Abstract
OBJECTIVE
To examine if the association between higher A1C and risk of cardiovascular disease (CVD) among adults with and without diabetes is modified by racial residential segregation.
RESEARCH DESIGN AND METHODS
The study used a case-cohort design, which included a random sample of 2,136 participants at baseline and 1,248 participants with incident CVD (i.e., stroke, coronary heart disease [CHD], and fatal CHD during 7-year follow-up) selected from 30,239 REasons for Geographic And Racial Differences in Stroke (REGARDS) study participants originally assessed between 2003 and 2007. The relationship of A1C with incident CVD, stratified by baseline diabetes status, was assessed using Cox proportional hazards models adjusting for demographics, CVD risk factors, and socioeconomic status. Effect modification by census tract-level residential segregation indices (dissimilarity, interaction, and isolation) was assessed using interaction terms.
RESULTS
The mean age of participants in the random sample was 64.2 years, with 44% African American, 59% female, and 19% with diabetes. In multivariable models, A1C was not associated with CVD risk among those without diabetes (hazard ratio [HR] per 1% [11 mmol/mol] increase, 0.94 [95% CI 0.76–1.16]). However, A1C was associated with an increased risk of CVD (HR per 1% increase, 1.23 [95% CI 1.08–1.40]) among those with diabetes. This A1C-CVD association was modified by the dissimilarity (P < 0.001) and interaction (P = 0.001) indices. The risk of CVD was increased at A1C levels between 7 and 9% (53–75 mmol/mol) for those in areas with higher residential segregation (i.e., lower interaction index). In race-stratified analyses, there was a more pronounced modifying effect of residential segregation among African American participants with diabetes.
CONCLUSIONS
Higher A1C was associated with increased CVD risk among individuals with diabetes, and this relationship was more pronounced at higher levels of residential segregation among African American adults. Additional research on how structural determinants like segregation may modify health effects is needed.
Introduction
The effect of blood glucose levels, as measured by glycated hemoglobin (A1C), on the risk of cardiovascular disease (CVD) among those with diabetes is well-established (1). In a meta-analysis conducted among individuals with type 2 diabetes, Zhang et al. (1) demonstrated a positive dose-response trend with a 17% increased risk of CVD for each 1% (11 mmol/mol) increase in A1C. Moreover, higher A1C has been associated with an increased risk of CVD among those without diabetes (2). In a more recent systematic review and meta-analysis, a 1% (11 mmol/mol) increase in A1C also increased the risk of first ever stroke by 17% and 12% among individuals with and without diabetes, respectively (3). However, this relationship between A1C and CVD risk in those with diabetes is impacted by multiple factors, and additional research is needed.
Community-level social determinants, such as socioeconomic factors, built environment characteristics and patterns of discrimination, reflect differential access to resources, and may have adverse effects on diabetes and CVD risk that go beyond differences in individual-level factors (4–14). Racial residential segregation, an institutional form of racism, has been identified as a primary driver of racial disparities in health in the U.S. (8). Understanding the potential health effects of residential segregation is complex, as there may be negative health effects related to isolation and limited resources, or there could be protective health effects emanating from greater social support received from being present in clustered neighborhoods that are more racially similar (9). Given this complex interplay, differing findings have been reported for the association of racial residential segregation with diabetes and CVD risk (9,10). For example, racial residential segregation was associated with a 12% higher hazard of CVD among Black adults, while there was no association among White adults or Hispanic adults in the Multi-Ethnic Study of Atherosclerosis (11). In contrast, racial residential segregation was not associated with incident diabetes or diabetes management among Black adults in the Coronary Artery Risk Development in Young Adults study (12). While the previous studies focused on the effect of racial residential segregation directly, less is known about whether racial residential segregation may modify known associations, such as between A1C and CVD risk. The influence of racial residential segregation needs to be understood in a larger context, as it is likely that such segregation may impact multiple pathways that can influence the relationship between glycemic control and CVD outcomes in those with and without diabetes. Therefore, the objective of this population-based study was to investigate whether the association between A1C and risk of CVD among adults with and without diabetes varied by community-level racial residential segregation.
Research Design and Methods
The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study is a population-based, prospective cohort study of 30,239 Black and White adults aged >45 years at baseline (2003–2007) that oversampled Black participants and adults residing in the southeastern “Stroke Belt” region of the U.S. A detailed description of the REGARDS study has been reported previously (15). Briefly, demographics and medical history were obtained via a computer-assisted telephone interview; anthropometrics, blood pressure (BP), and blood and urine samples were obtained during a subsequent in-home examination. Participants were contacted every 6 months during 7 years of follow-up. Written informed consent was obtained from participants, and the Institutional Review Boards of participating institutions approved the study methods.
The current study used a nested case-cohort design to allow for greater efficiency as recommended by Prentice (16). This design consisted of a race/sex/age-stratified random sample of the entire cohort from baseline and incident CVD events that occurred during follow-up. To use the stratification, at baseline, participants were given a random number and divided into 20 strata based on age, race, and sex. In each stratum, participants were randomly selected to fulfill the desired distribution: 50% Black, 50% White, 50% women, 50% men, and age groups: 20%, 45–54; 20%, 55–64; 25%, 65–74; 25%, 75–84; and 10%, ≥85 years. Incident events in this random sample were included in analyses in addition to those who developed incident CVD in the full cohort. After excluding participants with missing data for A1C, racial residential segregation indices, diabetes, and covariates, there were 2,136 participants in the stratified random sample at baseline, plus 1,248 participants with incident CVD events that occurred during follow-up (Fig. 1).
Figure 1.
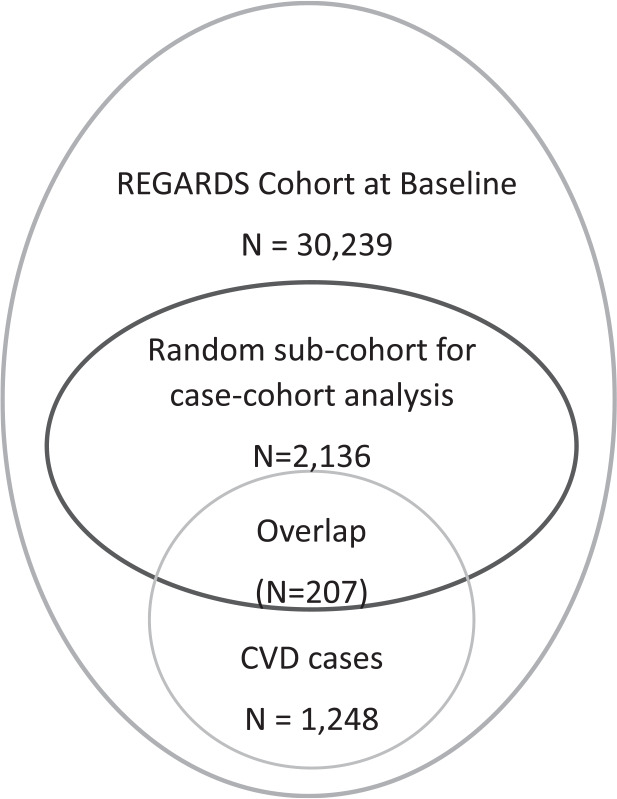
Study population.
A1C
A1C was measured using packed cells from EDTA and SCAT-1 tubes that had been stored at –80°C since baseline collection (2003–2007). The assays for A1C were completed using a turbidimetric inhibition immunoassay by Roche Diagnostics at the University of Vermont Laboratory for Clinical Biochemistry Research. Based on laboratory data, the results obtained from this assay were highly correlated (r = 0.89) with traditional fresh whole-blood methodologies for measuring A1C.
Incident CVD
The primary outcome was incident CVD, defined as stroke, nonfatal myocardial infarction (MI), or coronary heart disease (CHD) death. Participants or their proxies were contacted every 6 months to assess hospitalizations and vital status. Medical records were retrieved for potential stroke and CVD-related hospitalizations and deaths. All events were expert-adjudicated by medical review teams.
Stroke events were defined based on the World Health Organization definition (17) for those with focal neurologic symptoms lasting >24 h or concordant with the American Heart Association guidelines (18) with supportive neuroimaging for those with symptoms lasting <24 h.
MI was assessed following published guidelines (19,20) based on the presence of signs or symptoms suggestive of ischemia; a rising and/or falling pattern in cardiac troponin or creatine phosphokinase-MB over ≥6 hours with a peak value greater than or equal to twice the upper limit of normal (diagnostic cardiac enzymes); and electrocardiogram changes consistent with ischemia or MI, guided by the Minnesota code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia (21,22).
Data from medical records, interviews with next of kin, death certificates, and the National Death Index were collected and reviewed by adjudicators to determine if the death was a CHD death.
Racial Residential Segregation
Using the approach described by Massey and Denton (23), REGARDS participants’ geocoded residential addresses at baseline were linked to the 2000 U.S. census data to calculate the following racial residential segregation measures at the census tract level: dissimilarity index, isolation index, and interaction index.
The dissimilarity index is a measure of the evenness of racial distributions across a spatial unit and ranges from 0 to 1, with higher scores indicating more racial residential segregation. This measure can be interpreted as the proportion of Black adults within a census tract that would have to change residence for the census tract to have the same percentage of Black adults as the county as a whole. The isolation index is a measure of the degree to which Black adults are only exposed to one another and ranges from 0 to 1, with higher scores indicating more racial residential segregation. The interaction index quantifies the extent to which Black adults interact with White adults and ranges from 0 to 1; however, unlike the two previous measures, higher scores of the interaction index indicate less racial residential segregation. Indices of exposure, such as the interaction index, measure the extent to which minority and majority members physically confront one another by virtue of sharing a common residential area. For any spatial area, the degree of minority exposure to the majority population may be conceptualized as the likelihood of their sharing the same neighborhood (23). While the dissimilarity index is invariant to the relative size of the Black population in each unit, the isolation and interaction indices depend on the absolute numbers of Black and White persons in a spatial unit. For this analysis, to clarify relationships, these three indices were divided into tertiles (low, medium, and high) of racial residential segregation, and the interaction index was specifically reverse coded to reflect the same direction as the dissimilarity and isolation indices.
Covariates
Age, race, age–race interaction, sex, education (less than high school education, high school graduate, some college, and college graduate or higher), annual household income (<$20,000/year, $20,000–$35,000/year, $35,000–$75,000/year, >$75,000/year, or refused to answer), and smoking (never smoker, former smoker, or current smoker) were self-reported.
BP was assessed as the average of two measurements obtained with an aneroid sphygmomanometer after being in the seated position for at least 5 min with both feet on the floor. Height and weight were measured via standard procedures, and BMI was computed as the body weight (kilograms) divided by height (meters squared). hs-CRP and total and HDL cholesterol were measured using previously described methods (13). Prevalent diabetes at baseline was defined as fasting glucose ≥126 mg/dL (or a nonfasting glucose ≥200 mg/dL for those who did not fast) or use of oral or injectable diabetes medications.
Statistical Analysis
For this case-cohort study design, the subcohort selection was stratified by age, race, and sex as previously described (24). Given this stratified case-cohort design, all statistical analyses accounted for the sampling probabilities of each participant. Raw frequencies and weighted descriptive statistics were applied to summarize the subcohort population to weight it back to the original REGARDS cohort. Cox proportional hazards models were used to estimate associations between A1C and CVD events. To allow for nonlinear relationships between A1C and CVD, restricted cubic splines were used. Using the planned subcohort, the study had at least 80% power to detect a hazard ratio (HR) of ≥1.135 when examining A1C dichotomized at the observed median value, with even higher power when A1C is examined as a continuous measure as in the cubic splines (25). Statistical interactions between A1C (splines) and racial residential segregation measures (tertiles) were tested to determine whether these segregation measures modified the association between A1C and CVD. To visualize these relationships, plots of the estimated HRs across the range of A1C were created, with and without the residential segregation interactions. Given the differing distributions of A1C among those with and without diabetes at baseline, analyses were a priori stratified by baseline diabetes status. Cause-specific hazard methods were used to account for the competing risk of death (26). All main effects were assessed at the 0.05 level of significance, and interaction terms were tested at the 0.10 level of significance. Statistical analyses were conducted in R 3.5.1 (27,28).
Results
Patients/Events
The study included 2,136 participants in a stratified random sample from the REGARDS cohort at baseline and 1,248 participants who developed incident CVD (Fig. 1). Descriptive statistics, adjusted for sampling weight, for the random subcohort are provided in Table 1 stratified by diabetes status at baseline. In this random subcohort, the mean age was 64.2 ± 9.1 years, 44% were Black, 59% were women, and 19% had diabetes. In this random subcohort, 30 stroke and 27 CHD events occurred among the 408 participants with diabetes; 65 stroke and 85 CHD events occurred among the 1,728 participants without diabetes during a median follow-up of 7.0 years.
Table 1.
Weighted descriptive characteristics of the random subcohort, stratified by baseline diabetes status
| Overall (N = 2,136) | Baseline diabetes | ||
|---|---|---|---|
| Without diabetes (N = 1,728) | With diabetes (N = 408) | ||
| Age (years), mean (SD) | 64.2 (9.1) | 64.0 (9.1) | 65.3 (8.9) |
| Sex (%) | |||
| Female | 58.6 | 58.4 | 59.3 |
| Male | 41.4 | 41.6 | 40.7 |
| Race (%) | |||
| Black | 44.1 | 40.5 | 60.0 |
| White | 55.9 | 59.5 | 40.0 |
| Region (%) | |||
| Stroke Belt area | 34.5 | 34.7 | 33.8 |
| Stroke Belt Buckle area | 19.1 | 18.7 | 21.0 |
| Non-Belt area | 46.4 | 46.6 | 45.2 |
| Smoker (%) | |||
| Current | 13.5 | 13.8 | 12.0 |
| Never | 49.3 | 48.9 | 51.1 |
| Past | 37.2 | 37.3 | 36.9 |
| Income levels (%) | |||
| <$20,000 | 22.8 | 22.3 | 25.0 |
| $20,000–$34,000 | 30.0 | 30.6 | 27.5 |
| $35,000–$74,000 | 17.2 | 18.5 | 11.3 |
| ≥$75,000 | 16.2 | 14.5 | 23.9 |
| Refused to answer | 13.8 | 14.1 | 12.3 |
| Education levels (%) | |||
| Less than high school | 11.1 | 9.62 | 17.7 |
| High school graduate | 24.3 | 23.2 | 29.0 |
| Some college | 27.7 | 28.5 | 23.8 |
| College graduate and above | 37.0 | 38.7 | 29.4 |
| Systolic BP (mmHg), mean (SD) | 127 (16.2) | 126 (15.7) | 131 (17.5) |
| BMI (kg/m2), mean (SD) | 29.4 (5.93) | 28.7 (5.67) | 32.4 (6.12) |
| LDL cholesterol (mg/dL), mean (SD) | 117 (34.4) | 120 (33.9) | 104 (33.4) |
| HDL cholesterol (mg/dL), mean (SD) | 52.6 (15.9) | 53.6 (16.1) | 48.0 (14.3) |
| CRP (mg/L), median (inner quartiles) | 2.21 (0.98–4.78) | 2.03 (0.94–4.55) | 2.88 (1.39–6.72) |
| Incident CVD, (% yes)* | 8.25 | 7.32 | 12.4 |
| Dissimilarity index, median (interquartile range) | 0.53 (0.39–0.63) | 0.53 (0.39–0.63) | 0.53 (0.41–0.63) |
| Interaction index, median (interquartile range) | 0.41 (0.30–0.58) | 0.41 (0.30–0.59) | 0.40 (0.28–0.52) |
| Isolation index, median (interquartile range) | 0.49 (0.33–0.66) | 0.48 (0.32–0.66) | 0.52 (0.36–0.68) |
| Glycosylated hemoglobin (A1C) (%), median (interquartile range) | 5.97 (5.63–6.36) | 5.86 (5.57–6.17) | 7.00 (6.38–7.87) |
| Insulin usage (% yes) | 4.27 | 0.00 | 23.6 |
| Oral glucose-lowering medications (% yes) | 13.6 | 0.00 | 74.9 |
| Statin therapy (% yes) | 25.8 | 22.4 | 41.0 |
Values for continuous variables are weighted mean (weighted SD) or weighted median (weighted interquartile range). Values for categorical variables are percentages.
Incident CVD was defined stroke, CHD, or fatal CHD during 7-year follow-up.
A1C and CVD Risk
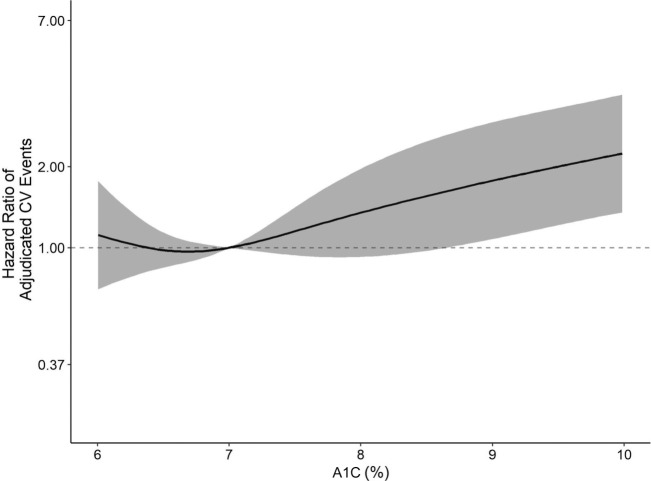
Among people without diabetes, the HR associated with each 1-percentage-point (11 mmol/mol) increase in A1C was 1.12 (95% CI 0.94–1.33) in the crude model and attenuated to 0.94 (95% CI 0.76–1.16) in the fully adjusted model (Table 2). However, among individuals with diabetes, each 1-percentage-point (11 mmol/mol) increase in A1C was associated with a 23% increased CVD risk (HR 1.23 [95% CI 1.08–1.40]) in fully adjusted models (Table 2). The HR spline for the relationship of continuous A1C and incident CVD events in participants with and without diabetes from the fully adjusted model is given in Fig. 2 (for those with diabetes) and Supplementary Fig. 1 (for those without diabetes).
Table 2.
Crude and sequentially adjusted HRs for each 1% increase in glycosylated hemoglobin (A1C) and the risk for adjudicated cardiovascular events* during follow-up among participants with and without diabetes
| Model** | Without diabetes | With diabetes |
|---|---|---|
| Crude (unadjusted) | 1.12 (0.94, 1.33) | 1.20 (1.07, 1.35) |
| + Demographic | 1.10 (0.91, 1.33) | 1.23 (1.09, 1.39) |
| + CV risk factors | 0.97 (0.79, 1.19) | 1.23 (1.08, 1.39) |
| + SES (income/education) | 0.95 (0.77, 1.17) | 1.21 (1.07, 1.38) |
| + Residential segregation indices | 0.94 (0.76, 1.16) | 1.23 (1.08, 1.40) |
Cardiovascular events are defined as stroke, nonfatal MI, or CHD death.
Demographics include sex, race, age, and race–age interaction; CV risk factors include BMI, smoking status, systolic and diastolic BP, LDL and HDL, and logarithm of CRP; socioeconomic status (SES) includes income level and education level; and residential segregation indices include tertiles of dissimilarity index, isolation index, and interaction index.
Figure 2.
HR for the association of A1C and adjudicated CVD events in fully adjusted model, including residential segregation, in participants with diabetes.
Racial Residential Segregation Influence on A1C-CVD Relationship
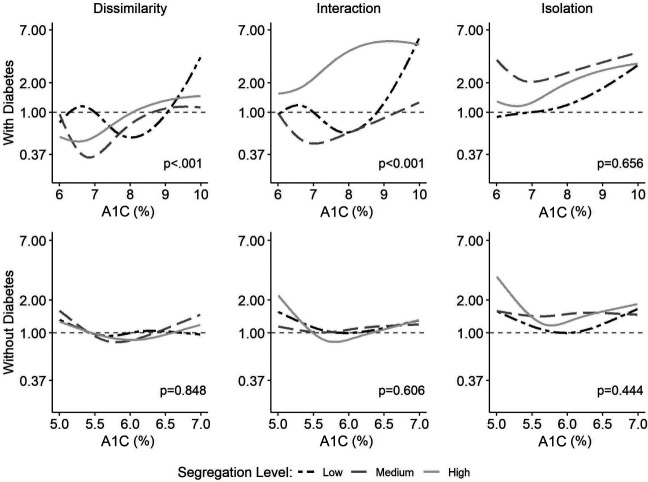
The splines for the A1C-CVD association by tertiles of racial residential segregation are presented in Fig. 3. Among those without diabetes, there were no statistically significant effect modifications by any of the segregation measures. By contrast, among those with diabetes, comparison of the A1C-CVD association across each segregation measure revealed different associations by tertiles of the dissimilarity index (P < 0.001) and the interaction index (P < 0.001).
Figure 3.
HRs for the association between A1C and CVD according to tertiles of racial residential segregation measures (dissimilarity, interaction, and isolation), stratified by diabetes status.
Interaction Index in Individuals With Diabetes
When examining residential segregation as measured by the interaction index in those with diabetes, the association of A1C with CVD events had a larger magnitude of effect for those in the highest tertile for segregation compared with those in the lowest tertile, even at moderate A1C levels between 7% (53 mmol/mol) and 9% (75 mmol/mol). By contrast, there was limited impact on the relationship between A1C and CVD outcomes among those with A1C values <9% (75 mmol/mol) for those in the lowest tertile of racial residential segregation. Only among those with A1C values of ≥9% (75 mmol/mol) for those in the lowest tertile of lower residential segregation did the slope for the interaction term rise sharply. In addition, in exploratory analyses, we examined the relationship between residential segregation as measured by the interaction index and measured CV risk factors, and these findings are given in Supplementary Table 1. There were only modest differences in CV risk factors across residential segregation tertiles.
Dissimilarity and Isolation Indices in Individuals With Diabetes
Although the slopes were statistically significantly different, the pattern was not as consistent across the tertiles of the dissimilarity measure of residential segregation. Among participants with diabetes in the highest segregation tertile, there was a modest rise in slope, evident among those with A1C values approaching 9% (75 mmol/mol), whereas there was a steep rise in slope for those in the lowest segregation tertile. The risk of CVD was lower for those in the medium and high segregation tertiles at lower A1C values (<8% [64 mmol/mol]), with the slope increasing at A1C values >9% (75 mmol/mol). There were no statistically significant differences in the pattern of relationships across tertiles when segregation was measured using the isolation index. In race-stratified analyses, the modifying effect of residential segregation on the A1C-CVD relationship was pronounced among Black participants with diabetes, whereas a modest effect was observed among White participants (Supplementary Fig. 2).
Conclusions
In the current study, higher A1C was associated with an increased risk for CVD among adults with diabetes, but not among adults without diabetes. Additionally, the association of A1C with CVD varied by residential segregation such that those in areas with the highest segregation, as measured by the interaction index, had a higher risk of CVD as A1C increased.
Our finding of an association between increasing A1C values and incident CVD among adults with diabetes is consistent with prior research and similar in magnitude to effect estimates reported in two meta-analyses (1,29). These findings add additional evidence to the link between poor glycemic control and CVD events and raise continued questions regarding whether efforts to improve A1C values may help reduce CVD events, as illustrated in the UK Prospective Diabetes Study (UKPDS) by Stratton et al. (30). However, our study did not identify an association between increasing A1C values and incident CVD outcomes among adults without diabetes. This is in contrast to the report by Mitsios et al. (3), which demonstrated increased ischemic stroke risk with elevated A1C values in individuals without diabetes, and the article by Selvin et al. (2), which demonstrated increased risk for both coronary artery disease and stroke in individuals without diabetes. The contrasting findings may have resulted from differences in the study population and their baseline CVD risk and/or from differences in A1C measurement techniques.
Our findings demonstrated that racial residential segregation modified the association between A1C and CVD events among those with diabetes. The most consistent finding occurred for the interaction index measure—a measure of the potential for interracial interaction—and showed that there was an increased CVD risk at A1C values between 7% (53 mmol/mol) and 9% (75 mmol/mol) for those in the highest tertile of racial residential segregation. This is important because, among individuals with diabetes and A1C values >7% (53 mmol/mol), the majority have values between 7% (53 mmol/mol) and 8% (64 mmol/mol) (31). The dissimilarity index also showed that the A1C-CVD association varied by residential segregation. At lower levels of A1C, there was not a marked increase in risk of CVD in any of the tertiles of residential segregation. When A1C values were approaching 9% (75 mmol/mol), there was a small increase in CVD risk for those in the medium and high tertiles of residential segregation. While these indices are all measures of racial residential segregation, they capture specific aspects of the community and are distinct in what characteristics they reflect. Rather than simply measuring segregation as departure from an ideal of “evenness” as illustrated in the dissimilarity index, the interaction index attempts to measure the experience of segregation as felt by the average minority or majority member. In our study, residential segregation as measured by both evenness of distribution (i.e., dissimilarity index) and the potential for exposure across racial groups (i.e., interaction index) modified the A1C-CVD association.
Few studies have investigated residential segregation among adults with diabetes. A prior study reported that racial residential segregation, measured by percentage of each race group in a given geographic area, was associated with higher age-adjusted diabetes mortality rates in Chicago (32). Additionally, racial residential segregation was not associated with incident diabetes or management of A1C, BP, or cholesterol among those with diabetes (12,32). Prio studies have shown that racial residential segregation can result in concentrated poverty in communities that leads to limited education and employment opportunities and less access to healthy food, physical activity environments, and health care (33–39). This suggests that, even beyond traditional individual-level risk factors, the patterning of segregation in a neighborhood may pose unmeasured biological or behavioral stressors and/or may influence the environment such that attempts to follow a healthy lifestyle, acquire needed medications, and see needed providers are challenged (40–43).
In a large social experiment in the U.S., the Moving to Opportunity study (6), invited women with children who lived in public housing developments located in high-poverty urban areas were randomized to one of the following arms: no housing voucher, a traditional housing voucher with no restrictions, or a housing voucher to move to a low-poverty area. At follow-up, the prevalence of BMI ≥35 kg/m2 and diabetes was modestly lower for those who received the housing voucher to move to a low-poverty area compared with those who did not receive a housing voucher. While this study did not specifically address patterns of racial residential segregation, women who received the voucher to move to a low-poverty area were more likely to reside in census tracts that had a lower proportion of people below the federal poverty line and a lower proportion of people who identified as a member of a racial/ethnic minority group compared with those who did not receive a voucher. This raises important questions about the potential for modifying neighborhood environments to affect health outcomes.
The strengths of the current study include the use of adjudicated CVD events as the primary outcome measure, investigation of residential segregation measures of evenness (dissimilarity index) and exposure (integration index and isolation index), and a study design with participants oversampled from the southeastern U.S. However, the current study has several limitations. The study used publicly available census data from the year 2000, measured shortly before REGARDS study participants were recruited in 2003–2007. However, relevant rmeasures such as duration of exposure in segregated environments, the local context of segregation in participant’s neighborhoods that were smaller than a census tract, or the lived experience of residential segregation among participants was not assessed. This analysis did not have measures of individual social support and did not directly measure the potential that some racially segregated enclaves may have been associated with increased social support that may be linked to improved outcomes. As such, caution is recommended in extrapolating these findings to other populations/settings.
In conclusion, higher A1C increased the risk of CVD among those with diabetes but not those without diabetes. Among those with diabetes, living in areas with higher segregation as measured by the interaction index appeared to modestly but significantly modify this risk relationship, particularly at A1C values between 7% (53 mmol/mol) and 9% (75 mmol/mol), a glycemic control level for a significant proportion of individuals with diabetes. Additional research on how structural determinants may modify health effects is needed.
Article Information
Acknowledgments. The authors thank the other investigators, staff, and participants of the REGARDS Study for the valuable contributions. A full list of participating REGARDS investigators and institutions can be found at https://www.regardsstudy.org.
Funding. This research project is supported by the National Institute of Neurological Disorders and Stroke, National Institute on Aging, National Institutes of Health, and Department of Health and Human Services cofunded cooperative agreement U01 NS041588. Additional support was provided by Centers for Disease Control and Prevention cooperative agreement U01DP006302.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Institute on Aging, Centers for Disease Control and Prevention, or Department of Health and Human Services.
Duality of Interest. D.M.C. and S.P.P. have received research grant funding from Novo Nordisk Pharmaceuticals for research unrelated to the present investigation. A.P.C. has received research funding from Amgen, Inc. for unrelated work. No other potential conflicts of interest were reported.
Author Contributions. D.L.L. and B.G. completed data analysis and assisted with interpretation of findings. D.M.C. interpreted findings and wrote and edited the manuscript. S.P.P., A.C., M.M.S., S.E.J., V.J.H., G.H., and A.P.C. contributed to content and reviewed and edited the manuscript. D.M.C. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. This study was presented at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.14055185.
References
- 1. Zhang Y, Hu G, Yuan Z, Chen L. Glycosylated hemoglobin in relationship to cardiovascular outcomes and death in patients with type 2 diabetes: a systematic review and meta-analysis. PLoS One 2012;7:e42551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 2010;362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mitsios JP, Ekinci EI, Mitsios GP, Churilov L, Thijs V. Relationship between glycated hemoglobin and stroke risk: a systematic review and meta-analysis. J Am Heart Assoc 2018;7:e007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010;1186:125–145 [DOI] [PubMed] [Google Scholar]
- 5. Walker RJ, Smalls BL, Egede LE. Social determinants of health in adults with type 2 diabetes--contribution of mutable and immutable factors. Diabetes Res Clin Pract 2015;110:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med 2011;365:1509–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams DR, Collins C. Racial residential segregation: a fundamental cause of racial disparities in health. Public Health Rep 2001;116:404–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kershaw KN, Pender AE. Racial/ethnic residential segregation, obesity, and diabetes mellitus. Curr Diab Rep 2016;16:108–118 [DOI] [PubMed] [Google Scholar]
- 10. Kershaw KN, Albrecht SS. Racial/ethnic residential segregation and cardiovascular disease risk. Curr Cardiovasc Risk Rep 2015;9:1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-level racial/ethnic residential segregation and incident cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis. Circulation 2015;131:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mayne SL, Loizzo L, Bancks MP, et al. Racial residential segregation, racial discrimination, and diabetes: the Coronary Artery Risk Development in Young Adults study. Health Place 2020;62:102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez-Roux AV. Neighborhood disadvantage, poor social conditions, and cardiovascular disease incidence among African American adults in the Jackson Heart Study. Am J Public Health 2016;106:2219–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci 2016;351:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howard VJ, Cushman M, Pulley L, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–143 [DOI] [PubMed] [Google Scholar]
- 16. Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11 [Google Scholar]
- 17. Stroke --1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke 1989;20:1407–1431 [DOI] [PubMed] [Google Scholar]
- 18. Sacco RL, Kasner SE, Broderick JP, et al.; American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism . An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:2064–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thygesen K, Alpert JS, White HD, et al.; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction . Universal definition of myocardial infarction. Circulation 2007;116:2634–2653 [DOI] [PubMed] [Google Scholar]
- 20. Luepker RV, Apple FS, Christenson RH, et al.; AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation 2003;108:2543–2549 [DOI] [PubMed] [Google Scholar]
- 21. Prineas R, Crow R, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Boston, MA, Wright-OSG, 1982 [Google Scholar]
- 22. Prineas R, Crow R, Zhang ZM. Minnesota Code Manual of Electrocardiographic Findings. 2nd edition. London, U.K., Springer-Verlag, 2010 [Google Scholar]
- 23. Massey DS, Denton NA. The dimensions of residential segregation. Social Forces 1988;67:281–315 [Google Scholar]
- 24. Cushman M, Judd SE, Howard VJ, et al. N-terminal pro-B-type natriuretic peptide and stroke risk: the reasons for geographic and racial differences in stroke cohort. Stroke 2014;45:1646–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics 2004;60:1015–1024 [DOI] [PubMed] [Google Scholar]
- 26. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Core Team . R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing, 2018. Accessed 24 February 2021. Available from https://www.R-project.org/ [Google Scholar]
- 28. Harrell FE Jr. rms: Regression Modeling Strategies. R package version 5.1-2, 2018. Accessed 24 February 2021. Available from https://CRAN.R-project.org/package=rms
- 29. Selvin E, Marinopoulos S, Berkenblit G, et al. Meta-analysis: glycosylated hemoglobin and cardiovascular disease in diabetes mellitus. Ann Intern Med 2004;141:421–431 [DOI] [PubMed] [Google Scholar]
- 30. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hoerger TJ, Segel JE, Gregg EW, Saaddine JB. Is glycemic control improving in U.S. adults? Diabetes Care 2008;31:81–86 [DOI] [PubMed] [Google Scholar]
- 32. Hunt BR, Whitman S, Henry CA. Age-adjusted diabetes mortality rates vary in local communities in a metropolitan area: racial and spatial disparities and correlates. Diabetes Care 2014;37:1279–1286 [DOI] [PubMed] [Google Scholar]
- 33. Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med 2002;22:23–29 [DOI] [PubMed] [Google Scholar]
- 34. Morland K, Filomena S. Disparities in the availability of fruits and vegetables between racially segregated urban neighbourhoods. Public Health Nutr 2007;10:1481–1489 [DOI] [PubMed] [Google Scholar]
- 35. Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern Med 2015;175:1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lleras C. Race, racial concentration, and the dynamics of educational inequality across urban and suburban schools. Am Educ Res J 2008;45:886–912 [Google Scholar]
- 37. Anderson LM, Charles JS, Fullilove MT, Scrimshaw SC, Fielding JE; Task Force on Community Preventive Services . Providing affordable family housing and reducing residential segregation by income. A systematic review. Am J Prev Med 2003;24(Suppl.):47–67 [DOI] [PubMed] [Google Scholar]
- 38. Dubowitz T, Subramanian SV, Acevedo-Garcia D, Osypuk TL, Peterson KE. Individual and neighborhood differences in diet among low-income foreign and U.S.-born women. Womens Health Issues 2008;18:181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lopez R. Black-white residential segregation and physical activity. Ethn Dis 2006;16:495–502 [PubMed] [Google Scholar]
- 40. Coulon SJ, Velasco-Gonzalez C, Scribner R, et al. Racial differences in neighborhood disadvantage, inflammation and metabolic control in black and white pediatric type 1 diabetes patients. Pediatr Diabetes 2017;18:120–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cummings DM, Kirian K, Howard G, et al. Consequences of comorbidity of elevated stress and/or depressive symptoms and incident cardiovascular outcomes in diabetes: results from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. Diabetes Care 2016;39:101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Laveist TA. Segregation, poverty, and empowerment: health consequences for African Americans. Milbank Q 1993;71:41–64 [PubMed] [Google Scholar]
- 43. Pickett KE, Wilkinson RG. People like us: ethnic group density effects on health. Ethn Health 2008;13:321–334 [DOI] [PubMed] [Google Scholar]