education level, health insurance, follow-up visit by a specialist, and specialty of the study physician. Other factors directly related to diabetes included disease duration, diabetes education, BMI, microvascular and macrovascular complications, self-management (self-monitoring of blood glucose and self-titration of insulin), insulin treatment, OGLD treatment, number of OGLDs, HbA1c level, and fasting blood glucose level. Log-linearity was assessed for the quantitative variables. If the assumption of log-linearity was not met, the corresponding categorical variable was considered in the model. Variables significant at a P value of ≤0.20 were considered for multivariate logistic regression models. A backward approach was used to test each potential risk factor in the multivariate model adjusted on the country expressed as the odds ratio with 95% CI. A P value of ≤0.05 (two-tailed) was considered to be significant. Interactions between significant variables selected by the multivariate model were tested.
Results
Complete PHQ-9 questionnaire data were collected from 9,865 eligible patients. Among them, 2,280 patients had type 1 diabetes, and 7,585 patients had type 2 diabetes, comprising 4,729 treated with OGLDs alone, 1,892 treated with OGLDs plus insulin, and 964 treated with insulin alone.
Patient Characteristics
Patients with type 2 diabetes were older than those with type 1 diabetes. Both groups had similar proportions of women (type 1 diabetes: 52.5%; type 2 diabetes subgroups: 52.0–54.9%). The mean ± SD disease duration was 11.8 ± 9.2 years in the type 1 diabetes group and ranged from 6.8 ± 6.1 to 12.6 ± 8.6 years in the type 2 diabetes subgroups. The proportion of patients with the last recorded HbA1c <7% (<53 mmol/mol) was 22.6% in the type 1 diabetes group. Among those with type 2 diabetes, HbA1c <7% (<53 mmol/mol) was recorded in 41.1% in the OGLD-only subgroup, in 16.5% in the OGLDs-plus-insulin subgroup, and in 18.5% in the insulin-only subgroup (Table 1). Obesity, hypertension, and dyslipidemia were more common in the type 2 diabetes group than in the type 1 diabetes group. In the latter group, 46.8% and 9.1% of patients had microvascular and macrovascular complications, respectively. Among patients with type 2 diabetes, the respective corresponding figures were 36.8% and 16.0% in the OGLDs-only subgroup, 64.3% and 28.4% in the OGLDs-plus-insulin subgroup, and 77.0% and 44.7% in the insulin-only subgroup.
Depressive Symptoms
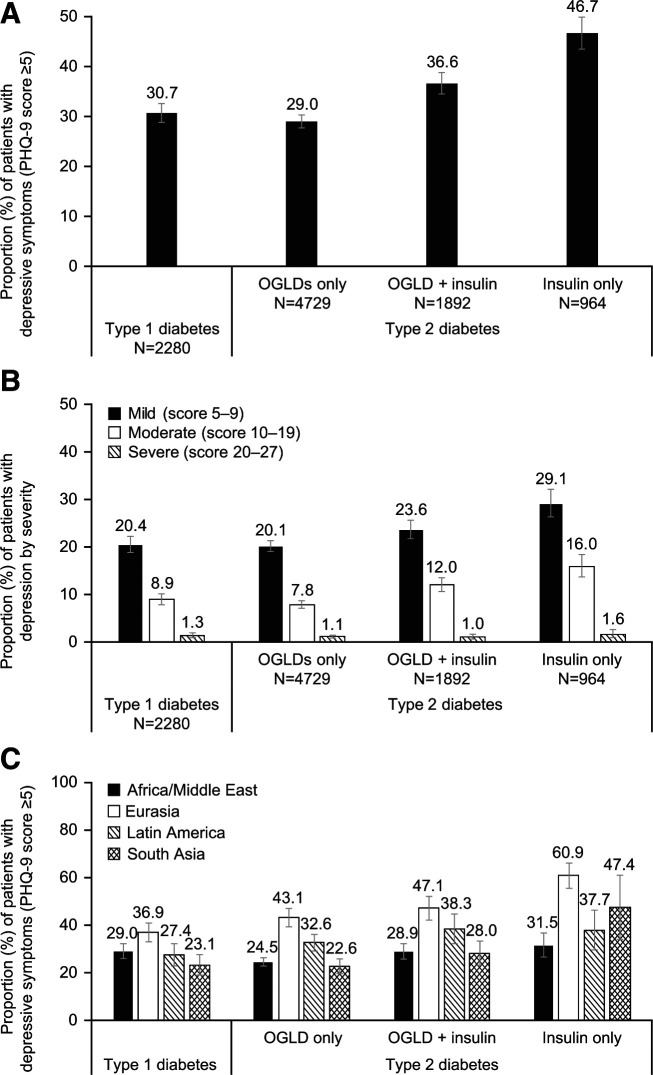
In the type 1 diabetes group, 30.7% (95% CI 28.8, 32.6) reported depressive symptoms (PHQ-9 score ≥5). Overall, 20.4% (95% CI 18.8, 22.2) of patients with type 1 diabetes reported mild symptoms (PHQ-9 score 5–9), 8.9% (95% CI 7.8, 10.1) reported moderate symptoms (PHQ-9 score 10–19), and 1.3% (95% CI 0.9, 1.9) reported severe symptoms (PHQ-9 score ≥20) (Fig. 1A and B). In patients with type 2 diabetes, 33.1% (95% CI 32.1, 34.2) reported depressive symptoms, 22.1% (95% CI 21.2, 23.0) reported mild symptoms, 9.9% (95% CI 9.3, 10.6) reported moderate symptoms, and 1.2% (95% CI 0.9, 1.4) reported severe symptoms (PHQ-9 score ≥20) (Fig. 1A and B). Higher proportions of insulin-treated patients with type 2 diabetes reported depressive symptoms compared with those treated with OGLDs only (36.6% [95% CI 34.5, 38.8] of patients receiving OGLDs plus insulin and 46.7% [95% CI 43.5, 49.9] of patients receiving insulin only vs. 29.0% [95% CI 27.7, 30.3] of patients receiving OGLDs only).
Figure 1.
Proportion of patients reporting depressive symptoms overall (A), by severity (B), and by geographical region (C). Error bars indicate 95% CI values. Africa/Middle East: Algeria, Cameroon, Egypt, Jordan, Lebanon, Morocco, Kingdom of Saudi Arabia, Senegal, Tunisia, and United Arab Emirates; Eurasia: Georgia, Kazakhstan, Russia, Ukraine, and Uzbekistan; Latin America: Argentina, Colombia, and Venezuela; and South Asia: India and Pakistan. Turkey was included in the overall sample but was not included in the regional analyses.
Eurasia had the highest proportion of patients reporting any depressive symptoms (PHQ-9 score ≥5) compared with Africa/Middle East, Latin America, or South Asia (Fig. 1C). In Eurasia and South Asia, respectively, ∼20% of patients treated with insulin reported a high score (PHQ-9 score ≥10), suggestive of major depression (Supplementary Table 1).
In the whole study population, Caucasians, women, and patients with microvascular or macrovascular complications were more likely to have depressive symptoms (PHQ-9 score ≥5). For both the type 1 and type 2 diabetes groups, patients with depressive symptoms also had longer diabetes duration and were more likely to have poor glycemic control, hypertension, and dyslipidemia than those without depressive symptoms (PHQ-9 score <5). Among all groups, except patients with type 2 diabetes receiving insulin only, depressive symptoms (PHQ-9 score <5) were more common in those with older age and lower education levels and were more likely to have obesity, hypertension, dyslipidemia, and poor glycemic control than those who did not have depressive symptoms. In the type 1 diabetes group, those living in a rural area or those not performing self-management behaviors were more likely to have depressive symptoms (PHQ-9 score ≥5). In patients with type 2 diabetes, depressive symptoms were more likely in those who were obese (Table 2).
Independent Risk Factors of Depressive Symptoms
After adjusting for confounders, among both the type 1 and type 2 diabetes groups, the presence of microvascular and macrovascular complications and female sex were independent risk factors for depressive symptoms (PHQ-9 score ≥5). For patients with type 1 diabetes, poor glycemic control and living in a rural area were further independent risk factors. Among patients with type 2 diabetes treated with OGLDs only, depressive symptoms (PHQ-9 score ≥5) were associated with poor glycemic control and older age. Among patients with type 2 diabetes treated with OGLDs plus insulin, depressive symptoms (PHQ-9 score ≥5) tended to be associated with low education level (Table 3).
Conclusions
The IDMPS aims to gather real-word data to identify knowledge and care gaps in order to inform health care decision makers to develop and implement strategies for optimizing diabetes care provision. In the fifth wave (2011), we focused on the diabetes–depression link and found a high prevalence of depressive symptoms in patients with type 1 or type 2 diabetes. We found 1 in 4 patients reported mild depressive symptoms (PHQ-9 score 5–9) and 1 in 10 reported moderately severe symptoms (PHQ-9 score 10–19). The highest prevalence of depressive symptoms (PHQ-9 score ≥5) was reported in insulin-treated patients with type 2 diabetes.
There were close associations between depressive symptoms (PHQ-9 score ≥5) and poor control of cardiovascular risk factors, including obesity, hypertension, dyslipidemia, and glycemic control. After adjusting for confounders, in both patients with type 1 or type 2 diabetes, we found female sex and the presence of microvascular or macrovascular complications were independent risk factors for depressive symptoms (PHQ-9 score ≥5). Living in rural areas (in patients with type 1 diabetes) and low educational levels (in patients with type 2 diabetes treated with OGLDs plus insulin) were also independent risk factors for depressive symptoms (PHQ-9 score ≥5). Poor glycemic control was a risk factor for depressive symptoms (PHQ-9 score ≥5) in patients with type 1 diabetes or type 2 diabetes treated with OGLDs only but not in insulin-treated patients with type 2 diabetes. Older age was an independent risk factor for depressive symptoms (PHQ-9 score ≥5), but only in patients with type 2 diabetes treated with OGLDs only.
Our findings are similar to a meta-analysis of 42 published studies involving 21,351 patients with type 1 or type 2 diabetes in which 31.0% of patients reported depressive symptoms and 11.4% had major depression. However, in this meta-analysis, minor depression was assessed using self-report scales, whereas major depressive disorder was measured using diagnostic interviews (1). Our results also align with population-based studies using the PHQ-9 questionnaire from the U.S. and Saudi Arabia. In the U.S. study of 4,193 patients with type 1 or type 2 diabetes, 12% had major depression and 8.5% had minor depression (3). In the study from Saudi Arabia of 385 people with type 2 diabetes, 37.6% had depressive symptoms (PHQ-9 score ≥10), with 4.2% reporting severe depression (20).
In our analysis, poor glycemic control was an independent risk factor for depressive symptoms (PHQ-9 score ≥5) in both patients with type 1 or type 2 diabetes. Our results concurred with some (21–23) but not all studies (24). This may be due to multidimensional factors such as host factors, treatment regimens, health care systems, quality of care, and ongoing support that may influence glycemic control (15). Poor control of hypertension and dyslipidemia were also found to be associated with depressive symptoms in the univariate analysis. Poor cardiometabolic risk profile in patients with diabetes and depression may further increase their long-term risk of complications (25,26). Indeed, cross-sectionally, we found that microvascular and macrovascular complications were independently associated with depressive symptoms (PHQ-9 score ≥5). Results from a meta-analysis assessing comorbid depression and risk of cardiac events/mortality in people with diabetes also showed that the risk of cardiovascular mortality, coronary heart disease, and stroke was significantly elevated (P ≤ 0.001 for all) in people with diabetes and comorbid depression (27). In patients with depression and negative emotions, poor adherence to diet, physical activity, and medication may contribute to suboptimal risk factor control (28). While depressive symptoms may be a consequence of low self-esteem due to poor control and complications, depression itself may be a barrier to intensified interventions for glycemic control and prevention of complications. In a randomized clinical trial including 214 patients with type 2 diabetes and depression, multidisciplinary care significantly improved both emotional health and cardiometabolic risk factors compared with usual care (29).
In this analysis, women and patients from low socioeconomic backgrounds were at increased risk of depressive symptoms. Studies have shown that these same individuals were less likely to achieve glycemic targets (30,31), raising the possibility that the co-occurrence of depressive symptoms might adversely affect self-management and glycemic control. In line with previous reports (32,33), this analysis found older age was associated with depressive symptoms, especially in patients with type 2 diabetes treated with OGLDs only. That said, older patients do not always present with the typical depressive symptoms, which may hinder the diagnosis of depression (34).
Living in a rural area was also a risk factor for development of depressive symptoms (PHQ-9 score ≥5) in patients with type 1 diabetes in our study. This may be due to scarcity of resources in such areas (e.g., less access to a diabetes care team/center, less contact with peers, less access to state-of-the-art self-monitoring) and potentially, poor knowledge about their disease, particularly for patients with type 1 diabetes.
Limitations
This is the first study measuring the prevalence of depressive symptoms using the same questionnaire and cutoff values in a large multinational population of patients with type 1 or type 2 diabetes living in developing countries. However, our results should be considered with caution. Observational studies are limited by selection bias and confounding factors. The lack of prospective and interventional data, a comparative group, and the cross-sectional nature of the study precludes assessment of causality, although these real-world data are applicable to a broader population (35). Differences in the size of relevant treatment subgroups, for example, by region or country or treatment type, precluded any formal statistical analyses. We would also note that in cases where statistical analyses were conducted (e.g., univariate/multivariate logistic regression analyses for identification of risk factors for depression), differences in sample sizes between subgroups could have led to different statistical power to detect possible associations and may have had an impact on results. For example, larger populations were available for patients with type 1 diabetes and patients with type 2 diabetes treated with OGLDs only versus OGLDs plus insulin or insulin only.
The PHQ-9 questionnaire is a common tool used to identify depressive symptoms in primary care settings (19). In the International Diabetes Federation practice guidelines, PHQ scores were recommended for screening, followed by a full evaluation by a specialist as appropriate. The performance of different screening tools can vary with the PHQ-9 questionnaire, yielding a higher prevalence of depression, especially for severe depression, compared with other tools such as the Hospital Anxiety and Depression Scale (36). PHQ-9 response rates may also be influenced by patient education level (37). In this light, diagnostic interviews remain the gold standard for diagnosis of a depressive disorder, although they are time consuming and require qualified clinicians for their administration. In busy settings, even the administration of PHQ-9 may not be feasible. A recent meta-analysis found that a shortened version of the PHQ questionnaire, the PHQ-2 questionnaire, which comprises two questions relating to “little interest or pleasure in doing things” and “feeling down, depressed, or hopeless,” had similar validity to that of the PHQ-9 and may be useful in underresourced areas (38).
In conclusion, using real-world data collected from developing countries, we confirmed similarly high proportions of patients with type 1 or type 2 diabetes with mild (∼20%) and moderate to severe (∼10%) depressive symptoms as observed in developed countries, especially in women and those with low socioeconomic status as well as older individuals and those with poor cardiometabolic control and complications. These results add to the body of evidence that supports the need to perform routine screening for depressive symptoms in all patients with type 1 or type 2 diabetes. Support should be provided alongside multidisciplinary care to identify and alleviate sources of depression. Patients reporting major depressive symptoms should be referred for psychologic or psychiatric evaluation. This type of multidisciplinary approach may help to reduce the double burden of diabetes and depression.
Article Information
Acknowledgments. The authors thank the physicians and patients who participated in the study. They also acknowledge medical writing and editorial assistance provided by Yann Bourhis, of ICON PLC, and Hannah Brown, of Fishawack Communications Ltd, a Fishawack Health company, whose service was funded by Sanofi. Coordination of the development of this manuscript and assistance with the revision was provided by Cecile Baradez of Sanofi.
Duality of Interest. The study was funded by Sanofi. Support with editing the manuscript was provided by Fishawack Communications Ltd (Knutsford, Cheshire, U.K.), a Fishawack Health company, funded by Sanofi. P.A., J.J.G., H.I., F.L., A.R., J.C.M., M.S., and J.C.N.C. are all members of the IDMPS steering committee and have received honoraria for travel grants and speakers’ fees by Sanofi. Y.B. is an employee of ICON PLC contracted by Sanofi. J.-M.C. is an employee of Sanofi. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. All authors contributed to the drafting of the paper, analysis and interpretation of data, and provided final approval to submit. Y.B. performed statistical analyses. P.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 52nd European Association for the Study of Diabetes (EASD) Annual Meeting, Munich, Germany,12–16 September 2016.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14098679.
References
- 1. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 2. Roy T, Lloyd CE. Epidemiology of depression and diabetes: a systematic review. J Affect Disord 2012;142(Suppl.):S8–S21 [DOI] [PubMed] [Google Scholar]
- 3. Katon W, von Korff M, Ciechanowski P, et al. Behavioral and clinical factors associated with depression among individuals with diabetes. Diabetes Care 2004;27:914–920 [DOI] [PubMed] [Google Scholar]
- 4. Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 2006;49:837–845 [DOI] [PubMed] [Google Scholar]
- 5. Mezuk B, Eaton WW, Albrecht S, Golden SH. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care 2008;31:2383–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pouwer F, Nefs G, Nouwen A. Adverse effects of depression on glycemic control and health outcomes in people with diabetes: a review. Endocrinol Metab Clin North Am 2013;42:529–544 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez JS, Peyrot M, McCarl LA, et al. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care 2008;31:2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lustman PJ, Clouse RE. Depression in diabetic patients: the relationship between mood and glycemic control. J Diabetes Complications 2005;19:113–122 [DOI] [PubMed] [Google Scholar]
- 9. Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464–470 [DOI] [PubMed] [Google Scholar]
- 10. Katon W, Fan MY, Unützer J, Taylor J, Pincus H, Schoenbaum M. Depression and diabetes: a potentially lethal combination. J Gen Intern Med 2008;23:1571–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimbro LB, Mangione CM, Steers WN, et al. Depression and all-cause mortality in persons with diabetes mellitus: are older adults at higher risk? Results from the Translating Research Into Action for Diabetes Study. J Am Geriatr Soc 2014;62:1017–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Diabetes Federation . IDF Clinical Practice Recommendations for Managing Type 2 Diabetes in Primary Care, 2018. Accessed 7 March 2020. Available from https://idf.org/e-library/guidelines/128-idf-clinical-practice-recommendations-for-managing-type-2-diabetes-in-primary-care.html
- 13. American Diabetes Association . Introduction: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S1–S2 [DOI] [PubMed] [Google Scholar]
- 14. International Diabetes Federation . IDF Diabetes Atlas 2019, 9th edition, 2019. Accessed 6 November 2020. Available from https://www.diabetesatlas.org/en/resources/ [PubMed]
- 15. Chan JCN, Gagliardino JJ, Baik SH, et al.; IDMPS Investigators . Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS). Diabetes Care 2009;32:227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aschner P, Gagliardino JJ, Ilkova H, et al. Persistent poor glycaemic control in individuals with type 2 diabetes in developing countries: 12 years of real-world evidence of the International Diabetes Management Practices Study (IDMPS). Diabetologia 2020;63:711–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gagliardino JJ, Aschner P, Baik SH, et al.; IDMPS investigators . Patients’ education, and its impact on care outcomes, resource consumption and working conditions: data from the International Diabetes Management Practices Study (IDMPS). Diabetes Metab 2012;38:128–134 [DOI] [PubMed] [Google Scholar]
- 18. Gagliardino JJ, Atanasov PK, Chan JCN, et al. Resource use associated with type 2 diabetes in Africa, the Middle East, South Asia, Eurasia and Turkey: results from the International Diabetes Management Practice Study (IDMPS). BMJ Open Diabetes Res Care 2017;5:e000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albasheer OB, Mahfouz MS, Solan Y, et al. Depression and related risk factors among patients with type 2 diabetes mellitus, Jazan area, KSA: a cross-sectional study. Diabetes Metab Syndr 2018;12:117–121 [DOI] [PubMed] [Google Scholar]
- 21. Ciechanowski PS, Katon WJ, Russo JE, Hirsch IB. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry 2003;25:246–252 [DOI] [PubMed] [Google Scholar]
- 22. Wagner JA, Abbott GL, Heapy A, Yong L. Depressive symptoms and diabetes control in African Americans. J Immigr Minor Health 2009;11:66–70 [DOI] [PubMed] [Google Scholar]
- 23. Werremeyer A, Maack B, Strand MA, Barnacle M, Petry N. Disease control among patients with diabetes and severe depressive symptoms. J Prim Care Community Health 2016;7:130–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor S, McDermott R, Thompson F, Usher K. Depression and diabetes in the remote Torres Strait Islands. Health Promot J Austr 2017;28:59–66 [DOI] [PubMed] [Google Scholar]
- 25. Gami AS, Witt BJ, Howard DE, et al. Metabolic syndrome and risk of incident cardiovascular events and death: a systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol 2007;49:403–414 [DOI] [PubMed] [Google Scholar]
- 26. Nathan DM; DCCT/EDIC Research Group . The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care 2014;37:9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Farooqi A, Khunti K, Abner S, Gillies C, Morriss R, Seidu S. Comorbid depression and risk of cardiac events and cardiac mortality in people with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2019;156:107816. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Y, Ting RZ, Yang W, et al.; China Depression in Chinese Patients with Type 2 Diabetes (DD2) Study Group . Depression in Chinese patients with type 2 diabetes: associations with hyperglycemia, hypoglycemia, and poor treatment adherence. J Diabetes 2015;7:800–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010;363:2611–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cheng LJ, Wang W, Lim ST, Wu VX. Factors associated with glycaemic control in patients with diabetes mellitus: a systematic literature review. J Clin Nurs 2019;28:1433–1450 [DOI] [PubMed] [Google Scholar]
- 31. Kautzky-Willer A, Kosi L, Lin J, Mihaljevic R. Gender-based differences in glycaemic control and hypoglycaemia prevalence in patients with type 2 diabetes: results from patient-level pooled data of six randomized controlled trials. Diabetes Obes Metab 2015;17:533–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rodríguez Calvín JL, Zapatero Gaviria A, Martín Ríos MD. Prevalence of depression in type 2 diabetes mellitus. Rev Clin Esp 2015;215:156–164 [DOI] [PubMed] [Google Scholar]
- 33. Mir K, Mir K, Malik I, Shehzadi A. Prevalence of co-morbid depression in diabetic population. J Ayub Med Coll Abbottabad 2015;27:99–101 [PubMed] [Google Scholar]
- 34. Park M, Reynolds CF III. Depression among older adults with diabetes mellitus. Clin Geriatr Med 2015;31:117–137, ix [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hannan EL. Randomized clinical trials and observational studies: guidelines for assessing respective strengths and limitations. JACC Cardiovasc Interv 2008;1:211–217 [DOI] [PubMed] [Google Scholar]
- 36. Hansson M, Chotai J, Nordstöm A, Bodlund O. Comparison of two self-rating scales to detect depression: HADS and PHQ-9. Br J Gen Pract 2009;59:e283–e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reddy P, Philpot B, Ford D, Dunbar JA. Identification of depression in diabetes: the efficacy of PHQ-9 and HADS-D. Br J Gen Pract 2010;60:e239–e245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levis B, Sun Y, He C, et al.; Depression Screening Data (DEPRESSD) PHQ Collaboration . Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA 2020;323:2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]