Abstract
OBJECTIVE
Children exposed to gestational diabetes mellitus (GDM) or maternal obesity in utero have an increased propensity to develop obesity. Little is known about the mechanisms underlying this phenomenon. We aimed to examine relationships between exposure to GDM or maternal obesity and daily energy intake (EI), brain responses to food cues within reward regions, and adiposity in children.
RESEARCH DESIGN AND METHODS
Participants were 159 children ages 7–11 years. Repeated 24-h recalls were conducted to assess mean daily EI. A subset of children (n = 102) completed a food cue task in the MRI scanner. A priori regions of interest included the orbital frontal cortex (OFC), insula, amygdala, ventral striatum, and dorsal striatum. Adiposity measurements, BMI z-scores, percent body fat, waist-to-height ratio (WtHR), and waist-to-hip ratio (WHR) were assessed.
RESULTS
Exposure to GDM was associated with greater daily EI, and children exposed to GDM diagnosed before 26 weeks gestation had greater OFC food cue reactivity. Children exposed to GDM also had larger WHR. Results remained significant after adjusting for child’s age and sex, maternal education and race/ethnicity, maternal prepregnancy BMI, and child’s physical activity levels. Furthermore, children who consumed more daily calories had greater WHR, and the relationship between GDM exposure and WHR was attenuated after adjustment for daily EI. Prepregnancy BMI was not significantly related to daily EI or food cue reactivity in reward regions. However, prepregnancy BMI was significantly related to all adiposity measurements; results remained significant for BMI z-scores, WtHR, and WHR after controlling for child’s age and sex, maternal education and race/ethnicity, maternal GDM exposure, and child’s physical activity levels.
CONCLUSIONS
Exposure to GDM in utero, in particular before 26 weeks gestation, is associated with increased EI, enhanced OFC food cue reactivity, and increased WHR. Future study with longitudinal follow-up is merited to assess potential pathways of daily EI and food cue reactivity in reward regions on the associations between GDM exposure and childhood adiposity.
Introduction
The rate of childhood obesity has more than tripled over the past four decades, increasing from 5% in 1978 to 18.5% in 2016 (1). Pediatric obesity has a high prevalence of persistence into adulthood, increasing the risk for type 2 diabetes, cardiovascular disease, and cancer (2–4). Therefore, risk factors contributing to childhood obesity are important to identify to guide early interventions and to mitigate these alarming trends.
Children exposed to gestational diabetes mellitus (GDM) or maternal obesity in utero have an increased propensity to develop obesity (5,6). However, the mechanisms underlying the relationship between fetal exposure to GDM and/or maternal obesity and increased obesity risk are poorly understood. Excessive food intake plays a central role in the development of obesity. Thus, increased energy intake (EI) may be one factor contributing to an increased risk for children exposed to GDM and/or maternal obesity to develop obesity, a hypothesis that we set out to test in the current study.
A growing body of research indicates that food intake is the result of a dynamic interplay among homeostatic and reward systems (7,8). A key neural region that supports the homeostatic system is the hypothalamus, which is critical for metabolic regulation of hunger and satiety. Key neural regions in the reward system include the striatum, amygdala, insula, and orbital frontal cortex (OFC). These reward regions are critical for incentive motivational effects of food rewards. The neuroscience of the obesity field has made substantial progress in understanding brain mechanisms underlying homeostatic and reward regulation of eating and how dysfunctions in these systems contribute to obesity (9–14). To date, accumulating evidence suggests two major central nervous system contributors to obesity: 1) altered hypothalamic structure and function (15–22) and 2) altered responses (hyper- or hyporesponsiveness) to palatable food or food cues potentiated by the brain’s reward network (23–42). We do not yet know how abnormalities in brain reward systems contribute to obesity risk in children exposed to GDM or maternal obesity. It is possible that alterations in the brain reward system induced by exposure to GDM or maternal obesity during early fetal development may lead to increases in food intake, resulting over time in obesity. Animal and human studies have suggested that intrauterine exposure to maternal diabetes or maternal obesity leads to abnormal organization of the hypothalamic feeding circuits, and this leads to increased food intake and predisposes offspring to obesity (43–48). Furthermore, in a human imaging study (48), children exposed to GDM before 26 weeks of gestation exhibited altered hypothalamic activity compared with unexposed children, suggesting that the timing and/or severity of GDM exposure played an important role in the development of homeostatic circuitry. Little is known about whether GDM or maternal obesity exposure is related to altered activation in the brain’s reward systems.
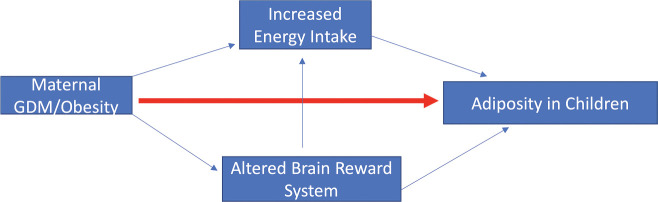
We hypothesized that intrauterine exposure to GDM or maternal obesity may be associated with greater daily EI and food cue reactivity in reward regions, which in turn leads to greater adiposity in children (Fig. 1). While formal testing of our hypothesis will require longitudinal studies, in this study, we conducted data analyses in a well-characterized cross-sectional cohort to provide preliminary data to examine our conceptual model.
Figure 1.
Proposed pathways for the link between maternal GDM/obesity exposure and increased adiposity in children.
Research Design and Methods
Participants
Children between the ages of 7 and 11 years, who were born at a Kaiser Permanente Southern California (KPSC) hospital, with documented exposure to maternal GDM or normal glucose levels during pregnancy were recruited for the BrainChild Study (48). GDM-exposed children were recruited first, then unexposed children were recruited by frequency matching with GDM-exposed participants on the child’s age and sex and maternal ethnicity/race. The KPSC electronic medical record (EMR) was used as a source for recruiting potential participants. Children were excluded if they had a history of medical or psychiatric disorders, used medications known to alter metabolism, had contraindications or complications preventing brain MRI scan acquisition, or were left handed to avoid potential effects of handedness on the brain findings, which could introduce variability into the results. As well, children exposed to preexisting maternal diabetes were excluded by ICD-9-CM diagnosis code 250 and/or use of antidiabetic medications prior to pregnancy. Neighborhood household income and maternal education at birth were obtained from the EMR.
Each participating institutional review board approved this study (University of Southern California [USC] # HS-14-00034 and KPSC # 10282). Participants’ parents gave written informed consent, while children provided informed assent.
Maternal Exposure
Diagnosis of GDM was based on laboratory glucose values confirming a plasma glucose level ≥200 mg/dL from the 50-g glucose challenge test or at least two plasma glucose values meeting or exceeding the following values on the 100-g or 75-g oral glucose tolerance test: fasting, 95 mg/dL; 1 h, 180 mg/dL; 2 h, 155 mg/dL; and 3 h, 140 mg/dL (49). Gestational age at GDM diagnosis was calculated using the date of the first glucose test result that met the GDM diagnosis criteria, date of delivery, and gestational age at delivery available in the EMR. KPSC follows the American Congress of Obstetricians and Gynecologists guidelines for screening for GDM (50). Maternal prepregnancy BMI was calculated from maternal height and weight measurements closest to last menstrual period from the EMR.
In-Person Visits
This study included two in-person visits. An overview of the study design is presented in Supplementary Fig. 1.
First Visit
The first visit occurred at the Clinical Research Unit of the USC Diabetes and Obesity Research Institute. Adiposity measurements, including BMI, BMI z-scores (age- and sex-specific SD scores), percent body fat (%BF), waist circumference, waist-to-hip ratio (WHR), and waist-to-height ratio (WtHR) were assessed. Details of adiposity assessment are included in the Supplementary Materials. Tanner stage was assessed by physical examination and/or by a validated sex-specific assessment questionnaire for children and parents. Sixty-two children had both self-report and physician evaluations of Tanner staging, with a correlation coefficient of 0.86.
Dietary Intake.
In-person 24-h dietary recalls were administered by trained staff to measure daily EI. The average number of recalls per person was two. Three participants did not have dietary intake data, leaving a sample size for dietary intake of n = 156. A standardized protocol that was based on the “multiple pass” method was used for dietary recall in which a face-to-face interview with the child (assisted with his/her parent) was performed. The multiple pass method is an attempt to limit the extent of underreporting (51). Participants were asked to provide information on all foods, drinks, and dietary supplements consumed in the past 24 h; details such as time of day, source and type of food, portion size, and preparation method were captured to provide a comprehensive report of dietary intake. Nutrition data were analyzed using the Nutrition Data System for Research, a program developed by the Nutrition Coordinating Center at the University of Minnesota (52). Mean daily EI was derived and used in this study. Data from dietary recalls were manually checked for quality and outliers. We excluded dietary recall records that exceeded 3 SDs of the expected mean value on the basis of a regression of body weight versus daily EI (53,54). Using this method, two recalls were excluded. After removing outliers, repeated dietary recalls were averaged to estimate mean daily EI.
Physical Activity.
Physical activity was assessed using the 3-Day Physical Activity Recall (55,56), and moderate to vigorous physical activity (MVPA) was the physical activity variable included in this study. MVPA was classified on the basis of the activity’s MET determined by the compendium of energy expenditure (55,56). Activities with METs ≥3 were classified as MVPA. The final output was average minutes per day spent in MVPA (square root transformed as a result of the skewed distribution).
Second Visit
The second visit occurred at the USC Dana and David Dornsife Cognitive Neuroimaging Center. Height, weight, and dietary intake data were collected again during this visit. Participants were trained on a mock scanner to familiarize them with the MRI procedures in a Siemens MAGNETOM Prisma Fit 3T MRI scanner with a 20-channel head coil. All participants were scanned between 8:00 a.m. and 10:00 a.m. after a 12-h overnight fast, which helped to provide a standardized time since last meal intake. Participants completed a food cue task in the scanner while brain signal was simultaneously collected during blood-oxygen-level-dependent functional scanning. A high-resolution structural image was also collected to be used for registration. Fifty-three of these participants’ food cue data were included in a prior report (57). Imaging data acquisition parameters can be found in Luo et al. (57). Details of food cue task and imaging analysis are included in the Supplementary Materials.
Statistical Analysis
Maternal Exposure and Food Intake
Generalized linear models (GLMs) were used to examine relationships between exposure to GDM or maternal obesity and mean daily EI. We started models without any adjustment of covariates (model 0), then with adjustment for demographic variables, including the child’s age and sex, maternal education (categorical variable college or not), and maternal race/ethnicity (categorical variable Hispanic or not) (model 1), followed by additional adjustment for maternal other exposure (i.e., prepregnancy BMI as a covariate for GDM models and GDM as a covariate for prepregnancy BMI models) (model 2), and then further adjustment for child’s physical activity level (i.e., MVPA) (model 3). Since >90% of children were prepubertal, we did not control for puberty status in our models.
GDM exposure was modeled in two ways: 1) yes or no to exposure and 2) GDM diagnosed at ≤26 weeks (early exposed), >26 weeks (late exposed), and unexposed while following the same temporal cutoff values from prior studies (48,58). Prepregnancy BMI was modeled as a continuous variable (in every 5 units) and used as an index of in utero exposure to varying levels of maternal obesity. Because a prior study showed sex-specific effects of prenatal exposure to impaired glucose tolerance on food intake (59), we further tested for interactions between GDM/maternal obesity exposures and child’s sex on food intake. Analysis was stratified by sex if the interaction was found to be significant.
Maternal Exposure and Food Cue Reactivity
Region-of-Interest Analysis.
Anatomical, bilateral a priori regions of interest (ROIs) of the OFC, insula, amygdala, and ventral and dorsal striatum were defined using the Harvard-Oxford Cortical and Subcortical Structural Atlas, with probability threshold ≥50%. Percent signal change in each ROI was extracted from food versus nonfood contrasts using Featquery. GLMs were used to examine relationships between maternal exposure and food cue reactivity in each ROI (unadjusted for multiple comparisons) using the same analysis approach as described above.
Whole-Brain Analysis.
Whole-brain exploratory analysis was performed to examine relationships between maternal exposure to GDM or maternal obesity and food cue reactivity with and without covariates in the models. All whole-brain analyses were corrected for multiple comparisons using Z >3.1 and P < 0.05.
Maternal Exposure and Adiposity
GLMs were used to investigate relationships between exposure to GDM or maternal obesity and adiposity measures. The same analysis approach as described above was used.
Daily EI, Brain Food Cue Reactivity, and Adiposity
Regression analyses were performed to examine relationships among daily EI, brain food cue reactivity, and adiposity measures. All analyses were performed using SAS 9.4 statistical software (SAS Institute, Cary, NC). Results with P < 0.05 were considered significant.
Results
Participant Characteristics
One hundred fifty-nine children were enrolled in this study, 0.6% were underweight, 56.6% were of normal weight, 14.5% were overweight, and 28.3% were obese. GDM-exposed children were younger than unexposed children (details see Table 1). Groups were not significantly different in other demographic variables except for maternal education (Table 1). A subset of children (n = 102) was included in the final imaging data analysis, and their characteristics are reported in Supplementary Table 1. There were no significant differences in any demographic variables between children included in the imaging analysis and those excluded (Supplementary Table 2).
Table 1.
Participant characteristics
| Characteristic | Overall (N = 159) | GDM* (n = 87) | Unexposed (n = 72) | P value** |
|---|---|---|---|---|
| Child | ||||
| Age (years) | 8.5 ± 0.96 | 8.4 ± 0.84 | 8.7 ± 1.05 | 0.02 |
| Sex | 0.88 | |||
| Female | 96 (60.4) | 53 (60.9) | 43 (59.7) | |
| Male | 63 (39.6) | 34 (39.1) | 29 (40.3) | |
| Tanner staging | 0.86 | |||
| 1 | 144 (90.6) | 80 (92) | 64 (88.9) | |
| 2 | 10 (6.3) | 5 (5.7) | 5 (6.9) | |
| 3 | 4 (2.5) | 2 (2.3) | 2 (2.8) | |
| 4 | 1 (0.6) | 0 (0.0) | 1 (1.4) | |
| BMI (kg/m2) | 19.1 ± 4.19 | 19.5 ± 4.71 | 18.5 ± 3.40 | 0.13 |
| BMI z-score | 0.8 ± 1.10 | 0.9 ± 1.15 | 0.7 ± 1.03 | 0.21 |
| Total body fat (%)*** | 25.4 ± 8.73 | 26.5 ± 9.49 | 24.2 ± 7.57 | 0.11 |
| Waist circumference (cm) | 64.9 ± 11.15 | 66.0 ± 12.15 | 63.6 ± 9.72 | 0.18 |
| Hip circumference (cm) | 73.9 ± 9.72 | 74.2 ± 10.50 | 73.5 ± 8.75 | 0.65 |
| Height (cm) | 132.3 ± 8.72 | 131.5 ± 8.04 | 133.3 ± 9.44 | 0.21 |
| WHR | 0.87 ± 0.06 | 0.89 ± 0.06 | 0.86 ± 0.05 | 0.01 |
| WtHR | 0.49 ± 0.07 | 0.50 ± 0.08 | 0.48 ± 0.06 | 0.03 |
| EI (kcal/day) | 1,741.4 ± 386.15 | 1,664.2 ± 367.23 | 1,805.9 ± 391.83 | 0.02 |
| MVPA (min/day) | 142.03 ± 97.15 | 136.23 ± 98.21 | 146.69 ± 96.61 | 0.31 |
| OFC food cue reactivity (% signal change) | 0.024 ± 0.15 | –0.004 ± 0.15 | 0.05 ± 0.15 | 0.07 |
| Maternal | ||||
| Prepregnancy BMI group | 0.85 | |||
| Normal weight (<25 kg/m2) | 39 (24.5) | 20 (23.0) | 19 (26.4) | |
| Overweight (≥25 and <30 kg/m2) | 48 (30.2) | 26 (29.9) | 22 (30.6) | |
| Obese (≥30 kg/m2) | 72 (45.3) | 41 (47.1) | 31 (43.1) | |
| Race/ethnicity | 0.24 | |||
| Hispanic | 92 (57.9) | 55 (63.2) | 37 (51.4) | |
| Non-Hispanic Black | 21 (13.2) | 9 (10.3) | 12 (16.7) | |
| Non-Hispanic White | 32 (20.1) | 14 (16.1) | 18 (25.0) | |
| Other | 14 (8.8) | 9 (10.3) | 5 (6.9) | |
| Income group at birth ($) | 0.56 | |||
| <30,000 | 25 (15.7) | 14 (16.1) | 11 (15.3) | |
| 30,000–50,000 | 48 (30.2) | 24 (27.6) | 24 (33.3) | |
| 50,000–70,000 | 46 (28.9) | 29 (33.3) | 17 (23.6) | |
| 70,000–90,000 | 22 (13.8) | 12 (13.8) | 10 (13.9) | |
| ≥90,000 | 16 (10.1) | 8 (9.2) | 8 (11.1) | |
| Missing | 2 (1.3) | 0 (0.0) | 2 (2.8) | |
| Education | 0.01 | |||
| High school or less | 35 (22.0) | 27 (31.0) | 8 (11.1) | |
| Some college | 50 (31.4) | 26 (29.9) | 24 (33.3) | |
| College and postgraduate | 72 (45.3) | 34 (39.1) | 38 (52.8) | |
| Unknown | 2 (1.3) | 0 (0.0) | 2 (2.8) |
Data are mean ± SD or n (%). Boldface indicates significance at P < 0.05.
Among the 87 GDM-exposed children, 29 were diagnosed ≤26 weeks and 58 diagnosed >26 weeks.
Categorical variables by χ2 or Fisher exact test, continuous variables by t test.
One child is missing %BF.
Prenatal Exposures and Child Daily EI
Group comparison results are presented in mean group difference ± SE throughout. GDM-exposed children consumed 1,850.9 ± 41.31 daily calories, and unexposed children consumed 1,664.25 ± 45.20 daily calories; the group difference was significant (141.65 ± 61.23 kcal/day; P = 0.022). The group differences became more pronounced after controlling for demographic covariates (GDM: 1,838.81 ± 42.63 kcal/day; unexposed: 1,661.92 ± 45.51 kcal/day; group difference: 176.89 ± 62.38 kcal/day; P = 0.005). Additional adjustment of prepregnancy BMI had minimal effects on results (GDM: 1,838.23 ± 42.88 kcal/day; unexposed: 1,662.68 ± 45.84 kcal/day; group difference: 175.56 ± 63.00 kcal/day; P = 0.006). Results remained significant after additional adjustment of child MVPA (GDM: 1,832.82 ± 43.15 kcal/day; unexposed: 1,667.68 ± 46.47 kcal/day; group difference: 165.13 ± 63.89 kcal/day; P = 0.011). When we limited the data analysis to children who had quality imaging data, GDM exposure remained significantly associated with higher EI (GDM: 1,826.95 ± 50.32 kcal/day; unexposed: 1,655.12 ± 50.81 kcal/day; group difference: 171.83 ± 71.75 kcal/day; P = 0.02) (Supplementary Table 3). The early GDM-exposed group and late GDM-exposed group had greater EI than unexposed children (early exposed: 1,859.79 ± 76.26 kcal/day; late exposed: 1,819.95 ± 52.63 kcal/day; unexposed: 1,666.71 ± 46.65 kcal/day; early exposed vs. unexposed: 193.08 ± 91.31 kcal/day, P = 0.036; late exposed vs. unexposed: 153.24 ± 69.79 kcal/day, P = 0.030) (Fig. 2A) after adjusting for demographic variables as well as prepregnancy BMI and child MVPA. Prepregnancy BMI was not significantly associated with daily EI in all models (all P > 0.1). Detailed results are presented in Table 2.
Figure 2.
A: Adjusted mean daily EI in the unexposed group, late-exposed group (GDM diagnosed >26 weeks), and early-exposed group (GDM diagnosed ≤26 weeks) (n= 71 for unexposed group, n = 29 for early-exposed group, and n = 56 for late-exposed group). Three participants had missing physical activity data, leaving n = 69 for unexposed group, n = 29 for early-exposed group, and n = 55 for late-exposed group. Both the early-exposed and the late-exposed groups were significantly different from the unexposed group in daily EI. B: Brain ROI analysis demonstrates adjusted mean % signal change in the OFC responses to food vs. non-food cues in the unexposed group, late-exposed group, and early-exposed group (n = 49 for unexposed group, n = 13 for early-exposed group, and n = 40 for late-exposed group). Three participants had missing physical activity data, leaving n = 47 for unexposed group, n = 13 for early-exposed group, and n = 39 for late-exposed group. The early-exposed group was significantly different from the unexposed group in OFC the food cue reactivity. However, GDM exposure as a whole was not significantly different from the unexposed group in the OFC responses to food vs. non-food cues. Covariates included child’s age and sex, maternal education, maternal race/ethnicity, maternal prepregnancy BMI, and child’s MVPA. *P < 0.05. LS, least significant.
Table 2.
Relationships between prenatal exposures and daily EI in children
| Model | GDM vs. unexposed | Prepregnancy BMI | ||
|---|---|---|---|---|
| β (SE)* | P value | β (SE)* | P value | |
| Model 0 | 141.65 (61.23) | 0.022 | 5.04 (20.84) | 0.809 |
| Model 1 | 176.89 (62.38) | 0.005 | 10.55 (21.21) | 0.62 |
| Model 2 | 175.56 (63.00) | 0.006 | 3.84 (20.89) | 0.854 |
| Model 3 | 165.13 (63.89) | 0.011 | 2.52 (20.98) | 0.905 |
Model 0, unadjusted; model 1, adjusted for child’s age and sex, maternal education, and maternal race/ethnicity; model 2, model 1 + maternal other exposure (prepregnancy BMI for GDM or GDM status for prepregnancy BMI); model 3, model 2 + child’s physical activity level. Boldface indicates significance at P < 0.05.
Regression coefficient (SE) from linear regression models. For GDM-related results, a positive β means greater daily EI in the GDM group than in the unexposed group, whereas a negative β means the opposite data pattern. For prepregnancy BMI-related results, a positive β means a positive relationship between prepregnancy BMI and daily EI, whereas a negative β means a negative relationship.
Prenatal Exposures and Child Brain Reward Responses to Food Cues
ROI Analysis
GDM exposure was marginally associated with greater OFC responses to food cues (0.054 ± 0.030% signal change; P = 0.071). Adjusting for demographic variables as well as prepregnancy BMI and child MVPA attenuated the results (all P > 0.1) (Table 3). The early GDM-exposed group versus the unexposed group had greater OFC food cue reactivity (0.115 ± 0.047% signal change; P = 0.015), and the results remained significant after controlling for demographic covariates (0.120 ± 0.049% signal change; P = 0.016). Additional adjustment for prepregnancy BMI had minimal effects on the results (0.119 ± 0.050% signal change; P = 0.019). Results remained significant after additional adjustment for child MVPA (0.115 ± 0.051% signal change; P = 0.026) (Fig. 2B). The late GDM-exposed group was not different from unexposed children in all models (all P > 0.1). We did not observe significant group differences in other brain ROIs (all P > 0.1) (Supplementary Tables 4–7). Prepregnancy BMI was not related to food cue reactivity in any of the brain ROIs (all P > 0.1) (Table 3 and Supplementary Tables 3–6).
Table 3.
Relationships between prenatal exposures and OFC responses to food cues (relative to non-food cues)
| Model | GDM vs. unexposed | Prepregnancy BMI | ||
|---|---|---|---|---|
| β (SE)* | P value | β (SE)* | P value | |
| Model 0 | 0.054 (0.030) | 0.071 | 0.006 (0.011) | 0.611 |
| Model 1 | 0.052 (0.031) | 0.102 | 0.005 (0.011) | 0.663 |
| Model 2 | 0.051 (0.032) | 0.106 | 0.005 (0.011) | 0.693 |
| Model 3 | 0.050 (0.032) | 0.124 | 0.005 (0.012) | 0.683 |
Model 0, unadjusted; model 1, adjusted for child’s age and sex, maternal education, and maternal race/ethnicity; model 2, model 1 + maternal other exposure (prepregnancy BMI for GDM or GDM status for prepregnancy BMI); model 3, model 2 + child’s physical activity level.
Regression coefficient (SE) from linear regression models. For GDM-related results, a positive β means greater OFC responses to food vs. non-food cues in the GDM group than in the unexposed group, whereas a negative β means the opposite data pattern. For prepregnancy BMI-related results, a positive β means a positive relationship between prepregnancy BMI and OFC responses to food vs. non-food cues, whereas a negative β means a negative relationship.
Whole-Brain Analysis
As expected, brain regions involved in reward signaling, including the OFC, insula, amygdala, dorsal striatum, and ventral striatum, were among those that showed greater responses to food vs. non-food cues in children (Supplementary Fig. 2 and Supplementary Table 8). We did not observe significant relationships between maternal exposure (i.e., GDM and maternal obesity separately) and food cue reactivity in the whole-brain analysis, adjusted for multiple comparisons across the whole brain.
Prenatal Exposures and Adiposity
GDM exposure was associated with greater WtHR (0.024 ± 0.011; P = 0.028) and WHR (0.023 ± 0.009; P = 0.012). Results remained significant for WHR (0.019 ± 0.009; P = 0.038) but were attenuated for WtHR (0.019 ± 0.011; P = 0.080) after adjusting for demographic variables, prepregnancy BMI, and child MVPA. There were no significant group differences in BMI z-scores or %BF in all models (all P > 0.1). The early GDM-exposed children had a larger WtHR (0.036 ± 0.015; P = 0.018) and WHR (0.043 ± 0.012; P < 0.001) than unexposed children, and results remained significant for WHR (0.035 ± 0.013; P = 0.008) but not WtHR (0.021 ± 0.016; P > 0.1) after controlling for demographic variables, prepregnancy BMI, and child MVPA.
Prepregnancy BMI was associated with BMI z-scores (β = 0.17, SE 0.06; P = 0.004), %BF (β = 0.97, SE 0.46; P = 0.04), WtHR (β = 0.012, SE 0.004; P = 0.001), and WHR (β = 0.0085, SE 0.003; P = 0.005). Results remained significant for BMI z-scores (β = 0.16, SE 0.06; P = 0.007), WtHR (β = 0.011, SE 0.004; P = 0.003), and WHR (β = 0.008, SE 0.003; P = 0.012) and were attenuated for %BF (β = 0.89, SE 0.47; P = 0.06) after adjusting for demographic variables, GDM exposure, and child MVPA. Results from fully adjusted models are presented in Table 4; results from other models are presented in Supplementary Tables 9–12.
Table 4.
Relationships between maternal exposure and adiposity measures
| Adiposity measurement | GDM vs. unexposed | Prepregnancy BMI | ||
|---|---|---|---|---|
| β (SE)* | P value | β (SE)* | P value | |
| BMI z-score | 0.16 (0.18) | 0.374 | 0.16 (0.06) | 0.007 |
| %BF | 2.00 (1.43) | 0.164 | 0.89 (0.47) | 0.062 |
| WHR | 0.019 (0.011) | 0.08 | 0.011 (0.004) | 0.003 |
| WtHR | 0.019 (0.009) | 0.038 | 0.008 (0.003) | 0.012 |
Results adjusted for child’s age and sex, maternal education, maternal race/ethnicity, maternal exposure (maternal prepregnancy BMI [for GDM exposures] or maternal GDM status as a three-categorical variable [for prepregnancy BMI]), and child’s physical activity level. Boldface indicates significance at P < 0.05.
Regression coefficient (SE) from linear regression models. For GDM-related results, a positive β means greater adiposity measurements in the GDM group than in the unexposed group, whereas a negative β means the opposite data pattern. For prepregnancy BMI-related results, a positive β means a positive relationship between prepregnancy BMI and each adiposity measurement, whereas a negative β means a negative relationship.
Relationships Among OFC Food Cue Reactivity, Daily EI, and Adiposity
An increase of 1,000 kcal in daily EI was associated with a 0.028 increase in WHR, adjusting for child’s age, sex, and MVPA and maternal education and race/ethnicity (β = 0.028; P = 0.02). No other significant relationships among OFC food cue reactivity, daily EI, and adiposity were observed.
For the relationship between GDM exposure and WHR, we performed a data analysis adjusting for EI. The relationship between GDM exposure and WHR was no longer significant after further adjustment of daily EI in addition to demographic variables, prepregnancy BMI, and child MVPA (mean group difference was reduced from 0.019 ± 0.009 [P = 0.038] before adjusting daily EI to 0.015 ± 0.010 [P = 0.106] after adjusting for daily EI). Daily EI explained 36% of the association between GDM exposure and WHR, suggesting that daily EI may, in part, play a mediating role in the relationship between GDM exposure and WHR in children. We did not observe any significant interactions of GDM or maternal obesity exposure and sex on food intake, food cue reactivity, and adiposity (all P > 0.1).
Conclusions
In this study, we investigated the effects of in utero exposure to GDM or maternal obesity on daily food intake and food cue reactivity in brain reward regions in children 7–11 years of age. We observed that exposure to GDM, but not maternal obesity, was associated with greater daily EI, and children exposed to GDM diagnosed ≤26 weeks gestation exhibited greater OFC food cue reactivity compared with unexposed children. These results remained significant after adjusting for child’s age and sex, maternal education, maternal ethnicity/race, prepregnancy BMI, and child’s physical activity levels. The data suggest an independent effect of GDM exposure during early fetal development on daily EI and the OFC responses to appetitive food cues, thereby potentially contributing to the risk for development of obesity.
After adjusting for important confounders, we observed that GDM-exposed children consumed 165 more daily calories than unexposed children, and the early GDM-exposed group consumed the highest number of daily calories. Furthermore, children who consumed more daily calories had greater WHR. The relationship between GDM exposure and WHR was attenuated after adjustment for daily EI, and daily EI explained 36% of the association between GDM exposure and WHR in children. These findings suggest that daily EI may partially mediate the relationship between GDM exposure and WHR in children. Other mechanisms for the link between GDM exposure and WHR that could be explored further include epigenetic changes, alterations in the brain networks involved in energy homeostasis, and/or the brain inhibition control system. Taken together, these results suggest that increased food intake may be one factor contributing to obesity development among children exposed in utero to GDM.
Prior studies that investigated relationships between GDM exposure and food intake observed nonsignificant findings (60,61). For example, in a prospective study conducted by Catalano et al. (60) in which daily EI was assessed using 3-day dietary logs, self-reported by parents, the authors did not find group differences in daily EI between offspring exposed to GDM (n = 37) and unexposed children (n = 52) 6–11.9 years of age. In a retrospective study of 82 children exposed to GDM and 379 unexposed children between 6 and 13 years old, Crume et al. (61) used the Block Kids Food Questionnaire to measure daily EI and did not observe significant group differences. In our study, in-person repeated 24-h dietary recalls were administered to the child with assistance of his/her parent by trained staff. Our sample included 87 GDM-exposed children and 72 unexposed children between 7 and 11 years old. Differences in the types of dietary assessments and/or child demographics may have contributed to the discrepancies in these findings. We did not observe a significant interaction between GDM exposure and sex on daily EI, whereas sex-specific effects were observed in a prior behavioral study such that adolescent girls prenatally exposed to mothers with an impaired glucose tolerance during pregnancy (compared with normal glucose tolerance) had a higher self-reported score of eating in the absence of hunger while boys showed the opposite pattern (59). Future studies are warranted to robustly examine potential sex differences in the effects of GDM exposure on feeding behavior in offspring.
In addition, we found that children exposed to GDM before 26 weeks of gestation had greater OFC responses to food cues compared with unexposed children. However, GDM as a whole was only marginally associated with the OFC food cue reactivity. We speculate, on the basis of prior evidence (48,58), that exposure to GDM during early gestation (i.e., before 26 weeks gestation) may have a larger effect on brain development, and thus, exposure to GDM before 26 weeks of gestation would have a larger effect on the child’s brain reward system than GDM exposure later in gestation (i.e., after 26 weeks gestation). The OFC plays an important role in reward processing, specifically the incentive motivational effects of food rewards. A meta-analysis reported that the OFC was among the regions implicated in the processing of appetitive food cues in youth (62). Prior studies showed that greater OFC food cue reactivity predicted greater food consumption in children (63) and greater future weight gain in adolescents (25). Prior animal and human studies indicated that GDM exposure disrupted the brain’s hypothalamic circuitry, which subsequently led to overeating and obesity (43–48). Because the hypothalamus and mesolimbic areas (e.g., OFC) are structurally and functionally connected (19,64,65), it is possible that the OFC may also be vulnerable to early life insults, such as in utero exposure to GDM. Indeed, there is suggestive evidence from animal studies that the fetal programming of feeding behavior involves the OFC (66). Although there is suggestive evidence of heightened OFC responses to food cues in children exposed to GDM diagnosed ≤26 weeks gestation, this finding needs to be replicated in a larger sample size.
The effects of the timing of GDM exposure on behavioral and brain measures that we observed here were consistent with our prior work, which showed that children exposed to GDM before 26 weeks gestation had altered hypothalamic responses to glucose that predicted future weight gain (48). While genetic factors may contribute to the intergenerational effects of obesity, the effects of the timing of GDM exposure found here indicate a specific role for the intrauterine environment in the programming of neurobehavioral systems underlying obesity risk.
We previously reported that children exposed to GDM had larger WHR than unexposed children, but groups did not differ in BMI z-scores or %BF in the BrainChild cohort, suggesting that GDM exposure has a larger impact on abdominal fat distribution than overall adiposity during childhood (48). Here, we additionally showed a positive relationship between daily EI and WHR, suggesting that greater food intake may be related to abdominal fat accumulation. Although a formal mediation analysis was not performed, our exploratory analysis showed that the relationship between GDM exposure and WHR was attenuated after controlling for daily EI, suggesting that daily EI may, in part, play a mediating role of the link between GDM exposure and WHR. Future study is needed to robustly test this mediation model.
Contrary to our hypothesis, maternal prepregnancy BMI was not significantly related to daily food intake or food cue reactivity within brain reward regions in children. We speculate that other neural pathways (e.g., metabolic signaling pathway [48], inhibition control pathway [67]) may underlie the effects of prenatal exposure to maternal obesity on risk for obesity in offspring. Indeed, prior studies have shown that intrauterine exposure to maternal obesity was associated with altered glucose-linked hypothalamic signaling (48), which could lead to dysregulation of energy balance. Alternatively, exposure to maternal obesity may affect food intake and brain food cue reactivity indirectly through GDM exposure.
Given that daily EI, brain food cue reactivity, and adiposity were all collected at the same time in the child participants, we were not able to tease apart temporal relationships among these variables. A number of important child and maternal covariates were controlled for in our models, but other uncontrolled variables (e.g., early feeding practices, parental dietary patterns) may contribute to the observed group differences. While the focus of this study is brain reward regions engaged during the food cue task, we recognize that there might be other important brain regions that we did not examine that could be affected by exposure to GDM or maternal obesity.
In conclusion, exposure to GDM in utero, in particular before 26 weeks gestation, is associated with increased EI, enhanced OFC food cue reactivity, and increased WHR in children. This work provides biological insights into potential pathways by which exposure to GDM in utero leads to increased risk for obesity in offspring. Future studies that include a larger sample size and/or longitudinal design are merited to assess whether greater daily EI and/or OFC food cue reactivity mediate the relationship between GDM exposure and childhood adiposity.
Article Information
Acknowledgments. The authors thank the volunteers who participated in these studies. We also thank Ana Romero (University of Southern California, LosAngeles, CA) for assistance with study coordination, Mayra Martinez and Janet Mora-Marquez (Kaiser Permanente Southern California, Los Angeles, CA) for recruitment, and the Dana and David Dornsife Cognitive Neuroimaging Center at USC, especially Bosco Tjan and J.C. Zhuang, for assistance with developing MRI protocols.
Funding. This work was supported by an American Diabetes Association Pathway Accelerator Award (1-14-ACE-36; K.A.P., principal investigator) and in part by the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, grants K01-DK-115638 (S.L., principal investigator), R01-DK-116858 (A.H.X and K.A.P., principal investigators), and R03-DK-103083 (K.A.P., principal investigator). A Research Electronic Data Capture database was used for this study, which is supported by the Southern California Clinical and Translational Science Institute through U.S. Department of Health and Human Services (DHHS) grant UL1-TR-001855. P.M.T. is funded in part by DHHS grant U54-EB-020403.
Duality of Interest. P.M.T. received grant support from Biogen, Inc., for research unrelated to this manuscript. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.L., A.H.X., and K.A.P. contributed to the study concept and design and drafted the manuscript. T.C. and A.H.X. performed the statistical analysis. All authors contributed to analysis or interpretation of data and critically revised the manuscript for intellectual content. A.H.X. and K.A.P. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in oral form at the 80th Scientific Sessions of the American Diabetes Association, Virtual, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14109674.
A.H.X. and K.A.P. contributed equally as senior authors.
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity Among Adults and Youth: United States, 2015-2016. NCHS Data Brief 2017;(288):1–8 [PubMed] [Google Scholar]
- 2. Singh AS, Mulder C, Twisk JW, van Mechelen W, Chinapaw MJ. Tracking of childhood overweight into adulthood: a systematic review of the literature. Obes Rev 2008;9:474–488 [DOI] [PubMed] [Google Scholar]
- 3. Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr 2010;91:1499S–1505S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pi-Sunyer FX. Health implications of obesity. Am J Clin Nutr 1991;53(Suppl. 6):1595S–1603S [DOI] [PubMed] [Google Scholar]
- 5. Kim SY, Sharma AJ, Callaghan WM. Gestational diabetes and childhood obesity: what is the link? Curr Opin Obstet Gynecol 2012;24:376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heslehurst N, Vieira R, Akhter Z, et al. The association between maternal body mass index and child obesity: a systematic review and meta-analysis. PLoS Med 2019;16:e1002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berthoud H-R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 2011;21:888–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron 2002;36:199–211 [DOI] [PubMed] [Google Scholar]
- 9. Berthoud H-R, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol 2008;59:55–92 [DOI] [PubMed] [Google Scholar]
- 10. Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev 2013;37:2047–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Curr Drug Abuse Rev 2011;4:182–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res 2010;1350:43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab 2012;23:250–260 [DOI] [PubMed] [Google Scholar]
- 14. Volkow ND, Wang G-J, Baler RD. Reward, dopamine and the control of food intake: implications for obesity. Trends Cogn Sci 2011;15:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsuda M, Liu Y, Mahankali S, et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–1806 [DOI] [PubMed] [Google Scholar]
- 16. Del Parigi A, Gautier JF, Chen K, et al. Neuroimaging and obesity: mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci 2002;967:389–397 [PubMed] [Google Scholar]
- 17. Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schur EA, Melhorn SJ, Oh SK, et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23:2142–2148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kullmann S, Heni M, Linder K, et al. Resting-state functional connectivity of the human hypothalamus. Hum Brain Mapp 2014;35:6088–6096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jastreboff AM, Sinha R, Arora J, et al. Altered brain response to drinking glucose and fructose in obese adolescents. Diabetes 2016;65:1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Puig J, Blasco G, Daunis-I-Estadella J, et al. Hypothalamic damage is associated with inflammatory markers and worse cognitive performance in obese subjects. J Clin Endocrinol Metab 2015;100:E276–E281 [DOI] [PubMed] [Google Scholar]
- 22. Sewaybricker LE, Schur EA, Melhorn SJ, et al. Initial evidence for hypothalamic gliosis in children with obesity by quantitative T2 MRI and implications for blood oxygen-level dependent response to glucose ingestion. Pediatr Obes 2019;14:e12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luo S, Romero A, Adam TC, Hu HH, Monterosso J, Page KA. Abdominal fat is associated with a greater brain reward response to high-calorie food cues in Hispanic women. Obesity (Silver Spring) 2013;21:2029–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shearrer GE, Stice E, Burger KS. Adolescents at high risk of obesity show greater striatal response to increased sugar content in milkshakes. Am J Clin Nutr 2018;107:859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19:1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Verdejo-Román J, Vilar-López R, Navas JF, Soriano-Mas C, Verdejo-García A. Brain reward system’s alterations in response to food and monetary stimuli in overweight and obese individuals. Hum Brain Mapp 2017;38:666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stice E, Yokum S. Gain in body fat is associated with increased striatal response to palatable food cues, whereas body fat stability is associated with decreased striatal response. J Neurosci 2016;36:6949–6956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite 2012;58:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simon JJ, Skunde M, Hamze Sinno M, et al. Impaired cross-talk between mesolimbic food reward processing and metabolic signaling predicts body mass index. Front Behav Neurosci 2014;8:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boutelle KN, Wierenga CE, Bischoff-Grethe A, et al. Increased brain response to appetitive tastes in the insula and amygdala in obese compared with healthy weight children when sated. Int J Obes 2015;39:620–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jastreboff AM, Lacadie C, Seo D, et al. Leptin is associated with exaggerated brain reward and emotion responses to food images in adolescent obesity. Diabetes Care 2014;37:3061–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bruce AS, Holsen LM, Chambers RJ, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes 2010;34:1494–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stice E, Spoor S, Bohon C, Small DM. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science 2008;322:449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiol Behav 2009;97:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gearhardt AN, Yokum S, Stice E, Harris JL, Brownell KD. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci 2014;9:932–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stice E, Spoor S, Bohon C, Veldhuizen MG, Small DM. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J Abnorm Psychol 2008;117:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. Neuroimage 2010;50:1618–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci 2015;35:10316–10324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yokum S, Stice E. Weight gain is associated with changes in neural response to palatable food tastes varying in sugar and fat and palatable food images: a repeated-measures fMRI study. Am J Clin Nutr 2019;110:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoeckel LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 2008;41:636–647 [DOI] [PubMed] [Google Scholar]
- 42. Stice E, Yokum S, Burger KS, Epstein LH, Small DM. Youth at risk for obesity show greater activation of striatal and somatosensory regions to food. J Neurosci 2011;31:4360–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes 2009;33:115–122 [DOI] [PubMed] [Google Scholar]
- 44. Franke K, Harder T, Aerts L, et al. ‘Programming’ of orexigenic and anorexigenic hypothalamic neurons in offspring of treated and untreated diabetic mother rats. Brain Res 2005;1031:276–283 [DOI] [PubMed] [Google Scholar]
- 45. Plagemann A, Harder T, Lindner R, et al. Alterations of hypothalamic catecholamines in the newborn offspring of gestational diabetic mother rats. Brain Res Dev Brain Res 1998;109:201–209 [DOI] [PubMed] [Google Scholar]
- 46. Plagemann A, Harder T, Janert U, et al. Malformations of hypothalamic nuclei in hyperinsulinemic offspring of rats with gestational diabetes. Dev Neurosci 1999;21:58–67 [DOI] [PubMed] [Google Scholar]
- 47. Steculorum SM, Bouret SG. Maternal diabetes compromises the organization of hypothalamic feeding circuits and impairs leptin sensitivity in offspring. Endocrinology 2011;152:4171–4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Page KA, Luo S, Wang X, et al. Children exposed to maternal obesity or gestational diabetes mellitus during early fetal development have hypothalamic alterations that predict future weight gain. Diabetes Care 2019;42:1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. American Diabetes Association . Gestational diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 50. Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol 2013;122:406–416 [DOI] [PubMed] [Google Scholar]
- 51. Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–1144 [DOI] [PubMed] [Google Scholar]
- 52. Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed 1989;30:47–57 [DOI] [PubMed] [Google Scholar]
- 53. Dorton HM, Luo S, Monterosso JR, Page KA. Influences of dietary added sugar consumption on striatal food-cue reactivity and postprandial GLP-1 response. Front Psychiatry 2018;8:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alves JM, Zink J, Chow T, et al. Contributions of prenatal exposures and child lifestyle to insulin sensitivity. J Clin Endocrinol Metab 2020;105:2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–1581 [DOI] [PubMed] [Google Scholar]
- 56. Pate RR, Ross R, Dowda M, Trost SG, Sirard JR. Validation of a 3-day physical activity recall instrument in female youth. Pediatr Exerc Sci 2003;15:257–265 [Google Scholar]
- 57. Luo S, Alves J, Hardy K, et al. Neural processing of food cues in pre-pubertal children. Pediatr Obes 2019;14:e12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiang AH, Wang X, Martinez MP, et al. Association of maternal diabetes with autism in offspring. JAMA 2015;313:1425–1434 [DOI] [PubMed] [Google Scholar]
- 59. Derks IPM, Hivert MF, Rifas-Shiman SL, et al. Associations of prenatal exposure to impaired glucose tolerance with eating in the absence of hunger in early adolescence. Int J Obes 2019;43:1903–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr 2011;158:941–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van Meer F, van der Laan LN, Adan RAH, Viergever MA, Smeets PAM. What you see is what you eat: an ALE meta-analysis of the neural correlates of food viewing in children and adolescents. Neuroimage 2015;104:35–43 [DOI] [PubMed] [Google Scholar]
- 63. Adise S, Geier CF, Roberts NJ, White CN, Keller KL. Is brain response to food rewards related to overeating? A test of the reward surfeit model of overeating in children. Appetite 2018;128:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hirose S, Osada T, Ogawa A, et al. Lateral-medial dissociation in orbitofrontal cortex-hypothalamus connectivity. Front Hum Neurosci 2016;10:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lemaire J-J, Frew AJ, McArthur D, et al. White matter connectivity of human hypothalamus. Brain Res 2011;1371:43–64 [DOI] [PubMed] [Google Scholar]
- 66. Alves MB, Dalle Molle R, Desai M, Ross MG, Silveira PP. Increased palatable food intake and response to food cues in intrauterine growth-restricted rats are related to tyrosine hydroxylase content in the orbitofrontal cortex and nucleus accumbens. Behav Brain Res 2015;287:73–81 [DOI] [PubMed] [Google Scholar]
- 67. Shapiro ALB, Moore BF, Sutton B, et al. In utero exposure to maternal overweight or obesity is associated with altered offspring brain function in middle childhood. Obesity (Silver Spring) 2020;28:1718–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]