Abstract
The sesquiterpene cyclase epi-isozizaene synthase (EIZS) catalyzes the cyclization of farnesyl diphosphate to form the tricyclic precursor of the antibiotic albaflavenone. The hydrophobic active site is largely defined by aromatic residues that direct a multistep reaction sequence through multiple carbocation intermediates. The previous substitution of polar residues for a key aromatic residue, F96, converts EIZS into a high-fidelity sesquisabinene synthase: the F96S, F96M, and F96Q variants respectively generate 78%, 91%, and 97% sesquisabinene A. Here, we report high-resolution X-ray crystal structures of two of these reprogrammed cyclases. The structures of the F96M EIZS–Mg2+3–risedronate and F96M EIZS–Mg2+3–inorganic pyrophosphate-benzyltriethylammonium cation complexes reveal structural changes in the F96 aromatic cluster that redirect the cyclization pathway leading from the bisabolyl carbocation intermediate in catalysis. The structure of the F96S EIZS–Mg2+3–neridronate complex reveals a partially occupied inhibitor and an enzyme active site caught in transition between open and closed states. Finally, three structures of wild-type EIZS complexed with the bisphosphonate inhibitors neridronate, pamidronate, and risedronate provide a foundation for understanding binding differences between wild-type and variant enzymes. These structures provide new insight regarding active site flexibility, particularly with regard to the potential for subtle expansion and contraction to accommodate ligands of varying sizes as well as bound water molecules. Additionally, these structures highlight the importance of conformational changes in the F96 aromatic cluster that could influence cation-π interactions with carbocation intermediates in catalysis.
Graphical Abstract

Introduction
Terpenes, also known as terpenoids, currently comprise the largest and most structurally diverse family of natural products.1, 2 The vast chemodiversity of the terpenoid family is fundamentally rooted in the activity of terpene cyclases that catalyze the first committed steps of myriad biosynthetic pathways. Terpene cyclases are responsible for the most complex chemical transformations found in nature, since nearly two-thirds of substrate carbon atoms typically undergo changes in chemical bonding during a single enzyme-catalyzed reaction.3–6 Importantly, many terpene natural products are commercially useful,7–11 e.g., as drugs,12, 13 insecticides,14–17 aromatherapies,18 and alternative biofuels,19, 20 and have more recently been explored in the craft beer21 and cannabis industries.22, 23 Thus, understanding the molecular mechanisms of terpene cyclases in the generation of commercially valuable natural products is important across a wide range of natural sciences, including chemistry, biology, ecology, and bioengineering.
The sesquiterpene cyclase epi-isozizaene synthase (EIZS) adopts the characteristic class I terpene synthase fold (Figure 1) and catalyzes the metal-dependent cyclization of farnesyl diphosphate (FPP) to form epi-isozizaene and coproduct inorganic pyrophosphate (Figure 2) as the first committed step of albaflavenone biosynthesis in Streptomyces coelicolor.24–26 The three-dimensional contour of the enzyme active site plays a vital role as a template to ensure that the flexible FPP substrate binds with a catalytically productive conformation. The active site contour is largely defined by aromatic residues, particularly the aromatic triad F95, F96, and F198. In addition to serving a template function, these residues also serve as electrostatic catalysts by stabilizing reactive carbocation intermediates through cation-π interactions. Notably, the active site contour can be remolded by mutagenesis to reprogram the cyclization cascade to generate alternative products. Such alternative products can derive from enhanced generation of minor products already produced by the wild-type enzyme, or from completely new biosynthetic pathways introduced by the remolded active site contour.
Figure 1.
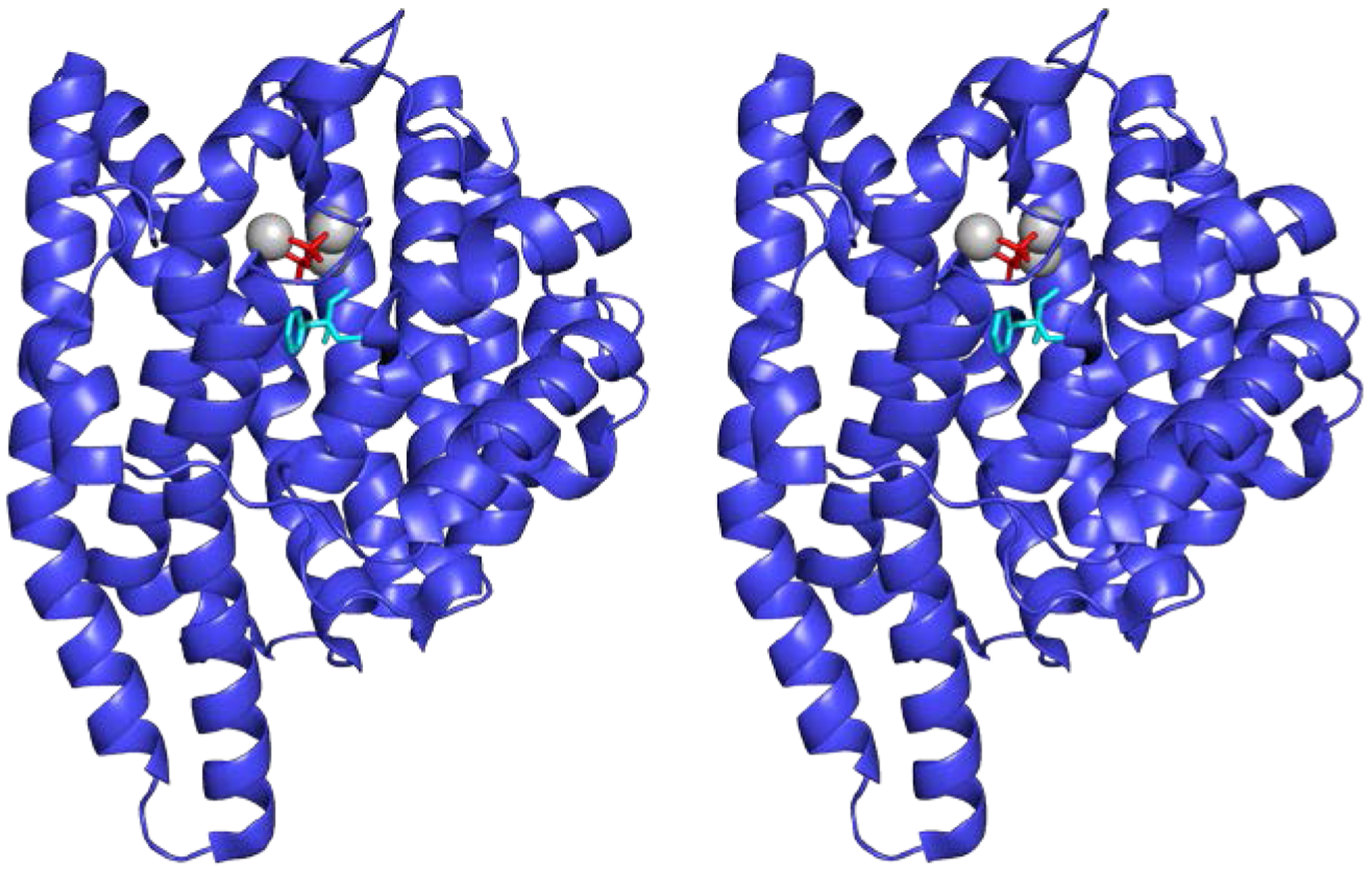
Stereoview showing the structure of epi-isozizaene synthase (EIZS) from S. coelicolor (PDB 3KB9). This sesquiterpene cyclase adopts the characteristic α-fold of a class I terpene cyclase. The binding of 3 Mg2+ ions (gray spheres) and inorganic pyrophosphate (red stick figure) stabilizes the closed active site conformation. The benzyltriethylammonium cation (BTAC, cyan stick figure) mimics a carbocation intermediate in catalysis and binds deep in the hydrophobic active site.
Figure 2:

(A) Catalytic mechanisms of wild-type EIZS and F96(S,M,Q) EIZS variants. Catalysis is initiated by ionization and isomerization of farnesyl diphosphate (OPP = diphosphate) to form (3R)-nerolidyl diphosphate, the cisoid conformer of which then undergoes re-ionization and cyclization to generate the (4R)-bisabolyl cation. A 1,2-hydride shift leads to the (7S)-homobisabolyl cation, which serves as a branch point for wild-type and F96X variants. Spirocyclization of the compressed conformation of the (7S)-homobisabolyl cation leads to formation of the (1S,4R,5R)-acorenyl cation in wild-type EIZS (blue curved arrows). The acorenyl cation then undergoes further cyclization, ring contraction, 1,2-methyl migration, and deprotonation to yield epi-isozizaene (blue curved arrows). In the F96X variants, an extended conformation of the (7S)-homobisabolyl cation intermediate yields a strained [3.1.0] bridged bicyclic cation, deprotonation of which yields sesquisabinene A (red curved arrows). (B) Molecular structures of ligands studied in EIZS complexes reported in this work.
Wild-type EIZS is a high-fidelity cyclase at lower temperature, generating 99% epi-isozizaene at 4°C; however, EIZS becomes more promiscuous at higher temperatures, generating 79% epi-isozizaene along with 5 minor products at 30°C.24, 27 To date, 40 EIZS variants with remolded active site contours have been characterized, yielding a product library five times larger than that of the wild-type cyclase; certain variants generate cyclic sesquiterpenes for which naturally occurring cyclases are not yet known.24, 27, 28 Remarkably, EIZS can be converted into nine different sesquiterpene synthases in which epi-isozizaene generation is switched off and the generation of alternative sesquiterpene products is switched on. For example, EIZS variants F96S, F96M, and F96Q generate sesquisabinene A with yields of 78%, 91%, and 97%, respectively (Figure 2).28
Here, we present six high-resolution X-ray crystal structures that illuminate aspects of the reprogrammed cyclization cascade leading to sesquisabinene A generation. The structure of the F96M EIZS–Mg2+3–PPi–benzyltriethylammonium cation (BTAC) complex reveals structural changes that likely accommodate the binding of the bisabolyl carbocation intermediate, since BTAC partially mimics this key intermediate in the cyclization cascade (molecular structures of all ligands studied are shown in Figure 2B). The structure of the F96M EIZS–Mg2+3–risedronate complex reveals a fully occupied bisphosphonate inhibitor complex. The structure of the F96S EIZS–Mg2+3–neridronate complex reveals a partially occupied inhibitor and features reflecting the transition between open and closed active site conformations. Finally, three structures of wild-type EIZS complexed with the bisphosphonate inhibitors neridronate, pamidronate, and risedronate establish points of comparison for understanding ligand binding differences between wild-type and variant enzymes. These structures reveal subtle active site flexibility as it expands or contracts to accommodate ligands of varying sizes as well as bound water molecules. Additionally, these structures highlight conformational changes in the F96 aromatic cluster that accompany active site closure and catalysis.
Materials and Methods
Reagents.
The chemicals used in buffers or crystallization conditions were purchased from Fisher Scientific, Millipore Sigma, or Hampton Research and used without further purification.
Overexpression and purification of EIZS variants.
Variant EIZS proteins were expressed and purified as previously described.24 Briefly, BL21 (DE3) Escherichia coli containing the eizs-variant overexpression plasmid were grown in lysogeny broth containing 50 μg/mL kanamycin at 37°C with shaking (250 rpm). Protein expression was induced when the OD600 reached ~0.5 by the addition of isopropyl β-d-thiogalactopyranoside (IPTG) to a final concentration of 100 μM. Purification was performed using a HiTrap® TALON® (GE Healthcare) column for affinity gel filtration chromatography followed by size-exclusion chromatography. Enzymes were concentrated to desired values and stored at −80°C until further use.
Crystallization.
Crystals of wild-type EIZS complexed with 2 mM benzyltriethyl-ammonium chloride and 2 mM pyrophosphate were grown using hanging drop vapor diffusion as previously described.24 These crystals were used for seeding and were manually crushed inside the drop. Mother liquor (10 μL) was added to the drop and the drop was aspirated; this procedure was repeated 10 times to remove all microcrystals. The collected seed solution was vortexed with a PTFE seed bead (Hampton) and further diluted 10-fold to make a seed stock for subsequent crystallization experiments. Crystallization experiments were performed by the sitting drop vapor diffusion method at pH 6.0–7.5. Briefly, 100 nL of protein solution was added to 100 nL of precipitant solution and 25 nL of a 1:100 dilution of crystallization seed stock. The 225 nL drops were equilibrated against 100 μL of precipitant solution. Crystals reached maximum dimensions after 5 days, were cryoprotected with 20% (v/v) glycerol, and flash frozen in liquid nitrogen. Protein and precipitant solutions are listed in Table S1.
Crystallographic data collection and structure determination.
Diffraction data were collected remotely on beamline 17-ID-1 AMX at the National Synchrotron Light Source II (NSLS-II, Brookhaven, NY) at Brookhaven National Laboratory, and on beamline 24-ID-C at the Northeastern Collaborative Access Team facility (NE-CAT) at the Advanced Photon Source at Argonne National Laboratory (Lemont, IL). Data sets were indexed, integrated, and scaled using iMosflm29 and processed using AIMLESS.30 A molecular replacement solution was determined with PHASER31 using wild-type EIZS (PDB 3KB9)24 as a search probe for wild-type and F96M structures, and using the structure of unliganded F96S EIZS (PDB 6AXO)28 as a search probe for the structure of the F96S EIZS–Mg2+3–neridronate complex. For each structure determination, manual model building was achieved with COOT32 and refinement was performed with PHENIX.REFINE.33 Water molecules were iteratively placed during refinement and edited using the 2mFo-DFc electron density map contoured at 1.5σ after each round of refinement. The electron density map of each structure revealed 3 Mg2+ ions and the specified ligand in the active site, atomic coordinates of which were included during the later stages of refinement. Glycerol molecules and sulfate ions were observed and modeled in the electron density map of each structure. N- and C-terminal residues are missing in each structure, presumably due to molecular disorder. Additionally, some residues lacked complete electron density for side chains, mainly on the protein surface where they are likely disordered, and were not modelled in full. In the F96S EIZS-Mg2+3-neridronate complex, electron density is sparse for residues in helices B, C, and K, along with the C-terminal loop, so these structural elements were not modeled. Disordered polypeptide segments and amino acid side chains are listed in Table S2. Data collection and refinement statistics for each structure are recorded in Table 1.
Table 1:
Crystallographic data collection and refinement statistics for EIZS variants.
| WT-EIZS 3 Mg2+, Neridronate | WT-EIZS 3 Mg2+, Pamidronate | WT-EIZS 3 Mg2+, Risedronate | F96M-EIZS 3 Mg2+, PPi, BTAC | F96M-EIZS 3 Mg2+, Risedronate | F96S-EIZS 3 Mg2+, Neridronate | |
|---|---|---|---|---|---|---|
| Unit Cell | ||||||
| Space Group | P21 | P21 | P21 | P21 | P21 | P21 21 2 |
| a, b, c (Å) | 51.5, 46.8, 74.8 | 52.5, 46.9, 75.5 | 53.0, 47.0, 75.5 | 51.6, 47.0, 75.1 | 52.8, 47.1, 75.4 | 46.7, 76.2 108.3 |
| α, β, γ (°) | 90.0, 98.3, 90.0 | 90.0, 96.7, 90.0 | 90.0, 96.1, 90.0 | 90.0, 97.8, 90.0 | 90.0, 96.3, 90.0 | 90.0, 90.0, 90.0 |
| Data Collection | ||||||
| Laboratory | NSLS-II | NE-CAT | NE-CAT | NSLS-II | NE-CAT | NE-CAT |
| Beamline | 17-ID-1 AMX | 24-ID-C | 24-ID-C | 17-ID-1 AMX | 24-ID-C | 24-ID-C |
| Detector | Dectris Eiger 9M | Dectris Pilatus 6M-F | Dectris Pilatus 6M-F | Dectris Eiger 9M | Dectris Pilatus 6M-F | Dectris Pilatus 6M-F |
| Resolution (Å) | 1.4 | 1.9 | 2.2 | 1.3 | 1.5 | 1.6 |
| Total/unique no. of reflections | 225,592/69,464 | 88,651/28,497 | 50,959/18,422 | 284,360/84,334 | 192,231/58,620 | 326,916/51,746 |
| Rmergea,b | 0.098 (0.539) | 0.120 (0.697) | 0.195 (0.534) | 0.092 (0.529) | 0.100 (0.852) | 0.127 (1.097) |
| Rpima,c | 0.063 (0.347) | 0.081 (0.480) | 0.138 (0.393) | 0.058 (0.328) | 0.065 (541) | 0.055 (0.461) |
| CC1/2a,d | 0.994 (0.559) | 0.988 (0.578) | 0.919 (0.606) | 0.995 (0.571) | 0.982 (0.694) | 0.997 (0.692) |
| I/σ(I)a | 6.3 (2.2) | 8.5 (4.2) | 4.3 (3.2) | 6.3 (2.2) | 6.1 (2.1) | 8.9 (2.0) |
| Redundancya | 3.2 (3.3) | 3.1 (3.1) | 2.8 (2.7) | 3.4 (3.5) | 3.3 (3.3) | 6.3 (6.5) |
| Completeness (%) | 99.8 (100.0) | 98.3 (98.4) | 97.0 (96.5) | 96.3 (93.6) | 99.1 (99.7) | 99.8 (100.0) |
| Refinement | ||||||
| Reflections used in refinement/test set | 69,429/6,902 | 28,445/2,797 | 18,373/1,840 | 84,302/8,219 | 58,547/5,853 | 51,644/5,110 |
| Rworka,e | 0.194 (0.276) | 0.185 (0.251) | 0.218 (0.259) | 0.177 (0.244) | 0.215 (0.314) | 0.189 (0.282) |
| Rfreea,e | 0.239 (0.322) | 0.229 (0.313) | 0.281 (0.339) | 0.197 (0.268) | 0.246 (0.343) | 0.210 (0.298) |
| Number of nonhydrogen atoms | ||||||
| Solvent | 228 | 183 | 90 | 199 | 229 | 249 |
| Average B factors (Å2) | ||||||
| Solvent | 27 | 28 | 19 | 23 | 29 | 30 |
| RMS deviation from ideal geometry | ||||||
| Angles (°) | 1.2 | 1.1 | 1.2 | 1.1 | 1.1 | 1.1 |
| Ramachandran plot (%) | ||||||
| Outliers | 0 | 0 | 0 | 0 | 0 | 0.65 |
| PDB Accession Code | ||||||
| PDB ID: | 7KJF | 7KJ8 | 7KJ9 | 7KJG | 7KJD | 7KJE |
Values in parentheses refer to data in the highest shell.
, where is the average intensity calculated for reflection hkl from replicate measurements.
where is the average intensity calculated for reflection hkl from replicate measurements and N is the number of reflections.
Pearson correlation coefficient between random half-datasets.
for reflections contained in the working set. |Fo| and |Fc| are the observed and calculated structure factor amplitudes, respectively. Rfree is calculated using the same expression for reflections contained in the test set held aside during refinement.
Structure superpositions and active site cavity determination.
Superpositions of EIZS variant structures were calculated using the CCE alignment algorithm in PyMOL (PyMOL Molecular Graphics System, version 2.0, Schrödinger, LLC) and the enclosed active site contour was generated using GetCleft from the NRGsuite plugin.34 Within the GetCleft software, the volume of the active site cavity was calculated. The cavity was defined by space below the trinuclear Mg2+ cluster, which included the pyrophosphate binding space.
Modeling Enzyme-Intermediate Complexes.
Molecular modelling was performed using the closed conformations of wild-type EIZS (PDB 3KB9) and F96M EIZS (PDB 7KJG) each bound with three Mg2+ ions, PPi, and BTAC. In each structure, the active-site contour was generated around the BTAC cation in PyMOL using GetCleft from the NRGsuite plugin.34 Atomic coordinates of the (7S)-homobisabolyl cation, the (1S,4R,5R)-acorenyl cation, and the sesquisabinene cation were created by converting their SMILES string generated in ChemDraw Professional 17.1 using eLBOW.35 Coot32 was then used to adjust the molecular geometries of the cation intermediates. After removal of the BTAC cation, the intermediates were modeled into the active site. Docked carbocation intermediates were centered on the location of the BTAC cationic charge observed in the original structure. Further minor adjustments were performed using PyMOL to manually optimize the fit of the ligand in the active site contour.
Results and Discussion
F96M EIZS–Mg2+3–PPi-BTAC complex.
F96M EIZS generates sesquisabinene A with 91% fidelity in addition to three minor products with 39% specific activity relative to wild-type EIZS.28 The catalytic mechanisms of wild-type EIZS and F96M EIZS each proceed through the homobisabolyl carbocation intermediate, after which the cyclization pathways diverge.
The 1.3 Å resolution crystal structure of the F96M EIZS–Mg2+3–PPi–BTAC complex reveals that the PPi anion coordinates to Mg2+ ions (Figure 3A), in similar fashion to that previously observed in other EIZS complexes.24, 27, 28 Additionally consistent with previous observations, the PPi anion accepts hydrogen bonds from R194, K247, R338, and Y339. Since the diphosphate group of FPP likely makes a similar array of interactions in the precatalytic Michaelis complex, it is likely that three metal ions and three basic residues are needed to activate the substrate diphosphate group.24 Moreover, these interactions are usually invariant when the hydrophobic active site contour is remolded by mutagenesis, as exemplified by comparison with the corresponding complex with wild-type EIZS (Figure 3B). Thus, the three-dimensional active site contour can be remolded without perturbing catalytic residues required for substrate activation and initiation of the cyclization cascade.
Figure 3.

(A) Stereoview of the F96M EIZS–Mg2+3–PPi–BTAC complex, showing Polder omit maps of the BTAC cation (green mesh, 4σ), inorganic pyrophosphate (orange mesh, 4σ), Mg2+ ions (blue mesh, 4σ), and M96 (ruby mesh, 4σ). Atoms are color coded as follows: C = violet (protein), or green (BTAC); N = blue; O = red; P = orange; Mg2+ ions = magenta spheres. (B). Stereoview showing the superposition of the F96M EIZS–Mg2+3–PPi–BTAC complex (color-coded as in (A)) with the wild-type EIZS–Mg2+3–PPi–BTAC complex (blue; BTAC, cyan; PDB 3KB9). Minimal structural differences are observed for active site aromatic residues and BTAC.
Superposition of the F96M and wild-type EIZS–Mg2+3–PPi–BTAC complexes reveals no major differences in overall protein conformations, as reflected by the root-mean-square deviation (RMSD) of 0.25 Å for 336 Cα atoms between the two structures. The side chain of M96 adopts a conformation that closely aligns with part of the F96 side chain in the wild-type enzyme (Figure 3B). No significant differences are observed in the binding mode of BTAC between the wild-type or variant enzyme active sites. Since the generation of both epi-isozizaene and sesquisabinene A proceeds through a common bisabolyl carbocation intermediate, and since BTAC partially mimics the bisabolyl carbocation intermediate, it is reasonable to expect that the binding mode of BTAC would remain essentially unperturbed between wild-type EIZS and F96M EIZS. The quaternary ammonium cation of BTAC makes cation-π interactions with F95, F96, and F198 in the active site of wild-type EIZS;24 cation-π interactions with 95 and F198 are maintained in the active site of F96M EIZS.
The active site volume in the F96M EIZS-Mg2+3-PPi-BTAC complex increases to 414 Å3, compared with 392 Å3 measured for the corresponding complex with the wild-type enzyme. The van der Waals volumes of methionine and phenylalanine are 175 Å3 and 202 Å3, respectively,36 so the 22 Å3 increase in active site volume is approximately consistent with the 27 Å3 increase expected for the substitution of a smaller methionine side chain for the larger phenylalanine side chain. Comparison of the enclosed active site contours in wild-type EIZS and F96M EIZS and modeling of the homobisabolyl cation in these contours reveals that the additional active site volume conferred by the F96M substitution loosens the conformational constraints on the homobisabolyl cation such that the pendant isoprenoid group cannot be held adjacent to the carbocation, thereby disabling acorenyl cation formation and enabling formation of the strained [3.1.0] bicyclic sesquisabinene cation (Figure 4).
Figure 4.
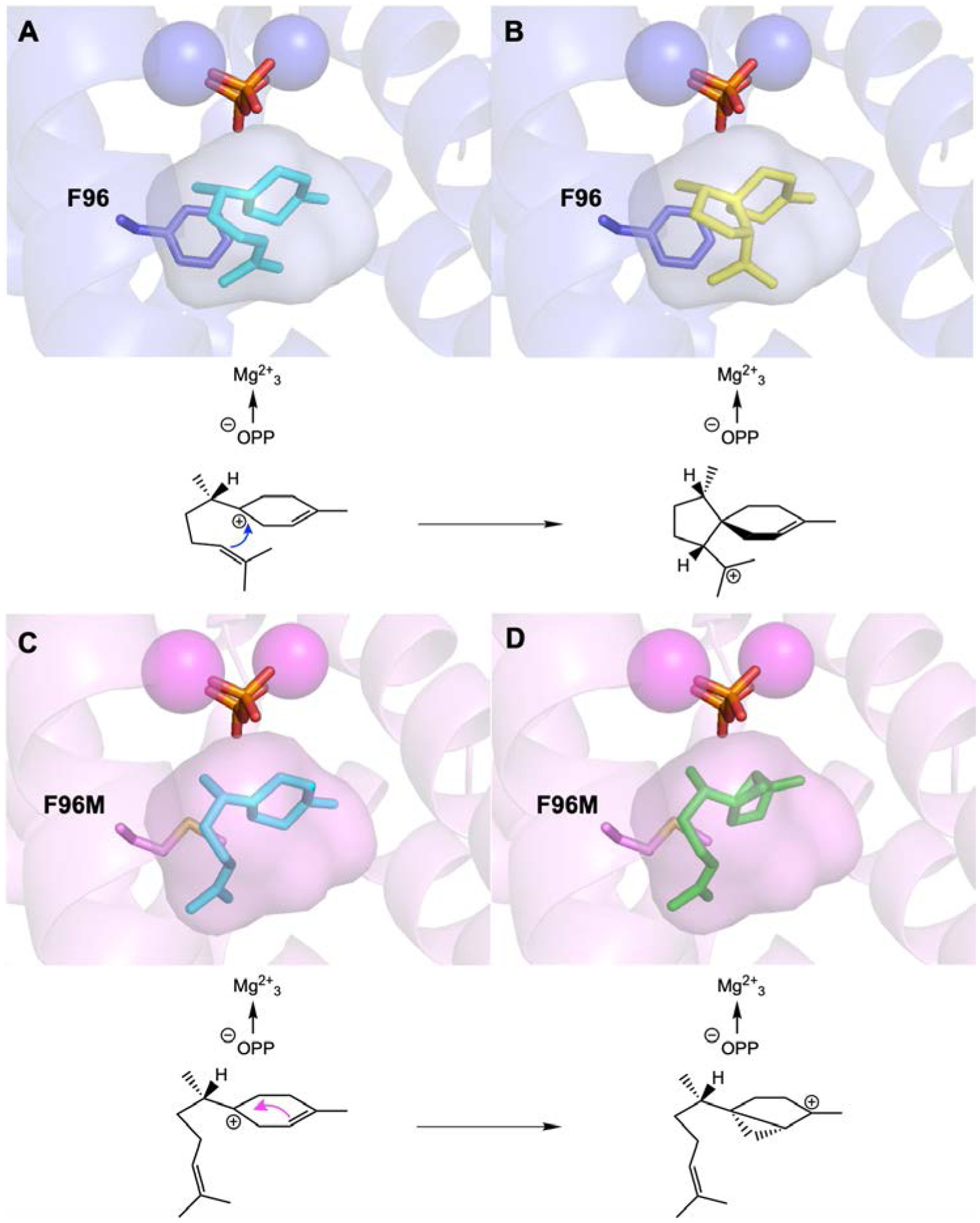
Models of wild-type EIZS and F96M EIZS complexed with carbocation intermediates. Wild-type EIZS (PDB 3KB9) with the (7S)-homobisabolyl cation (cyan) (A) and the (1S,4R,5R)-acorenyl cation (yellow) (B) docked in the active site. This reaction sequence leads to epi-isozizaene formation. F96M EIZS (PDB 7KJG) with the (7S)-homobisabolyl cation (cyan) (C) and the (1S,5S,6S)-sesquisabinyl cation (green) (D) docked in the active site. This reaction sequence leads to sesquisabinene A formation. The active site contour of F96M is slightly larger and is unable to hold the isoprenoid tail of the homobisabolyl cation sufficiently close for acorenyl cation formation.
F96M and wild-type EIZS–Mg2+3–bisphosphonate complexes.
The crystal structure of the F96M EIZS–Mg2+3–risedronate complex (Figure 5A) is generally identical to that of the wild-type EIZS–Mg2+3–risedronate complex (RMSD = 0.17 Å for 336 Cα atoms between the two structures) (Figure 5B). The bisphosphonate moiety engages in metal coordination and hydrogen bond interactions that are nearly identical to those observed for the PPi anion in Figure 3. Thus, the stable bisphosphonate moiety of the inhibitor is an effective surrogate for the phosphoanhydride coproduct of the cyclization reaction. The hydroxyl group of the inhibitor makes a water-mediated hydrogen bond interaction with Y339.
Figure 5.

(A) Stereoview of the F96M EIZS–Mg2+3–risedronate complex, showing Polder omit maps of risedronate (green mesh, 4σ), Mg2+ ions (blue mesh, 4σ), and M96 (ruby mesh, 4σ). Atoms are color coded as follows: C = violet (protein), or green (risedronate); N = blue; O = red; P = orange; Mg2+ ions = magenta spheres. (B) Stereoview showing the superposition of the F96M EIZS–Mg2+3–risedronate complex (color-coded as in (A)) with the wild-type EIZS–Mg2+3–risedronate complex (blue; risedronate, cyan). Minimal structural differences are observed.
The pyridine ring of the inhibitor makes water-mediated hydrogen bonds that are slightly different between the wild-type and F96M complexes (active site water molecules are illustrated for all complexes in Figure 6). In the wild-type EIZS–Mg2+3–risedronate complex, a single water molecule hydrogen bonds to the pyridine nitrogen. In contrast, the additional active site volume resulting from the F96M substitution allows for the binding of an additional water molecule, which forms a hydrogen bond with the water molecule that is hydrogen bonded to the pyridine nitrogen atom of risedronate (Figure 6B,E). The new water molecule also forms a hydrogen bond with S92 at the base of the active site. The side chain of S92 undergoes a conformational change such that it is oriented toward the vacant space resulting from the F96M substitution. In both structures, the pyridine ring binds in an aromatic crevice adjacent to M96 that is defined by F95, F198, and W203. Since the pyridine ring binds in the region where the positively charged quaternary ammonium group of BTAC binds, it could be stabilized as the pyridinium cation (pKa ≈ 5) by cation-π interactions. The side chain of M96 adopts a generally similar conformation to that observed in the BTAC complex.
Figure 6:
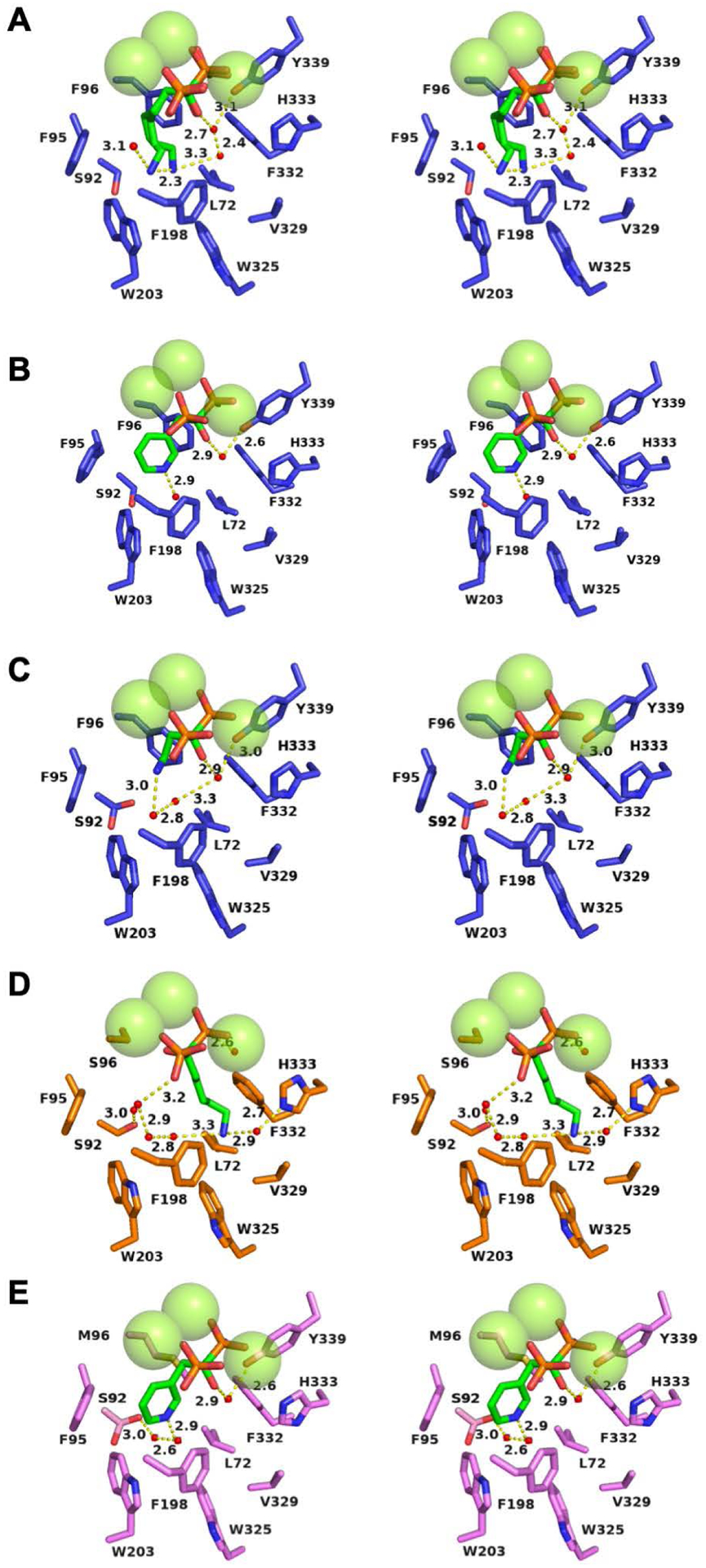
Water molecules and solvent hydrogen bond networks in EIZS variants complexed with 3 Mg2+ ions and bisphosphonate inhibitors. Atoms are color-coded as follows: C = blue (wild-type EIZS), orange (F96S EIZS), violet (F96M EIZS), or green (inhibitors); N = blue; O = red; P = orange; water molecules = small red spheres; Mg2+ ions = large green spheres. Hydrogen bonds are indicated by yellow dashed lines and distances are reported in Å. (A) Wild-type EIZS–neridronate complex; (B) wild-type EIZS–risedronate complex; (C) wild-type EIZS–pamidronate complex (note that S92 adopts two alternative conformations); (D) F96S EIZS–neridronate complex; (E) F96M EIZS–risedronate complex (note that S92 adopts two alternative conformations).
The structure of the wild-type EIZS-Mg2+3-pamidronate complex was determined to ascertain how a bisphosphonate inhibitor with a smaller substituent would be accommodated in the active site (Figure S1A). No major conformational changes in the protein structure are observed, and the RMSD is 0.18 Å for 336 Cα atoms between the wild-type EIZS complexes with risedronate and pamidronate (Figure S1B). However, interesting differences in active site volumes are observed. The substitution of the smaller methionine side chain for F96 results in a 48 Å3 increase in active site volume, from 341 Å3 to 389 Å3 in the wild-type and F96M EIZS–risedronate complexes, respectively. Additionally, a slight reduction in active site volume is observed for wild-type EIZS complexes with risedronate and pamidronate, from 341 Å3 to 333 Å3, respectively. This reflects some degree of flexibility in the active site, such that it can slightly expand or contract to better accommodate inhibitors with larger or smaller substituents. Three ordered water molecules bind in the void space of the active site in this complex (Figure 6C).
F96S and wild-type EIZS–Mg2+3–neridronate complexes.
F96S EIZS generates sesquisabinene A with 78% fidelity in addition to three minor products with 22% specific activity relative to wild-type EIZS.28 Previously, F96S EIZS was crystallized in orthorhombic space group P212121 in the presence of Mg2+, PPi, and BTAC [0.5 μL solution of 5 mg/mL protein, 300 mM NaCl, 20 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 2 mM sodium pyrophosphate, 2 mM BTAC, 2 mM TCEP, and 10% glycerol added to a sitting drop of 0.6 μL solution of 1.0 M ammonium citrate tribasic and 0.1 bis-Tris propane (pH 7.0)].28 Despite crystallization in the presence of ligands, the enzyme crystallized in the unliganded state with an open active site conformation.
Here, F96S EIZS crystallizes in orthorhombic space group P21212 in the presence of 10 mM MgCl2 and 2 mM neridronate (Table S1) to yield the 1.6 Å resolution structure of the complex with 3 Mg2+ ions and neridronate (Figure 7A). Notably, the structure of this complex reveals significant disorder for residues A57–Y69 and G337–N355 in helices B and K, respectively, located adjacent to the active site (Table S2). Residues A57–L67 and G337–N355 in these helices similarly exhibit disorder in unliganded F96S EIZS.28 Additionally, part of helix H, helix H-α−1, and the loop connecting the two, which are responsible for capping the active site, are found in both open and closed conformations (Figure S2). Thus, we conclude that the active site is caught in transition between open and closed states.
Figure 7.
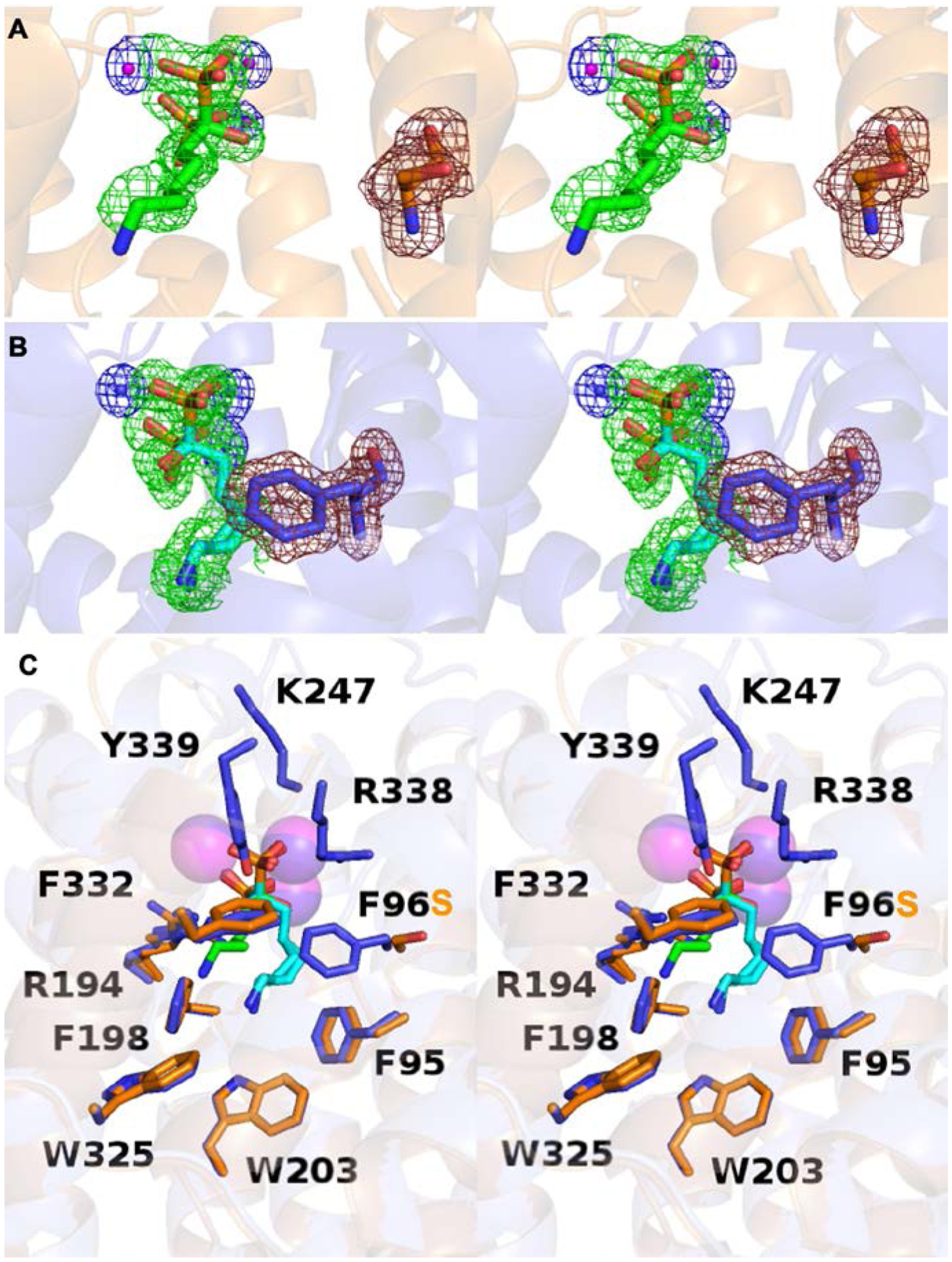
(A) Stereoview of the F96S EIZS–Mg2+3–neridronate complex, showing Polder omit maps of neridronate (green mesh, 3σ), Mg2+ ions (blue mesh, 4σ), and S96 (ruby mesh, 4σ). Atoms are color coded as follows: C = orange (protein) or green (risedronate); N = blue; O = red; P = orange; Mg2+ ions = magenta spheres. (B) Stereoview of the wild-type EIZS–Mg2+3–neridronate complex, showing Polder omit maps of neridronate (green mesh, 3σ), Mg2+ ions (blue mesh, 4σ), and F96 (ruby mesh, 4σ). Atoms are color coded as follows: C = blue (protein) or cyan (risedronate); N = blue; O = red; P = orange; Mg2+ ions = blue spheres. (C) Stereoview showing the superposition of the F96S and wild-type EIZS–Mg2+3–neridronate complexes (blue and orange, respectively). Minimal structural differences are observed.
In comparison, the wild-type EIZS–Mg2+3–neridronate complex crystallizes in monoclinic space group P21 under similar conditions to yield the 1.4 Å resolution structure of the fully closed active site conformation complexed with 3 Mg2+ ions and neridronate (Figure 7B). While some disordered residues are observed in this structure (Table S2), helices B and K are fully ordered, and helices H, H-α−1, and the connecting loop adopt a fully closed conformation.
The measured active site volume in the F96S EIZS-Mg2+3-neridronate complex increases to 940 Å3, compared with 395 Å3 measured for the corresponding complex with the wild-type enzyme. However, this inordinately large change in active site volume is artifactual and arises from the missing residues of the B and K helices which ordinarily comprise one side of the active site.
Superposition of the F96S and wild-type EIZS–Mg2+3–neridronate complexes reveals similar overall protein conformations (RMSD = 0.70 Å for 304 Cα atoms between the two structures) (Figure 7C). However, a substantial difference is observed for the binding orientation of neridronate, which is rotated 180° such that the alcohol and aminoalkyl substituents of the phosphonate are switched. This is the only EIZS variant in which the bisphosphonate inhibitor binds with this alternative orientation. The side chains of R338 and Y339 would be expected to form hydrogen bonds with the phosphonate but are disordered in the complex with F96S EIZS, but the lack of these hydrogen bond interactions is not the likely cause of the alternative inhibitor binding mode. Instead, the F96S substitution weakens cation-π interactions between the amino group of neridronate and the remaining residues of the aromatic triad, F95 and F198. Consequently, the alternative orientation of the amino group of neridronate allows for an alternative constellation of cation-π interactions with aromatic residues F332, W325, H333, W203, and F198. Three ordered hydrogen bonded water molecules fill the remaining volume of the enclosed active site cavity in both wild-type and F96S EIZS complexes with neridronate (Figure 6A,D).
Discussion
Myriad cyclization products can be generated from FPP, the universal substrate of sesquiterpene cyclases. The “instructions” for generating a specific product are encoded in the three-dimensional contour of the cyclase active site, which serves as the template for catalysis. The cyclization cascade can be reprogrammed by remolding the active site contour to direct alternative reaction pathways. This can be done without significant compromise of catalytic activity as long as residues important for substrate activation are preserved, i.e., residues that coordinate to the catalytically obligatory metal ions. The structure-based redesign of terpene cyclase active sites is an important goal as “designer cyclases” are considered for use in synthetic biology approaches for generating high-value terpenoids. Progress toward this goal is achieved by understanding structure-function relationships for cyclase variants that form alternative cyclization products.
EIZS serves as a paradigm system for studying cyclase structure-function relationships due to the ease of preparing crystals of wild-type and variant enzymes that diffract to high resolution. The active site of EIZS undergoes a transition from an open to a closed conformation to form a three-dimensional contour that serves as the template for catalysis.24, 27, 28 Thus, X-ray crystal structures of the closed conformation are preferred for studying structure-function relationships. The current study highlights two different aspects of structure-function relationships in cyclase variants: the influence of aromatic clusters on ligand binding in the active site, and the binding of water molecules trapped in the closed active site conformation.
Aromatic clusters.
The conformational change of F96 between open and closed states was noted upon structural comparison of the wild-type EIZS–Mg2+3–PPi–BTAC complex and unliganded EIZS.24, 27 Interestingly, F96 is part of an extended cluster of aromatic residues that also includes Y63, Y69, F95, F198, W203, W325, and F332, most of which contribute to the surface of the active site contour or abut residues that do. Structural changes in the aromatic cluster accompany active site closure in the wild-type enzyme. In particular, F96 is located at the edge of this cluster and interacts most closely with Y63 and Y69 in the open active site conformation, and with Y69 and F332 in the closed conformation (Figures 8A–C). Interactions among these and other aromatic residues in the cluster are perturbed by the F96S substitution (Figures 8D, 8E), but not as much by the F96M substitution (Figures 8F). The F96 substitutions trigger structural changes that remold the active site contour and reprogram the cyclization cascade.
Figure 8.
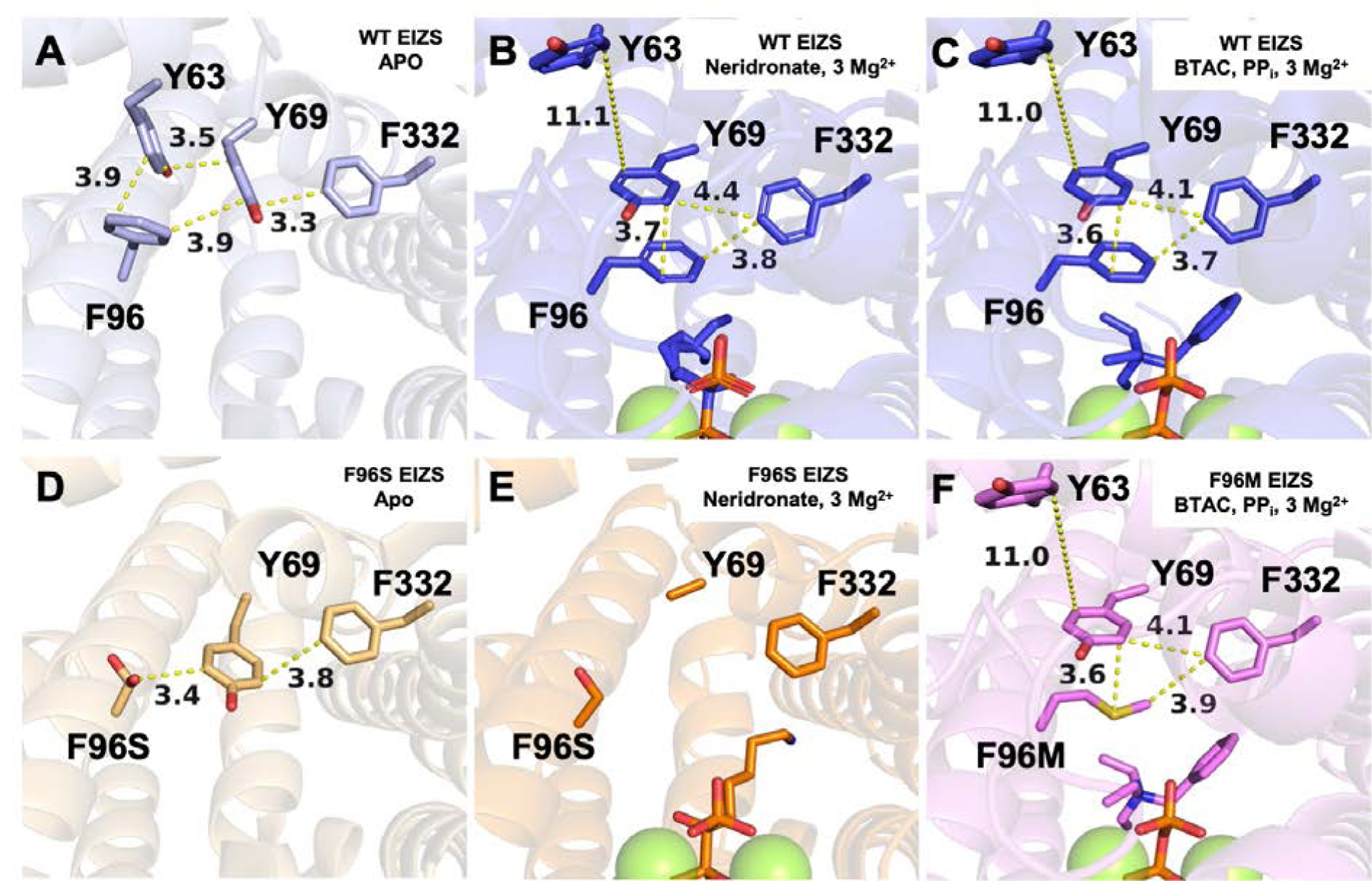
Crystal structures of (A) unliganded wild-type EIZS (PDB 4LTV), (B) wild-type EIZS–Mg2+3–neridronate complex (PDB 7KJF), (C) wild-type EIZS–Mg2+3–PPi–BTAC complex (PDB 3KB9), along with (D) unliganded F96S EIZS (PDB 6AXO), (E) F96S EIZS–Mg2+3–neridronate complex (PDB 7KJE), and (F) F96M EIZS–Mg2+3–PPi–BTAC complex (PDB 7KJG). These structures highlight an aromatic π-stacking network between Y63 of helix B, Y69 of helix C, F96 of helix D, and F332 of helix J. The F96M substitution maintains aromatic π-stacking interactions in the closed active site conformation (F). However, the F96S substitution disrupts the π-stacking network in the open, unliganded conformation (D) and in the liganded conformation (E).
In unliganded wild-type EIZS, the aromatic side chain of Y63 makes an edge-to-face interaction with F96 and an offset π-stacking interaction with Y69 (Figure 8A). F96 also forms an edge-to-face interaction with Y69, as does F332 on the opposing side. Upon ligand binding, interactions in this aromatic cluster change significantly (Figure 8B,C). The side chain of F96 moves to establish one wall of the active site, and Y69 rotates approximately 90° to form an offset π-stacking interaction with F96. The side chain of F332 packs against Y69 and F96. Finally, Y63 moves away from Y69, shifting by 11 Å away from the aromatic cluster.
The aromatic side chain of F96 appears to be a molecular toggle for the conformational change leading to active site closure. Interestingly, the F96M substitution preserves the interaction network through the formation of methionine S-π aromatic interactions (Figure 8F); such interactions are commonly observed in protein structures.37–39 However, the F96S substitution cannot sustain this interaction network in the open and closed conformations (Figure 8D,E). The inability of the S96 side chain to form π-stacking or S-π interactions may account for the observed disorder of helix B and Y69 in helix C (Figure 8E). The inability of S96 to enable proper active site closure may be responsible for compromised activity in F96S EIZS compared with F96M EIZS. The resultant volume increase as well as loosening of the active site contour in F96S EIZS disables acorenyl cation formation and enables transannular C–C bond formation for sesquisabinene generation.28 In F96M EIZS, increased active site volume alone similarly directs the cyclization pathway toward sesquisabinene (Figure 4).28
The aromatic triad consisting of F95, F96, and F198 is important for the binding of the pendant amino groups of phosphonate inhibitors through cation-π interactions. The short alkylammonium substituent of pamidronate is fixed above this aromatic triad and donates a hydrogen bond to a water molecule that is involved in a hydrogen bond network with other active site water molecules (Figure 6C). In contrast, the longer alkylammonium substituent of neridronate is found in two conformations that diverge at the β-carbon; the ammonium cation of each interacts with two aromatic residues in the triad, with one situated between F95 and F198 and the other between F96 and F198. The ammonium cation interacting with F95 and F198 also donates a hydrogen bond to a water molecule. The ammonium cation of the alternative neridronate conformation donates a hydrogen bond to an additional water molecule. Finally, the pyridine ring substituent of risedronate extends into the middle of the aromatic triad, in similar positioning to that observed for the ammonium cation of BTAC.
Trapped water molecules.
Interestingly, 2–5 ordered water molecules are observed in the active site of each EIZS–bisphosphonate inhibitor complex (Figure 6). Indeed, ordered water molecules have been observed in the F95M, F95Q, F198A, and W203Y EIZS complexes with BTAC.24, 27, 28 If the binding of water accompanies substrate binding in the closed active site conformation of EIZS or its variants, it is possible that carbocation intermediates could be quenched to generate sesquiterpene alcohol products.24, 27, 28, 40 If active site water molecules do not react with carbocation intermediates, they can serve as part of the active site contour and contribute to its template function.40
A trend in ligand size and active site volume is observed for the different bisphosphonate inhibitors bound in the wild-type EIZS active site (Figure 9). The size of the active site cavity is smallest when pamidronate (the smallest bisphosphonate inhibitor studied) is bound, and largest in size when complexed with neridronate (the largest bisphosphonate inhibitor studied). Ordered water molecules fill active site void space in each enzyme-inhibitor complex, and additional water molecules can bind if active site volume is increased through mutagenesis (Figure 6). For example, the F96M EIZS–Mg2+3–risedronate complex contains an extra active site water molecule in comparison with the wild-type EIZS–Mg2+3–risedronate complex.
Figure 9:
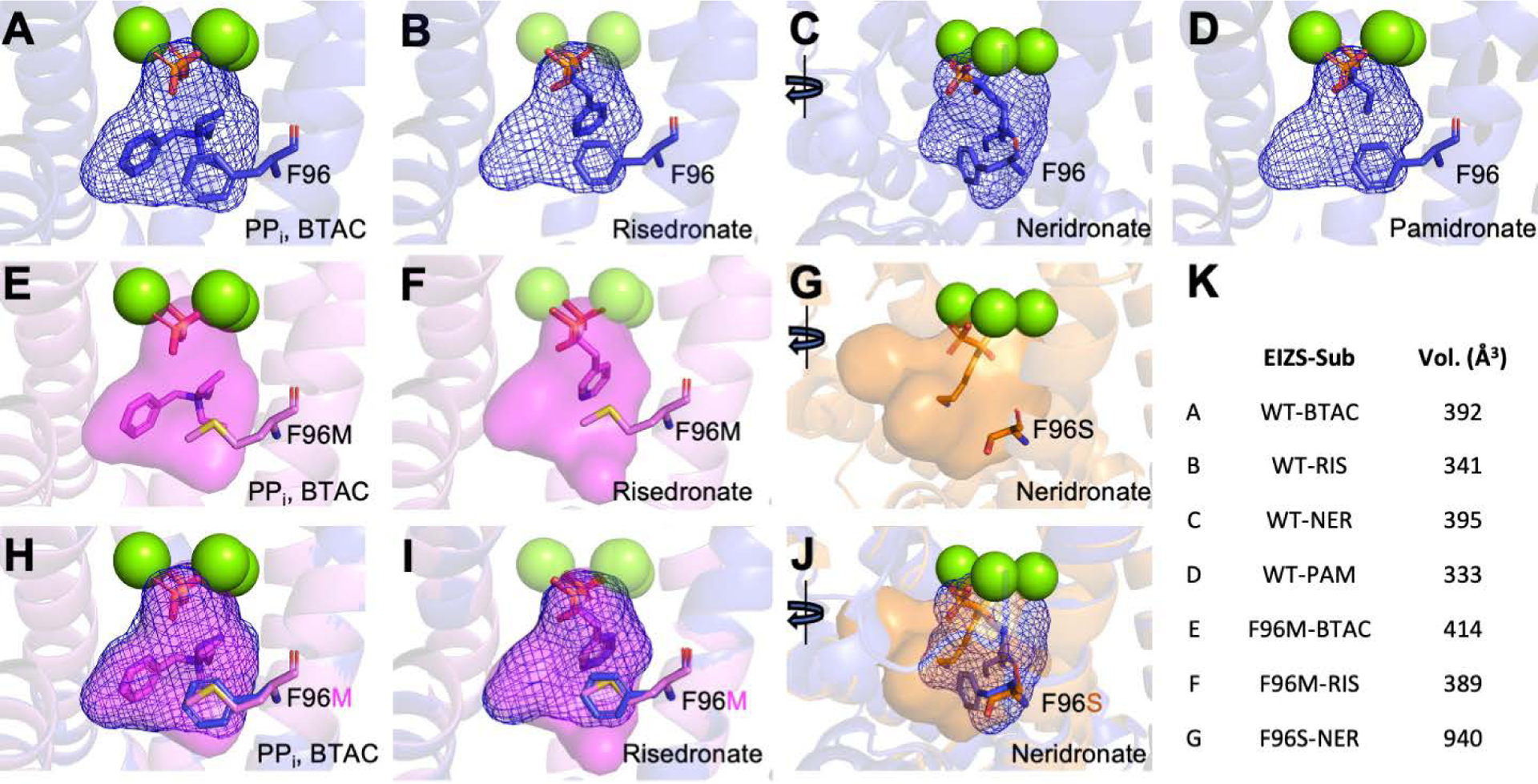
The active site contour of each EIZS variant calculated by GetCleft in the NRGSuite plugin in Pymol.34 The active site cavities were calculated under the three bound Mg2+ ions. Active site contours of wild-type EIZS bound with (A) pyrophosphate and BTAC, (B) risedronate, (C) neridronate, and (D) pamidronate, are highlighted by blue mesh. The contours of F96M EIZS are displayed as a transparent violet surface bound with (E) pyrophosphate and BTAC and (F) risedronate, whereas the partially open active site of F96S EIZS cavity is displayed as a transparent orange surface bound with (G) neridronate. Overlay of the mutant cavities with the respective wild type contours are depicted in (H–J). The calculated volume (Å3) of each active site cavity is displayed in (K).
A more dramatic change is observed in the F96S EIZS–Mg2+3–neridronate complex. Mutation and disruption of the F95-F96-F198 aromatic triad by the F96S substitution gives rise to an alternative binding mode for the bisphosphonate group of neridronate, which is rotated 180° to allow the positively charged ammonium group to bind in an alternative aromatic cluster on the opposite side of the active site. Here, too, the resulting void space is occupied by ordered water molecules (Figures 6A,D).
Conclusions
Terpene cyclases catalyze the most complex carbon-carbon bond-forming reactions in nature, and the multistep cyclization cascades catalyzed by these enzymes are very sensitive to conformational control as enforced by the three-dimensional active site contour. Structure-function relationships outlined here highlight the catalytic function of the F95-F96-F198 aromatic triad, as well as a more extended aromatic cluster encompassing these residues and others in the surrounding protein structure. Aromatic residues undergo conformational changes upon the binding of a Mg2+3–PPi or Mg2+3–phosphonate cluster, and these changes likely accompany the binding of the FPP diphosphate group as well. Additionally, these aromatic residues are also capable of stabilizing bound cations through cation-π interactions, whether the cations are carbocation intermediates in catalysis or positively-charged ammonium ions. The π-stacking network involving F96 (Figure 8) is responsible for important intermolecular and intramolecular interactions in both the unliganded and liganded states of the enzyme. Many of the aromatic residues identified in the π-stacking network are conserved in other class I terpene cyclases.
The crystal structures reported herein additionally highlight the capacity for engineering new water binding sites in active site mutants of EIZS. Active site water molecules can contribute to the active site contour during catalysis, but they can also participate in catalysis by quenching carbocation intermediates to generate sesquiterpene alcohol products. It is intriguing that such hydroxylated products were not identified in product array analyses of F96 variants in which the active site was sufficiently enlarged to contain additional water molecules in addition to the bound substrate. A water molecule must be in the right location and orientation to react with the empty 2p orbital of a fleeting carbocation intermediate; otherwise, the water molecule will simply be an inert bystander in the chemistry of catalysis. There remains much to learn with regard to water management strategies in the active site of EIZS, and our future work will focus on exploring and exploiting the reactivity of water in engineered terpene cyclases.
Supplementary Material
ACKNOWLEDGMENTS
This work is based upon research conducted at the Northeastern Collaborative Access Team beamlines, which are funded by the National Institute of General Medical Sciences from the National Institutes of Health (P30 GM124165). The Pilatus 6M detector on beamline 24-ID-C is funded by a NIH-ORIP HEI grant (S10 RR029205). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Finally, this work is also based on research conducted at beamline 17-ID-1 (AMX) of the National Synchrotron Light Source II, a DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under Contract DE-SC0012704. The Center for BioMolecular Structure (CBMS) is primarily supported by the National Institutes of Health, NIGMS, through a Center Core P30 Grant (P30GM133893) and by the DOE Office of Biological and Environmental Research (KP1605010).
Funding
We thank the National Institutes of Health for grant GM56838 in support of this research.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: https://doi.org/10.1021/acs.biochem.0c00876
Table S1, crystallization conditions; Table S2, summary of final protein structures; Figure S1, structure of the wild-type EIZS–Mg2+3–pamidronate complex; Figure S2, structure of the F96S EIZS–Mg2+3–neridronate complex.
Accession Codes
The atomic coordinates and crystallographic structure factors of EIZS variants complexes with the inhibitors shown in Figure 1b have been deposited in the Protein Data Bank (www.rcsb.org) with accession codes as follows: 7KJ8, 7KJ9, 7KJD, 7KJE, 7KJF, 7KJG.
The authors declare no competing financial interests.
References
- (1).Buckingham J, Ed. (1994) Dictionary of Natural Products. Chapman & Hall, London. [Google Scholar]
- (2).Buckingham J, Cooper CM, Purchase R (2015) Natural Products Desk Reference. CRC Press, Taylor & Francis Group, Boca Raton, 253 pp. [Google Scholar]
- (3).Christianson DW (2006) Structural biology and chemistry of the terpenoid cyclases. Chem. Rev 106, 3412–3442. [DOI] [PubMed] [Google Scholar]
- (4).Christianson DW (2017) Structural and chemical biology of terpenoid cyclases. Chem. Rev 117, 11570–11648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Gao Y, Honzatko RB, and Peters RJ (2012) Terpenoid synthase structures: a so far incomplete view of complex catalysis. Nat. Prod. Rep 29, 1153–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Tian B, Poulter CD, and Jacobson MP (2016) Defining the product chemical space of monoterpenoid synthases. PLoS Comput. Biol 12, e1005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Gershenzon J, and Dudareva N (2007) The function of terpene natural products in the natural world. Nat. Chem. Biol 3, 408–414. [DOI] [PubMed] [Google Scholar]
- (8).Pichersky E, Noel JP, and Dudareva N (2006) Biosynthesis of plant volatiles: nature’s diversity and ingenuity. Science 311, 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Quin MB, Flynn CM, and Schmidt-Dannert C (2014) Traversing the fungal terpenome. Nat. Prod. Rep 31, 1449–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Yazaki K, Arimura GI, and Ohnishi T (2017) ‘Hidden’ terpenoids in plants: their biosynthesis, localization and ecological roles. Plant Cell Physiol. 58, 1615–1621. [DOI] [PubMed] [Google Scholar]
- (11).Tetali SD (2019) Terpenes and isoprenoids: a wealth of compounds for global use. Planta 249, 1–8. [DOI] [PubMed] [Google Scholar]
- (12).Schiff PB, Fant J, and Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277, 665–667. [DOI] [PubMed] [Google Scholar]
- (13).Tu Y (2016) Artemisinin – a gift from traditional chinese medicine to the world (Nobel lecture). Angew. Chem. Int. Ed. Engl 55, 10210–10226. [DOI] [PubMed] [Google Scholar]
- (14).Scalerandi E, Flores GA, Palacio M, Defago MT, Carpinella MC, Valladares G, Bertoni A, and Palacios SM (2018) Understanding synergistic toxicity of terpenes as insecticides: contribution of metabolic detoxification in Musca domestica. Front. Plant. Sci 9, 1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Panella NA, Dolan MC, Karchesy JJ, Xiong Y, Peralta-Cruz J, Khasawneh M, Montenieri JA, and Maupin GO (2005) Use of novel compounds for pest control: insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. J. Med. Entomol 42, 352–358. [DOI] [PubMed] [Google Scholar]
- (16).Little DB, and Croteau RB (2002) Alteration of product formation by directed mutagenesis and truncation of the multiple-product sesquiterpene synthases δ-selinene synthase and γ-humulene synthase. Arch. Biochem. Biophys 402, 120–135. [DOI] [PubMed] [Google Scholar]
- (17).Bleeker PM, Diergaarde PJ, Ament K, Schutz S, Johne B, Dijkink J, Hiemstra H, de Gelder R, de Both MT, Sabelis MW, Haring MA, and Schuurink RC (2011) Tomato-produced 7-epizingiberene and R-curcumene act as repellents to whiteflies. Phytochemistry 72, 68–73. [DOI] [PubMed] [Google Scholar]
- (18).Setzer WN (2009) Essential oils and anxiolytic aromatherapy. Nat. Prod. Commun 4, 1305–1316. [PubMed] [Google Scholar]
- (19).Mewalal R, Rai DK, Kainer D, Chen F, Kulheim C, Peter GF, and Tuskan GA (2017) Plant-derived terpenes: a feedstock for specialty biofuels. Trends Biotechnol. 35, 227–240. [DOI] [PubMed] [Google Scholar]
- (20).Zargar A, Bailey CB, Haushalter RW, Eiben CB, Katz L, and Keasling JD (2017) Leveraging microbial biosynthetic pathways for the generation of ‘drop-in’ biofuels. Curr. Opin. Biotechnol 45, 156–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Steenackers B, De Cooman L, and De Vos D (2015) Chemical transformations of characteristic hop secondary metabolites in relation to beer properties and the brewing process: a review. Food Chem. 172, 742–756. [DOI] [PubMed] [Google Scholar]
- (22).Andre CM, Hausman JF, and Guerriero G (2016) Cannabis sativa: the plant of the thousand and one molecules. Front. Plant. Sci 7, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Booth JK, Page JE, and Bohlmann J (2017) Terpene synthases from Cannabis sativa. PLoS One 12, e0173911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Aaron JA, Lin X, Cane DE, and Christianson DW (2010) Structure of epi-isozizaene synthase from Streptomyces coelicolor A3(2), a platform for new terpenoid cyclization templates. Biochemistry 49, 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lin X, and Cane DE (2009) Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor. Mechanism and stereochemistry of the enzymatic formation of epi-isozizaene. J. Am. Chem. Soc 131, 6332–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Lin X, Hopson R, and Cane DE (2006) Genome mining in Streptomyces coelicolor: molecular cloning and characterization of a new sesquiterpene synthase. J. Am. Chem. Soc 128, 6022–6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Li R, Chou WK, Himmelberger JA, Litwin KM, Harris GG, Cane DE, and Christianson DW (2014) Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 53, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Blank PN, Barrow GH, Chou WKW, Duan L, Cane DE, and Christianson DW (2017) Substitution of aromatic residues with polar residues in the active site pocket of epi-isozizaene synthase leads to the generation of new cyclic sesquiterpenes. Biochemistry 56, 5798–5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Battye TG, Kontogiannis L, Johnson O, Powell HR, and Leslie AG (2011) iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr 67, 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Evans PR, and Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr 69, 1204–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, and Read RJ (2007) Phaser crystallographic software. J. Appl. Crystallogr 40, 658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Emsley P, Lohkamp B, Scott WG, and Cowtan K (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr 66, 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, and Zwart PH (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr 66, 213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Gaudreault F, Morency LP, and Najmanovich RJ (2015) NRGsuite: a PyMOL plugin to perform docking simulations in real time using FlexAID. Bioinformatics 31, 3856–3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Moriarty NW, Grosse-Kunstleve RW, and Adams PD (2009) electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr 65, 1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Harpaz Y, Gerstein M, and Chothia C (1994) Volume changes on protein folding. Structure 2, 641–649. [DOI] [PubMed] [Google Scholar]
- (37).Weber DS, and Warren JJ (2019) The interaction between methionine and two aromatic amino acids is an abundant and multifunctional motif in proteins. Arch. Biochem. Biophys 672, 108053. [DOI] [PubMed] [Google Scholar]
- (38).Ringer AL, Senenko A, and Sherrill CD (2007) Models of S/π interactions in protein structures: comparison of the H2S benzene complex with PDB data. Protein Sci. 16, 2216–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Valley CC, Cembran A, Perlmutter JD, Lewis AK, Labello NP, Gao J, and Sachs JN (2012) The methionine-aromatic motif plays a unique role in stabilizing protein structure. J. Biol. Chem 287, 34979–34991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Blank PN, Barrow GH, and Christianson DW (2019) Crystal structure of F95Q epi-isozizaene synthase, an engineered sesquiterpene cyclase that generates biofuel precursors β- and γ-curcumene. J. Struct. Biol 207, 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


