Abstract
Purpose
The purpose of this study was to determine whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is detectable in the aqueous of asymptomatic individuals presenting for ophthalmic surgery.
Design
Prospective cross-sectional study.
Methods
Setting and participants: all patients undergoing anterior segment surgery at an ambulatory surgical center (ASC) belonging to a tertiary academic center in South Florida during a 102-day period between June and September 2020 received nasal swab testing for SARS-CoV-2 and underwent a relevant review of symptoms prior to surgery, with negative results required for both in order to proceed with surgery. Main outcomes and measurements: a small sample of aqueous humor (approximately 0.2 cc) was acquired at the beginning of anterior segment surgery from all participants. Aqueous humor was analyzed for SARS-CoV-2 viral ribonucleic acid (RNA) using real-time reverse transcriptase polymerase chain reaction. Demographic information was acquired from participants for secondary analyses.
Results
A total of 70 samples were acquired. Of those, 39 samples were excluded due to insufficient material or inconclusive results. Of 31 samples that were successfully analyzed, 6 (19.4%) demonstrated detectable SARS-CoV-2 RNA. None of the 6 individuals (0%) with detectable viral RNA in aqueous humor reported symptoms during the year, compared to 2 of 25 individuals (8%) with negative samples (P = 1). Positive samples were distributed throughout the study period, including both the first and the last days of enrollment.
Conclusions
The presence of SARS-CoV-2 viral RNA in aqueous despite negative nasal swab testing confirmed its presence beyond the blood-ocular barrier in asymptomatic individuals and raises the possibility that the virus may persist in immunoprivileged spaces despite an absence of symptoms.
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is associated with a spectrum of pathological changes. Changes include anosmia, cough, fever, respiratory distress, and death in severe cases.1 At the time of this writing, approximately 100 million people have been infected, and more than 2 million have died worldwide.2
While initial attention to the ocular milieu in patients infected with SARS-CoV-2 was directed to the ocular surface,3 , 4 recent reports have demonstrated evidence of viral ribonucleic acid (RNA) and proteins within the anterior segment,5, 6, 7 suggesting that SARS-CoV-2 may enter the eye and that viral proteins may remain in the intraocular space after viremia has ended.5
Elective anterior segment ophthalmic surgeries, particularly cataract surgery, constitute a large proportion of ambulatory surgery performed each year in the United States. Although guidelines for hospitals and ambulatory surgical centers (ASC) continue to evolve during the ongoing COVID-19 pandemic, patients undergoing elective cataract surgery and other anterior segment surgeries must generally be asymptomatic and/or demonstrate evidence of a negative result for nasal swab tests for SARS-CoV-2.
To date, it is unclear whether viral RNA from SARS-CoV-2 can be detected in the aqueous humor of asymptomatic individuals without known history of COVID-19. This study assessed aqueous humor of patients undergoing elective ophthalmic surgery in Florida, a state with a relatively high prevalence of COVID-19 in the United States, for presence of SARS-CoV-2.
MATERIALS AND METHODS
This prospective cross-sectional study was approved by the Institutional Research Board of the University of Miami and adhered to the principles of the Declaration of Helsinki. All patients provided informed consent to participate in the study.
INCLUSION CRITERIA
Patients undergoing elective anterior segment surgery at an ASC belonging to a tertiary academic center in South Florida by a single surgeon (E.K.) were invited to participate in the study. All participants required negative nasal swab testing 24-72 hours before the procedure.
EXCLUSION CRITERIA
We excluded cases in which a sufficient volume of aqueous humor could be not be sampled.
QUESTIONNAIRE
Patients were asked about COVID-19 history prior to clinical visits, and surgery was deferred if they described a history of COVID-19 or active symptoms. A questionnaire was administered 3-5 days prior to the scheduled surgery date, asking patients if they had been previously tested for SARS-CoV-2 before surgery-related testing and whether they had experienced symptoms suggestive of COVID-19, including cough, fever, shortness of breath, and loss of taste and/or smell. If patients answered yes to either question, they were asked for the date of prior COVID-19 testing.
PARTICIPANTS
A total of 70 participants contributed samples. For all 70 participants, samples were collected from only 1 eye from each participant. These samples were acquired over 8 surgical days during a 102-day period from June 19, 2020, to September 29, 2020, from 51 cataract surgeries, 12 keratoplasty cases, 2 pterygium cases, and 5 other anterior segment cases. Of those participants, 33 (52.9%) were women, and the median age was 72 (range: 37-93; interquartile range [IQR]: 65, 77) years old.
SARS-CoV-2 TESTING
Within 24-72 hours prior to surgery, all patients underwent nasal swab testing for SARS-CoV-2 and tested negative. Patients were also contacted within 72 hours of surgery to confirm the absence of symptoms to proceed.
Aqueous humor was drawn at the beginning of cataract surgery. Using a 1-cc syringe and a 30-G cannula, approximately 0.2 cc of aqueous humor was drawn and sent immediately to the laboratory for analysis. Of the 70 samples acquired, 29 had insufficient volume and were unable to be analyzed. Ten samples produced indeterminate results and were excluded from analysis. A total of 31 samples were successfully analyzed.
The Centers for Disease Control and Prevention (CDC) 2019-nCoV real-time reverse transcription polymerase chain reaction (rRT-PCR) diagnostic panel and protocol-recommended primer and probe sets N1, N2 were used to detect different regions of the SARS-CoV-2 nucleocapsid gene, and a third primer and probe set was used to detect human RNase P. Appropriate SARS-CoV-2-positive, -negative, and human RNA controls as well as the 2019-nCov CDC Emergency Use Authorization (EUA) kits were purchased from Integrated DNA Technologies, Inc. (Coralville, IA, USA). RNA was isolated from the aqueous humor samples using the Qiagen RNeasy Plus Mini kit (Fisher Scientific, Waltham, Massachusetts, USA). The Promega Go Taq Probe 1-Step RT-qPCR system (Promega, Madison, Wisconsin, USA) for viral RNA reverse transcription into cDNA. Sample cDNA was amplified using the Mic qPCR cycler from Bio Molecular System software version 2.8 (Queensland, Australia).
Results and assay performance were interpreted in accordance with the manufacturers’ instructions and CDC recommendations. Results were reviewed and accepted only after all controls were performed as expected
STATISTICAL ANALYSIS
Medians and interquartile ranges (IQR) were used for descriptive statistics. Fisher exact test and Wilcoxon rank sum test were used to compare participants with and without positive samples.
RESULTS
ANALYSIS OF SAMPLES
A total of 31 samples were analyzed. Among donors of samples that were analyzed and excluded, there were no difference in median age (73 vs. 71 years old; P = .49) or sex (45% vs. 59%; P = .34)
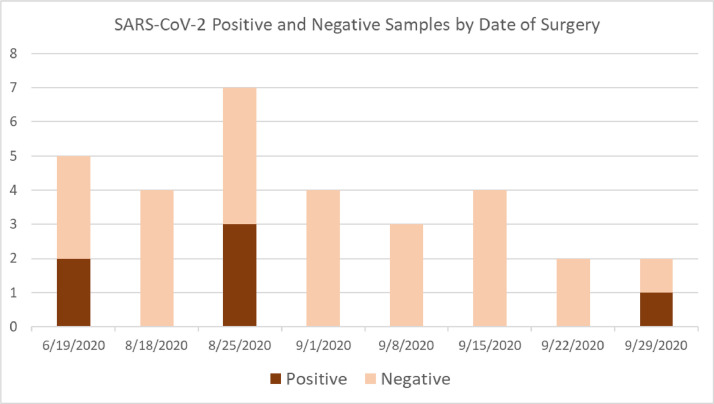
Viral RNA was detected in 6 of 31 samples analyzed (19.4%). There were no statistically significant differences in median age (72 vs. 75 years old; P = .73) or associations with female sex (2 or 6; 33% vs. 12 of 25; 48%; P = .66) between positive and negative samples, respectively. Positive samples were acquired during 3 of the 8 surgical days, as shown in Figure 1 .
Figure 1.
Aqueous humor was drawn from eyes during anterior segment surgery during 8 surgical days over a 102-day period in a tertiary care center in Florida in 2020. Samples from 6 individuals tested positive and were distributed throughout the study period.
QUESTIONNAIRE
Of patients with successfully analyzed samples, 0 of 6 positive patients (0%) had previously undergone SARS-CoV-2 testing, compared to 7 of 25 negative patients (28%; P = .29).
When asked about cough, fever, shortness of breath, and loss of taste and/or smell in the preceding months, 0 of 6 patients who tested positive (0%) confirmed such symptoms compared to 2 of 25 patients who tested negative (8%; P = 1.0). A sample of the questionnaire is included in the Supplemental Material.
DISCUSSION
In this study, asymptomatic individuals presenting for elective ophthalmic surgery without a known history or symptoms of COVID-19 provided aqueous samples in which SARS-CoV-2 viral genetic material was detected.
To date, reports of SARS-CoV-2 genetic material or proteins within the anterior chamber have included individuals with a known history of SARS-CoV-2. Nucleocapsid protein antigens of SARS-CoV-2 were found during cataract surgery in the iris and trabecular meshwork of a patient previously infected with COVID-19.5 In a series of 11 post mortem corneas from patients who died from COVID-19, 6 demonstrated evidence of viral RNA, and 1 aqueous humor sample tested positive.6 This study is the first to detect SARS-CoV-2 in the aqueous humor of living individuals and demonstrates that it may be present despite an absence of symptoms.
These data also raise the possibility of viral persistence in immunoprivileged spaces. In the case above,5 viral proteins were present despite timing, 2 months after known infection. The report highlighted the possibility that SARS-CoV-2 proteins may be found inside the eye and persist after the infection has seemingly cleared from circulation. In these positive cases, all tested negative by nasal swab within 72 hours of the aqueous sample, suggesting that the infection they experienced might have occurred in the past. In addition, all 6 patients answered no to previous COVID-19 related symptoms or previous positive tests according to the questionnaire. This is not surprising or conflicting, as COVID-19 infections frequently occur in so-called “asymptomatic” patients.8
Jin and associates9 recently reported the case of a patient who experienced acute graft rejection and later tested positive for SARS-CoV-2 by PCR 5 days after the onset of ocular symptoms. The corneal endothelium is devoid of vasculature and receives its nourishment from the aqueous humor. This suggests that the aqueous humor may play a role in the rejection of the corneal endothelium in certain patients who have been infected with COVID-19. The presence of viral genetic material in aqueous humor, as shown in this study, is consistent with this theory.
Considering that millions of cataract surgeries are performed in the world each year, these findings carry potential implications for this elective surgery. Assessment of aerosolization during phacoemulsification in ex vivo models suggest that potential spread through the air is likely negligible10 , 11 but may be dependent on incision size.12 It is encouraging that there are no known cases of transmission during cataract surgery to date. Although we do not believe the results should be a cause for alarm, proper use of personal protective equipment, sterile surgical technique, and sterilization practices are particularly important. Of note, all 6 of the patients who had SARS-CoV-2 viral genetic material detected in the aqueous humor, did well in their post-operative course following surgery, with positive visual outcomes and without evidence of inflammation by the postoperative 1-month visits.
In two previous reports from patients with known history of COVID-19, the case report of an individual with concurrent herpetic stromal keratitis13 and a post mortem series of 16 individuals,14 did not identify viral RNA in the aqueous humor. Given the limited numbers of samples in those reports, the present data do not contradict these findings but rather offer a more complete understanding that SARS-CoV-2 may be present in a low but substantial proportion of people who have experienced COVID-19.
In the setting of an emerging infectious disease, questions arise regarding safety of surgery, and prospective studies can be helpful to generate the statistical power necessary to answer such questions. One limitation of this study was the large number of samples that were unable to be analyzed. This reflects the reality of conducting a prospective study in the setting of an emerging infectious disease, in the absence of commercially available assays for testing aqueous humor for SARS-CoV-2. Although the possibility of false positive and false negative results must be accounted for, the data suggest that contamination is an unlikely contributor to the positive results, as they were distributed across surgical dates and tested on separate days.
Florida was chosen for its relatively high rate of COVID-19 incidence15 at the time of the study. Further research is needed to determine the duration of viral persistence in the eye after the initial infection. As found from the Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT)16 and Partnership for Research on Ebola Virus in Liberia (PREVAIL) 717 studies, the Ebola virus could be found in the aqueous humor in survivors of the disease, months following the infection.
Another limitation of this study is that antibody testing was not performed at the time of surgery. However, given the fact that patients may take approximately 1 to 3 weeks to develop antibodies following exposure to the virus,18 a negative antibody test would not necessarily rule out a positive aqueous sample.
Increasing evidence points to a long-term, post-COVID syndrome among survivors of COVID-19. Future studies will be needed to identify whether pathological changes include the eye and the extent to which viral persistence may contribute to such findings.
In conclusion, this study found that SARS-CoV-2 viral genetic material can be present in aqueous humor, including in asymptomatic individuals. The length of viral persistence, if present, is unknown and requires further study.
Acknowledgments
ALL AUTHORS HAVE COMPLETED AND SUBMITTED THE ICMJE FORM FOR DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST and none were reported.
Funding/Support: This study was funded in part by the Florida Lions Eye Bank.
Financial Disclosures The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Supplemental Material available at AJO.com.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajo.2021.05.008.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020 Feb 20;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins University and Medicine Coronavirus Resource Center. Available at: https://coronavirus.jhu.edu/. Accessed 24 January 2021.
- 3.Wu P, Duan F, Luo C, et al. Characteristics of ocular findings of patients with coronavirus disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138(5):575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seah IYJ, Anderson DE, Kang AEZ, et al. Assessing viral shedding and infectivity of tears in coronavirus disease 2019 (COVID-19) patients. Ophthalmology. 2020;127(7):977–979. doi: 10.1016/j.ophtha.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Diao B, Liu Y, Zhang W, Wang G, Chen X. Severe acute respiratory syndrome coronavirus 2 nucleocapsid protein in the ocular tissues of a patient previously infected with coronavirus disease 2019. JAMA Ophthalmol. 2020;138(11):1201–1204. doi: 10.1001/jamaophthalmol.2020.3962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casagrande M, Fitzek A, Spitzer MS, et al. Presence of SARS-CoV-2 RNA in the cornea of viremic patients with COVID-19. JAMA Ophthalmol. 2021;139(4):383–388. doi: 10.1001/jamaophthalmol.2020.6339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sawant OB, Singh S, Wright RE, et al. Prevalence of SARS-CoV-2 in human post-mortem ocular tissues. Ocul Surf. 2021;19:322–329. doi: 10.1016/j.jtos.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Z, Xu Y, Sun C, et al. A systematic review of asymptomatic infections with COVID-19. J Microbiol Immunol Infect. 2021;54(1):12–16. doi: 10.1016/j.jmii.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin SX, Juthani VV. Acute corneal endothelial graft rejection with coinciding COVID-19 infection. Cornea. 2021;40(1):123–124. doi: 10.1097/ICO.0000000000002556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee H, Naveed H, Ashena Z, Nanavaty MA. Aerosol generation through phacoemulsification. J Cataract Refract Surg. 2020;46(9):1290–1296. doi: 10.1097/j.jcrs.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 11.Dickie D, Koshy ZR. Aerosol generation from high speed ophthalmic instrumentation and the risk of contamination from SARS COVID19. Eye (Lond) 2020;34(11):1954–1955. doi: 10.1038/s41433-020-1000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darcy K, Elhaddad O, Achiron A, et al. Reducing visible aerosol generation during phacoemulsification in the era of Covid-19. Eye (Lond) 2020;26:1–6. doi: 10.1038/s41433-020-1053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts HW, Akram H, Myerscough J. Negative polymerase chain reaction for SARS-CoV-2 in aqueous sample of patient with confirmed SARS-CoV-2 and recurrence of herpetic stromal keratitis. J Cataract Refract Surg. 2020;46(12):e61–e63. doi: 10.1097/j.jcrs.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 14.List W, Regitnig P, Kashofer K, Gorkiewicz G, et al. Occurrence of SARS-CoV-2 in the intraocular milieu. Exp Eye Res. 2020;201 doi: 10.1016/j.exer.2020.108273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rui R, Tian M, Tang M-L, et al. Analysis of the Spread of COVID-19 in the USA with a spatio-temporal multivariate time series model. Int J Environ Res Public Health. 2021;18(2):774. doi: 10.3390/ijerph18020774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shantha JG, Mattia JG, Goba A, et al. Ebola Virus Persistence in Ocular Tissues and Fluids (EVICT) study: reverse transcription-polymerase chain reaction and cataract surgery outcomes of Ebola survivors in Sierra Leone. EBioMedicine. 2018;30:217–224. doi: 10.1016/j.ebiom.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eghrari AO, Shantha JG, Ross RD, et al. Efficacy and safety outcomes of cataract surgery in survivors of Ebola virus disease: 12-month results from the PREVAIL VII study. Trans Vis Sci Tech. 2021;10(1):32. doi: 10.1167/tvst.10.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Interim Guidelines for COVID-19 antibody testing in clinical and public health settings. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed 26 January 2021
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.