Abstract
OBJECTIVE
Better preconception metabolic and nutritional health are hypothesized to promote gestational normoglycemia and reduce preterm birth, but evidence supporting improved outcomes with nutritional supplementation starting preconception is limited.
RESEARCH DESIGN AND METHODS
This double-blind randomized controlled trial recruited from the community 1,729 U.K., Singapore, and New Zealand women aged 18–38 years planning conception. We investigated whether a nutritional formulation containing myo-inositol, probiotics, and multiple micronutrients (intervention), compared with a standard micronutrient supplement (control), taken preconception and throughout pregnancy could improve pregnancy outcomes. The primary outcome was combined fasting, 1-h, and 2-h postload glycemia (28 weeks gestation oral glucose tolerance test).
RESULTS
Between 2015 and 2017, participants were randomized to control (n = 859) or intervention (n = 870); 585 conceived within 1 year and completed the primary outcome (295 intervention, 290 control). In an intention-to-treat analysis adjusting for site, ethnicity, and preconception glycemia with prespecified P < 0.017 for multiplicity, there were no differences in gestational fasting, 1-h, and 2-h glycemia between groups (β [95% CI] loge mmol/L intervention vs. control −0.004 [−0.018 to 0.011], 0.025 [−0.014 to 0.064], 0.040 [0.004–0.077], respectively). Between the intervention and control groups there were no significant differences in gestational diabetes mellitus (24.8% vs. 22.6%, adjusted risk ratio [aRR] 1.22 [0.92–1.62]), birth weight (adjusted β = 0.05 kg [−0.03 to 0.13]), or gestational age at birth (mean 39.3 vs. 39.2 weeks, adjusted β = 0.20 [−0.06 to 0.46]), but there were fewer preterm births (5.8% vs. 9.2%, aRR 0.43 [0.22–0.82]), adjusting for prespecified covariates.
CONCLUSIONS
Supplementation with myo-inositol, probiotics, and micronutrients preconception and in pregnancy did not lower gestational glycemia but did reduce preterm birth.
Introduction
Suboptimal metabolic and nutritional health around conception and during pregnancy have important implications for pregnancy outcomes, fetal growth, adiposity, and long-term offspring health (1). Adverse effects of higher maternal glucose concentrations increase across the continuum of maternal glycemia (2,3), and micronutrient insufficiency is highly prevalent in women. Interventions that optimize glycemia and nutritional status are thought to improve pregnancy and offspring outcomes, but supportive evidence from intervention studies is sparse.
Pregnancy is a state of relative maternal insulin resistance, promoting glucose transfer to the fetus (4). Physiological insulin resistance and impaired insulin secretion can be accentuated by individual genetic and environmental vulnerabilities and lead to gestational diabetes mellitus (GDM) (5). The global GDM incidence is rising, estimated at 14% (6). Following GDM diagnosis, lifestyle changes, oral hypoglycemic drugs, and insulin can improve some short-term obstetric outcomes (7) but cannot fully mitigate pregnancy and offspring adversity (8). Risk reduction strategies have thus shifted toward GDM prevention. However, population trials of dietary, physical activity, or combined lifestyle measures, mostly beginning in the first half of gestation, have had limited impact on preventing GDM (9,10). This has led to postulations that preconception interventions could be more effective and that alternative approaches are required.
Small clinical trials have suggested that supplementation with myo-inositol or probiotics from early pregnancy may be beneficial; myo-inositol is a naturally occurring six-carbon polyol with insulin sensitizing actions arising from functions relating to many second messenger signaling pathways and endogenous insulin-mimetic factors (11). Meta-analysis of women given myo-inositol supplementation from the end of the first trimester reported reductions in GDM, gestational glycemia, and preterm birth (12). Similarly, meta-analysis of studies of probiotics (Lactobacillus and/or Bifidobacterium species) from early pregnancy showed improved insulin sensitivity (13). One trial of probiotics taken from the first trimester reported improved glucose tolerance and reduced GDM (14). Low intakes and insufficiencies of several micronutrients (vitamin B6, vitamin B12, riboflavin, zinc) are prevalent in pregnancy and have been linked with glucose intolerance and pregnancy outcomes (15–17), but there are few intervention studies (18). Vitamin D deficiency has also been linked with GDM and preterm birth (19), but a trial of vitamin D supplementation starting in early pregnancy showed no preventive effects on pregnancy complications (20). Another trial of a nutritional supplement (containing protein, polyunsaturated fatty acids, and micronutrients without inositols or probiotics) in low-resource settings showed improved birth length but no difference in preterm birth compared with no supplementation, with no difference between the group starting supplementation preconception and the group starting in early pregnancy; glycemia outcomes were, however, not reported (21).
Dysglycemia and maternal micronutrient insufficiency preconception or in early pregnancy are common in the general population and thought to influence the risk of adverse pregnancy outcomes (1,5,17,22). We hypothesized that a myo-inositol, probiotic, and micronutrient nutritional supplement commencing before pregnancy could collectively lower maternal glycemia and improve pregnancy outcomes across the general population. We therefore undertook an international, multicenter, double-blind randomized controlled trial (the Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health [NiPPeR] study [23]) to investigate whether intervention with a nutritional supplement containing myo-inositol, probiotics, and additional micronutrients (vitamins D, B6, and B12; riboflavin; and zinc), compared with a standard preconception micronutrient supplement, taken before and during pregnancy would promote improved maternal pregnancy glycemia and outcomes.
Research Design and Methods
This international, multicenter, double-blind randomized controlled trial recruited women who were planning to conceive within the next 6 months. Women were recruited in Singapore, Auckland (New Zealand), and Southampton (U.K.), primarily from the community (Fig. 1). Our trial was approved by the U.K., Singapore, and New Zealand research ethics services at each site (Southampton: Health Research Authority National Research Ethics Service Committee South Central Research Ethics Committee reference 15/SC/0142; the National Healthcare Group Domain Specific Review Board Singapore reference 2015/00205; and the Health and Disability Ethics Committee New Zealand reference 15/NTA/21), with confirmation from the relevant regulatory authorities that the formulation was not an investigational medicinal product. Trial oversight and monitoring were provided by an independent data and safety monitoring committee.
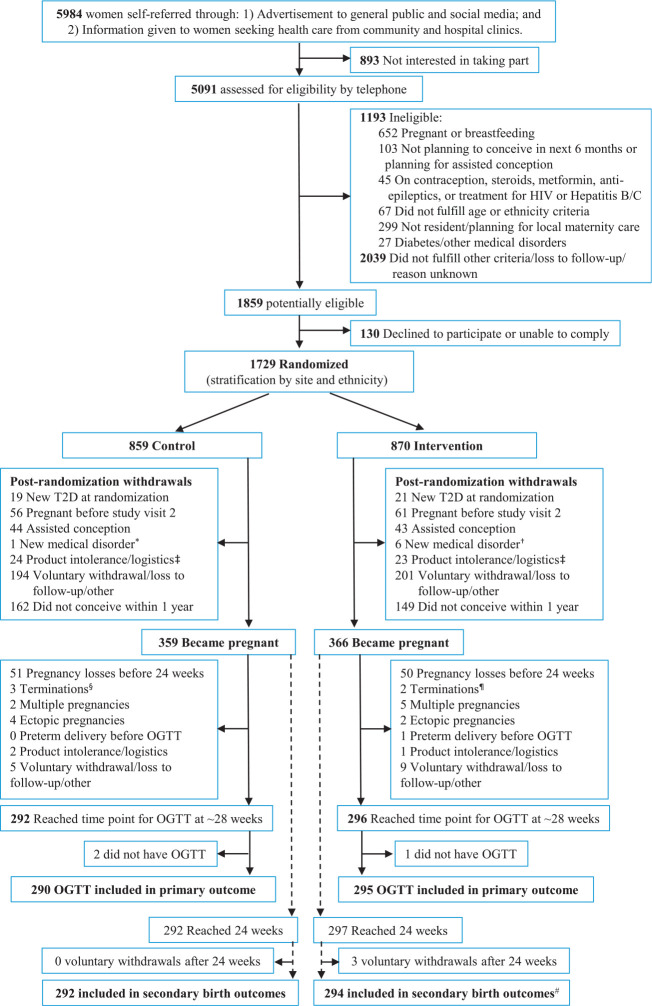
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram outlining participant flow. *Premature ovarian failure. †New-onset Graves’ disease, hemoglobinopathy with iron overload, prolactinoma, endometrial polyp, endometrial atypia, breast cancer. ‡Withdrew because product may contain animal remnants, no storage space in refrigerator, participant suspicion of product-related symptoms. §Includes two cases of trisomy 21, Klinefelter syndrome. ¶Includes hypoplastic left heart syndrome, unknown reason in private clinic. #Includes one stillbirth and one neonatal death. T2D, type 2 diabetes.
Participants
On the basis of our previous population-based Southampton Women’s Survey our initial target was 1,800 recruits to have 600 established pregnancies to study. Recruitment was stopped at 1,729 women when it became clear that the projected number of pregnancies would exceed our target (final conceptions n = 725) (Fig. 1).
Women were eligible for trial enrollment if they were aged 18–38 years, were planning to conceive within 6 months, and had future maternity care at the recruiting centers. In Singapore, women had to be of homogeneous or mixed Chinese, Malay, or Indian ethnicity. A priori, women conceiving within 1 year were followed through pregnancy and beyond. Women were excluded if they were pregnant or lactating at recruitment; were undergoing assisted conception (apart from taking clomiphene or letrozole alone); had known serious food allergy or preexisting type 1 or type 2 diabetes, were using oral, implanted, or intrauterine contraception or taking metformin, systemic steroids, or anticonvulsants; or were undergoing treatment for HIV or hepatitis B or C in the past month. Participants provided written informed consent.
The Formulation
The intervention and control formulations were packaged as a powder in sachets and stored at 2–6°C until made up in water and taken twice daily, with similar sensory characteristics. Formulations were produced by S.I.I.T. (Milan, Italy). Ingredients common to control and intervention formulations were folic acid 400 μg/day, iron 12 mg/day, calcium 150 mg/day, iodine 150 μg/day, and β-carotene 720 μg/day; the intervention additionally included myo-inositol 4 g/day, vitamin D 10 μg/day, riboflavin 1.8 mg/day, vitamin B6 2.6 mg/day, vitamin B12 5.2 μg/day, zinc 10 mg/day, and probiotics (Lactobacillus rhamnosus NCC 4007 [CGMCC 1.3724] and Bifidobacterium animalis species lactis NCC 2818 [CNCM I-3446]; average survival counts remained within target over the shelf life of the refrigerated product) (24). Quantities were either standard amounts on the basis of previous trials (myo-inositol, probiotics) (12,14), amounts enhanced above those available in over-the-counter products (vitamin B6, vitamin B12, riboflavin), U.K. recommended daily allowance amounts for pregnant women (vitamin D, zinc, folic acid, iodine), or minimal amounts for micronutrients linked with potential detrimental effects at higher doses (iron, β-carotene, calcium).
Randomization and Procedures
Participants were randomly assigned in a 1:1 ratio to the control or intervention groups through the electronic study database (23), with stratification by site and ethnicity to ensure balanced allocation to the groups. Throughout the trial, participants, investigators, clinicians, and fieldworkers were unaware of the trial group assignments.
Following a baseline 75-g oral glucose tolerance test (OGTT), anthropometric measurements, and questionnaire ascertainment of the women’s characteristics, trial formulations were initiated before conception and continued until the end of pregnancy. Participants were instructed to contact the trial team as soon as they had a positive urinary pregnancy test, which was then confirmed by an ultrasonographic examination at 6–8 weeks gestation. Once pregnant, the women were followed up with questionnaires, for resupply of trial formulations, with a 20-week fetal anomaly scan, and with a 28-week OGTT. Plasma glucose was collected in antiglycolytic buffered tubes and transported on an ice slurry to the laboratory within 30 min using standardized protocols across sites. Glucose measurements using the glucose oxidase method were undertaken by a single laboratory at each site, with uniform external quality assurance per the Royal College of Pathologists of Australasia Quality Assurance Program. Plasma 25-hydroxyvitamin D and serum insulin concentrations (fasting, 30 min, and 120 min at preconception baseline; fasting and 30, 60, 90, and 120 min at the 28-week OGTT) were batch analyzed by liquid chromatography-tandem mass spectrometry (Bevital, Bergen, Norway) and an electrochemiluminescence immunoassay (cobas; Roche Diagnostics), respectively. The HOMA for insulin resistance (HOMA2-IR) (https://www.OCDEM.ox.ac.uk) (25) and Matsuda index measure of insulin sensitivity (http://mmatsuda.diabetes-smc.jp/xpoints.html) (26) were calculated.
Antenatal, peripartum, and neonatal outcomes were ascertained from medical records. Adherence to the trial formulation was ascertained by sachet counting. Good adherence was defined a priori as at least 60% of the sachets taken.
Outcomes
The primary outcome was fasting and/or 1-h and/or 2-h plasma glucose concentrations following a 75-g OGTT at 28 weeks gestation (a priori specification included all conducted between 24 and 32 weeks). On the basis of prior systematic reviews, principal prespecified secondary outcomes were GDM (defined by International Association of Diabetes and Pregnancy Study Groups criteria [27]), large for gestational age at birth (using sex- and gestational age–specific Royal College of Paediatrics and Child Health 2009 U.K.-World Health Organization growth charts [28]), and preterm birth; other secondary outcomes are shown in Table 3 and Supplementary Table 2. Gestational age was determined using a prespecified algorithm (29) using menstrual data (date of last menstrual period [LMP], self-reported cycle regularity, mean cycle lengths in past 3 months), with first trimester fetal ultrasonographic crown-rump length measurement used if >7 days discrepancy between LMP and scan dates, uncertain LMP date, irregular cycles, or hormonal contraception use within past 3 months.
Table 3.
Secondary outcomes of pregnancy complications, delivery events, and neonatal outcomes with the NiPPeR intervention compared with control
| Control | Intervention | Effect of intervention | |
|---|---|---|---|
| Pregnancy complications | RR (95% CI)† | ||
| GDM* (denominator: all those who completed OGTT at 24–32 weeks) | 64/283 (22.6) | 73/294 (24.8) | 1.22 (0.92–1.62) (n = 545) |
| Miscarriages <24 weeks gestation (denominator: all those who became pregnant after the second preconception visit) | 51/359 (14.2) | 50/366 (13.7) | 0.91 (0.62–1.33) (n = 688) |
| Congenital abnormalities‡ (denominator: all reaching 7 weeks) | 16/314 (5.1) | 15/330 (4.5) | 0.83 (0.35–1.96) (n = 557) |
| Severe nausea and vomiting of pregnancy§ (denominator: all reaching 7 weeks) | 51/305 (16.7) | 43/322 (13.4) | 0.86 (0.57–1.30) (n = 553) |
| Hypertensive disorders of pregnancy, both preeclampsia‖ and pregnancy-induced hypertension¶ (denominator: all pregnancies reaching ≥24 weeks) | 14/292 (4.8) | 12/294 (4.1) | 1.19 (0.55–2.59) (n = 557) |
| Delivery outcomes (denominator: all live births ≥24 weeks unless otherwise stated) | Mean difference (95% CI)# or RR (95% CI)# | ||
| Gestational age at birth in decimal weeks | 39.2 (1.74) | 39.3 (1.78) | 0.20 (−0.06 to 0.46) (n = 553) |
| All preterm deliveries (<37 weeks) (spontaneous labor onset: iatrogenic, n:n) | 27/292 (9.2) (12:15)†† | 17/293 (5.8) (8:9)‡‡ | 0.43 (0.22–0.82) (n = 553) |
| Late preterm deliveries (34 weeks+0 days to 36 weeks+6 days) (spontaneous labor onset: iatrogenic, n:n) | 22/292 (7.5) (11:11) | 13/293 (4.4) (6:7) | 0.41 (0.20–0.85) (n = 553) |
| PPROM | 19/280 (6.8) | 8/277 (2.9) | 0.39 (0.16–0.97) (n = 526) |
| Preterm deliveries associated with PPROM (spontaneous labor onset: iatrogenic, n:n) | 17/280 (6.1) (8:9) | 5/277 (1.8) (2:3) | 0.21 (0.06–0.69) (n = 526) |
| Cesarean section delivery (elective: emergency, n:n) | 85/292 (29.1) (41:44) | 84/293 (28.7) (34:50) | 0.99 (0.76–1.28) (n = 553) |
| Major postpartum hemorrhage (>1-L blood loss, denominator: all pregnancies reaching ≥24 weeks) | 24/292 (8.2) | 9/294 (3.1) | 0.44 (0.20–0.94) (n = 554) |
| Neonatal outcomes (denominator: all live births ≥24 weeks) | Mean difference (95% CI)# or RR (95% CI)# | ||
| Birth weight (kg) | 3.30 (0.54) | 3.33 (0.55) | 0.05 (−0.03 to 0.13) (n = 553) |
| Large for gestational age (>90th centile adjusted for sex and gestational age**) | 22/292 (7.5) | 21/293 (7.2) | 0.94 (0.54–1.63) (n = 555) |
| Small for gestational age (<10th centile adjusted for sex and gestational age**) | 21/292 (7.2) | 24/293 (8.2) | 1.34 (0.79–2.29) (n = 555) |
| Admission to neonatal unit | 19/290 (6.6) | 24/293 (8.2) | 1.11 (0.57–2.17) (n = 550) |
| Neonatal hypoglycemia requiring dextrose treatment | 24/292 (8.2) | 19/293 (6.5) | 0.79 (0.43–1.48) (n = 553) |
| Neonatal septicemia (positive blood culture) | 0/287 (0) | 2/288 (0.7) | Insufficient to analyze |
Data are mean (SD) or n (%) unless otherwise indicated. RR, risk ratio.
According to International Association of Diabetes and Pregnancy Study Groups criteria (fasting glucose ≥5.1 mmol/L or 1-h glucose ≥10.0 mmol/L or 2-h glucose ≥8.5 mmol/L) (24); includes only women with complete OGTT data at all three time points.
Adjusted for site, ethnicity, maternal age, preconception BMI, household income level, parity, preconception smoking, preconception baseline fasting glucose, family history of diabetes, and offspring’s sex (not applicable for miscarriages).
Includes anomalies in the following categories: in the control group, four cases of karyotypic/multiple anomalies, two cardiovascular, six genitourinary, two respiratory, two musculoskeletal; in the intervention group, five cases of karyotypic/multiple anomalies, three cardiovascular, four genitourinary, three musculoskeletal.
Requiring admission to the hospital for intravenous rehydration with or without significantly deranged biochemistry or weight loss.
Preeclampsia defined as hypertension in pregnancy associated with significant proteinuria or evidence of multisystem disorder; there were no differences in incidence between study groups.
Pregnancy-induced hypertension defined as isolated nonproteinuric hypertension in a previously normotensive woman or aggravation of hypertension during pregnancy; there were no differences in incidence between study groups.
Adjusted for site, ethnicity, maternal age, preconception BMI, household income level, parity, smoking during pregnancy, offspring sex (except for large and small for gestational age), and (where data were available) 28 weeks gestation fasting glucose.
By Royal College of Paediatrics and Child Health 2009 U.K.-World Health Organization growth charts (25). Use of respective local population charts, Fenton growth charts, and World Health Organization INTERGROWTH-21st charts did not materially alter results.
Iatrogenic preterm births include cases of induction of labor and nonlabor cesarean section. Indications for iatrogenic delivery in the control group were as follows: five for PPROM alone, four for PPROM plus another indication (previous cesarean section, vasa previa, breech presentation, maternal medical condition), five for placental-associated conditions (intrauterine growth restriction with or without preeclampsia or placental abruption), and one maternal medical condition.
Indications for iatrogenic delivery in the intervention group were as follows: three for PPROM alone, four for placental-associated conditions (intrauterine growth restriction with or without preeclampsia or placental abruption), one maternal medical condition, and one fetal anomaly with breech presentation.
Statistical Analysis
Considering the composite multiple end point primary outcome of plasma glucose at three 75-g OGTT time points, the prespecified level of statistical significance was set as P < 0.017 (i.e., 0.05 divided by 3). With a sample size of 600 (300 in each group), a two-sided test with α = 0.017, and 80% power, the detectable differences in fasting, 1-h, and 2-h glucose concentrations between groups were 0.12, 0.45, and 0.34 mmol/L, respectively (each with a standardized effect size of 0.265 SDs using values reported in the Hyperglycemia and Adverse Pregnancy Outcome [HAPO] study [30]). Such magnitudes of glycemic change are expected to have clinically appreciable effects on neonatal size and adiposity and long-term offspring health (2,3).
Glucose values were loge transformed to achieve approximately normal distributions before using these values for analysis. Analysis of the primary outcome used linear regression on the intention-to-treat data set (all randomized participants who provided an OGTT at 24–32 weeks). Group (control or intervention) was included as a predictor and regressions adjusted for site, ethnicity, and corresponding preconception glycemia to account for potential imbalance between treatment arms among pregnancies that reached 24–32 weeks gestation. Subsequent regression models were additionally adjusted for prespecified factors thought to be important predictors of outcomes and for other factors not balanced across control and intervention groups and believed to be prognostic; these are listed in the relevant tables of results. Likewise, HOMA2-IR and Matsuda indices were loge transformed, and comparisons at 28 weeks were similarly adjusted but with corresponding preconception values instead of preconception glycemia. Estimates of differences (β) between the groups are presented with 95% CIs; t tests on loge glucose were also conducted. Group comparisons were performed in two prespecified special interest subgroups: women who were overweight or obese (defined using ethnic-specific thresholds of BMI >23 kg/m2 for Asians, including Chinese, Indians, Pakistani, Bangladeshi, Malay, and mixed Asian, and >25 kg/m2 for non-Asians, including White Caucasian, Polynesian, Black, and mixed Asian-non-Asian) and women with documented evidence of dysglycemia before conception (defined as at least one of the following: GDM in a previous pregnancy or preconception baseline first visit raised HbA1c [≥5.7% (39 mmol/mol)], impaired fasting glucose [5.6–6.9 mmol/L], or impaired glucose tolerance [2-h glucose 7.8–11.0 mmol/L]) (31).
The statistical analysis plan did not include correction for multiple comparisons for secondary or other outcomes; therefore, results for these outcomes are reported as point estimates and 95% CIs and should not be used to infer definitive treatment effects. Analyses were performed using Stata 15.1 software (StataCorp, College Station, TX).
Results
Between 3 August 2015 and 12 May 2017, 1,729 women were recruited and randomly assigned to either the control (n = 859) or the intervention (n = 870) group. Pregnancies fulfilling the study criteria and reaching 28 weeks gestation were achieved in 588 women, 292 (34%) of 859 and 296 (34%) of 870 in the control and intervention groups, respectively (Fig. 1); 585 (99.5%) of 588 had an OGTT and provided the primary outcome of glycemia at 28 weeks gestation (median [interquartile range] 27.7 weeks [27.2–28.3]). Median BMI and other baseline characteristics were similar in the two study groups providing the primary outcome, except fewer women in the intervention group were obese, nulliparous, or had a family history of diabetes (Table 1).
Table 1.
Baseline preconception characteristics of women who provided a primary outcome
| Control (n = 290) | Intervention (n = 295) | |
|---|---|---|
| Age (years) | 30.14 (3.30) | 30.53 (3.40) |
| BMI (kg/m2) | 23.75 (21.34–27.5) | 23.65 (21.16–26.23) |
| Overweight* | 68 (23.5) | 89 (30.3) |
| Obese* | 61 (21.0) | 40 (13.6) |
| Ethnic origin | ||
| White Caucasian | 167 (57.6) | 180 (61.0) |
| Chinese | 73 (25.2) | 72 (24.4) |
| South Asian (Indian, Pakistani, Bangladeshi) | 15 (5.2) | 15 (5.1) |
| Malay | 12 (4.1) | 11 (3.7) |
| Other (mixed, Black, Polynesian) | 23 (7.9) | 17 (5.8) |
| Site | ||
| New Zealand† | 116 (40.0) | 113 (38.3) |
| Singapore | 82 (28.3) | 84 (28.5) |
| U.K.‡ | 92 (31.7) | 98 (33.2) |
| Nulliparous | 200 (69.0) | 171 (58.0) |
| Smoker | 12 (4.2) | 12 (4.1) |
| Family history of type 2 diabetes | 79 (27.2) | 56 (19.1) |
| Household income quintile | ||
| 5 (lowest) | 5 (1.7) | 2 (0.7) |
| 4 | 20 (6.9) | 24 (8.1) |
| 3 | 69 (23.8) | 54 (18.3) |
| 2 | 95 (32.8) | 109 (37.0) |
| 1 (highest) | 91 (31.4) | 92 (31.2) |
| Not available | 10 (3.5) | 14 (4.8) |
| Preconception plasma glucose (OGTT) (mmol/L) | ||
| Fasting | 4.85 (4.52–5.18) | 4.85 (4.63–5.18) |
| 30 min | 7.81 (6.71–8.90) | 7.70 (6.60–9.01) |
| 2 h | 5.40 (4.41–6.38) | 5.51 (4.63–6.27) |
Data are mean (SD), median (interquartile range), or n (%).
Defined using ethnic-specific thresholds for overweight and obesity: BMI ≥23 to <27.5 and ≥27.5 kg/m2, respectively, for Asians, including Chinese, Indians, Pakistani, Bangladeshi, Malay, mixed Asian; BMI ≥25 to <30 and ≥30 kg/m2, respectively, for non-Asians, including White Caucasian, Polynesian, Black, mixed Asian-non-Asian.
72.9% White Caucasian, 16.6% any Asian, 10.5% other.
94.8% White Caucasian, 2.6% any Asian, 2.6% other.
Comparisons of unadjusted plasma glucose values at the three OGTT time points between the control and intervention groups were not significantly different (Table 2). In the primary outcome intention-to-treat analysis adjusting for site, ethnicity, and matched preconception glucose values (where available), plasma glucose values did not differ between study groups at each of the three time points (P > 0.017) (Table 2). Full adjustment as prespecified provided similar results (Table 2). The incidence of GDM was similar between study groups (Table 3). Sensitivity analyses excluding 32 participants who were subsequently found not to fulfill the eligibility criteria or did not have good adherence gave similar results (Supplementary Table 1).
Table 2.
Primary outcome of maternal OGTT plasma glucose values at 28 (24–32) weeks gestation
| OGTT time point | Control | Intervention | β (95% CI) for loge glucose (loge mmol/L) | |||
|---|---|---|---|---|---|---|
| n | Plasma glucose (mmol/L) | n | Plasma glucose (mmol/L) | Adjusted* | Fully adjusted† | |
| Fasting | 290 | 4.41 (4.08–4.63) | 295 | 4.30 (4.08–4.63) | −0.004 (−0.018 to 0.011) | 0.0002 (−0.014 to 0.014) |
| P value | — | 0.55 | 0.63 | 0.98 | ||
| 1 h | 283 | 8.02 (6.60–9.23) | 294 | 8.24 (6.93–9.45) | 0.025 (−0.014 to 0.064) | 0.036 (−0.003 to 0.074) |
| P value | — | 0.26 | 0.22 | 0.07 | ||
| 2 h | 287 | 6.49 (5.51–7.70) | 295 | 6.60 (5.84–8.02) | 0.040 (0.004–0.077) | 0.043 (0.006–0.081) |
| P value | — | 0.03 | 0.03 | 0.02 | ||
Data are median (interquartile range [unadjusted]) unless otherwise indicated. All P values (t tests on loge-transformed glucose and linear regressions) were not significant ≥0.017 (a priori statistical significance is P < 0.017).
Loge glucose at 24–32 weeks adjusted for site, ethnicity, and baseline loge glucose (for fasting and 2 h only; baseline 1-h glucose not available); n = 584 and 578 for fasting and 2-h glucose, respectively, as a result of missing values for corresponding preconception glucose.
Loge glucose at 24–32 weeks adjusted for site, ethnicity, maternal age, prepregnancy BMI, preconception smoking, parity, family history of diabetes, and baseline loge glucose (for fasting and 2 h only; baseline 1-h glucose not available); n = 581, 574, and 575 for fasting, 1-h, and 2-h glucose, respectively, as a result of missing data.
Glycemia outcomes were examined in two special interest subgroups specified a priori where it was hypothesized that the intervention could have a greater effect. Among women who were overweight or obese before conception (n = 258), intervention did not alter fasting and 1-h glycemia; 2-h glycemia was higher in the intervention group (adjusted β = 0.076 [95% CI 0.020–0.131] loge mmol/L, equivalent to 0.53 mmol/L glucose) but with no increased risk of GDM (Supplementary Table 1). In women with documented dysglycemia before conception (n = 94), glycemia at 28 weeks and GDM incidences were similar between study groups (Supplementary Table 1). Interaction terms in the fully adjusted models including all women showed no evidence of differential effects on 28-week glycemia in response to the intervention among the three study sites and among ethnicities. As measures of insulin resistance and insulin sensitivity, respectively, HOMA2-IR (adjusted β = −0.022 [−0.090 to 0.046]) and Matsuda index (adjusted β = 0.001 [−0.068 to 0.070] at 28 weeks were also similar between study groups.
Adjusting for covariates, there was a lower incidence of preterm birth (<37 weeks gestation) in the intervention group (adjusted risk ratio [aRR] 0.43 [95% CI 0.22–0.82]) (Table 3). There were similar trends in both spontaneous and iatrogenic preterm births. The effect of the intervention was principally observed for late preterm births (34–36 completed weeks gestation, aRR 0.41 [0.20–0.85]) and preterm births associated with preterm prelabor rupture of membranes (PPROM) (aRR 0.21 [0.06–0.69]), with the incidence of PPROM itself also reduced (aRR 0.39 [0.16–0.97]) (Table 3). There were no differences in mean gestational age at delivery, neonatal unit admissions, and neonatal septicemia (Table 3).
The incidence of major postpartum hemorrhage (>1-L blood loss) was lower in the intervention group (aRR 0.44 [95% CI 0.20–0.94]); this reduction was not explained by cesarean section delivery rates or birth weight, which were similar between study groups (Table 3). There were no differences between groups for the secondary outcomes of miscarriage, congenital anomaly, severe nausea and vomiting of pregnancy, hypertensive disorders of pregnancy, intrauterine death, neonatal death, neonatal hypoglycemia, and other neonatal complications (Table 3 and Supplementary Table 2).
Among women who provided the primary and birth outcomes, overall supplement adherence was good, with 80.7% having 80–100% adherence, 15.9% having 60–80% adherence, and only 3.4% having <60% adherence averaged from recruitment to delivery. Adherence was similar in the control and intervention groups. As a further indication of good adherence, 25-hydroxyvitamin D concentrations in the intervention and control groups were similar at the preconception baseline but higher in the intervention compared with the control group at the 28-week OGTT (median 92.8 vs. 63.0 nmol/L). Among all randomized women, withdrawals because of perceived minor side effects from the supplement were similar in both groups (8.3% control, 7.5% intervention), as were other serious adverse events (2.3% control, 2.8% intervention) (Supplementary Table 3).
Conclusions
In this international, multicenter, randomized controlled trial, nutritional formulation enriched with myo-inositol, probiotics, and multiple micronutrients, commenced preconception and continued throughout pregnancy, did not result in lowered maternal glycemia at 28 weeks gestation. There were no significant effects on the incidence of GDM and large-for-gestational-age infants. Intervention reduced preterm birth, affirming findings from previous myo-inositol trials. We also found a reduction in the incidence of major postpartum hemorrhage.
Three previous trials of open-label myo-inositol taken from early pregnancy to prevent GDM in women in Italy focused on discrete high-risk groups for dysglycemia, namely those who were overweight or obese or with a family history of type 2 diabetes (32). All showed a similar reduction in GDM, with an overall odds ratio (OR) of 0.34, as well as lower fasting, 1-h, and 2-h glycemia in a 24–28-week OGTT (32). A further small trial among women with impaired fasting glycemia in early pregnancy reported a large reduction in GDM risk (relative risk 0.127) alongside lower fasting and 1-h glycemia (33). These observations contrast with the finding of no difference in glycemia at 28 weeks gestation with our intervention. However, our study intervention was administered double-blinded over two important periods—preconception and pregnancy—in a general population of women planning pregnancy across multiple centers and ethnicities, excluding those with existing or newly diagnosed type 1 or 2 diabetes preconception. Subgroup analysis of overweight and obese women or those with documented dysglycemia did not show any benefit of our intervention on glycemia, although the trial was not powered to do so. Our results, however, are consistent with an Irish trial of a lower dose of myo-inositol combined with d-chiro-inositol in women with a family history of type 1 or 2 diabetes, which showed no impact on glycemia (34).
In another small trial, dietary counseling and probiotics in pregnant women improved glycemia (OR for elevated glucose 0.31) and insulin sensitivity (14); these findings are discordant with ours despite using the same probiotic combination. However, a meta-analysis of 10 trials of probiotic supplementation in pregnancy found no difference in fasting glycemia (despite a reduction in HOMA-IR) (13), and a recently completed trial showed no difference in GDM rates, with slightly higher fasting glycemia (35). Inconsistent findings may be attributable to different populations and concurrent use of different combinations of prenatal supplements.
Meta-analysis of the group of three Italian myo-inositol studies found a reduction in fetal macrosomia (OR 0.38) and large for gestational age (OR 0.52) (32) in contrast to the finding of no difference with our intervention. The same meta-analysis also found a reduction in preterm birth (OR 0.44) (32), with the separate Irish inositol trial of a lower myo-inositol dose observing a nonstatistically significant trend of fewer preterm births in the intervention group (2% vs. 7%, P = 0.11) (34). Another meta-analysis of trials of multiple micronutrient supplements concluded that the supplements probably also lead to a slight reduction in preterm birth (aRR 0.95 [95% CI 0.90–1.01]) (36). In contrast, none of the probiotic trials reported a change in preterm birth rates. Nonetheless, findings of a meta-analysis of myo-inositol trials (12) are consistent with our demonstration of a reduction in preterm birth. Furthermore, our finding of a reduction particularly in PPROM and PPROM-associated preterm births in the intervention group indicates that this is the likely explanation for reduced prematurity. Approximately 30% of preterm births are preceded by PPROM, of which 60–70% occur late preterm after 34 weeks gestation (37). PPROM is postulated to break down the barrier to ascending pathogens, resulting in intrauterine infection, increased inflammation, and the triggering of preterm labor. In our trial, there was only a reduction in preterm births with intervention without any associated difference in clinically detectable infections between study groups. Potential mechanisms for a preventive effect on PPROM-associated preterm births in our study may include anti-inflammatory effects of myo-inositol (38) and a contribution from the potential synergistic effect of micronutrients, including zinc and vitamin D (39). Our results of specifically a reduction in late preterm births is still clinically significant since prematurity survivors in this group constitute the majority of cases of neurodevelopmental disability associated with preterm delivery (40); thus, the supplement could potentially be impactful.
Our observation that intervention was associated with a reduction in major postpartum hemorrhage is novel and has not previously been reported with myo-inositol, probiotics, or the micronutrients enriched in the supplement used. Since this observation is not explained by differences in cesarean section rates, parity, or birth size, this effect may be mediated by other factors, such as length of labor, myometrial contractility, or blood coagulation, which remain to be examined. Of note, our study found no difference in hypertensive disorders of pregnancy, which is in contrast to a probiotic trial reporting an increased trend of preeclampsia (35) possibly because of counteraction by other components in our intervention. However, our result of a lack of effect on hypertensive disorders is consistent with the myo-inositol trials and a vitamin D trial (20) that also reported no difference.
Collectively available data suggest that further studies are required to determine whether there are subpopulations, dose regimens, or intervention commencement time points when myo-inositol and probiotics may lower maternal glycemia. Conversely, there appears to be a potential benefit of myo-inositol–containing supplements in reducing preterm birth. Whether the other components of our intervention could play an additive role in preterm birth reduction is unclear. Assessment of longitudinal changes in levels of myo-inositol and the other components may shed further light on potential pathways of effect, which may pave the way for the design of more definitive trials in the future.
In contrast to most previously published myo-inositol and probiotic trials, major strengths of our study are its double-blind design and inclusion of multiethnic women from three different continents. Nevertheless, generalizability is limited by the lack of Latina and Native American Indians and only a few Black and Polynesian participants, by less than half of participants being overweight/obese unlike typical U.S. and Western populations, and by our trial being conducted in high-resource settings. Microbiome data were not available to confirm viability of the probiotic in participant samples, and sachet counts provide a limited measure of adherence to the intervention; good adherence is, however, suggested by higher plasma 25-hydroxyvitamin D concentrations in the intervention group at 28 weeks gestation. Another limitation is that we studied a combination of myo-inositol and probiotics with micronutrients. Previous studies have generally examined these individually or as a less complex formulation (e.g., myo-inositol with vitamin D) (12). We cannot exclude the possibility that constituents of the supplement may have moderated individual effects in lowering maternal glycemia or that intervening in the general population (vs. a high-risk population) or commencing intervention preconception (vs. early pregnancy) altered the impact on gestational glycemia. In conclusion, our trial showed that supplementation with myo-inositol, probiotics, and multiple micronutrients preconception and in pregnancy did not lower gestational glycemia but did reduce preterm birth.
Article Information
Acknowledgments. The authors thank the participants and their families for their enthusiastic involvement in the study, the study research staff and hospital clinical staff at participating centers and operational support staff for contributions to the trial, and the members of the independent data monitoring and safety committee for invaluable contributions and for overseeing the conduct of the trial.
Funding and Duality of Interest. Public good funding for this investigator-led study is through the Medical Research Council (U.K.) (MRC) as part of an MRC award to the MRC Lifecourse Epidemiology Unit (MC_UU_12011/4), the Singapore National Research Foundation, the National Medical Research Council (SG) (NMRC) (NMRC/TCR/012-NUHS/2014), the National University of Singapore (NUS), the Agency for Science, Technology and Research (SG) as part of the Growth, Development and Metabolism Programme of the Singapore Institute for Clinical Sciences (H17/01/a0/005), and as part of Gravida, a New Zealand Government Centre of Research Excellence. Funding for provision of the intervention and control drinks and to cover aspects of the fieldwork for the study was provided by Société Des Produits Nestlé S.A. under a research agreement with the University of Southampton, Auckland UniServices Ltd., Singapore Institute for Clinical Sciences, National University Hospital Singapore PTE Ltd., and NUS. K.M.G. is supported by the National Institute for Health Research (NIHR) (Senior Investigator Award NF-SI-0515-10042), NIHR Southampton 1000DaysPlus Global Nutrition Research Group (17/63/154), and NIHR Southampton Biomedical Research Center (IS-BRC-1215-20004); the British Heart Foundation (RG/15/17/3174); and the European Union (Erasmus + Programme Early Nutrition eAcademy Southeast Asia 573651-EPP-1-2016-1-DE-EPPKA2-CBHE-JP and ImpENSA 598488-EPP-1-2018-1-DE-EPPKA2-CBHE-JP). K.M.G. has received reimbursement for speaking at conferences sponsored by companies selling nutritional products. K.M.G., S.J.B., Y.S.C., W.C., and S.-Y.C. are part of an academic consortium that has received grants from Abbott Nutrition, Nestlé S.A., Danone, and BenevolentAI Bio Ltd. outside the submitted work. K.M.G., Y.S.C., W.C., and S.-Y.C. report grants from Société Des Produits Nestlé S.A. during the conduct of the study and are co-inventors on patent filings by Nestlé S.A. relating to the NiPPeR intervention or its components. S.-Y.C. is supported by a Singapore NMRC Clinician Scientist Award (NMRC/CSA-INV/0010/2016) and has received reimbursement and honoraria into her research funds from Nestlé S.A. for a half-day consultancy and for speaking at a conference. No other potential conflicts of interest relevant to this article were reported.
The funders had no role in the data collection and analysis and the decision to submit for publication.
Author Contributions. K.M.G., S.J.B., S.E.-H., T.K., H.N., W.C., and S.-Y.C. contributed to the data collection and assimilation. K.M.G., S.J.B., W.C., and S.-Y.C. contributed to the statistical analysis and vouch for the accuracy and completeness of the data and analyses and for the fidelity of the trial to the protocol. K.M.G., P.N.B., and Y.S.C. conceptualized and designed the study. K.M.G., W.C., and S.-Y.C. led the writing of the manuscript. All authors contributed to the interpretation of the data, critical revision of the manuscript, and approval of the final manuscript for submission. K.M.G. and S.J.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Members of the NiPPeR Study Group are listed in the supplementary material online.
Clinical trial reg. nos. NCT02509988, clinicaltrials.gov and U1111-1171-8056, www.who.int/clinical-trials-registry-platform
This article contains supplementary material online at https://doi.org/10.2337/figshare.13874705.
W.C. and S.-Y.C. contributed equally to this article.
Contributor Information
Collaborators: NiPPeR Study Group, Veronica Boyle, Shirong Cai, Ryan Carvalho, Julie Ann Guiao Castro, Mary Cavanagh, Hsin Fang Chang, Claudia Chi, Caroline E. Childs, Mary F. Chong, Cathryn Conlon, Cyrus Cooper, Paula Costello, Vanessa Cox, Marilou Ebreo, Judith Hammond, Nicholas C. Harvey, Richard Holt, Hazel M. Inskip, Mrunalini Jagtap, Neerja Karnani, Gene Jeon, Yung Seng Lee, Karen Lillycrop, See Ling Loy, Pamela A. Mahon, Chiara Nembrini, Sharon Ng, Justin M. O’Sullivan, Judith Ong, Gernalia Satianegara, Lynette Pei-Chi Shek, Shu E. Soh, Irma Silva-Zolezzi, Karen Tan, Vicky Tay, Rachael Taylor, Elizabeth Tham, Mya Thway Tint, Mark Vickers, Clare Wall, Gladys Woon, Wong Jui-Tsung Ray, Wei Ying, Mei Ling Chang, and Hannah Yong
References
- 1. Farrar D, Simmonds M, Bryant M, et al. Hyperglycaemia and risk of adverse perinatal outcomes: systematic review and meta-analysis. BMJ 2016;354:i4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 3. Lowe WL Jr., Lowe LP, Kuang A, et al.; HAPO Follow-up Study Cooperative Research Group . Maternal glucose levels during pregnancy and childhood adiposity in the Hyperglycemia and Adverse Pregnancy Outcome follow-up study. Diabetologia 2019;62:598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lain KY, Catalano PM. Metabolic changes in pregnancy. Clin Obstet Gynecol 2007;50:938–948 [DOI] [PubMed] [Google Scholar]
- 5. Sacks DA, Hadden DR, Maresh M, et al.; HAPO Study Cooperative Research Group . Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study. Diabetes Care 2012;35:526–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Diabetes Federation . IDF Diabetes Atlas, 8th edition. Brussels, Belgium, International Diabetes Federation, 2017 [Google Scholar]
- 7. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486 [DOI] [PubMed] [Google Scholar]
- 8. Damm P, Houshmand-Oeregaard A, Kelstrup L, Lauenborg J, Mathiesen ER, Clausen TD. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 2016;59:1396–1399 [DOI] [PubMed] [Google Scholar]
- 9. Poston L, Bell R, Croker H, et al.; UPBEAT Trial Consortium . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767–777 [DOI] [PubMed] [Google Scholar]
- 10. Rönö K, Grotenfelt NE, Klemetti MM, et al. Effect of a lifestyle intervention during pregnancy-findings from the Finnish Gestational Diabetes Prevention trial (RADIEL). J Perinatol 2018;38:1157–1164 [DOI] [PubMed] [Google Scholar]
- 11. Watkins OC, Yong HEJ, Sharma N, Chan S-Y. A review of the role of inositols in conditions of insulin dysregulation and in uncomplicated and pathological pregnancy. Crit Rev Food Sci Nutr. 7 December 2020 [Epub ahead of print]. DOI: 10.1080/10408398.2020.1845604 [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Lv Y, Li Z, Sun L, Guo W. The efficacy of myo-inositol supplementation to prevent gestational diabetes onset: a meta-analysis of randomized controlled trials. J Matern Fetal Neonatal Med 2019;32:2249–2255 [DOI] [PubMed] [Google Scholar]
- 13. Zheng J, Feng Q, Zheng S, Xiao X. The effects of probiotics supplementation on metabolic health in pregnant women: an evidence based meta-analysis. PLoS One 2018;13:e0197771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laitinen K, Poussa T; Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group . Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 2009;101:1679–1687 [DOI] [PubMed] [Google Scholar]
- 15. Chen Q, Feng Y, Yang H, et al. A vitamin pattern diet is associated with decreased risk of gestational diabetes mellitus in Chinese women: results from a case control study in Taiyuan, China. J Diabetes Res 2019;2019:5232308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lai JS, Pang WW, Cai S, et al. High folate and low vitamin B12 status during pregnancy is associated with gestational diabetes mellitus. Clin Nutr 2018;37:940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rogne T, Tielemans MJ, Chong MF, et al. Associations of maternal vitamin B12 concentration in pregnancy with the risks of preterm birth and low birth weight: a systematic review and meta-analysis of individual participant data. Am J Epidemiol 2017;185:212–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sahariah SA, Potdar RD, Gandhi M, et al. A daily snack containing leafy green vegetables, fruit, and milk before and during pregnancy prevents gestational diabetes in a randomized, controlled trial in Mumbai, India. J Nutr 2016;146:1453S–1460S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Gong Y, Xue H, Xiong J, Cheng G. Vitamin D and gestational diabetes mellitus: a systematic review based on data free of Hawthorne effect. BJOG 2018;125:784–793 [DOI] [PubMed] [Google Scholar]
- 20. Corcoy R, Mendoza LC, Simmons D, et al. The DALI vitamin D randomized controlled trial for gestational diabetes mellitus prevention: no major benefit shown besides vitamin D sufficiency. Clin Nutr 2020;39:976–984 [DOI] [PubMed] [Google Scholar]
- 21. Hambidge KM, Westcott JE, Garcés A, et al.; Women First Preconception Trial Study Group . A multicountry randomized controlled trial of comprehensive maternal nutrition supplementation initiated before conception: the Women First trial. Am J Clin Nutr 2019;109:457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stephenson J, Vogel C, Hall J, et al.; Preconception Partnership . Preconception health in England: a proposal for annual reporting with core metrics. Lancet 2019;393:2262–2271 [DOI] [PubMed] [Google Scholar]
- 23. Godfrey KM, Cutfield W, Chan SY, Baker PN; NiPPeR Study Group . Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health (“NiPPeR”): study protocol for a randomised controlled trial. Trials 2017;18:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr 2010;103:1792–1799 [DOI] [PubMed] [Google Scholar]
- 25. Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 1998;21:2191–2192 [DOI] [PubMed] [Google Scholar]
- 26. DeFronzo RA, Matsuda M. Reduced time points to calculate the composite index. Diabetes Care 2010;33:e93. [DOI] [PubMed] [Google Scholar]
- 27. Metzger BE, Gabbe SG, Persson B, et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole TJ, Williams AF; RCPCH Growth Chart Expert Group . Revised birth centiles for weight, length and head circumference in the UK-WHO growth charts [published correction appears in Ann Hum Biol 2011;38:241]. Ann Hum Biol 2011;38:7–11 [DOI] [PubMed] [Google Scholar]
- 29. Pike KC, Crozier SR, Lucas JSA, et al.; Southampton Women’s Survey Study Group . Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax 2010;65:1099–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lowe LP, Metzger BE, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care 2012;35:574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S13–S28 [DOI] [PubMed] [Google Scholar]
- 32. Santamaria A, Alibrandi A, Di Benedetto A, et al. Clinical and metabolic outcomes in pregnant women at risk for gestational diabetes mellitus supplemented with myo-inositol: a secondary analysis from 3 RCTs. Am J Obstet Gynecol 2018;219:300.e1–300.e6 [DOI] [PubMed] [Google Scholar]
- 33. Matarrelli B, Vitacolonna E, D’Angelo M, et al. Effect of dietary myo-inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. J Matern Fetal Neonatal Med 2013;26:967–972 [DOI] [PubMed] [Google Scholar]
- 34. Farren M, Daly N, McKeating A, Kinsley B, Turner MJ, Daly S. The prevention of gestational diabetes mellitus with antenatal oral inositol supplementation: a randomized controlled trial. Diabetes Care 2017;40:759–763 [DOI] [PubMed] [Google Scholar]
- 35. Callaway LK, McIntyre HD, Barrett HL, et al. Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 2019;42:364–371 [DOI] [PubMed] [Google Scholar]
- 36. Keats EC, Haider BA, Tam E, Bhutta ZA. Multiple-micronutrient supplementation for women during pregnancy. Cochrane Database Syst Rev 2019;3:CD004905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008;371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Unver N, Delgado O, Zeleke K, et al. Reduced IL-6 levels and tumor-associated phospho-STAT3 are associated with reduced tumor development in a mouse model of lung cancer chemoprevention with myo-inositol. Int J Cancer 2018;142:1405–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kucukaydin Z, Kurdoglu M, Kurdoglu Z, Demir H, Yoruk IH. Selected maternal, fetal and placental trace element and heavy metal and maternal vitamin levels in preterm deliveries with or without preterm premature rupture of membranes. J Obstet Gynaecol Res 2018;44:880–889 [DOI] [PubMed] [Google Scholar]
- 40. Blencowe H, Lee AC, Cousens S, et al. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr Res 2013;74(Suppl. 1):17–34 [DOI] [PMC free article] [PubMed] [Google Scholar]