Abstract
Although in vitro fertilization (IVF) is associated with adverse perinatal outcomes, there is increasing concern about the long-term and sex-specific health implications. Augmenting our IVF mouse model to longitudinally investigate metabolic outcomes in offspring from optimal neonatal litter sizes, we found sex-specific metabolic outcomes in IVF offspring. IVF-conceived females had higher body weight and cholesterol levels compared to naturally conceived females, whereas IVF-conceived males had higher levels of triglycerides and insulin, and increased body fat composition. Through adult liver transcriptomics and proteomics, we identified sexually dimorphic dysregulation of the sterol regulatory element-binding protein (SREBP) pathways that are associated with the sex-specific phenotypes. We also found that global loss of DNA methylation in placenta was linked to higher cholesterol levels in IVF-conceived females. Our findings indicate that IVF procedures have long-lasting sex-specific effects on metabolic health of offspring and lay the foundation to utilize the placenta as a predictor of long-term outcomes.
Keywords: assisted reproductive technologies, developmental origins of health and disease, long-term health, metabolic outcomes, sex-specific
1. ∣. INTRODUCTION
Over the past 40 years, assisted reproductive technologies (ART), including in vitro fertilization (IVF), have become a highly successful treatment for infertility. Globally, there has been a consistent and steady increase in the use of ART,1 now contributing to over 9 million births worldwide.2 Although the majority of IVF births are uncomplicated, IVF is associated with adverse perinatal outcomes, including higher risk of congenital anomalies, preterm birth, low birth weight, perinatal mortality, small for gestational age, and imprinting disorders, as well as hypertensive and placental disorders during pregnancy.3-8
Beyond perinatal outcomes, it is critical to consider the success and safety of IVF within the context of developmental origins of health and disease (DOHaD). DOHaD posits that suboptimal environments and exposures during critical periods of embryo development can predispose individuals to health complications later in life.9 An unavoidable circumstance is that IVF procedures and manipulations occur during preimplantation development, a period of tightly coordinated physiological and epigenetic changes. IVF opens this critical window of embryo development to a synthetic, ex vivo environment that can perturb the coordination of embryo development in ways that may affect health and disease later in life. Because IVF has only been in the clinical setting for just over 40 years, the current epidemiological evidence is limited. Nevertheless, subclinical indicators of cardiometabolic alterations have been identified in human cohort studies of children and young adults conceived with IVF.8,10
Investigating the effects of ART in fertile mouse models circumvents issues raised by underlying infertility and thereby affords the ability to dissect the effects of individual or collective ART procedures on long-term health outcomes. In agreement with human cohort studies, mouse models also demonstrate an array of cardiometabolic alterations in offspring conceived with IVF and other ART procedures. Long-lasting effects of IVF observed in mouse models, include increased body weight, increased fasting glucose, impaired glucose tolerance, insulin resistance, and higher blood pressure,11-17 some of which occur in a sex-specific manner. However, the evidence from mouse models has yet to define clear and consistent phenotypes or underlying pathways that contribute to these phenotypes.
Here, we exploit our mouse IVF model with a neonatal fostering paradigm, which controls for neonatal litter size, to conduct longitudinal metabolic assessments of IVF and naturally conceived offspring between 12 weeks and 39-weeks-of-age. As early as 12-weeks-of-age, we detect distinct, sex-specific phenotypes that persist through adulthood. Examination of adult hepatic transcriptome and proteome identified pathways involved in these sex-specific phenotypes, in particular, sexually dimorphic dysregulation of sterol regulatory-binding element proteins (SREBPs). We also find that global dysregulation of DNA methylation in the placenta is linked to the IVF-female phenotype.
2. ∣. MATERIALS AND METHODS
2.1 ∣. Animals
CF1 female mice (Envigo), B6SJL male mice (Jackson Laboratory), and CD1 vasectomized male mice (Charles River) were housed in polysulfone cages in a pathogen-free facility on a 12-12 light-dark cycle. All animals had access to ad libitum water and standard chow (Laboratory Autoclavable Rodent Diet 5010, LabDiet, St. Louis, MO, USA), unless fasting for metabolic assays (discussed below). All animal work in this study was conducted with the approval of the Institutional Animal Care and Use Committee at the University of Pennsylvania (Protocol Number: 803545).
2.2 ∣. Generation of natural and IVF offspring
For the natural control group (Nat), naturally cycling CF1 females were mated overnight with B6SJL males and embryonic day (E) 0.5 was noted as the day on which a copulatory plug was observed. Development occurred in vivo until E18.5.
Optimized protocols recommended by Jackson Laboratory18 were used to generate the IVF group. CF1 female mice were superovulated with 5 IU of eCG followed by 5 IU of hCG administered 48 h later. Approximately 14 hours after injection of hCG, cumulus-egg complexes were collected from oviducts and IVF was conducted using EmbryoMax Human Tubal Fluid (1×) medium (HTF, EMD MilliporeSigma, Burlington, MA, USA) with capacitated mature spermatozoa collected from the cauda epididymis and vas deferens of B6SJL male mice. After 4 hours, eggs were washed in HTF media followed by culture in EmbryoMax KSOM media (1×) containing ½ Amino Acids (KSOM+AA; EMD MilliporeSigma, Burlington, MA, USA) in an incubator at optimized conditions (37°C, 5% CO2, 5% O2, 90% N2) under mineral oil suitable for embryo culture (MilliporeSigma, Burlington, MA, USA). After 4 days of development in culture, fully expanded blastocysts of similar morphology were briefly washed in HEPES-buffered minimum essential medium (MEM) prior to transferring to 2.5-day pseudo-pregnant recipient females using nonsurgical embryo transfer (NSET) devices (ParaTechs, Lexington, KY, USA). Each pseudo-pregnant female (generated by mating CF1 females with vasectomized CD1 males) received 10 blastocysts. The day of embryo transfer was defined as E4 and IVF blastocysts developed in vivo from E4 to E19.
2.3 ∣. Fostering of natural and IVF offspring
To account for differences in developmental rates between Nat and IVF fetuses, cesarean sections were performed at E18.5 (Nat) and E19 (IVF) to obtain both the fetus and placenta. We also employed the following fostering protocol to minimize the effects of litter size on growth and adult metabolism.19 We generated the foster mother and her native litter by mating a CF1 female with a CF1 stud male. The foster mother gave birth to her native litter < 36 hours prior to fostering IVF or Nat neonates into the native litter. To begin fostering, the foster mother was removed from the cage shortly before cesarean delivery of the IVF and Nat neonates, which were quickly weighed and given a unique tattoo identifier to match neonates with their placentas. The neonates were then immediately integrated into the foster mother’s native litter. The native litter was culled so that the average total litter size (native plus foster neonates) was 10 neonates/foster mother. The foster mother was returned to the cage as soon as the fostering procedure was complete.
Sample size for this study was estimated based on our previous work and that of others.13,20,21 We produced a total of 13 IVF-females and 19 IVF-males from 19 IVF-litters and 13 Nat-females and 12 Nat-males from 9 Nat-litters. A replication cohort was generated in the same manner and aged to 12-weeks. At 12-weeks, metabolic phenotyping was performed on 16 IVF-females and 12 IVF-males from 7 litters and 12 Nat-females and 12 Nat-males from 4 litters.
2.4 ∣. Metabolic phenotyping
2.4.1 ∣. Body weight
Using a calibrated digital scale, body weights were measured at birth and every week from 1-week through 39-weeks-of-age.
2.4.2 ∣. Intraperitoneal glucose tolerance test (IPGTT)
Beginning at 12-weeks-of-age, IPGTTs were performed by an expert technician blinded to the experimental group of each mouse every 4 weeks until and 28-weeks-of-age, and again at 39 weeks (ie, end of life). In line with optimal conditions for assessing glucose tolerance,22 mice were fasted for 6 h during the light cycle (0800-1300) on alpha-dri bedding with ad libitum access to water. IPGTTs were performed by an expert technician blinded to the experimental group of each mouse. Baseline (t = 0-min) blood glucose levels were obtained by tail snip from conscious unrestrained mice using a hand-held glucometer (ReliOn). Subsequently, the mice received an IP injection of 20% dextrose (2 g/Kg body weight) and blood glucose levels were measured at t = 15-, 30-, 60-, and 120-minutes post-injection.
2.4.3 ∣. Serum hormone and lipid assay analysis
During the IPGTT at t = 0 minutes, whole blood was collected from the tail snip in 6-h fasted mice, placed into appropriate serum separator tubes, centrifuged and serum aliquoted and stored at −20°C for subsequent assay measurements. All assays were performed by an expert technician from the Radioimmunoassay and Biomarker Core (UPenn) who was blinded to the experimental group of each mouse. Insulin analysis was performed using a mouse insulin ELISA assay from ALPCO (Salem, NH, USA). Lipids were assayed from serum with enzymatic colorimetric assays using the following reagent kits: Triglycerides and total cholesterol from Stanbio (Boerne, TX, USA). For the 12-week cohort, total, HDL, and LDL/VLDL were obtained using the Abcam HDL and LDL/ VLDL Cholesterol Assay Kit (ab65390; Cambridge, UK).
2.4.4 ∣. Body composition, blood pressure, and heart rate measurements
At 39 weeks (ie, end of life), body weight and body composition were measured in ad-lib fed conscious mice using EchoMRI 3-in-1 system nuclear magnetic resonance spectrometer (Echo Medical Systems, Houston, TX, USA) to determine whole body lean and fat mass. Subsequently, systolic and diastolic blood pressure and heart rate were measured by noninvasive tail-cuff volume pressure recording (VPR) using the CODA 8 BP System (Kent Scientific Corporation, Torrington, CT, USA). Briefly, mice were placed into an animal holder on a warming platform and tails were threaded into an O-cuff and then a VPR cuff. Before initiating the blood pressure measurement, a noncontact infrared thermometer was used to confirm that the temperature at the base of the tail was 32-35°C. Subsequently, two runs of 30 measurement cycles were performed to obtain measurement averages. All assays were performed by an expert technician blinded to the experimental group of each mouse.
2.5 ∣. Tissue collection
At the time of fostering (see above), placentas were dissected, cleaned, and weighed, and then bisected through the umbilical attachment. Half of the placenta was snap frozen in liquid nitrogen and stored at −80°C until processed for DNA and RNA isolation (see below). The other half was fixed in 10% of phosphate-buffered formalin for future histological analyses.
At 12 weeks and 39 weeks, mice were euthanized via CO2 and cervical dislocation. All vital organs, including liver, were collected and snap frozen in liquid nitrogen. A portion of the left lobe of the liver was fixed in 10% of phosphate-buffered formalin for histological analyses.
2.6 ∣. DNA and RNA isolation
DNA and RNA were simultaneously isolated from one-quarter of snap frozen placentas and snap frozen liver by using phenol-chloroform extraction and TRIzol (Invitrogen, CA, USA), respectively, as previously described.23
2.7 ∣. Bisulfite pyrosequencing and luminometric methylation Assay (LUMA)
Using 1 μg of bisulfite-treated DNA, DNA methylation was measured at the imprinting control region (ICR) of several imprinted genes by bisulfite pyrosequencing as previously described.23,24
Global DNA methylation at repetitive elements was measured by LUMA using 500 ng of genomic DNA as previously described.23,24
2.8 ∣. RNA sequencing
We performed RNA sequencing on a random subset of liver samples from the 39-week cohort (n = 3/sex/group, from 11 litters). Total RNA (4 pg) was used as input for poly-A selection and mRNA-sequencing library synthesis using KAPA mRNA-Seq library synthesis kit and KAPA Single-Indexed adapter kit (Kapa Biosystems, Wilmington, MA, USA). Library quality control was conducted using Agilent 2100 Bioanalyzer system, High Sensitivity dsDNA Qubit system and NEBNext Library Quantification Kit (New England Biolabs, Ipswich, MA, USA). Sequencing was performed on NextSeq 500 platform (Illumina, San Diego, CA).
RNA-seq reads were trimmed for quality using Trimmomatic (version 0.32).25 Illumina TruSeq3-PE primers, leading and trailing low-quality (below quality 3) and N base calls were trimmed, and reads were scanned using a 4-bp sliding window and trimmed when the average quality per base dropped below 15. Reads with at least 30-bp in length following this quality control process were retained and used for subsequent analyses. The resulting reads were then aligned to mm10 reference genome assembly using STAR (version 2.3.0e),26 allowing read pairs to align no farther than 2000-bp apart and keeping alignments with a mapping quality score MAPQ greater than 1 for genome-wide transcript counting. FeatureCounts (version 1.5.0)27 was used to generate a matrix of mapped fragments per RefSeq annotated gene. Numbers for total sequenced, aligned, and counted reads for each sample are listed in Supplemental Table S1. Analysis for differential gene expression was performed using DESeq228 with the cutoff of FDR < 0.05 (Supplemental Data set 1). Clustering and heatmap of differentially expressed genes were generated using heatmaply R package. The raw sequencing data reported in this work have been deposited in the NCBI Gene Expression Omnibus under accession number GSE158029 (reviewers’ token: uvwdgeemnnilhsr).
2.9 ∣. Quantitative mass spectrometry
2.9.1 ∣. Sample preparation
The same subset of samples used for RNA sequencing was also used for liver proteomics with the addition of two random samples/sex/group (n = 5/sex/group). A section of liver tissue was lysed by adding 2X packed tissue volumes of lysis buffer (6 M guanidine-HCl, 100 mM TEAB pH = 8.0) followed by sonication using a probe sonicator at 4°C. Protein estimation was performed using a Bradford assay (Bio-Rad) with BSA as the standard. A 50-μg aliquot of each sample was volume-adjusted to 25 μL (with 6 M guanidine-HCl, 100 mM TEAB pH = 8.0, 10 mM DTT), denatured and reduced while mixing on a ThermoMixer C at 60°C for 20 minutes followed by cysteine alkylation using 40 mM of iodoacetamide in the dark for 20 minutes. Prior to proteolytic digestion, guanidine-HCl wad diluted to 1 M using 100 mM TEAB pH = 8.0. Samples were digested overnight at ambient temperature using 1 μg of Trypsin (Promega, Madison, WI, USA). Prior to LCMS, digested peptides were desalted using in-house made desalting-tips,29 dried using vacuum centrifugation and resuspended into 50 μL of 2% acetonitrile, 0.1% formic acid.
2.9.2 ∣. Liquid chromatography
Approximately 1 μg of peptides were loaded and pre-concentrated onto an Acclaim PepMap 100 C18 pre-column (0.3 mm × 5 mm, 5 μm) using 0.05% of trifluoroacetic acid in water followed by loading onto an analytical, in-house-packed fused silica capillary, C18 column (75 μm × 30 cm, 2.4 μm ReproSil-Pur Dr Maisch GmbH) using a Dionex Ultimate 3000 RSLCnano high-performance liquid chromatographic system at a flow rate of 300 nL/min. Mobile phase A consisted of an aqueous solution of 0.1% of formic acid and mobile phase B as 0.1% formic acid in 80% acetonitrile. Peptides were separated using a linear gradient from 5% to 35% B over 90 min followed by a gradient ramp to 60% B over 10 minutes. The system was washed in 95% B for 15 minutes and re-equilibrated to initial conditions for 19 minutes.
2.9.3 ∣. Mass spectrometry
Eluting peptides were injected into a Thermo Q-Exactive HFX and acquired using data-independent acquisition (DIA) with the chromatogram library workflow as previously described.30,31 An MS survey scan was collected in centroid mode for the mass range of 385 m/z to 1015 m/z (60,000 MS1 resolution, automatic gain control (AGC) 1E6 ions, 60 ms max ion injection time). The MS1 scan was followed by precursor isolation windows for fragmentation with the normalized collision energy (NCE) set to 27 and the default charge state was set to 3. Proteome profiling was performed using single-injection DIA mass spectrometry (15 000 fragment ion resolution, 1E6 AGC, 20 ms max IT) using 75-8 m/z staggered precursor isolation windows (4 m/z after demultiplexing) with optimized window placement.32 The precursor isolation windows were generated using EncyclopeDIA’s built-in window scheme wizard31 using the following settings Window scheme – staggered/overlapping DIA; Phospho enriched – FALSE; number of windows – 75; start m/z – 400; stop m/z – 1000; margin width – 0.
To generate the chromatogram library, an aliquot of all peptide samples was pooled and analyzed using six gas phase fractionation DIA (GPF-DIA) fractions. Each of the six GPF-DIA injections were acquired using different precursor mass ranges (500-600 mz, 600-700 m/z, 700-800 m/z, 800-900 m/z 900-1000 m/z) at 30 000 MS2 resolution using 25-4 m/z staggered precursor isolation windows (2 m/z after demultiplexing) with optimized window placement. The GPF-DIA window scheme was also generated in EncyclopeDIA using the GPF-specific mass range and number of windows-25.
2.9.4 ∣. Spectral library
A Prosit predicted spectral library was generated for the mouse proteome (https://www.proteomicsdb.org/prosit/).33 The input for the Prosit predicted spectral library was created using EncyclopeDIA’s “Convert FASTA to Prosit CSV” wizard using the complete mouse proteome (Mouse Uniprot FASTA downloaded 2019-08-21; 17015 entries). The charge range was set to 2-3, maximum missed cleavage was set to 1, m/z range: 396.4-1002.7; Default NCE-35; Default Charge-3.
2.9.5 ∣. DIA data processing
DIA raw files were converted to mzML and demultiplexed using MSConvert34,35 (ProteoWizard version 3.0.20084.721dd2c95) with 10 ppm accuracy and searched using EncyclopeDIA (v 0.9.0)30,31 using default settings (Normal target/decoy approach, nonoverlapping DIA, fragmentation set to CID/HCD (B/Y ions), 10 ppm precursor mass tolerance, 10 ppm fragment mass tolerance, 10 ppm library mass tolerance, Percolator v3-01, minimum of 3 quantitative ions).
2.9.6 ∣. Data analysis
Peptides below 1% FDR were used for the analysis. Peptide abundances were log2-transformed and normalized by equalizing the medians. Peptides with a coefficient of variation below 50% were used for protein estimation (see below). Protein abundance summarization was performed using Tukey’s median polish.36 Statistical analysis was performed using a Two-way ANOVA and a pairwise comparison using a TukeyHSD post hoc test. All P values reported were corrected for multiple hypothesis testing. All of the analysis was performed using R (version 4.0.0).
2.9.7 ∣. Coefficient of variation
Technical replicates of the pooled sample were acquired throughout the acquisition of the experimental samples. Technical replicates are used to calculate peptide level coefficient of variation. Peptides with a CV less than 50% were used to estimate protein abundance.
2.9.8 ∣. Tissue preparation for lipids extraction
Pieces of about 5 mg of frozen liver tissues were cut on a tile kept in dry ice with a new blade kept in dry ice. The tissue was added to low retention Eppendorf tube prepared with 0.6 mL 80% methanol (MeOH) and 20 μL SPLASH LIPIDOMIX (diluted 1:1 with methanol from original #330707 Avanti solution) (Avanti Polar Lipids, Alablaster, AL) and kept in dry ice. Samples were pulse sonicated for 30x half-second pulses on ice and kept on ice for 20 minutes for metabolite extraction. Each tube was then vortexed 3× 30 seconds. The tissue homogenate was moved to a 10 mL glass Pyrex tube with screw cap. The Eppendorf tubes were rinsed with 0.5 mL methanol and added to same glass tube. Five mL methyl tert-butyl ether (MTBE) was added to each of the tubes and the tubes were shaken vigorously for 30 minutes. A 1.2 mL aliquot of water was added to each tube and vortexed for 30 seconds. Centrifugation for 10 minutes @ 1000 ×g created two phases. The top clear phase was moved to a clean glass Pyrex tube and dried down under nitrogen. A 200 μL MTBE/MeOH = 1/3 (v/v) was used to resuspend the residue. The sample was spun down at 10 000 ×g for 10 minutes at 4°C and only the top 100 μL were transferred to a HPLC vial for LC-MS analysis. A pooled sample was created by mixing 10 μL of each resuspended sample and 3 μL injections were made. Optima grade methanol, water, acetonitrile, methyl tert-butyl ether, and 2-propanol were from Thermo Fisher Scientific (Pittsburg, PA). Gasses were supplied by Airgas (Philadelphia, PA).
2.9.9 ∣. Liquid chromatography high resolution-mass spectrometry (LC-HRMS) for lipids
Separations were conducted on an Ultimate 3000 (Thermo Fisher Scientific) using an Ascentis Express C18, 2.1 × 150 mm 2.7 μm column (Sigma-Aldrich, St. Louis, MO). The flow rate was 0.4 m /min, solvent A was water:acetonitrile (4:6 v/v) with 0.1% of formic acid and 10 mM of ammonium formate and solvent B was acetonitrile:isopropanol (1:9 v/v) with 0.1% of formic acid and 10 mM of ammonium formate. The gradient was as follows: 10% B at 0 minutes, 10% B at 1 minute, 40% B at 4 minutes, 75% B at 12 minutes, 99% B at 21 minutes, 99% B at 24 minutes, 10% B at 24.5 minutes, and 10% at 30 minutes. Separations were performed at 55°C.
For the HRMS analysis, a recently calibrated QE Exactive-HF mass spectrometer (Thermo Fisher Scientific) was used in positive ion mode with an HESI source. The operating conditions were: spray voltage at 3.5 kV; capillary temperature at 285°C; auxiliary temperature 370°C; tube lens 45. Nitrogen was used as the sheath gas at 45 units, the auxiliary gas at 10 units and sweep gas was 2 units. The same MS conditions were used in negative ionization mode, but with a spray voltage at 3.2 kV. Control extraction blanks were made in the same way using only the solvents instead of the tissue homogenate. The control blanks were used for the exclusion list with a threshold feature intensity set at 1e10^5. Untargeted analysis and targeted peak integration were conducted using LipidsSearch 4.2 (Thermo Fisher Scientific).
An external mass calibration was performed using the standard calibration mixture approximately every 3 days. All samples were analyzed in a randomized order in full scan MS that alternated with MS2 of top 20, with HCD scans at 30, 45, or 60 eV. Full scan resolution was set to 120 000 in the scan range between m/z 250 and 1800. The pool sample was run every 15 samples. The coefficient of variation of less than 20% of the pooled sample was used to assess a robust signal for TGs. Calibration curves were prepared in the range of 2 ng to 500 ng for cholesterol, and were plotted against the area ratio of the dehydration product (m/z 369.3513) to the corresponding internal standard. The top 16 abundant TGs were identified based on their MS2 data from the LipidsSearch library and quantification was done from the full scan. The areas were normalized based on the 0.7 nmoles of TG internal standard (15:0/18:1D7/15:0) added per sample (as found in the SPLAH LIPIDOMIX). All amounts were normalized to the original tissue weight.
2.10 ∣. Real-time PCR
Using all samples from the 39-week cohort, total RNA was isolated from frozen livers under TRIzol (Invitrogen, CA, USA) conditions. To ensure RNA free of genomic DNA all samples underwent DNase treatment (Roche, Germany) according to the manufacture’s instructions; briefly, 1500 ng of RNA isolated from livers were treated with 1.5 μL of DNase followed by first-strand synthesis using Superscript III reverse transcriptase (Invitrogen, CA, USA) and random hexamer primers (Roche, Germany). All real-time PCR reactions were performed using 10 μL reactions consisting of 5.0 μL of SYBR green master mix (Applied Biosystems, MA, USA), 0.2 μL of 10 μM Forward primer, 0.2 μL of 10 μM Reverse primer, and 4.6 μL of cDNA (Final concentration: 1.09 ng/μL; primers listed in Supplemental Table S2). Cycle thresholds (Cts) were detected using QuantStudio 7 Flex Real-Time PCR System (Life Technologies, CA, USA). Reaction efficiency (E) was estimated for each pair of primers using a standard curve and expression levels were quantified by measuring the Cts for each sample using the E(-Cts) method. All samples were run in triplicate. Relative expression was calculated using the quantified expression from the endogenous control NONO that had stable expression levels in mouse liver across multiple samples and experimental groups.
2.11 ∣. Western blot
Using all liver samples from the 12- and 39-week cohorts, protein analysis was performed by Western Blot. Tissues were homogenized in RIPA extraction buffer (Cell Signaling Technology, Danvers, MA, USA) supplemented with cOmplete EDTA-free Protease inhibitor cocktail (Roche, Germany) in a cold-room using a Diagenode Bioruptor UCD-200 at HI for 15 minutes (30 s on, 30 s off) and a controlled temperature of 4°C. Tissue lysates were cleared by centrifugation at 10 000 ×g for 10 min at 4°C and supernatants were transferred to new tubes. Protein concentrations were quantified by Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA) and 20 μg of protein lysate was used per sample. 2X Laemmli Sample Buffer (Bio-Rad, Hercules, CA, USA) with 5% of 2-Mercaptoethanol (Bio-Rad, Hercules, CA, USA) were added to protein lysates and denatured at 90°C for 5 minutes. Samples were loaded on a NuPAGE 4-12% Bis-Tris 1.5 mm Mini Protein Gel (Thermo Fisher Scientific, Waltham, MA, USA) alongside with PageRuler Plus Prestained Protein Ladder 10 to 250 kDa (Thermo Fisher Scientific, Waltham, MA, USA) and run using NuPAGE MOPS SDS buffer (Thermo Fisher Scientific, Waltham, MA, USA). The gels were transferred onto a PVDF Immobilon-P membrane (MilliporeSigma, Burlington, MA, USA) for 90 minutes at 400 mA constant current. The membranes were blocked for 1 hours in 5% of nonfat dairy milk in TBS-T (TBS Bio Rad with 0.1% of Tween-20; Bio-Rad, Hercules, CA, USA). After blocking the membranes were cut to separate the runs according to protein molecular weight of interest and each section was probed with primary antibodies diluted in 5% of nonfat dairy milk in TBS-T for GAPDH (1:5,000, Cell Signaling) and either SREBP-1 (1:1,000; Abcam, Cambridge, UK), SREBP-2 (1:1,000; Abcam, Cambridge, UK) or HMGCR (1:1,000; Thermo Fisher Scientific, Waltham, MA, USA) at 4°C overnight. Membranes were subsequently probed with HRP-conjugated secondary antibody (Invitrogen, CA, USA) for 2 hours at room temperature. Prior to imaging, membranes were exposed to Millipore Immobilon Western HRP (MilliporeSigma, Burlington, MA, USA) substrate for 20 seconds. Bands were visualized and analyzed using an Amersham Imager 600 (GE Healthcare Life Sciences, Marlborough, MA, USA). Levels of unmodified SREBP-1, SREBP-2, and HMGCR were determined relative to GAPDH levels and compared between treatments.
2.12 ∣. Liver histology
After fixation in 10% of phosphate-buffered formalin, a portion of the left lobe was dehydrated in ethanol and xylenes, embedded in paraffin wax and cut into 5 pm cross sections. In a random subset of 39-week liver (n = 4/sex/group), sections were stained with hematoxylin and eosin (H&E) and sent to the University of Pennsylvania Comparative Pathology Core for pathological analyses.
2.13 ∣. Statistical analyses
Statistical differences in the mean between groups were tested by two-tailed t tests for data obtained at a singular timepoint and the F-test was used to test the equality of variance between groups. For longitudinal metabolic measures, statistical differences in the mean between groups were tested by two-tailed Welch’s t tests and corrected for multiple comparisons using the Holm-Sidak method with a significance threshold of α = 0.05.
Overrepresentation tests were performed with PANTHER (pantherdb.org) using the GO biological processes annotation data set (released 2020-06-01).37,38 The correlations of placental DNA methylation and offspring phenotypes were determined using Pearson’s correlation.
3. ∣. RESULTS
3.1 ∣. IVF produces sex-specific metabolic phenotypes
At birth, neonates from IVF-pregnancies were heavier than those from Nat-pregnancies (Supplemental Table S3). Although some mouse models report lower birth in IVF offspring, our findings are consistent with others who find that IVF neonates are heavier than naturally conceived neonates. Consistent with numerous other studies, we found increased placental weight in IVF-pregnancies compared to natural.39-42 In line with these findings, we previously reported placental overgrowth in both males and females, with males weighing more.52
To determine the effects of IVF on metabolic phenotypes throughout the life span, we performed longitudinal metabolic phenotyping on IVF and Nat offspring. An important confounder of adult metabolic phenotypes is neonatal litter size. In particular, neonates nursed in small litters (3-4), which are commonly used in mouse models of ART, show increased body weight and adiposity in adulthood compared to neonates nursed in litters of 6-10.19 We controlled for the effects of neonatal litter size on adult metabolic phenotypes by maintaining an average total litter size (native plus foster neonates) of 10 neonates throughout nursing. Then, metabolic panels were performed at 12- , 16- , 20- , 24- , 28- , and 39-weeks-of-age which consisted of fasting serum measures of total cholesterol, triglycerides, and insulin along with glucose tolerance testing. All fasting serum measures and statistics are displayed in Supplemental Table S4.
As early as 12 weeks we identified sex-specific differences in metabolic phenotypes. Relative to Nat-females, IVF-females were significantly heavier from birth through 24 weeks, with the exception of 8 weeks (Figure 1A). IVF-females also had consistently higher cholesterol than Nat-females at all timepoints between 12 and 24 weeks and also displayed a trend toward higher cholesterol for the remaining timepoints (Figure 1B). No differences were detected between IVF and Nat-Females in fasting triglycerides, insulin, or glucose levels, or the glucose area under the curve (AUC; Figure 1C-F).
FIGURE 1.

Female metabolic phenotypes in naturally-conceived and IVF-conceived offspring through 39-weeks-of-age. Data from each timepoint are from n = 8-12 Nat-females and n = 14-16 IVF-females. Body weights were measured in un-fasted mice throughout the study A, Age 0 is equivalent to birth weight by cesarean section at E18.5 Nat and E19.0 IVF. At six timepoints, total cholesterol (B), total triglycerides (C), insulin (D), and glucose (E) were measured after 6 hours of fasting. IPGTTs were also performed at these same timepoints and analyzed by area-under-the-curve (AUC) (F). At the terminal timepoint (ie, 39 weeks), body composition was measured by nuclear magnetic resonance (G) and heart rate (H) and blood pressure (I) were measured by noninvasive tail-cuff. Black and gray dots represent Nat-conceived and IVF-conceived females, respectively. Lines represent the mean and error bars represent the standard deviation. Statistical significance was determined using two-tailed Welch’s t test with Holm-Sidak correction (A-F) and student’s t test (G-I). P value < .05 was considered significant (*)
Unlike females, we only found significant differences in body weight between IVF and Nat males at birth and 4 weeks (Figure 2A). No differences in serum cholesterol levels were observed between groups at any timepoint (Figure 2B). In contrast to females, IVF-males showed significantly higher triglyceride levels at 12 and 20 weeks and trends toward higher triglyceride levels were observed at other timepoints (Figure 2C). For insulin, IVF-males displayed significantly higher levels than Nat-males at 24, 28, and 39 weeks (Figure 2D). IVF-males also demonstrated significantly higher fasting glucose at 16 and 24 weeks, but no differences in glucose homeostasis as analyzed by AUC (Figures 2E,F).
FIGURE 2.

Male metabolic phenotypes in naturally-conceived and IVF-conceived offspring through 39-weeks-of-age. Data from each timepoint are from n = 9-13 Nat-males and n = 15-19 IVF-males. Body weights were measured in un-fasted mice throughout the study (A). Age 0 is equivalent to birth weight by cesarean section at E18.5 Nat and E19.0 IVF. At six timepoints, total cholesterol (B), total triglycerides (C), insulin (D), and glucose (E) were measured after 6 hours of fasting. IPGTTs were also performed at these same timepoints and analyzed by area-under- the-curve (AUC) (F). At the terminal timepoint (ie, 39 weeks), body composition was measured by nuclear magnetic resonance (G) and heart rate (H) and blood pressure (I) were measured by noninvasive tail-cuff. Black and gray dots represent Nat-conceived and IVF-conceived males, respectively. Lines represent the mean and error bars represent the standard deviation. Statistical significance was determined using two-tailed Welch’s t test with Holm-Sidak correction (A-F) and student’s t test (G-I). P value < .05 was considered significant (*)
To further characterize the observed metabolic phenotypes we measured body composition, blood pressure, and heart rate at 39 weeks, which was the terminal timepoint for this study (Supplemental Table S4). We found no difference in body fat percentage between IVF- and Nat-females (Figure 1G). Despite no significant difference in body weight at 39 weeks, IVF-males demonstrated significantly higher body fat percentage compared to Nat-males (Figure 2G). No differences were found in either sex for heart rate, mean arterial pressure (Figure 1H-I and 2H-I) or systolic and diastolic blood pressures.
3.2 ∣. Sex-specific effects of IVF on metabolic phenotypes are reflected in adult liver transcriptome and proteome
3.2.1 ∣. Adult liver transcriptome
To identify molecular mechanisms underlying the metabolic phenotypes, we performed RNA-seq on whole liver collected from IVF and Nat offspring at 39 weeks. Alignment of reads to reference sequence and number of mapped reads per sample are displayed in Supplemental Table S1. As the liver displays sexual dimorphism43,44 we analyzed female and male liver separately. Principle Component Analysis (PCA) using the expression profile of all genes did not show separation between female IVF and Nat samples, whereas males showed separation on PC2 (Supplemental Figure S1A,C). Although these findings indicate that expression differences between groups are small compared to individual differences, using a cutoff FDR < 0.05 we identified 12 differentially expressed genes (DEGs; seven upregulated and five downregulated) between IVF- and Nat-females, and 17 DEGs (nine upregulated and eight downregulated) between IVF- and Nat-males (Supplemental Figure S1B,D).
Given the low number of DEGs, we initially performed GO analysis using the top 100 transcripts based on P values. The top 10 overrepresented GO pathways in females (Table 1) suggest that IVF and Nat-females differ in pathways related to cholesterol biosynthesis (Mvd, Hmgcr, Fdft1) as well as steroid biosynthesis and metabolic processes (Hsd3b5, G6pc, Mvd, Hmgcr, Fdft1), highlighting the interrelatedness of cholesterol and steroid pathways. Of note, although not contributing to our top 10 GO pathways, Esr1 was upregulated in our RNA-seq data. In contrast to females, the top 10 overrepresented GO pathways in males (Table 1) suggest differences between IVF and Nat-male in several immune response and inflammation pathways (CRP, Saa3, Fn1, SERPINA3n, SERPINE1, Cd51, etc.). For both sexes, phosphorylation appears in our GO analysis and suggests widespread effects on regulatory events including cell division, growth, differentiation, and intercellular communication, in IVF offspring.
TABLE 1.
Overrepresented pathways in liver transcriptome
| GO biological process | Genes | Fold enrichment |
FDR |
|---|---|---|---|
| Female | |||
| Isoprenoid biosynthetic process (GO:0008299) | MVD, HMGCR, FDFT1 | 35.45 | 1 |
| Steroid biosynthetic process (GO:0006694) | HSD3B5, MVD, HMGCR, FDFT1 | 12.96 | 1 |
| Vascular endothelial growth factor receptor signaling pathway (GO:0048010) | BMPR2, MAPKAPK2, SULF1 | 24.10 | 1 |
| Sterol biosynthetic process (GO:0016126) | MVD, HMGCR, FDFT1 | 22.32 | 1 |
| Steroid metabolic process (GO:0008202) | G6PC, MVD, HMGCR, FDFT1 | 9.56 | 1 |
| Cholesterol biosynthetic process (GO:0006695) | MVD, HMGCR, FDFT1 | 18.83 | 1 |
| GO:0043066~negative regulation of apoptotic process | PKHD1, TSC22D1, ADORA1, CFLAR, HMGCR, CDC73, TBX3, MAP4K4 | 2.83 | 1 |
| Protein phosphorylation (GO:0006468) | CDK18, BMPR2, RPS6KC1, AKT3, MAPKAPK2, SGK3, NEK2, MAP4K4 | 2.79 | 1 |
| Eye morphogenesis (GO:0048592) | STAU2, COL5A2 | 80.36 | 1 |
| Phosphorylation (GO:0016310) | CDK18, BMPR2, RPS6KC1, AKT3, MAPKAPK2, SGK3, NEK2, MAP4K4 | 2.62 | 1 |
| Male | |||
| Response to stilbenoid (GO:0035634) | SAA3, GSTA2, GSTA1, SLC22A7 | 34.44 | 0.13 |
| Acute-phase response (GO:0006953) | CRP, SAA3, FN1, SERPINA3N | 24.60 | 0.18 |
| Wound healing (GO:0042060) | CRP, PTK7, SERPINE1, FN1, TGFBR2 | 11.45 | 0.19 |
| Cellular iron ion homeostasis (GO:0006879) | HPX, SCARA5, LCN2, CP | 19.13 | 0.19 |
| Complement activation, classical pathway (GO:0006958) | CRP, C1QA, HC, C1QC | 15.66 | 0.27 |
| Receptor-mediated endocytosis (GO:0006898) | IFITM3, SCARA5, LRP1, TGFBR2 | 14.35 | 0.28 |
| Cellular response to interleukin-1 (GO:0071347) | SAA3, SERPINE1, LCN2, FN1 | 10.76 | 0.55 |
| Response to virus (GO:0009615) | CREBZF, IFITM3, CDK6, LCN2 | 10.25 | 0.55 |
| Immune system process (GO:0002376) | IFITM3, CSF1R, C1QA, CD5L, LCN2, HC, C1QC | 3.93 | 0.60 |
| Protein phosphorylation (GO:0006468) | CAMK2B, CSF1R, CDK6, PTK7, PLK2, FGFR3, IGF1R, TGFBR2 | 2.99 | 0.98 |
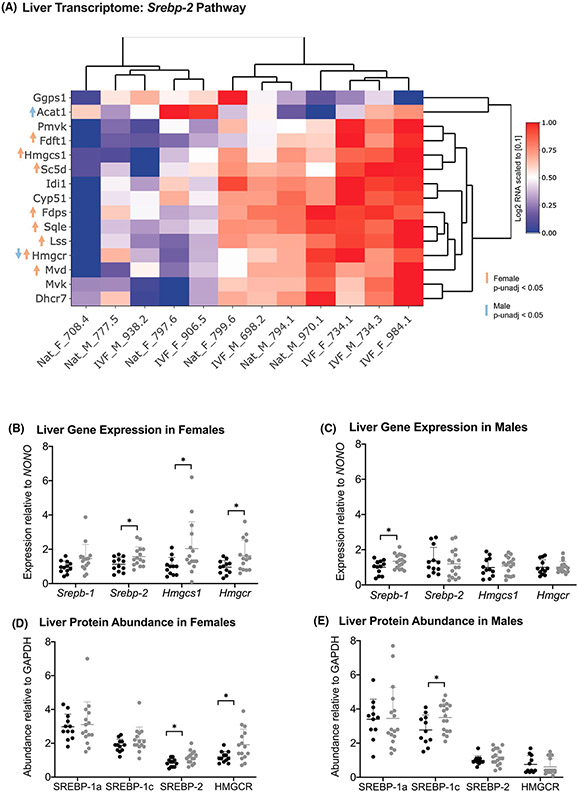
Next, based on the observed sex-specific phenotypes of cholesterol, triglycerides, and insulin in IVF offspring, we explored pathways regulated by sterol regulatory element-binding proteins (SREBPs), which act as master regulators of cholesterol and fatty acid synthesis in liver.45,46 Of the three mammalian isoforms, Srebp-2 preferentially activates genes involved in cholesterol metabolism, whereas Srebp-1c is activated by insulin and preferentially activates genes involved in fatty acid and triglyceride metabolism. Although SREBPs were not detected in our RNA-seq data, presumably reflecting very low abundance, heatmaps of genes within the Srepb-2 regulated pathway showed higher expression in IVF-female mice (unadjusted-p < 0.05; Figure 2A and Supplemental Figure 2A). No differences between IVF and Nat-females were observed in the Srebp-1c pathway (Supplemental Figure 2B). Within this small subsample, expression differences in the Srepb-2 pathway did not remain significant upon correcting for multiple comparisons (FDR < 0.05).
We further investigated these pathways among the entire cohort by performing real-time PCR and western blot of SREBPs and their primary targets. Again, we found sex-specific differences. Consistent with their high cholesterol phenotype, IVF-females exhibited higher transcript and protein abundance of Srebp-2 (Figure 3B,D). In the cholesterol biosynthetic path-way, Srebp-2 responsive genes include HMG-CoA synthase (Hmgcs1) and HMG-CoA reductase (Hmgcr).45 Transcript and protein abundance of these enzymes were elevated in IVF-females compared to Nat-females (Figure 3B,D), suggesting that the Srepb-2 pathway may contribute to the high cholesterol phenotype observed in IVF-females.
FIGURE 3.
Gene expression and protein levels in 39 week liver of naturally-conceived and IVF-conceived offspring. The heatmap demonstrates log2-transformed expression levels obtained from RNA-Seq of genes within the Srebp-2 pathway (A). mRNA expression levels of Srepb1, Srebp2, Hmgcs1, Hmgcr obtained through real-time PCR for females (B) and males (C). Downstream protein levels of SREPB isoforms 1a and 1c, SREBP-2 and HMGCR in females (D) and males (E). Black and gray dots represent Nat-conceived and IVF-conceived males, respectively. Lines represent the mean and error bars represent the standard deviation. Statistical significance was determined using student’s t test. P value < .05 was considered significant (*)
In contrast, we did not observe any differences in Srebp-2 transcript abundance in males, but rather PCR-based analyses showed upregulation of Srebp-1 in IVF-males (Figure 3C). When we evaluated downstream protein abundance of the two SREBP-1 isoforms (ie, SREBP-1a and SREBP-1c), we found that SREBP-1a expression, whose target genes increase both cholesterol and triglyceride biosynthesis,46 did not differ between IVF-and Nat-males. However, protein expression of SREBP-1c, which is activated by insulin and has downstream effects that stimulate production of triglycerides and phospholipids, was significantly higher in IVF-males compared to Nat-males (Figure 3E). These findings suggest that SREBPs are involved in development of IVF phenotypes and that the effects of IVF on the SREBP pathways are sex-specific and may underlie the sex-specific phenotypes.
3.2.2 ∣. Adult liver proteome
We also used liquid chromatography mass spectrometry-based proteomics to identify protein abundance in liver collected at 39 weeks from a subset of IVF and Nat offspring (n = 5/sex/group). We identified a total of 24 037 peptides with abundance above zero and a median peptide coefficient of variation (CV) of 18.22. Applying a cutoff CV of 50, the 24 037 peptides corresponded to 2924 proteins. The heatmap in Figure 4A demonstrates significant proteins in the liver proteome identified by two-way ANOVA.
FIGURE 4.
Liver proteome in 39 week naturally-conceived and IVF-conceived offspring. The heatmap demonstrates results from two-way ANOVA of log2-transformed protein expression levels obtained from liquid chromatography mass spectrometry (A). Hepatic content of cholesterol and triglycerides in 39 week females (B & D) and males (C & E). Each dot represents an individual. Black and gray dots represent Nat-conceived and IVF-conceived males, respectively. Lines represent the mean and error bars represent the standard deviation. Statistical significance was determined using student’s t test. P value < .05 was considered significant (*)
Two-way ANOVA identified 43 proteins with differential abundance between IVF-female and Nat-female (FDR < 0.05, 20 upregulated and 23 downregulated). Using the top 100 proteins based on P values, we performed gene ontology analysis with the PANTHER overrepresentation test. The top 10 overrepresented GO pathways in females (Table 2) suggest that IVF- females and Nat-females differ in pathways important for cholesterol biosynthesis (FDPS, HMGCS1), fatty acid metabolism (GCCH, ACAT1, ASCL5, GCDH, ABCD3, HSD17B4, etc.) and energy production pathways involving glucose 6-phosphate (GCK, HK1, PGD). Of particular relevance to the IVF-female phenotype of high cholesterol is the upregulation of FPDS and HMGCS1 that are involved in the farnesyl diphosphate biosynthetic process (GO:0045337), an important intermediate in conversion of mevalonate to cholesterol. Although FPDS was in the top 100 proteins identified by two-way ANOVA in females, differential abundance of FPDS did not remain significant after correction for multiple testing (FDR = 0.1187). However, consistent with our evaluation of gene expression (Figure 3) and further supporting involvement of genes responsive to Srebp-2, HMGCS1 displayed significantly greater abundance in IVF-females compared to Nat-females (log2 fold change = 1.4062, FDR = 0.0130).
TABLE 2.
Overrepresented pathways in liver proteome
| GO biological process | Proteins | Fold enrichment |
FDR |
|---|---|---|---|
| Female | |||
| Farnesyl diphosphate biosynthetic process (GO:0045337) | FDPS, HMGCS1 | >100 | 4.56E-02 |
| Fatty-acyl-CoA biosynthetic process (GO:0046949) | GCCH, ACAT1, ACSL5 | >100 | 2.72E-03 |
| Fatty-acyl-CoA metabolic process (GO:0035337) | GCDH, HSD17B4, ACAT1, ASCL5 | 56.55 | 6.82E-04 |
| Glucose 6-phosphate metabolic process (GO:0051156) | GCK, HK1, PGD | 33.48 | 3.53E-02 |
| Acyl-CoA biosynthetic process (GO:0071616) | GCDH, ACAT1, ACSL5 | 31.81 | 3.81E-02 |
| Thioester biosynthetic process (GO:0035384) | GCDH, ACAT1, ACSL5 | 31.81 | 3.75E-02 |
| Fatty acid beta-oxidation (GO:0006635) | GCDH, ABCD3, HSD17B4, ACAT1, ACOX2 | 21.64 | 1.96E-03 |
| Monocarboxylic acid catabolic process (GO:0072329) | HACL1, GCDH, ABCD3, HSD17B4, ASRGL1, ACAT1, ACOX2, PGD, LYPLA2 | 20.52 | 2.18E-06 |
| Fatty acid catabolic process (GO:0009062) | HACL1, GCDH, ABCD3, HSD17B4, ACAT1, ACOX2, LYPLA2 | 20.33 | 8.63E-05 |
| Acyl-CoA metabolic process (GO:0006637) | GCDH, GLYAT, HSD17B4, SCP, ACAT1, HMGCS1, ASCL5 |
19.03 | 1.13E-04 |
| Male | |||
| Vesicle fusion with Golgi apparatus (GO:0048280) | STX5, VTL1B, USO1 | 81.86 | 1.80E-02 |
| Arginine metabolic process (GO:0006525) | DDAH1, ASL, AGMAT | 43.66 | 4.91E-02 |
| Glutamine family amino acid metabolic process (GO:0009064) | DDAH1, ALDH4A1, DGLUCY, ASL, AGMAT | 19.15 | 1.65E-02 |
| Vesicle fusion (GO:0006906) | STX4, STX5, VTI1B, USO1, VAMP3 | 14.55 | 3.24E-02 |
| Alpha-amino acid catabolic process (GO:1901606) | DDAH1, ALDH4A1, ALDH6A1, AFMID, SHMT1 | 14.55 | 3.05E-02 |
| Organelle membrane fusion (GO:0090174) | STX4, STX5, VTI1B, USO1, VAMP3 | 13.47 | 4.08E-02 |
| Dicarboxylic acid metabolic process (GO:0043648) | ALDH4A1, DLUCY, ASL, IDH1, SHMT1 | 13.47 | 3.86E-02 |
| Cellular amino acid catabolic process (GO:0009063) | DDAH1, ALDH4A1, ALDH6A1, AFMID, SHMT1 | 12.13 | 4.73E-02 |
| Alpha-amino acid metabolic process (GO:1901605) | DDAH1, ALDH4A1, DGLUCY, ASL1, ALDH6A1, AFMID, AGMAT, SHMT1 | 10.65 | 2.43E-03 |
| Cellular amino acid metabolic process (GO:0006520) | DDAH1, ALDH4A1, DGLUCY, ASL1, ALDH6A1, AFMID, AGMAT, SHMT1 | 7.56 | 2.01E-02 |
In males, we identified 24 proteins with differential abundance between IVF-male and Nat-male (FDR < 0.05, 11 upregulated and 13 downregulated). Again, we performed an overrepresentation test in PANTHER using the top 100 proteins; Table 2 shows the top 10 overrepresented GO pathways. The identified pathways suggest potential differences in vesicle fusion processes (STX5, VTI1B, USO1) and amino acid metabolism (DDAH1, ALDH4A1, DGLUCY, ASL, AGMAT, SHMT1, etc.) between IVF- and Nat-males. For proteins involved in amino acid metabolism, several remained significantly different after correction for multiple testing (STX5, PDXDC1, IDH1, DGLUCY, and CYP2C55; FDR < 0.05), indicating that hepatic amino acid metabolism is one of the most pronounced differences between IVF- and Nat-males. As excess amino acids can be interconverted and metabolized directly into precursors for production of glucose and fatty acids, our findings suggest that disruption of amino acid metabolism in IVF-males may be a potential contributor to the IVF-male phenotype characterized by high triglycerides.
3.2.3 ∣. Adult liver lipid content
To examine differences in hepatic cholesterol and triglyceride content we performed liquid-chromatography- high resolution mass-spectrometry on livers obtained from mice 39 weeks old. We found no differences in hepatic cholesterol content between IVF and Nat for either sex and no differences in hepatic triglyceride content between IVF- and Nat- females (Figure 4B-D). However, in alignment with the IVF-male phenotype of elevated serum triglycerides and insulin and increased hepatic SREBP-1c abundance, IVF- males demonstrated significantly higher liver triglyceride content than Nat-males (Figure 4E).
3.2.4 ∣. Adult liver histology
Given the sexually dimorphic differences observed in liver, we performed liver histology in a random subset of offspring to assess hepatocellular swelling, clearing, atrophy, vacuolation, karyomegaly, and necrosis as well as sinusoidal inflammatory cell aggregates. Liver histology assessments performed at 39 weeks did not reveal marked differences. Many of the lesions noted in the liver samples are considered common background findings, such as the extramedullary hematopoiesis, portal inflammatory cell infiltrates, and inflammatory cell aggregates within the sinusoids with or without individual hepatocellular necrosis. These traits did not differ between sexes or groups. Both groups and sexes also exhibited expected age-related changes with no differences regardless of individual phenotypes.
3.3 ∣. Replication of findings in 12-week cohort
To replicate our findings in an independent cohort as well as determine if differences in liver proteins could be detected early on, we generated an additional cohort of IVF and Nat offspring and assessed them at 12-weeks-of-age. Similar to our findings from the original cohort, IVF- females from the l2 week cohort had significantly higher total cholesterol (P = .0022; Supplemental Figure S3A). For this cohort, we quantified HDL and combined LDL/VLDL fractions and found that both fractions were higher in IVF-females compared to Nat-females (HDL: P = .0174; LDL/VLDL: P = .0418). As with the original cohort, the 12-week cohort did not exhibit a significant difference in triglycerides between IVF- and Nat-Females (P = .682; Supplemental Figure S3B). Like our original cohort, IVF-males showed no differences in total, HDL, and LDL/VLDL cholesterol (Supplemental Figure S4A), but elevated triglycerides were again evident at 12 weeks (P = .0155; Supplemental Figure S4B).
We also confirmed sexually dimorphic protein expression of SREBPs and HMGCR. Combined with our results from 39 week liver, elevation of SREBP-2 and HMGCR in 12 week IVF-females (Supplemental Figure S3C) indicates that this pathway of cholesterol biosynthesis is disrupted early on and disruption persists through adulthood. Unlike our at 39-week cohort, SREBP-1 was elevated in 12 week IVF-females compared to Nat-females. As SREBP-1a and 1c were not quantified separately, it is unclear which isoform contributes to this increase. SREBP-1c preferentially activates fatty acid and triglyceride synthesis, SREBP-1a activates both triglyceride and cholesterol synthesis. Although we detected no differences in either isoform in 39 week liver, based on the high cholesterol phenotype, it is possible that the elevation may be transient and due to SREBP-1a.
As expected, no differences were observed in SREBP-2 or HMGCR in liver of 12 week males (Supplemental Figure S4C). SREBP-1 was significantly higher in IVF-males compared to Nat-males and given the results from our 39 week cohort, we hypothesize that this increase is driven by the preferential activator of triglyceride biosynthesis, SREBP-1c. At 12 weeks, neither females nor males demonstrated differences in hepatic lipid content between IVF and Nat (Supplemental Figures S3D-E and S4D-E, respectively).
3.4 ∣. IVF produces sexually dimorphic placental epigenotypes
In previous work, we and others have shown that IVF procedures induce placental epigenotypes characterized by reduced global DNA methylation and loss of monoallelic expression at imprinted genes.23,42,47-51 To obtain placental tissues and deliver neonates at similar developmental stages, Nat offspring were delivered at E18.5 and IVF offspring at E19. To assess whether placental epigenetic changes were sex-specific, we used LUMA (see methods)23,24,52 to measure global DNA methylation and pyrosequencing to measure DNA methylation at the regulatory imprinting control regions (ICRs) of four imprinted genes that are involved in regulation of placental and fetal development. As the ICRs of imprinted genes exhibit methylation on only one allele, non-allele-specific measurement yields roughly 50% methylation.
We performed non-allele-specific measurement of DNA methylation at the ICRs of two paternally methylated ICRs--H19/Igf2 and IgDMR (the ICR for the Dlk1/Gtl2 imprinted cluster)—as well as two maternally methylated ICRs—KvDMR1 (the ICR for the Kcnq1 cluster) and Peg3. We found sex-specific differences in global and ICR DNA methylation between IVF and Nat (Supplemental Table S5). IVF-females demonstrated significantly lower DNA methylation globally and at the ICRs of H19/Igf2 and Peg3, but no differences at KvDMR1 and IgDMR compared to Nat-females (Figure 5A). Conversely, IVF-males had lower DNA methylation at the Peg3 ICR but no differences globally or at the H19/Igf2 ICR, KvDMR1, or IgDMR compared to Nat-males (Figure 5B). These results support previous findings of loss of imprinting in placentas of IVF-conceived pregnancies and provides new evidence that these methylation changes are sex- and gene-specific.
FIGURE 5.

DNA methylation in naturally conceived and IVF-conceived placentas. Each data point represents an individual female placenta from n = 13 Nat-females and n = 16 IVF-females. Luminometric methylation assay was used to measure global DNA methylation. Bisulfite pyrosequencing was used to measure Imprinting control region DNA methylation in placenta at H19/Igf2, KvDMR1, IgDMR, and Peg3 in female (A) and (B) male placenta. Black and gray dots represent Nat-conceived and IVF-conceived males, respectively. Lines represent the mean and error bars represent the standard deviation. Statistical significance was determined using student’s t test. P value < .05 was considered significant (*)
3.5 ∣. Average cholesterol of IVF-females is correlated with global DNAm
Placental epigenetic marks regulate fetal development and variations in these marks have been proposed as biomarkers of early life growth and long-term health outcomes. To evaluate the potential of placental epigenetics marks as predictive biomarkers, we assessed whether the identified placental epigenotypes were associated with the sex-specific offspring outcomes. Accordingly, we used a cesarean delivery and fostering protocol that enabled us to distinguish which placenta belonged to which offspring by permanently marking each neonate with a unique identifier at birth. We then performed exploratory correlation analysis to evaluate whether or not placental DNA methylation was associated with the long-term phenotypes observed in this study. For serum measures, we used the average of the measures over time. Upon correlation analyses (Supplemental Figure S5), IVF-females demonstrated a correlation between average cholesterol and global placental DNA methylation. No other correlations were found between other measures of placental epigenotype and offspring metabolic phenotypes.
4. ∣. DISCUSSION
Throughout the field of developmental programming, evidence showing that prenatal exposures affect males and females differently53,54 continues to grow. In mouse, more than 600 transcripts are differentially expressed between the sexes as early as the blastocyst stage,55 suggesting that the early embryo can respond to ART procedures in a sex-specific manner. Here, we use a mouse model to show that ART procedures during preimplantation development result in differential effects based on sex and that these effects have long-lasting consequences on metabolic parameters and pathways in adulthood. To date, human epidemiologic studies find that the majority of individuals conceived with ART are healthy at birth and in childhood.56 However, because the oldest individuals conceived with ART are just now reaching their 40s, epidemiological studies are limited in their ability to distinguish adulthood outcomes.56 Our findings here suggest that while most individuals are healthy, metabolic effects may not emerge until later in life. Individuals who demonstrate altered lipid profiles in early adulthood should be monitored for long-term effects.
We and others find that IVF-females are heavier in adulthood compared to controls. Although we detect higher body weight at 12 weeks, Feuer et al14 did not observe higher body weight until 17weeks. Feuer et al, also noted IVF- females exhibit increased body fat and altered glucose homeostasis, whereas we do not. Although Feuer et al, note no differences in outcomes based on litter size, differences between their findings and ours may be due to a variety of other reasons including comparison groups. Feuer et al, use a flushed blastocyst control group with litter sizes ranging from 3 to 8. We and others have already identified how individual ART procedures, including those used to generate a flushed blastocyst control group, introduce iatrogenic changes to fetal and placental development23,42,48,52 and that the full IVF protocol demonstrates the most severe phenotypes. Therefore, our intent here was to take a clinically relevant approach by examining the full IVF protocol in comparison to natural conception.
In contrast to females, we find a novel and more severe metabolic phenotype in IVF-males characterized by high insulin, triglycerides, and body fat percentage. Abnormality in all three of these metabolic parameters occurs in development of severe metabolic disease.57,58 Hence, these findings are potentially clinically relevant and suggest that males conceived through IVF may be at increased risk for metabolic dysfunction. Others have found that male mice conceived through various ART procedures exhibit reduced life span with high-fat diet,59 insulin resistance,15 altered glucose homeostasis,15,17 increased blood pressure,11,12,59 or no differences compared to controls.13,14
One challenging aspect of long-term outcome studies, is that litter size can contribute to differences in outcomes between studies. We maintained an average neonatal litter size of 10, because it is a standard control size in studies evaluating the impact of neonatal litter size on metabolic pheontypes.19 Litter sizes reported in the studies cited here range from 2 to 8, noting that litter sizes < 5 are considered small. Small neonatal litter size is associated with offspring that exhibit increased food intake during lactation, leading to increased body weight and adiposity in adulthood.19 Thus, similar to dietary challenge models, studies of ART that use small litter sizes could present a postnatal “challenge” of increased food availability and intake that is inherent to the study design and potentially confounds the effects of ART. Other factors contributing to variations in outcomes between studies include differences in genetic background, comparison groups, IVF protocols, fasting protocols, and the use of high-fat dietary challenges.
To our knowledge, this is the first study in an animal model of ART to longitudinally evaluate triglyceride and cholesterol levels in IVF-conceived offspring and moreover, to assess sex differences, which we find. The few human studies evaluating triglyceride and cholesterol in children and young adults conceived with IVF have not demonstrated adverse lipid profiles.17,60 In fact, one study of children between the ages of 4- and 10-years-old demonstrated a favorable profile of lower triglycerides and increased HDL cholesterol.61 Many of the human studies assessing metabolic outcomes in IVF-conceived children are limited by lack of longitudinal evaluation, particularly because the oldest individuals conceived through ART are just entering their fourth decade of life. They are also limited by small sample sizes that do not allow for sex-specific analyses, thus, masking any sex-specific effects that may be present. Whereas studies in young adults and children conceived with IVF are essential, an important finding from our and other mouse models is that some phenotypes resulting from IVF may not present until early adulthood or later. Taken together, our findings highlight the necessity of longitudinal assessment across several parameters of cardiometabolic health including lipid profiles, and the need to evaluate these parameters in a sex-specific manner throughout life.
Because we found novel sex-specific differences in cholesterol, triglyceride and insulin levels, we used transcriptomics and proteomics to compare how IVF affects molecular mechanisms in the liver between females and males. We explored pathways regulated by sterol regulatory element-binding proteins (SREBPs), transcription factors that act as master regulators of cholesterol and fatty acid synthesis in liver. Of the three mammalian isoforms, SREBP-2 preferentially activates genes involved in cholesterol metabolism, whereas SREBP-1c is activated by insulin and preferentially activates genes involved in fatty acid and triglyceride metabolism. Given these differences, we hypothesized that we would see differences in these pathways based on sex.
Consistent with the high cholesterol phenotype found in IVF-females, transcriptomic analysis of the SREBP-2 pathway shows several genes that trended toward upregulation in females conceived through IVF (Fdft1, Hmgcs1, Sc5d, Fdps, Sqle, Lss, Hmgcr, and Mvd). Likewise, pathway analysis of the liver proteome from a subset of individuals indicates overrepresentation of proteins involved in cholesterol biosynthesis (GO:0045337; FDPS and HMGCS1); we confirmed upregulation of SREBP-2 and its main targets (ie, HMGCS1 and HMGCR) in the entire cohort. Because estrogen regulates Srebp-2 transcription through an estrogen response element,62 our finding of upregulated Esr1 in liver of IVF-females, suggests that estrogen dynamics are altered in IVF-females in a way that contributes to dysregulation of cholesterol homeostasis. Our findings lay the groundwork for further interrogating the estrogen-SREBP-2 relationship and the interrelated metabolic, endocrine and reproductive effects.
Beyond the SREBP pathways, our analyses of the female liver proteome also identified alterations in pathways of fatty acid metabolism (GO:0046949, GO:0035337, GO:0071616, GO:0006635, GO:0009062, GO:0006637). Although phenotypes differ, liver proteomics assessing ob/ob and db/db mice,63 high-fat diet in rat,64 and cholesterol-induced atherosclerosis in rabbit65 also show disturbances in lipid metabolism. With the diverse role of fatty acid metabolism, including dependence on substrates that are also involved in cholesterol biosynthesis, it is not surprising that fatty acid metabolism is commonly affected in various models of metabolic dysfunction, including IVF.
In line with the high insulin and triglyceride levels exhibited in IVF-males, we find dysregulation of hepatic SREBP-1c. Although our exploratory transcriptomic assessment of the SREBP-1c pathway did not show differences in gene expression between naturally and IVF-conceived males, locus-specific assessment of SREBP-1c in the entire cohort showed increased abundance of SREBP-1c. SREBP-1c acts as the master regulator of lipogenesis by preferentially activating the entire complement of genes involved in fatty acid and triglyceride synthesis.46,66,67 Insulin activates SREBP-1c by increasing transcription68 and by increasing the conversion of the inactive precursor to the active nuclear form.69 Like the IVF-males of this study, mouse models of insulin resistance, such as high-fat diet70 and ob/ob mice71 have also demonstrated higher levels of SREBP-1c. Our findings suggest that the higher insulin levels associated with IVF may trigger signaling events that increase hepatic SREBP-1c and lipogenesis, resulting in higher hepatic triglyceride levels. IVF-males demonstrate higher serum triglyceride levels as early as 12 weeks and higher insulin levels are not detected until 20 weeks. Even so, it is possible that the insulin-dependent SREBP-1c pathway may be more sensitive to insulin in IVF-males even in the absence of higher insulin levels at the earlier timepoints.
Although the majority of offspring conceived with IVF are generally healthy, our work and that of others suggest that those who develop adverse metabolic outcomes may not do so until later life. As such, an important task in optimizing IVF outcomes is to identify biomarkers of long-term health. The placenta has been proposed as an easily accessible tissue that may serve as one such biomarker at birth. Imprinted genes in the placenta regulate fetal development and altered DNA methylation of imprinted genes has been proposed as biomarkers of early life growth and long-term health outcomes. Additionally, we have previously shown placental tissue is more epigenetically sensitive to ART procedures than fetal tissue and locus-specific effects of IVF on DNA methylation in the placenta.23,42,52 We have also shown that DNA methylation changes in the placenta are driven by embryo culture and co-occur with vascular defects, suggesting that epigenetic and functional changes in the placenta impact nutrient exchange in fetal development.52
Here, our placental collection and fostering protocol provided the unique opportunity to assess whether placental epigenetic changes at birth could serve as biomarkers of future metabolic outcomes in offspring conceived with IVF. In correlating placental DNA methylation with sex-specific phenotypes, we found that global placental DNA methylation is associated with average serum total cholesterol in females conceived through IVF. To our knowledge, this is the first study to demonstrate a link between placental DNA methylation and long-term outcomes in offspring. This exploratory finding remains to be validated in a larger cohort and in a manner that accounts for cellular heterogeneity. Even so, this finding provides promise for the use of placental epigenetic biomarkers to identify individuals at birth who may be at risk for adverse metabolic outcomes later at life.
Overall, the findings presented here support the growing body of evidence that IVF contributes to altered metabolic health outcomes in adulthood,8,11-17 and that this effect occurs in a sex-specific manner. Although mouse is an excellent and efficient model of investigation that allows for the IVF procedures to be isolated from underlying fertility, the findings here may be limited in their translation to human outcomes. Because oxygen, temperature, pH, humidity, light, and culture conditions are not consistent across all models of IVF—nor are they standardized in the clinical setting—phenotypes and epigenotypes may be specific to these conditions, emphasizing the importance of standardization in clinical and preclinical settings. Moreover, our findings highlight the necessity of longitudinal and sex-specific monitoring of outcomes in individuals conceived with IVF and the need for continual efforts in optimizing IVF procedures to ensure the future health of offspring.
Supplementary Material
ACKNOWLEDGMENTS
We thank Paula Stein, Teri Ord, Monica Mainigi, and Christos Coutifaris for their technical advice with IVF procedures and helpful comments, Chris Krapp and Joanne Thorvaldsen for their technical expertise with molecular protocols. We also thank Xiaoyan Yin, Jennifer Rojas, and the Rodent Metabolic Phenotyping Core (supported in part by Penn Diabetes Research Center grant (P30-DK19525)) at the University of Pennsylvania for performing cardiometabolic assays. We acknowledge Charles-Antoine Assenmacher and the Comparative Pathology Core at the University of Pennsylvania for performing liver pathology assessment. We also thank Mary Putt for her statistical guidance and expertise.
Funding information
This work was funded by the National Institutes of Child Health and Human Development HD092266 and HD068157 (MSB), the National Institute of General Medical Sciences GM110174 (BAG), the National Institute of Nursing Research T32NR007100 (LN), Ruth L. Kirshstein National Service Award Individual Postdoctoral Fellowship HD089623 (LAV), and the National Institute of Environmental Health Sciences P30-ES013508 (Center for Excellence in Environmental Toxicology).
Abbreviations:
- ART
assisted reproductive technologies
- DEG
differentially expressed gene
- DNAm
deoxyribonucleic acid methylation
- IPGTT
intraperitoneal glucose tolerance test
- IVF
in vitro fertilization
- Nat
naturally conceived
Footnotes
CONFLICT OF INTERESTS
The authors declare no competing or financial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
DATA AVAILABILITY STATEMENT
The DIA raw files, FASTA, Prosit library, EncyclopeDIA Chromatogram library, and quant report are available at MassIVE (MSV000086177).
REFERENCES
- 1.Thoma ME, McLain AC, Louis JF, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324–1331.e1. 10.1016/j.fertnstert.2012.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Society of Human Reproduction and Embryology. ART Fact Sheet; 2020. https://www.eshre.eu/. Accessed April 8, 2020.
- 3.Luke B, Brown MB, Eisenberg ML, et al. In vitro fertilization and risk for hypertensive disorders of pregnancy: associations with treatment parameters. Am J Obstet Gynecol. 2020;222 (4): 350e1–350e13. 10.1016/j.ajog.2019.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from ivf/icsi: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. 10.1093/humupd/dms018 [DOI] [PubMed] [Google Scholar]
- 5.Stern JE, Liu C-L, Cabral HJ, et al. Birth outcomes of singleton vaginal deliveries to ART-treated, subfertile, and fertile primiparous women. J Assist Reprod Genet. 2018;35(9): 1585–1593. 10.1007/s10815-018-1238-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schieve LA, Cohen B, Nannini A, et al. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Matern Child Health J. 2007;11(6):517–525. 10.1007/s10995-007-0202-7 [DOI] [PubMed] [Google Scholar]
- 7.Hattori H, Hiura H, Kitamura A, et al. Association of four imprinting disorders and ART. Clin Epigenetics. 2019;11(1):21. 10.1186/s13148-019-0623-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrooman LA, Bartolomei MS. Can assisted reproductive technologies cause adult-onset disease? evidence from human and mouse. Reprod Toxicol. 2017;68:72–84. 10.1016/j.reprotox.2016.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker D The fetal and infant origins of adult disease. BMJ Br Med J. 1990;301(156):1111. 10.1136/bmj.301.6761.1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen M, Heilbronn LK. The health outcomes of human offspring conceived by assisted reproductive technologies (ART). J Dev Orig Health Dis. 2017;8(4):388–402. 10.1017/S2040174417000228 [DOI] [PubMed] [Google Scholar]
- 11.Watkins AJ, Platt D, Papenbrock T, et al. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA. 2007;104(13):5449–5454. 10.1073/pnas.0610317104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rexhaj E, Pireva A, Paoloni-Giacobino A, et al. Prevention of vascular dysfunction and arterial hypertension in mice generated by assisted reproductive technologies by addition of melatonin to culture media. Am J Physiol Circ Physiol. 2015;309(7):H1151–H1156. 10.1152/ajpheart.00621.2014 [DOI] [PubMed] [Google Scholar]
- 13.Scott KA, Yamazaki Y, Yamamoto M, et al. Glucose parameters are altered in mouse offspring produced by assisted reproductive technologies and somatic cell nuclear transfer. Biol Reprod. 2010;83:220–227. 10.1095/biolreprod.109.082826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuer SK, Liu X, Donjacour A, et al. Use of a mouse in vitro fertilization model to understand the developmental origins of health and disease hypothesis. Endocrinology. 2014;155(5):1956–1969. 10.1210/en.2013-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calle A, Miranda A, Fernandez-Gonzalez R, Pericuesta E, Laguna R, Gutierrez-Adan A. Male mice produced by in vitro culture have reduced fertility and transmit organomegaly and glucose intolerance to their male offspring. Biol Reprod. 2012;87:1–9. 10.1095/biolreprod.112.100743 [DOI] [PubMed] [Google Scholar]
- 16.Chen M, Wu L, Wu F, et al. Impaired glucose metabolism in response to high fat diet in female mice conceived by in vitro fertilization (IVF) or ovarian stimulation alone. Young M, ed. PLoS One. 2014;9(11):e113155. 10.1371/journal.pone.0113155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M, Wu L, Zhao J, et al. Altered glucose metabolism in mouse and humans conceived by IVF. Diabetes. 2014;63(10):3189–3198. 10.2337/db14-0103 [DOI] [PubMed] [Google Scholar]
- 18.Behringer R, Gertsentein M, Nagy K, Nagy A. Assisted reproduction. In: Manipulating the Mouse Embryo: A Laboratory Manual, 4th ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2014:604–608. [Google Scholar]
- 19.Parra-Vargas M, Ramon-Krauel M, Lerin C, Jimenez-Chillaron JC. Size does matter: litter size strongly determines adult metabolism in rodents. Cell Metab. 2020; 32(3):334–340. 10.1016/j.cmet.2020.07.014 [DOI] [PubMed] [Google Scholar]
- 20.Feuer SK, Donjacour A, Simbulan RK, et al. Sexually dimorphic effect of in vitro fertilization (IVF) on adult mouse fat and liver metabolomes. Endocrinology. 2014;155(11):4554–4567. 10.1210/en.2014-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donjacour A, Liu X, Lin W, Simbulan R, Rinaudo PF. In vitro fertilization affects growth and glucose metabolism in a sex-specific manner in an outbred mouse model1. Biol Reprod. 2014;90(4): 1–10. 10.1095/biolreprod.113.113134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrikopoulos S, Blair AR, Deluca N, Fam BC, Proietto J. Evaluating the glucose tolerance test in mice. 2008;295(6). http://www.physiology.org/. 10.1152/ajpendo.90617.2008. Accessed July 14, 2018. [DOI] [PubMed] [Google Scholar]
- 23.de Waal E, Mak W, Calhoun S, et al. In vitro culture increases the frequency of stochastic epigenetic errors at imprinted genes in placental tissues from mouse concepti produced through assisted reproductive technologies1. Biol Reprod. 2014;90(2):1–12. 10.1095/biolreprod.113.114785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Susiarjo M, Sasson I, Mesaros C, Bartolomei MS. Bisphenol A exposure disrupts genomic imprinting in the mouse. Kelsey G, ed. PLoS Genet. 2013;9(4):e1003401. 10.1371/journal.pgen.1003401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30(15):2114–2120. 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1): 15–21. 10.1093/bioinformatics/bts635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–930. 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 28.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-s eq data with DESeq2. Genome Biol. 2014;15(12). 10.1186/S13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2(8):1896–1906. 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- 30.Searle BC, Pino LK, Egertson JD, et al. Chromatogram libraries improve peptide detection and quantification by data independent acquisition mass spectrometry. Nat Commun. 2018;9(1):5128. 10.1038/s41467-018-07454-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Searle BC, Swearingen KE, Barnes CA, et al. Generating high quality libraries for DIA MS with empirically corrected peptide predictions. Nat Commun. 2020;11(1):1548. 10.1038/s41467-020-15346-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pino LK, Just SC, MacCoss MJ, Searle BC. Acquiring and analyzing data independent acquisition proteomics experiments without spectrum libraries. Mol Cell Proteomics. 2020;19(7):1088–1103. 10.1074/mcp.P119.001913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gessulat S, Schmidt T, Zolg DP, et al. Prosit: proteome-wide prediction of peptide tandem mass spectra by deep learning. Nat Methods. 2019;16(6):509–518. 10.1038/s41592-019-0426-7 [DOI] [PubMed] [Google Scholar]
- 34.Chambers MC, Maclean B, Burke R, et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat Biotechnol. 2012;30(10):918–920. 10.1038/nbt.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24(21):2534–2536. 10.1093/bioinformatics/btn323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi M, Chang C-Y, Clough T, et al. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics. 2014;30(17):2524–2526. 10.1093/bioinformatics/btu305 [DOI] [PubMed] [Google Scholar]
- 37.Carbon S, Mungall C. Gene ontology data archive. July 2018. 10.5281/ZENODO.3873405 [DOI] [Google Scholar]
- 38.Mi H, Muruganujan A, Huang X, et al. Protocol update for large-scale genome and gene function analysis with the PANTHER classification system (v.14.0). Nat Protoc. 2019;14(3):703–721. 10.1038/s41596-019-0128-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Collier AC, Miyagi SJ, Yamauchi Y, Ward MA. Assisted reproduction technologies impair placental steroid metabolism. J Steroid Biochem Mol Biol. 2009;116(1-2):21–28. 10.1016/j.jsbmb.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bloise E, Lin W, Liu X, et al. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153(7):3457–3467. 10.1210/en.2011-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui L, An L, Tan K, et al. Dynamic proteomic profiles of in vivo- and in vitro-produced mouse postimplantation extraembryonic tissues and placentas1. Biol Reprod. 2014;91(6). 10.1095/biolreprod.114.124248 [DOI] [PubMed] [Google Scholar]
- 42.de Waal E, Vrooman LA, Fischer E, et al. The cumulative effect of assisted reproduction procedures on placental development and epigenetic perturbations in a mouse model. Hum Mol Genet. 2015;24(24):6975–6985. 10.1093/hmg/ddv400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.García-Calzón S, Perfilyev A, de Mello VD, Pihlajamäki J, Ling C. Sex differences in the methylome and transcriptome of the human liver and circulating HDL-cholesterol levels. J Clin Endocrinol Metab. 2018;103(12):4395–4408. 10.1210/jc.2018-00423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roy AK, Chatterjee B. Sexual dimorphism in the liver. Annu Rev Physiol. 1983;45(1):37–50. 10.1146/annurev.ph.45.030183.000345 [DOI] [PubMed] [Google Scholar]
- 45.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109(9):1125. 10.1172/JCI15593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eberlé D, Hegarty B, Bossard P, Ferré P, Foufelle F. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie. 2004;86(11):839–848. 10.1016/j.biochi.2004.09.018 [DOI] [PubMed] [Google Scholar]
- 47.Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17(1):1–14. 10.1093/hmg/ddm280 [DOI] [PubMed] [Google Scholar]
- 48.Mann MRW. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131(15):3727–3735. 10.1242/dev.01241 [DOI] [PubMed] [Google Scholar]
- 49.Whidden L, Martel J, Rahimi S, Chaillet JR, Chan D, Trasler JM. Compromised oocyte quality and assisted reproduction contribute to sex-specific effects on offspring outcomes and epigenetic patterning. Hum Mol Genet. 2016;25(21):ddw293. 10.1093/hmg/ddw293 [DOI] [PubMed] [Google Scholar]
- 50.Chen S, Sun F-Z, Huang X, et al. Assisted reproduction causes placental maldevelopment and dysfunction linked to reduced fetal weight in mice. Sci Rep. 2015;5. 10.1038/srep10596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fortier AL, McGraw S, Lopes FL, Niles KM, Landry M, Trasler JM. Modulation of imprinted gene expression following superovulation. Mol Cell Endocrinol. 2014;388(1-2):51–57. 10.1016/j.mce.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 52.Vrooman LA, Rhon-Calderon EA, Chao OY, et al. Assisted reproductive technologies induce temporally specific placental defects and the preeclampsia risk marker sFLT1 in mouse. Development. 2020. 10.1242/dev.186551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013;145(1):R1–R13. 10.1530/REP-11-0489 [DOI] [PubMed] [Google Scholar]
- 54.Intapad S, Ojeda NB, Dasinger JH, Alexander BT. Sex differences in the developmental origins of cardiovascular disease. Physiology. 2014;29(2):122–132. 10.1152/physiol.00045.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kobayashi S, Isotani A, Mise N, et al. Comparison of gene expression in male and female mouse blastocysts revealed imprinting of the X-linked gene, Rhox5/Pem, at preimplantation stages. Curr Biol. 2006; 16(2): 166–172. 10.1016/j.cub.2005.11.071 [DOI] [PubMed] [Google Scholar]
- 56.Hart R, Norman RJ. The longer-term health outcomes for children born as a result of IVF treatment: Part I-General health outcomes. Hum Reprod Update. 2013;19(3):232–243. 10.1093/humupd/dms062 [DOI] [PubMed] [Google Scholar]
- 57.Erion KA, Corkey BE. Hyperinsulinemia: a cause of obesity? Curr Obes Rep. 2017;6(2):178–186. 10.1007/s13679-017-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moro E, Gallina P, Pais M, et al. World health organization criteria for the classification of diabetes. Metabolism. 2003;52:616619. 10.1053/meta.2003.50102 [DOI] [PubMed] [Google Scholar]
- 59.Rexhaj E, Paoloni-Giacobino A, Rimoldi SF, et al. Mice generated by in vitro fertilization exhibit vascular dysfunction and shortened life span. Journal of Clinical Investigation. 2013;123:12. 10.1172/JCI68943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pontesilli M, Painter RC, Grooten IJ, et al. Subfertility and assisted reproduction techniques are associated with poorer cardiometabolic profiles in childhood. Reprod Biomed Online. 2015;30(3). 10.1016/j.rbmo.2014.11.006 [DOI] [PubMed] [Google Scholar]
- 61.Miles HL, Hofman PL, Peek J, et al. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92(9):3441–3445. 10.1210/jc.2006-2465 [DOI] [PubMed] [Google Scholar]
- 62.Meng Y, Zong L. Estrogen stimulates SREBP2 expression in hepatic cell lines via an estrogen response element in the SREBP2 promoter. Cell Mol Biol Lett. 2019;24(1):65. 10.1186/s11658-019-0194-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nesteruk M, Hennig EE, Mikula M, et al. Mitochondrial-related proteomic changes during obesity and fasting in mice are greater in the liver than skeletal muscles. Funct Integr Genomics. 2014;14(1):245. 10.1007/S10142-013-0342-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sang J, Qu H, Gu R, et al. Proteomics study of the effect of high-fat diet on rat liver. Br J Nutr. 2019;122(9):1062–1072. 10.1017/S0007114519001740 [DOI] [PubMed] [Google Scholar]
- 65.Wang W, Chen Y, Bai L, et al. Transcriptomic analysis of the liver of cholesterol-fed rabbits reveals altered hepatic lipid metabolism and inflammatory response. Sci Rep. 2018;8(1):6437. 10.1038/s41598-018-24813-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horton JD, Bashmakov Y, Shimomura I, Shimano H. Regulation of sterol regulatory element binding proteins in livers of fasted and refed mice. Proc Natl Acad Sci. 1998;95(11):59875992. 10.1073/pnas.95.11.5987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Haas J, Miao JI, Chanda D, et al. Hepatic insulin signaling is required for obesity-dependent expression of SREBP-1c mRNA but not for feeding-dependent expression. Cell Metab. 2012;15(6):873–884. 10.1016/J.CMET.2012.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci USA. 1999;96:12737–12742. 10.1073/pnas.96.22.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci USA. 2003;100(6):3155–3160. 10.1073/pnas.0130116100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54(5):1314–1323. 10.2337/diabetes.54.5.1314 [DOI] [PubMed] [Google Scholar]
- 71.Shimomura I, Matsuda M, Hammer RE, Bashmakov Y, Brown MS, Goldstein JL. Decreased IRS-2 and Increased SREBP-1c lead to mixed insulin resistance and sensitivity in livers of lipodystrophic and ob/ob mice. Mol Cell. 2000;6(1):77–86. 10.1016/S1097-2765(05)00010-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The DIA raw files, FASTA, Prosit library, EncyclopeDIA Chromatogram library, and quant report are available at MassIVE (MSV000086177).