Summary
Brown and beige adipocytes, or thermogenic fat, were initially thought to be merely a thermogenic organ. However, emerging evidence suggests its multifaceted roles in the regulation of systemic glucose and lipid homeostasis. One of the important functions of thermogenic fat is as a “metabolic-sink” for glucose, fatty acids, and amino acids, which profoundly impacts metabolite clearance and oxidation. Importantly, lipids are not only the predominant fuel source used for thermogenesis, but also essential molecules for development, cellular signaling, and structural components. Here we review the multifaceted role of lipids in thermogenic adipocytes.
Introduction
The development of obesity is strongly related to the development of type 2 diabetes, cardiovascular disease, and several cancers. Obesity occurs from chronic energy imbalance where caloric intake exceeds caloric expenditure. The majority of excess energy is safely sequestered as triglycerides (TAG) in lipid droplets of adipose tissues. However, adipocytes have a finite storage capacity, which once full, redirect excess lipids for storage toward other tissues resulting in lipotoxicity (Lee et al., 1994; Unger et al., 2010). Several strategies to regulate energy balance and energy storage exist. One promising strategy to remove excess energy storage is to increase energy expenditure through the activation of thermogenic fat (Sidossis and Kajimura, 2015).
Thermogenic fat - brown adipocytes and the recruitable form, a.k.a., beige adipocytes - are the predominant tissue that is responsible for non-shivering thermogenesis. Non-shivering thermogenesis occurs by dissipating energy in the form of heat through mitochondrial uncoupling proton 1 (UCP1) and UCP1-independent pathways (Chouchani and Kajimura, 2019). When activated by external stimuli (e.g., cold exposure), thermogenic fat acts as a metabolic-sink for the uptake and disposal of glucose, lipids, and branched-chain amino acids (BCAA). Thus, enhancing the thermogenic activity and/or the development would effectively remove excess energy from the body.
Whereas the abundance of brown adipose tissue (BAT) is highest in newborns (Lean et al., 1986), compelling evidence demonstrates that adult humans possess both brown and beige adipocytes (Finlin et al., 2018; Lidell et al., 2013; Shinoda et al., 2015; Wu et al., 2012). Importantly, a recent epidemiological study demonstrates that the prevalence of thermogenic fat, as assessed by 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT), was significantly associated with a lower incidence of type 2 diabetes, dyslipidemia, coronary artery disease, cerebrovascular disease, congestive heart failure, and hypertension (Becher et al., 2021). In thermogenic fat, lipids are more than a fuel source: they have essential roles in triggering thermogenesis, cellular signaling, and organelle formation. Here, we discuss the current understandings of the multifaceted roles of lipids in thermogenic adipocytes.
Role of lipids in brown/beige fat thermogenesis
The best-characterized thermogenic mechanism in thermogenic fat is mitochondrial proton uncoupling through UCP1 (Cannon and Nedergaard, 2004). In general, the mitochondrial electron transport system exports protons to generate an energetic gradient across the inner mitochondrial membrane (IMM) to generate a thermodynamically favorable free energy (ΔG) for the synthesis of ATP by the ATP synthase (Neufer, 2018). However, UCP1, located on the IMM transmembrane, can hijack the energetic gradient across the IMM to convert the energy potential generated from electron transport chain proton pumping away from ATP generation and toward heat production (Smith et al., 1966). The process of uncoupling mitochondrial proton pumping generates large energy demand to maintain the electrical gradient of the mitochondrial membrane. To meet this high energetic demand, the mitochondrial electron transport system is highly activated, and macronutrient catabolism is increased to generate the reducing equivalents (e.g., NADH and FADH2) to meet the demand of membrane depolarization caused by UCP1 transport of protons into the mitochondrial matrix. At the molecular level, long-chain fatty acids (FAs) are required for UCP1 activity. Long-chain FAs directly bind to UCP1 protein, which overcome the inhibitory effect of purine nucleotides to UCP1, leading to activation of proton conductance in the mitochondrial inner membrane (Jezek et al., 1994; Urbankova et al., 2003; Winkler and Klingenberg, 1994). Long-chain FAs are negatively charged at a carboxyl end, and thus, the direct binding to UCP1 appears to trigger the H+ flux into the matrix like a fatty-acid anion/H+ symporter (Fedorenko et al., 2012).
Recent studies also highlight the role of UCP1-independent thermogenesis. This involves ATP-dependent futile cycling, such as Ca2+ cycling and creatine cycling (Ikeda et al., 2017; Kazak et al., 2015; Kazak et al., 2019; Tajima et al., 2020). In contrast to UCP1, substrate futile cycling requires active ATP synthesis, and thus, also requires active fuel oxidation in the mitochondria. The underlying mechanisms of substrate preference of fatty acids or other substrates for UCP1-independent thermogenesis remain unclear.
Essential fuels for thermogenesis:
Active uptake of glucose and fatty acids into thermogenic fat is a common distinguishing feature and is used as an effective method to characterize and quantify BAT depot volume in humans, as demonstrated by the studies using 18F-FDG-PET/CT (Cypess et al., 2009; Saito et al., 2009; van Marken Lichtenbelt et al., 2009; Virtanen et al., 2009) and 18F-fluoro-thiaheptadecanoic acid (18FTHA) PET-CT (Ouellet et al., 2012). While the glucose analog, 18F-FDG, has been the predominant molecule utilized to characterize and quantify thermogenic fat, it is important to recognize glucose is not the only molecule that is sequestered upon cold stimulation in thermogenic fat. As highlighted in this review, lipid metabolism is essential for the thermogenic response in BAT, and thus, the utilization of lipid analog 18FTHA, should be considered as an essential maker of BAT activity in humans. Together, glucose, lipid, and amino acid imaging can provide a comprehensive evaluation of the sequestering ability of thermogenic fat and in turn provide important insight into the role of thermogenic fat on systemic metabolism.
In response to cold stimuli, norepinephrine released from the sympathetic nerve terminals and subsequent activation of the β-adrenergic receptor (β-AR) induces a signaling cascade that stimulates lipolysis in adipocytes, which stimulates supplies free fatty acids to support the increased demand for electron transport chain reducing equivalents. The current dogma surrounding human BAT activation through β-AR signaling is that the signal transduction occurs through the β3-AR. Work conducted by Cypess et al. (2015), demonstrates that a 200 mg dose of mirabegron, a β3-AR specific agonist, augments human BAT activity as measured through 18F-FDG PET/CT. While follow up studies conducted by O’Mara et al. (2020) demonstrate that chronic treatment of 100 mg of mirabegron increased BAT activity and resting energy expenditure, while improving insulin sensitivity, without changes in body mass. On the other hand, a recent study by Blondin et al. (2020) reported that human BAT was activated by β2-AR stimulation. In differentiated human brown adipocytes, the mRNA expression of the β2- AR is the most highly expressed isoform and β2-AR mRNA expression is augmented when stimulated with norepinephrine. Furthermore, differentiated human primary adipocytes generate an increase in oxygen consumption when stimulated with formoterol, a selective β2-AR agonist, while the effect was blunted in the presence of β2-AR antagonist, ICI-118,551. While these results suggest that human thermogenic fat can be activated through β2-AR, continued research is needed to validate the significance of β2-AR relative to β3-AR in activation of human BAT.
The source of FAs for UCP1 uncoupling was initially thought to derive from de novo lipolysis within brown adipocytes, i.e., norepinephrine acts on β3-AR in brown adipocytes, leading to increased FAs synthesis for beta-oxidation. However, BAT-specific defects in lipolysis, such as genetic deletion of adipose triglyceride lipase (ATGL) or the ATGL-activating protein comparative gene identification-58 (CGI-58) by Ucp1-Cre, did not impair BAT thermogenesis in vivo. In contrast, deletion of ATGL or CGI-58 in both brown and white fat, by the use of adiponectin-Cre, significantly attenuates BAT thermogenesis (Schreiber et al., 2017; Shin et al., 2017). These data suggest that FAs released from WAT, rather than de novo lipolysis in BAT, are the primary fuel source for thermogenesis (Fig. 1). FFA transported to brown adipose tissue are taken up by plasma membrane fatty acid transport proteins such as CD36 or Fatty acid transport protein 1 (FATP1) (Bartelt et al., 2011; Wu et al., 2006). In mice, a loss of FATP1 is sufficient to inhibit fatty acid uptake into BAT and impair the thermogenic response to cold exposure, suggesting that active FA transport into BAT, rather than de novo lipolysis, is required for BAT thermogenesis (Wu et al., 2006). Besides lipolysis in WAT, FFA can be processed in the liver to acylcarnitine, which significantly contributes to optimal thermogenesis in BAT (Simcox et al., 2017).
Figure 1. Role of lipids in thermogenesis.
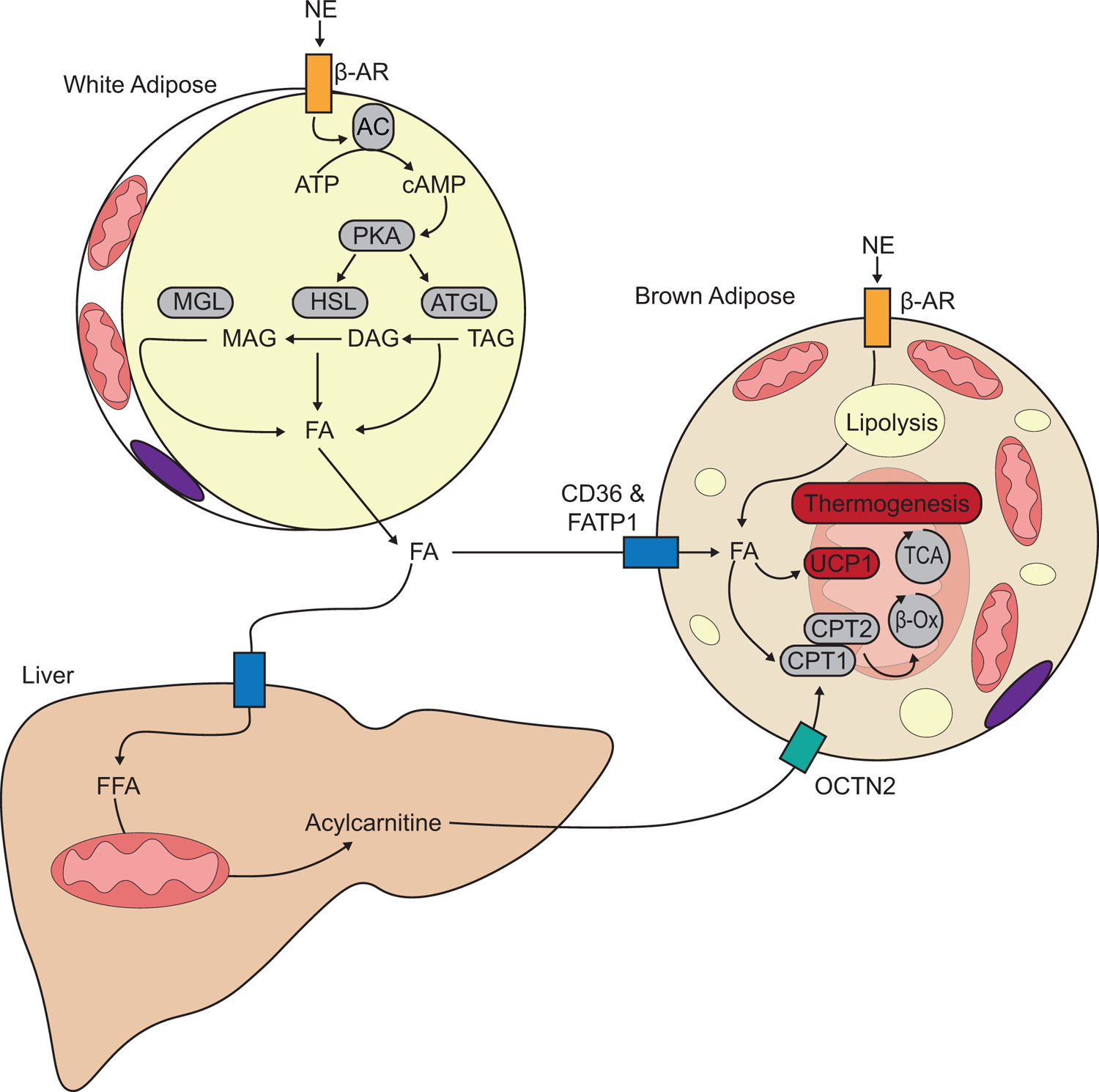
Cold-induced norepinephrine (NE) activation of β- adrenergic receptor (β-AR) induces a lipolytic signaling cascade with activates adenylate cyclase (AC) to produce cyclic-AMP (cAMP), which activates protein kinase A (PKA). PKA activates lipase activity and adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) and monoglyceride lipase breakdown triacylglyceride (TAG), diacylglyceride (DAG), monoacylglyceride (MAG) into fatty acids (FAs) and glycerol. FAs are taken up by brown/beige adipocytes and transported into the mitochondria through carnitine palmitoyltransferase 1 and 2 (CPT1, CPT2), where they undergo β-oxidation and feed into the tricarboxylic acid (TCA) cycle to support energy requirements of thermogenesis.
In addition to fatty acids, glucose and BCAA oxidation are essential for optimal thermogenesis. Panic et al. (2020) demonstrate that a lack of pyruvate entry into brown adipose tissue mitochondria is required for a robust thermogenic response to a cold challenge in mice. Likewise, Yoneshiro et al. (2019) demonstrate that thermogenic fat acts as a major metabolic-sink for BCAA and that active BCAA oxidation is required for optimal thermogenesis. Together, these studies suggest that one fuel alone is not sufficient for a robust thermogenic response without compensational adaptation, while each fuel source is regulated to work in concert to supply necessary reducing equivalents to meet the energetic demand to produce heat.
The role of lipids as signaling entities (lipokines)
Lipids are a diverse class of molecules and are more than fuel for cellular energy. For example, specific lipids are signaling molecules known as lipokines that mediate inter-organ or inter-cellular communication (Cao et al., 2008). The diverse structures of lipids allow for a potentially wide range of the currently uncharacterized function of lipokines. Lipid structure varies by the lipid properties, such as the lipid class, fatty acid chain length, and degrees of unsaturation. The diversity of these features allows for a wide range of lipids to be lipokines that act on specific receptors (Fig.2).
Figure 2. Lipid activated G protein-coupled receptors and mouse phenotypes.
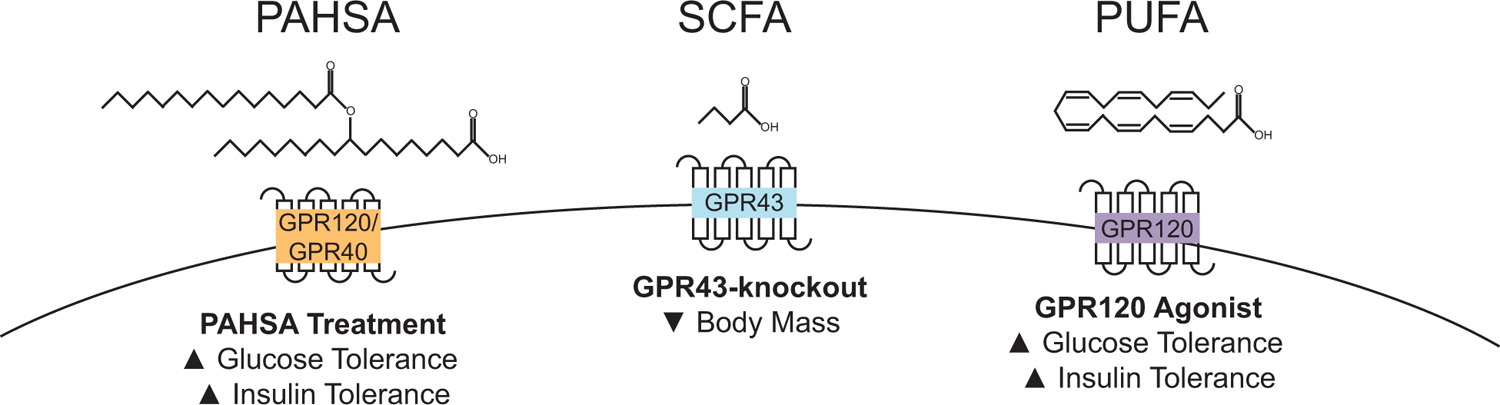
Short-chain fatty acids (SCFA) stimulate GPR43. Polyunsaturated fatty acids (PUFA) activate GPR120. PAHSAs activate adipose GPR120 and pancreatic β cell GPR40.
G-Protein Coupled Receptors:
G-protein coupled receptors (GPRs) can be stimulated by certain FFAs, where different species of FFA are ligands for different receptors (Vinolo et al., 2012). Short-chain fatty acids (SCFA) are lipid molecules less than 6 carbons long, and they are a ligand for GPR43 (Free fatty acid receptor 2, FFAR2). In brown adipocytes, GPR43 expression is markedly enhanced in differentiated cells. Stimulation of GPR43 in brown adipocytes increased expression of UCP1 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), whereas knockdown of GPR43 attenuated UCP1 expression and mitochondrial biogenesis in differentiated brown adipocytes (Hu et al., 2016). However, whole-body knockout of GPR43 in mice demonstrates discrepancies when compared to cell culture data. Mice with a whole-body knockout of GPR43 have lower body weight than controls when fed a high-fat diet. GPR43 whole-body knockout mice also have less lipid accumulation in the BAT and greater core body temperature after 20 weeks of high-fat diet feeding. However, GPR43 whole-body knockout mice consume more food and absorb less energy than control mice, suggesting that GPR43 signaling in the gastrointestinal tract is critical for calorie absorption (Bjursell et al., 2011). Future studies should examine mice with brown adipose tissue-specific knockout of GPR43 to determine the role of brown adipose tissue GPR43 signaling in core body temperature.
Medium-chain fatty acids (MCFA) are lipid molecules between 6–12 carbons long, and they activate GPR84. Tissue-specific expression of GPR84 is highest in skeletal muscle, but BAT and inguinal WAT both have high levels of GPR84 protein expression (Montgomery et al., 2019). GPR84 has been predominantly studied for its role in immune cell response (Recio et al., 2018). In mice, a whole-body knockout of GPR84 does have a minor reduction in glucose tolerance, but only when mice were fed an MCFA enriched diet. Body mass and energy expenditure are not affected by GPR84 loss (Montgomery et al., 2019).
The unsaturation or number of double bonds also change the shape of the lipid, and polyunsaturated omega-3 fatty acids stimulate GPR120 (FFAR4). In humans with obesity, GPR120 expression in adipose tissue is greater when compared to lean controls. A deleterious mutation of GPR120 (p.R270H) in humans was determined to be a risk factor for obesity in European populations (Ichimura et al., 2012). Also, GPR120 expression is elevated during brown adipocyte differentiation (Schilperoort et al., 2018) and in response to cold stimulation (Quesada-Lopez et al., 2016). A knockout of GPR120 in mice was sufficient to increase body weight gain with high-fat diet feeding and attenuate energy expenditure, glucose tolerance, and insulin tolerance (Ichimura et al., 2012). When fed a standard chow diet, mice with an absence of GPR120 have a mild impairment in glucose tolerance that is accompanied by a significant increase in circulating insulin, suggesting that GPR120 knockout mice are insulin resistant (Oh et al., 2010). GPR120 knockout mice additionally display mild cold intolerance that is accompanied by attenuated levels of UCP1 expression in inguinal WAT (Quesada-Lopez et al., 2016), while a knockout of GPR120 in brown adipocytes was sufficient to reduce Ucp1 expression in differentiated brown adipocytes (Schilperoort et al., 2018). Conversely, activation of GPR120 in white adipocytes with docosahexaenoic acid (DHA) potently increased glucose uptake and augmented GLUT4 translocation (Oh et al., 2010). Pharmacological activation of GPR120 with CpdA, a selective agonist, did improve glucose and insulin tolerance from a high-fat diet in wild-type mice without stimulating differences in body weight (Oh et al., 2014). A separate GPR120 agonist, TUG-891, also increases BAT activity, while treatment of TUG-891 to brown adipocytes did not increase Ucp1 expression compared to vehicle-treated cells. Mice treated with GPR120 agonist, GW9508, had augmented UCP1 protein expression in inguinal WAT (i.e., beige adipocytes) and greater oxygen consumption under basal conditions and when stimulated with CL316,243, a β-adrenergic agonist, when compared to untreated controls (Quesada-Lopez et al., 2016). Combined these studies suggest that treatment with a GPR120 agonist may improve glucose tolerance mediated, at least in part, through activation of thermogenic adipocytes.
Palmitic acid hydroxystearic acids (PAHSAs) are considered endogenous ligands for GPR120 in adipose (Yore et al., 2014) and GPR40 in pancreatic β cells (Syed et al., 2018). Acute administration of 5-PAHSA or 9-PAHSA was sufficient to lower basal glucose levels and improve glucose tolerance in mice on a high-fat diet (Yore et al., 2014). In cultured white adipocytes, treatment with 9-PAHSA induced a thermogenic transcriptional profile with upregulation of UCP1, PGC-1α, C/EBPβ, and PRDM16. In wild-type and ob/ob mice chronically treated with 9-PAHSA, white adipose tissue UCP1 and PGC-1α protein expression were upregulated in a GPR120-dependent manner (Wang et al., 2018). Chronic treatment of 5- and 9-PAHSA or 9-PAHSA in chow or high fat diet-fed mice did not alter body weight gain but did improve glucose and insulin tolerance, which are thought to be in part mediated through GPR40 signaling in pancreatic β cells (Syed et al., 2018). While chronic PAHSA treatment significantly improves glucose and insulin tolerance, which is observed with augmentation in insulin secretion mediated through GPR40 signaling, the role of PAHSAs in thermogenic adipose is an exciting area requiring more characterization.
Thermogenic lipokines:
Certain lipokines are shown to activate thermogenic fat via upregulating UCP1 expression or in a UCP1-independent fashion. Such lipids include N-acyl amino acids, 12-hydroxyeicosapentaenoic acid (12-HEPE), and 12,13-dihydroxy-9z-octadecenoic acid (12,13-diHOME).
Amino acids conjugated to fatty acids are known as N-acyl amino acids. These modified fatty acids have unusual signaling activity with metabolic consequences. A biosynthetic pathway for N-acyl amino acids is through the peptidase M20 domain containing 1 (PM20D1) protein, which has enzymatic activities to both synthesize and hydrolyze N-acyl amino acids. PM20D1 is enriched in UCP1-positive adipocytes, and mRNA levels are increased in brown adipose tissue with cold exposure (Long et al., 2016). Overexpression of PM20D1 increased energy expenditure, reduced fat mass, reduced food intake, and improved glucose tolerance in mice fed a high-fat diet. Additionally, studies with supplemented N-acyl amides mirrored the findings of PMD20D1 overexpression. Notably, N-acyl amino acids can be natural uncouplers that induce mitochondrial uncoupling respiration independent of UCP1 (Long et al., 2016). This action appears to be through mitochondrial ATP/ADP symporters, ANT1 and ANT2 (SLC25A4 and SLC25A5), although the mechanism of the action needs further investigation. In mice with a whole-body knockout of Pm20d1, energy expenditure and diet-induced body weight gain were not different from control mice. However, loss of Pm20d1 was sufficient to exacerbate glucose and insulin tolerance. Of note, Pm20d1 knockout mice did not have lower levels of N-acyl amino acids. Instead, several lipid species were upregulated compared to control, which corresponded with Pm20d1 knockout mice having an improved thermogenic response to acute cold stress that might be attributed to an elevation in N-oleoyl-glutamine (C18:1-Gln) (Long et al., 2018).
Oxylipins are a group of bioactive lipids which are synthesized from polyunsaturated omega-3 or omega-6 fatty acids (Barquissau et al., 2017). 12-HEPE is an oxylipin that is produced by 12-lipoxygenase (Alox12, 12-LOX) from the precursor fatty acid eicosapentaenoic acid (C20:5) (Hamberg, 1980). Cold exposure in humans and mice increased the abundance of circulating 12-LOX generated oxylipins (Kulterer et al., 2020; Leiria et al., 2019). While in mice multiple 12-LOX generated lipids were upregulated in cold, the genetic reduction of BAT only ablated cold-induced upregulation of 12-HEPE. Mice with Alox12-deletion in UCP1-positive cells have exacerbated tolerance to cold compared to controls. In brown adipocytes absent of Alox12, glucose uptake and glycolytic metabolism was impaired compared to controls. Consistent with impaired cold tolerance and glucose metabolism, Alox12-deletion in brown adipocytes resulted in drastically reduced maximal mitochondrial oxygen consumption. Daily treatment with 12(S)-HEPE for two weeks in mice was unable to alter body weight. However, 12(S)-HEPE treated mice had improved glucose and insulin tolerance compared to vehicle treated mice. Consistent with improved glucose and insulin tolerance, glucose uptake was greater in BAT and skeletal muscle of mice treated with 12(S)-HEPE (Leiria et al., 2019). Together these data support the role of 12-HEPE as a cold-induced thermogenic lipid which has positive outcomes on glucose uptake in skeletal muscle.
Another example of thermogenic lipokine is 12,13 diHOME that is released from brown adipose or white adipose tissue. This lipokine is induced by cold exposure and a downstream fatty acid metabolite of linoleic acid (C18:2). Plasma levels of 12,13 diHOME are elevated with cold exposure in humans and positively correlates with BAT activity. In mice, acute injection of 12,13 diHOME augments body temperature in response to a cold challenge and increases the uptake of fatty acids into BAT. The increase in fatty acid uptake to BAT was mediated through an increase in fatty acid transporters translocation. A chronic treatment of 12,13 diHOME was not sufficient to reduce body weight or ameliorate glucose tolerance, but did improve oral lipid tolerance in mice fed a high-fat diet. Additionally, chronic treatment did not increase BAT UCP1 mRNA expression, but did increase lipoprotein lipase (LPL) expression in BAT (Lynes et al., 2017). 12,13 diHOME as a fatty acid uptake stimulating endocrine molecule has also been observed in skeletal muscle (Stanford et al., 2018), suggesting that many 12,13 diHOME endocrine actions are yet to be characterized.
The role of lipids in membrane structure and function
Phospholipids, sphingolipids, and cholesterol are integral components of cellular membranes. Classically, they provide organizational barriers and structural support for membrane-bound proteins. However, membrane lipids are highly compartmentalized among cellular organelles, and their regulation is critical to cellular function (Funai et al., 2020). The composition of membrane lipids has been demonstrated to alter the structure, function (Laganowsky et al., 2014), and efficiency (Verkerke et al., 2019) of membrane-bound proteins. Further, phospholipids are responsive to environmental stressors such as exercise and inactivity (Heden et al., 2019). Membrane phospholipids in BAT mitochondria are also responsive to environmental cues, and BAT phospholipids increase in response to cold, while re-acclimation to room temperature will bring phospholipid levels back to pre-cold exposure (Ricquier et al., 1978; Strunecka et al., 1981). The metabolic phenotypes of mouse models with altered lipid species are summarized in Table. 1.
Table 1.
Summary of lipid modifying models and their metabolic outcomes.
| Lipid | Model | Metabolic Response | Source |
|---|---|---|---|
| Cardiolipin | Cls1 knockout | ▼Body Mass ▼ Insulin Tolerance ▼Cold Tolerance |
Sustaric et al., Cell Metab 2018 |
| Taz knockdown | ▼Body Mass ▼Cold Tolerance |
Johnson et al., Mol Metab 2019 | |
| Phosphatidylcholine | Pemt knockout | ▲Energy Expenditure ▼Body Mass ▼Fat Mass ▼Cold Tolerance |
Gao et al., J Lipid Res 2015 Johnson et al., Mol Metab 2019 |
| Ceramide | Cers6 knockout | ▲Energy Expenditure ▲Glucose Tolerance |
Turpin et al., Cell Metab 2014 |
| Sptlc2 knockout | ▲Energy Expenditure ▼Body Weight Gain ▼Fat Mass ▲Glucose Tolerance |
Chaurasia et al., Mol Metab 2020 | |
| Asah1 knockout | ▼ Energy Expenditure ▲ Body Weight Gain ▲ Fat Mass ▼Glucose Tolerance |
Chaurasia et al., Mol Metab 2020 | |
| 12,13-DiHOME | Acute Injection | ▲Cold Tolerance ▲Fatty acid uptake |
Lynes et al., Nat Med 2017 |
| 12-HEPE | Alox12 knockout | ▼Cold Tolerance ▼Glucose Uptake |
Osório Leiria et al., Cell Metab 2019 |
| 12(S)HEPE Treatment | ▲Glucose Tolerance ▲Insulin Tolerance |
Osório Leiria et al., Cell Metab 2019 | |
| PAHSA | 5-PAHSA or 9-PAHSA Acute Treatment | ▼Basal Glucose ▲Glucose Tolerance |
Yore et al., Cell 2014 |
| 5- and 9-PAHSA or 9-PAHSA Chronic Treatment | ▲Glucose Tolerance ▲Insulin Tolerance |
Syed et al., Cell Metab 2018 | |
| N-Acyl amino acids | PM20D1 overexpression (AAV) | ▲Energy Expenditure ▼Body Weight Gain ▼Fat Mass ▼Food Intake ▲Glucose Tolerance |
Long et al., Cell 2016 |
| C18:1-Leu, C18:1-Phe, or C20:4-Gly Supplementation | ▲Energy Expenditure ▼Body Weight Gain ▼Fat Mass ▼Food Intake ▲Glucose Tolerance |
Long et al., Cell 2016 | |
| Pm20d1 whole-body knockout | ▼ Glucose Tolerance ▼ Insulin Tolerance ▲ Cold Tolerance |
Long et al., PNAS 2018 |
Phosphatidylcholine (PC) is the most abundant phospholipid in mammalian cells (van der Veen et al., 2017). It is primarily synthesized through the Kennedy pathway, which produces new phospholipid molecules (DeLong et al., 1999). Additionally, PC can be formed by the methylation of phosphatidylethanolamine (PE) by the enzyme PE methyltransferase (PEMT) (Ridgway and Vance, 1987) or through the conversion of lyso-PC to PC by acyltransferases (Wang and Tontonoz, 2019). Phosphatidylcholine abundance in BAT is responsive to environmental stimuli such as cold exposure (Ricquier et al., 1978) and exercise (May et al., 2017). Whole-body deletion of PEMT increases energy expenditure and protects mice from diet-induced obesity (Jacobs et al., 2010). However, mice with whole-body deletion of PEMT are cold intolerant (Gao et al., 2015) and have PC species-specific changes (Johnson et al., 2020). PEMT expression increases during differentiation of brown adipocytes, and a knockdown of PEMT corresponds to a reduced thermogenic program, including the lower abundance of UCP1. However, BAT-specific knockout of PEMT does not result in a lower abundance of UCP1, cold intolerance, or changes in the species abundance of PC. In mice with a whole-body knockout of PEMT, alternative splicing of UCP1 occurs in a non-cell autonomous action, which generates a non-functional UCP1 protein (Johnson et al., 2020).
Cardiolipin is a unique class of phospholipids. Unlike other phospholipids, cardiolipin has two phosphatidic acid groups, which are connected through a glycerol backbone. Thus, creating a lipid with four acyl chains and a cone shape (Ikon and Ryan, 2017). Cardiolipin synthase generates nascent cardiolipin from cytidine diphosphate diacylglycerol (CDP-DAG) (Houtkooper et al., 2006). The acyl chains of the nascent cardiolipin are remodeled in a process catalyzed by taffazin to form mature cardiolipin molecules, which are predominately tetralinoleic (Xu et al., 2006). Cardiolipin is highly concentrated in the inner mitochondrial membrane where it is thought to be critical for the structure and function of many IMM proteins (Houtkooper and Vaz, 2008). The isolation of UCP1 has demonstrated that cardiolipin does bind to UCP1 (Lee et al., 2015). Additionally, biochemical studies utilizing reconstituted UCP1 demonstrate that the addition of cardiolipin alleviates the inhibition of UCP1 proton transport by purine nucleotide-binding (Klingenberg, 2009). In brown adipocytes, cardiolipin synthase gain of function was accompanied by an increase in UCP1 mRNA abundance as well as an increase in NE-induced oxygen consumption. Congruently, a reduction in cardiolipin synthase reduced NE-induced oxygen consumption in brown adipocytes (Sustarsic et al., 2018). However, reducing cardiolipin synthase did not reduce maximal respiration rates in brown adipocytes. In mice, fat-specific knockout of cardiolipin synthase impaired the ability of thermogenic adipose to respond to cold stimulus and these KO mice were cold intolerant. Interestingly, fat-specific loss of cardiolipin synthase did not result in augmented weight gain in mice fed a high-fat diet. Rather, the KO mice had attenuated weight gain with a high-fat diet feeding compared to controls. Loss of adipose cardiolipin synthase did cause metabolic inflexibility, and mice without adipose cardiolipin synthase were insulin intolerant (Sustarsic et al., 2018). Similar to mice with fat-specific cardiolipin synthase knockout, mice with whole-body taffazin knockdown are cold intolerant, although UCP1 protein levels were unchanged in mice with taffazin-knockdown (Johnson et al., 2020). These results may suggest a differential role between nascent cardiolipin and mature cardiolipin in mitochondrial function for thermogenesis that is not clearly characterized.
Ceramides, a lipid class of sphingolipids, are low in abundance compared to phospholipids, but have significant biological activity (Summers et al., 2019). Ceramide abundance increases with obesity, and inhibiting ceramide production has been demonstrated to improve metabolic health (Chaurasia et al., 2019). In human white adipose tissue, ceramide synthase 6 (CerS6) positively correlates with body mass index (BMI) and insulin resistance. A whole-body knockout of CerS6 was sufficient to protect mice from diet-induced obesity, increase energy expenditure levels, and protect glucose and insulin tolerance from effects of HFD feeding. The BAT-specific knockout of CerS6 was sufficient to increase energy expenditure levels and blunt exacerbated glucose tolerance with high-fat diet feeding (Turpin et al., 2014). Inhibiting ceramide synthesis through BAT-specific deletion of serine palmitoyltransferase subunit 2 (Sptlc2) also increased energy expenditure, reduced weight gain, and protected mice from diet-induced impairments in glucose and insulin intolerance. Conversely, increases in ceramide accumulation mediated through BAT-specific loss of acid ceramidase 1 (Asah1) lowered energy expenditure, increased diet-induced weight gain, and exacerbated glucose tolerance (Chaurasia et al., 2020). Together, these studies suggest that the regulation of ceramide abundance is critical to thermogenic function and systemic lipid and glucose homeostasis.
Perspectives: The best ability is the availability
Lipids serve thermogenic fat in many ways, from serving as structural support and their utilization as signaling entities to their most well-characterized role as a fuel source. While highlighted in this review is that lipids are essential for thermogenic fat, glucose and BCAA metabolism are also crucial for an optimal thermogenic response. Which raises the key question: how do cells determine the optimal fuels? The glucose-fatty acid cycle, or the Randle Cycle, is the biological process by which the optimal fuel selection of glucose to fatty acid oxidation is most commonly viewed and best understood (Randle et al., 1963). In the glucose-fatty acid cycle, as the fatty acid metabolism increases metabolite flux into the TCA cycle, there is an accumulation of acetyl-CoA, which inhibits pyruvate dehydrogenase complex (Hue and Taegtmeyer, 2009). Additionally, the availability of TCA intermediates induces a cascade, which results in transport of citrate out of the mitochondrial matrix and into the cytosol, where it has inhibitory effects on glycolysis (Garland et al., 1963). Thus, allowing for the influx of fatty acids into brown/beige adipocytes to predominate as an essential fuel source for thermogenesis.
An essential aspect of the glucose-fatty acid cycle is the importance of compartmentalization of metabolites between the cytosol and the mitochondrial matrix. As more metabolites are characterized as essential for optimal thermogenic response, it will be critical to developing a deeper understanding of fuel selection mechanisms. In this regard, accumulating evidence indicates the importance of the solute carrier family 25 (SLC25) family, a mitochondria-localized transporter family of proteins (Palmieri, 2014). The majority of proteins in the SLC25 family localize to the inner mitochondrial membrane and regulate mitochondrial matrix ion and metabolite flux. Included in the SLC25 family are UCP1–3 (SLC25A7–9), nicotinamide adenine dinucleotide transporter (SLC25A51), mitochondrial BCAA carrier (SLC25A44, MBC), and the carnitine-acylcarnitine translocase (SLC25A20, CACT). As an example, SLC25A44 is recently characterized as the MBC that is required for mitochondrial BCAA uptake and oxidation in BAT (Yoneshiro et al. (2019). The MBC determines the intracellular BCAA fate, i.e., mitochondria BCAA catabolism vs. anabolic reactions, such as protein synthesis. Thus, the utilization of inner mitochondrial membrane gatekeepers to study the relationship of subcellular metabolite fate may help us understanding the regulation of optimal fuel selection and metabolic flexibility.
Figure 3. Fuel selection during thermogenesis.
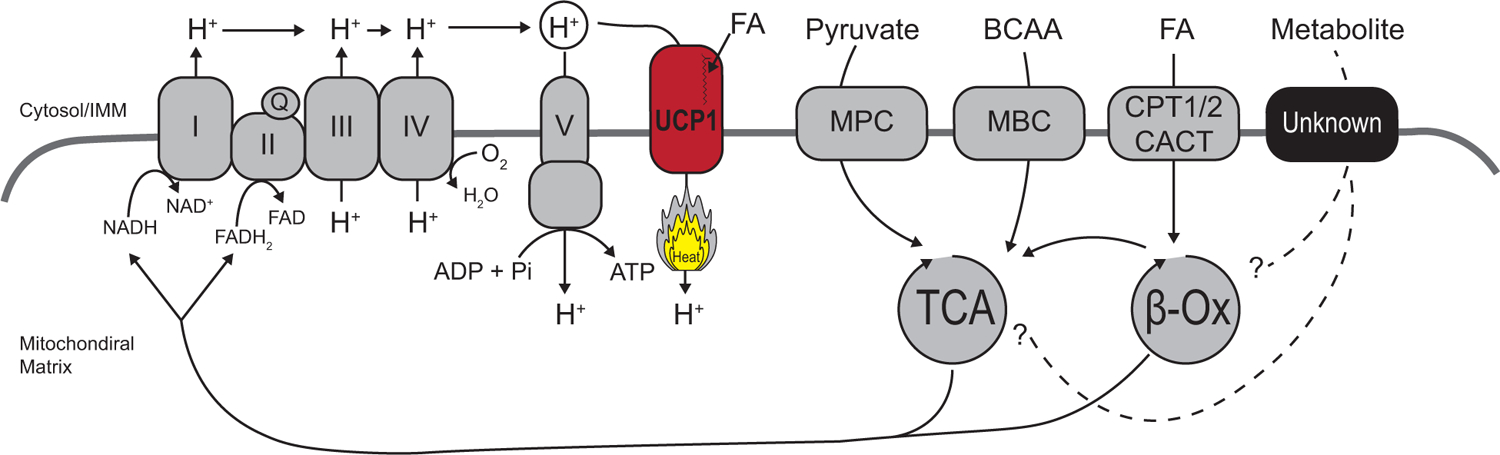
Glucose, fatty acids (FA), and branched-chain amino acids (BCAA) are necessary for optimal thermogenesis in brown and beige fat. A better understanding of metabolite gating into the mitochondrial matrix is needed to develop a comprehensive model of fuel selection. Genetic or pharmacological regulation of inner mitochondrial membrane (IMM) transport proteins allows for the control of the fate of metabolites from cytosolic actions and that of mitochondrial metabolism action.
Verkerke and Kajimura present a Review about the multifaceted role of lipids in thermogenic (brown and beige) adipocytes. They discuss lipids as a fuel source used for thermogenesis, and as essential molecules for development, cellular signaling, and structural components
Acknowledgements
This work was supported by NIH grants R01DK125283, R01DK125281, R01DK097441, R01DK127575, and DP1DK126160 to S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References
- Barquissau V, Ghandour RA, Ailhaud G, Klingenspor M, Langin D, Amri EZ, and Pisani DF (2017). Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 136, 3–11. [DOI] [PubMed] [Google Scholar]
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. (2011). Brown adipose tissue activity controls triglyceride clearance. Nature medicine 17, 200–205. [DOI] [PubMed] [Google Scholar]
- Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, Butler SD, Jiang CS, Vaughan R, Schoder H, et al. (2021). Brown adipose tissue is associated with cardiometabolic health. Nature medicine 27, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J, and Bohlooly YM (2011). Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab 300, E211–220. [DOI] [PubMed] [Google Scholar]
- Blondin DP, Nielsen S, Kuipers EN, Severinsen MC, Jensen VH, Miard S, Jespersen NZ, Kooijman S, Boon MR, Fortin M, et al. (2020). Human Brown Adipocyte Thermogenesis Is Driven by beta2-AR Stimulation. Cell metabolism 32, 287–300 e287. [DOI] [PubMed] [Google Scholar]
- Cannon B, and Nedergaard J (2004). Brown adipose tissue: function and physiological significance. Physiological reviews 84, 277–359. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, and Hotamisligil GS (2008). Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Tippetts TS, Mayoral Monibas R, Liu J, Li Y, Wang L, Wilkerson JL, Sweeney CR, Pereira RF, Sumida DH, et al. (2019). Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia B, Ying L, Talbot CL, Maschek JA, Cox J, Schuchman EH, Hirabayashi Y, Holland WL, and Summers SA (2020). Ceramides are necessary and sufficient for diet-induced impairment of thermogenic adipocytes. Mol Metab 45, 101145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouchani ET, and Kajimura S (2019). Metabolic adaptation and maladaptation in adipose tissue. Nat Metab 1, 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. (2009). Identification and importance of brown adipose tissue in adult humans. The New England journal of medicine 360, 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elia E, Kessler SH, Kahn PA, English J, Chatman K, Trauger SA, Doria A, et al. (2015). Activation of human brown adipose tissue by a beta3- adrenergic receptor agonist. Cell metabolism 21, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong CJ, Shen YJ, Thomas MJ, and Cui Z (1999). Molecular distinction of phosphatidylcholine synthesis between the CDP-choline pathway and phosphatidylethanolamine methylation pathway. J Biol Chem 274, 29683–29688. [DOI] [PubMed] [Google Scholar]
- Fedorenko A, Lishko PV, and Kirichok Y (2012). Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell 151, 400–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, Harfmann B, Jones KA, Johnson ZR, Westgate PM, et al. (2018). Human adipose beiging in response to cold and mirabegron. JCI Insight 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funai K, Summers SA, and Rutter J (2020). Reign in the membrane: How common lipids govern mitochondrial function. Curr Opin Cell Biol 63, 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, van der Veen JN, Fernandez-Patron C, Vance JE, Vance DE, and Jacobs RL (2015). Insufficient glucose supply is linked to hypothermia upon cold exposure in high-fat diet-fed mice lacking PEMT. J Lipid Res 56, 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland PB, Randle PJ, and Newsholme EA (1963). Citrate as an Intermediary in the Inhibition of Phosphofructokinase in Rat Heart Muscle by Fatty Acids, Ketone Bodies, Pyruvate, Diabetes, and Starvation. Nature 200, 169–170. [DOI] [PubMed] [Google Scholar]
- Hamberg M (1980). Transformations of 5,8,11,14,17-eicosapentaenoic acid in human platelets. Biochim Biophys Acta 618, 389–398. [DOI] [PubMed] [Google Scholar]
- Heden TD, Johnson JM, Ferrara PJ, Eshima H, Verkerke ARP, Wentzler EJ, Siripoksup P, Narowski TM, Coleman CB, Lin CT, et al. (2019). Mitochondrial PE potentiates respiratory enzymes to amplify skeletal muscle aerobic capacity. Sci Adv 5, eaax8352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Akbari H, van Lenthe H, Kulik W, Wanders RJ, Frentzen M, and Vaz FM (2006). Identification and characterization of human cardiolipin synthase. FEBS Lett 580, 3059–3064. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, and Vaz FM (2008). Cardiolipin, the heart of mitochondrial metabolism. Cell Mol Life Sci 65, 2493–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Kyrou I, Tan BK, Dimitriadis GK, Ramanjaneya M, Tripathi G, Patel V, James S, Kawan M, Chen J, et al. (2016). Short-Chain Fatty Acid Acetate Stimulates Adipogenesis and Mitochondrial Biogenesis via GPR43 in Brown Adipocytes. Endocrinology 157, 1881–1894. [DOI] [PubMed] [Google Scholar]
- Hue L, and Taegtmeyer H (2009). The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab 297, E578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, et al. (2012). Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, Chen Y, Lu X, Maretich P, et al. (2017). UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature medicine 23, 1454–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikon N, and Ryan RO (2017). Cardiolipin and mitochondrial cristae organization. Biochim Biophys Acta Biomembr 1859, 1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, et al. (2010). Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem 285, 22403–22413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezek P, Orosz DE, Modriansky M, and Garlid KD (1994). Transport of anions and protons by the mitochondrial uncoupling protein and its regulation by nucleotides and fatty acids. A new look at old hypotheses. The Journal of biological chemistry 269, 26184–26190. [PubMed] [Google Scholar]
- Johnson JM, Verkerke ARP, Maschek JA, Ferrara PJ, Lin CT, Kew KA, Neufer PD, Lodhi IJ, Cox JE, and Funai K (2020). Alternative splicing of UCP1 by non-cell-autonomous action of PEMT. Mol Metab 31, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, Vetrivelan R, Lu GZ, Laznik-Bogoslavski D, Hasenfuss SC, et al. (2015). A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazak L, Rahbani JF, Samborska B, Lu GZ, Jedrychowski MP, Lajoie M, Zhang S, Ramsay LC, Dou FY, Tenen D, et al. (2019). Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat Metab 1, 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M (2009). Cardiolipin and mitochondrial carriers. Biochim Biophys Acta 1788, 2048–2058. [DOI] [PubMed] [Google Scholar]
- Kulterer OC, Niederstaetter L, Herz CT, Haug AR, Bileck A, Pils D, Kautzky-Willer A, Gerner C, and Kiefer FW (2020). The Presence of Active Brown Adipose Tissue Determines Cold-Induced Energy Expenditure and Oxylipin Profiles in Humans. J Clin Endocrinol Metab 105. [DOI] [PubMed] [Google Scholar]
- Laganowsky A, Reading E, Allison TM, Ulmschneider MB, Degiacomi MT, Baldwin AJ, and Robinson CV (2014). Membrane proteins bind lipids selectively to modulate their structure and function. Nature 510, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean ME, James WP, Jennings G, and Trayhurn P (1986). Brown adipose tissue uncoupling protein content in human infants, children and adults. Clin Sci (Lond) 71, 291–297. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hirose H, Ohneda M, Johnson JH, McGarry JD, and Unger RH (1994). Beta-cell lipotoxicity in the pathogenesis of non-insulin-dependent diabetes mellitus of obese rats: impairment in adipocyte-beta-cell relationships. Proc Natl Acad Sci U S A 91, 10878–10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Willers C, Kunji ER, and Crichton PG (2015). Uncoupling protein 1 binds one nucleotide per monomer and is stabilized by tightly bound cardiolipin. Proc Natl Acad Sci U S A 112, 6973–6978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiria LO, Wang CH, Lynes MD, Yang K, Shamsi F, Sato M, Sugimoto S, Chen EY, Bussberg V, Narain NR, et al. (2019). 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab 30, 768–783 e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist Leinhard O, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, et al. (2013). Evidence for two types of brown adipose tissue in humans. Nature medicine 19, 631–634. [DOI] [PubMed] [Google Scholar]
- Long JZ, Roche AM, Berdan CA, Louie SM, Roberts AJ, Svensson KJ, Dou FY, Bateman LA, Mina AI, Deng Z, et al. (2018). Ablation of PM20D1 reveals N-acyl amino acid control of metabolism and nociception. Proc Natl Acad Sci U S A 115, E6937–E6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA, Lou J, Rao RR, Chang MR, Jedrychowski MP, et al. (2016). The Secreted Enzyme PM20D1 Regulates Lipidated Amino Acid Uncouplers of Mitochondria. Cell 166, 424–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, et al. (2017). The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nature medicine 23, 631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May FJ, Baer LA, Lehnig AC, So K, Chen EY, Gao F, Narain NR, Gushchina L, Rose A, Doseff AI, et al. (2017). Lipidomic Adaptations in White and Brown Adipose Tissue in Response to Exercise Demonstrate Molecular Species-Specific Remodeling. Cell Rep 18, 1558–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery MK, Osborne B, Brandon AE, O’Reilly L, Fiveash CE, Brown SHJ, Wilkins BP, Samsudeen A, Yu J, Devanapalli B, et al. (2019). Regulation of mitochondrial metabolism in murine skeletal muscle by the medium-chain fatty acid receptor Gpr84. FASEB J 33, 12264–12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufer PD (2018). The Bioenergetics of Exercise. Cold Spring Harb Perspect Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara AE, Johnson JW, Linderman JD, Brychta RJ, McGehee S, Fletcher LA, Fink YA, Kapuria D, Cassimatis TM, Kelsey N, et al. (2020). Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. The Journal of clinical investigation 130, 2209–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, and Olefsky JM (2010). GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Walenta E, Akiyama TE, Lagakos WS, Lackey D, Pessentheiner AR, Sasik R, Hah N, Chi TJ, Cox JM, et al. (2014). A Gpr120-selective agonist improves insulin resistance and chronic inflammation in obese mice. Nat Med 20, 942–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, and Carpentier AC (2012). Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. The Journal of clinical investigation 122, 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri F (2014). Mitochondrial transporters of the SLC25 family and associated diseases: a review. J Inherit Metab Dis 37, 565–575. [DOI] [PubMed] [Google Scholar]
- Panic V, Pearson S, Banks J, Tippetts TS, Velasco-Silva JN, Lee S, Simcox J, Geoghegan G, Bensard CL, van Ry T, et al. (2020). Mitochondrial pyruvate carrier is required for optimal brown fat thermogenesis. eLife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada-Lopez T, Cereijo R, Turatsinze JV, Planavila A, Cairo M, Gavalda-Navarro A, Peyrou M, Moure R, Iglesias R, Giralt M, et al. (2016). The lipid sensor GPR120 promotes brown fat activation and FGF21 release from adipocytes. Nat Commun 7, 13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, and Newsholme EA (1963). The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1, 785–789. [DOI] [PubMed] [Google Scholar]
- Recio C, Lucy D, Purvis GSD, Iveson P, Zeboudj L, Iqbal AJ, Lin D, O’Callaghan C, Davison L, Griesbach E, et al. (2018). Activation of the Immune-Metabolic Receptor GPR84 Enhances Inflammation and Phagocytosis in Macrophages. Front Immunol 9, 1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricquier D, Mory G, Nechad M, and Hemon P (1978). Effects of cold adaptation and re-adaptation upon the mitochondrial phospholipids of brown adipose tissue. J Physiol (Paris) 74, 695–702. [PubMed] [Google Scholar]
- Ridgway ND, and Vance DE (1987). Purification of phosphatidylethanolamine N-methyltransferase from rat liver. J Biol Chem 262, 17231–17239. [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, et al. (2009). High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilperoort M, van Dam AD, Hoeke G, Shabalina IG, Okolo A, Hanyaloglu AC, Dib LH, Mol IM, Caengprasath N, Chan YW, et al. (2018). The GPR120 agonist TUG-891 promotes metabolic health by stimulating mitochondrial respiration in brown fat. EMBO Mol Med 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Diwoky C, Schoiswohl G, Feiler U, Wongsiriroj N, Abdellatif M, Kolb D, Hoeks J, Kershaw EE, Sedej S, et al. (2017). Cold-Induced Thermogenesis Depends on ATGL-Mediated Lipolysis in Cardiac Muscle, but Not Brown Adipose Tissue. Cell metabolism 26, 753–763 e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Ma Y, Chanturiya T, Cao Q, Wang Y, Kadegowda AKG, Jackson R, Rumore D, Xue B, Shi H, et al. (2017). Lipolysis in Brown Adipocytes Is Not Essential for Cold-Induced Thermogenesis in Mice. Cell metabolism 26, 764–777 e765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda K, Luijten IH, Hasegawa Y, Hong H, Sonne SB, Kim M, Xue R, Chondronikola M, Cypess AM, Tseng YH, et al. (2015). Genetic and functional characterization of clonally derived adult human brown adipocytes. Nature medicine 21, 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidossis L, and Kajimura S (2015). Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. The Journal of clinical investigation 125, 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcox J, Geoghegan G, Maschek JA, Bensard CL, Pasquali M, Miao R, Lee S, Jiang L, Huck I, Kershaw EE, et al. (2017). Global Analysis of Plasma Lipids Identifies Liver-Derived Acylcarnitines as a Fuel Source for Brown Fat Thermogenesis. Cell metabolism 26, 509–522 e506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RE, Roberts JC, and Hittelman KJ (1966). Nonphosphorylating respiration of mitochondria from brown adipose tissue of rats. Science 154, 653–654. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Lynes MD, Takahashi H, Baer LA, Arts PJ, May FJ, Lehnig AC, Middelbeek RJW, Richard JJ, So K, et al. (2018). 12,13-diHOME: An Exercise-Induced Lipokine that Increases Skeletal Muscle Fatty Acid Uptake. Cell metabolism 27, 1111–1120 e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strunecka A, Olivierusova L, Kubista V, and Drahota Z (1981). Effect of cold stress and norepinephrine on the turnover of phospholipids in brown adipose tissue of the golden hamster (Mesocricetus auratus). Physiol Bohemoslov 30, 307–313. [PubMed] [Google Scholar]
- Summers SA, Chaurasia B, and Holland WL (2019). Metabolic Messengers: ceramides. Nat Metab 1, 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sustarsic EG, Ma T, Lynes MD, Larsen M, Karavaeva I, Havelund JF, Nielsen CH, Jedrychowski MP, Moreno-Torres M, Lundh M, et al. (2018). Cardiolipin Synthesis in Brown and Beige Fat Mitochondria Is Essential for Systemic Energy Homeostasis. Cell metabolism 28, 159–174 e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed I, Lee J, Moraes-Vieira PM, Donaldson CJ, Sontheimer A, Aryal P, Wellenstein K, Kolar MJ, Nelson AT, Siegel D, et al. (2018). Palmitic Acid Hydroxystearic Acids Activate GPR40, Which Is Involved in Their Beneficial Effects on Glucose Homeostasis. Cell metabolism 27, 419–427 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima K, Ikeda K, Tanabe Y, Thomson EA, Yoneshiro T, Oguri Y, Ferro MD, Poon ASY, and Kajimura S (2020). Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis. Nature communications 11, 1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, et al. (2014). Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell metabolism 20, 678–686. [DOI] [PubMed] [Google Scholar]
- Unger RH, Clark GO, Scherer PE, and Orci L (2010). Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta 1801, 209–214. [DOI] [PubMed] [Google Scholar]
- Urbankova E, Voltchenko A, Pohl P, Jezek P, and Pohl EE (2003). Transport kinetics of uncoupling proteins. Analysis of UCP1 reconstituted in planar lipid bilayers. The Journal of biological chemistry 278, 32497–32500. [DOI] [PubMed] [Google Scholar]
- van der Veen JN, Kennelly JP, Wan S, Vance JE, Vance DE, and Jacobs RL (2017). The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr 1859, 1558–1572. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, and Teule GJ (2009). Cold-activated brown adipose tissue in healthy men. The New England journal of medicine 360, 1500–1508. [DOI] [PubMed] [Google Scholar]
- Verkerke ARP, Ferrara PJ, Lin CT, Johnson JM, Ryan TE, Maschek JA, Eshima H, Paran CW, Laing BT, Siripoksup P, et al. (2019). Phospholipid methylation regulates muscle metabolic rate through Ca(2+) transport efficiency. Nat Metab 1, 876–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinolo MA, Hirabara SM, and Curi R (2012). G-protein-coupled receptors as fat sensors. Curr Opin Clin Nutr Metab Care 15, 112–116. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. (2009). Functional brown adipose tissue in healthy adults. The New England journal of medicine 360, 1518–1525. [DOI] [PubMed] [Google Scholar]
- Wang B, and Tontonoz P (2019). Phospholipid Remodeling in Physiology and Disease. Annu Rev Physiol 81, 165–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YM, Liu HX, and Fang NY (2018). 9-PAHSA promotes browning of white fat via activating G-protein-coupled receptor 120 and inhibiting lipopolysaccharide / NF-kappa B pathway. Biochem Biophys Res Commun 506, 153–160. [DOI] [PubMed] [Google Scholar]
- Winkler E, and Klingenberg M (1994). Effect of fatty acids on H+ transport activity of the reconstituted uncoupling protein. The Journal of biological chemistry 269, 2508–2515. [PubMed] [Google Scholar]
- Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. (2012). Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Kazantzis M, Doege H, Ortegon AM, Tsang B, Falcon A, and Stahl A (2006). Fatty acid transport protein 1 is required for nonshivering thermogenesis in brown adipose tissue. Diabetes 55, 3229–3237. [DOI] [PubMed] [Google Scholar]
- Xu Y, Malhotra A, Ren M, and Schlame M (2006). The enzymatic function of tafazzin. J Biol Chem 281, 39217–39224. [DOI] [PubMed] [Google Scholar]
- Yoneshiro T, Wang Q, Tajima K, Matsushita M, Maki H, Igarashi K, Dai Z, White PJ, McGarrah RW, Ilkayeva OR, et al. (2019). BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yore MM, Syed I, Moraes-Vieira PM, Zhang T, Herman MA, Homan EA, Patel RT, Lee J, Chen S, Peroni OD, et al. (2014). Discovery of a class of endogenous mammalian lipids with anti-diabetic and anti-inflammatory effects. Cell 159, 318–332. [DOI] [PMC free article] [PubMed] [Google Scholar]


