Purpose:
To understand the hemodynamic effect of angiotensin II as a vasopressor in patients with shock secondary to COVID-19 infection.
Methods:
A retrospective analysis was performed on all patients at a single center with COVID-19 infection and shock who were treated with angiotensin II. The hemodynamic response to angiotensin II was estimated by recording the mean arterial pressure, norepinephrine equivalent dose (NED) and urine output.
Results:
Ten patients with COVID-19 related shock were treated with angiotensin II. Over the initial 6 hours, the average the NED decreased by 30.4% (from 64.6 to 44 µg/min) without a significant change in the mean arterial pressure (0.7% decrease). Six patients experienced at least a 25% reduction in NED by 6 hours, and 2 experienced at least a 50% reduction.
Conclusions:
On average, the hemodynamic response to angiotensin II in COVID-19 related shock was favorable. Two patients had a marked rapid improvement. Given the relationship of SARS-CoV-2 with the renin-angiotensin-aldosterone system, further evaluation of angiotensin II for the treatment of COVID-19 related shock is warranted.
Keywords: COVID-19, shock, vasopressors
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic has caused significant illness and death across the world over the past several months. In addition to respiratory failure, shock related to sepsis or cytokine release syndrome may occur. The true prevalence of shock in this population is not clearly defined; however, shock seems to be a complicating factor in most patients who are admitted to the intensive care unit (ICU), and therefore the use of vasopressors in these critically ill patients is common.1–3 Angiotensin II has been shown to be an effective vasopressor in vasodilatory shock in the ATHOS 3 trial.4 The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus utilizes the angiotensin-converting enzyme 2 (ACE2) receptor to gain entry into host cells. Given this interaction with the renin-angiotensin-aldosterone system, some have proposed that angiotensin II as a vasopressor would be worth further scientific investigation.5
We have compiled our experience of angiotensin II use in 10 patients with shock secondary to COVID-19 and describe the hemodynamic response and outcomes.
METHODS
We conducted a review of all patients with COVID-19 who were treated with angiotensin II for shock at our institution. Using a pharmacy database, all patients who were treated with angiotensin II from March first, 2020 to April 25, 2020 at a single academic institution (Lahey Hospital and Medical Center, Burlington, MA) were reviewed. The Institutional review board at our institution approved this study. To be included in the analysis, patients were required to have tested positive for SARS-CoV-2 infection by polymerase chain reaction either with a nasopharyngeal or tracheal sample. No patients were excluded from our analysis. A thorough review of the electronic medical record was performed. Baseline characteristics, imaging, laboratory data, and hemodynamic data were tabulated. Hourly mean arterial pressure, vasopressor or inotrope dose, and urine output were tabulated starting at time −6 hours before the start of angiotensin II until +12 hours after angiotensin II was started. Given the small sample size and the case series design of this study, statistical analysis was not performed.
RESULTS
Ten patients were identified during the study period as being treated with angiotensin II and testing positive for SARS-CoV-2. The patients were 64.5 ± 6.15 years old on average and 10% were female. Patients on average tested positive 3.8 days before ICU admission and had been in the ICU for 17.6 hours before the initiation of angiotensin II. All 10 were intubated and receiving mechanical ventilation, 9 of the 10 underwent prone positioning at some point during their hospitalization. Angiotensin II was added as a third vasopressor in 2 (20%) patients and as a fourth vasopressor in 6 (60%) patients (second vasopressor and fifth vasopressor in 1 patient each). The starting dose of angiotensin II was 20 ng/kg/min in 7 (70%) patients (10 ng/kg/min in 1 patient, 30 ng/kg/min in 1 patient and 40 ng/kg/min in 1 patient). Seven patients had received tocilizumab, an interleukin-6-receptor inhibitor, and all 10 had received glucocorticoids. Of the 7 patients who had received tocilizumab, 5 had received this medication following resolution of shock and were no longer receiving angiotensin II at that time, 1 patient received tocilizumab 15 minutes before initiation of angiotensin II and died shortly thereafter, and 1 patient received tocilizumab 16 hours before angiotensin II initiation.
Selected baseline characteristics, the hemodynamic impact of angiotensin II within 6 hours, and outcomes are shown in Table 1. On average the norepinephrine equivalent dose decreased by 30.4% (from 64.6 to 44 µg/min) without a significant change in the mean arterial pressure (0.7% decrease). Six patients experienced at least a 25% reduction in norepinephrine equivalent dose by 6 hours, and 2 experienced at least a 50% reduction (Fig. 1). By the writing of this manuscript, 5 of the 10 patients (50%) have died (care was withdrawn in all 5 patients, 2 secondary to refractory shock and 3 secondary to refractory hypoxemia), none have been discharged alive and 5 (50%) remain in the hospital. One patient died within 6 hours after starting angiotensin II.
TABLE 1.
Demographic, Clinical, and Outcome Data for 10 Patients With COVID-19 and Shock Treated With Angiotensin II
| Age/Sex | Comorbid Conditions | ACE/ARB | Laboratory Data | Apache II Score | Treatment | Hemodynamic Response (From 0 to 6 Hours) | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Creatinine (mg/dL) | D-Dimer (ng/mL) | Troponin (ng/mL) | Lactic Acid (mmol/L) | Viral Directed Treatment | Immunomodulatory Therapy | Change in MAP in MAP 0 to 6 Hours | Change in Norepinephrine Equivalent Dose | |||||
| 69/M | HTN, CKD | No | 9.2 | 943 | 0.2 | 2.1 | 33 | AZ | GC | +44.6% | −76.1% | Alive in-hospital |
| 68/F | None | No | 1 | 2790 | 0.05 | 1.8 | 38 | HCQ/AZ/convalescent plasma | Tocilizumab/GC | +18.1% | −51.9% | Alive in-hospital |
| 60/M | HTN | No | 4.9 | >2000 | 1.19 | 2.8 | 34 | AZ/convalescent plasma | Tocilizumab/GC | +11.6% | −42.8% | Alive in-hospital |
| 58/M | HTN | Yes | 0.7 | >2000 | 0.01 | 4.5 | 33 | HCQ/AZ | Tocilizumab/GC | −19.2% | −39.2% | In-hospital death |
| 52/M | HTN, DM | Yes | 1.5 | >2000 | 0.22 | 8.9 | 33 | Convalescent plasma | Tocilizumab/GC | +17.6% | −26.1% | Alive in-hospital |
| 68/M | HTN | Yes | 1.5 | 262 | 0.07 | 1.7 | 32 | HCQ/AZ | Tocilizumab/GC | −4.8% | −24.7% | In-hospital death |
| 61/M | HTN | Yes | 1.1 | 1460 | 0.01 | 1.5 | 27 | HCQ/AZ | Tocilizumab/GC | −1.2% | −8.7% | In-hospital death |
| 58/M | HTN, DM | Yes | 3.3 | 552 | 0.08 | 1.7 | 26 | HCQ/AZ | GC | +19.4% | −2.4% | Alive in-hospital |
| 71/M | CKD, AF, solid organ transplantation, cancer | No | 5.1 | >2000 | 0.11 | 0.8 | 39 | None | GC | +1.5% | 0% | In-hospital death |
| 60/M | HTN | No | 1 | 1240 | 0.06 | 2.2 | 30 | HCQ/AZ | Tocilizumab/GC | Died 3 hours after initiation of angiotensin II | In-hospital death | |
Figure 1.
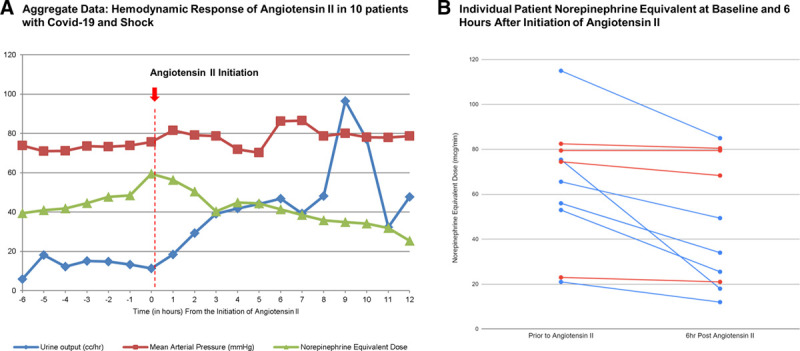
Mean arterial pressure, norepinephrine equivalent dose, over an 18-hour time period starting 6 hours before the initiation of angiotensin II in 10 patients with COVID-19 and shock (panel A). Individual hemodynamic response based on norepinephrine equivalent dose in 10 patients at time 0 compared to 6 hours after initiation of angiotensin II (panel B). Patients with a 25% or greater decrease in norepinephrine dose are denoted in blue.
DISCUSSION
Given the interaction of the SARS-CoV-2 virus with the renin-angiotensin-aldosterone system, dysregulation of this system may play a role in the vasodilatory shock associated with severe COVID-19 infection. However, there is no clearly identified mechanism to support this hypothesis.5 The severe acute respiratory SARS-CoV-2 virus utilizes the ACE2 receptor to gain entry into host cells.6 The ACE2 enzyme is responsible for conversion of angiotensin II to angiotensin (1,7), which has vasodilatory actions via both an increase in angiotensin (1,7) as well as a complimentary reduction in angiotensin II. ACE2 thereby balances the vasoconstriction and sodium retention effects exerted by activation of the renin-angiotensin-aldosterone system. Although there is no definite mechanism that has been elucidated to support a hypothesis that angiotensin II would be beneficial in shock related to COVID-19, given the interaction of the virus with the renin-angiotensin-aldosterone system further study of angiotensin II as a vasopressor in this clinical scenario would be worthwhile.7,8
Angiotensin II is a potent vasoconstrictor that has been shown to effectively increase mean arterial pressure in patients with vasodilatory shock enrolled in the ATHOS 3 trial.4 In this trial, patients with vasodilatory shock (mostly septic shock) who were already on norepinephrine at a dose of at least 0.2 µg/kg/min were randomized to an infusion of either angiotensin II or placebo. By 3 hours, the primary endpoint of an increase in baseline mean arterial pressure of at least than 10 mm Hg or to greater than 75 mm Hg occurred more frequently in the angiotensin II group than the placebo group (69.9% vs. 23.4%, odds ratio 7.95, P < 0.0001). Based on this short term favorable hemodynamic impact, angiotensin II was approved by the Food and Drug Administration in the United States of America in 2017 for the treatment of vasodilatory shock.
There has been 1 case report published thus far to our knowledge demonstrating a favorable response to therapy with angiotensin II in patients with shock in the setting of COVID-19 infection.9 Two patients in our series demonstrated a dramatic hemodynamic response to angiotensin II with a greater than 50% decline in norepinephrine equivalent dose within 6 hours. This “sensitivity” to angiotensin II had also been reported in a subset of patients in the ATHOS 3 trial which evaluated the use of angiotensin II in vasodilatory shock.10 It remains possible that there is a subgroup of patients who respond favorably to angiotensin II and therefore there may be reason to use angiotensin II earlier in the course of septic shock related to COVID-19 in certain patients. Further study is necessary to determine if there is a role for the earlier use of angiotensin II as a vasopressor in patients with vasodilatory shock secondary to COVID-19 infection.
DISCLOSURES
Nothing to declare.
REFERENCES
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020; 323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grasselli G, Zangrillo A, Zanella A, et al. ; COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020; 323:1574–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020; 382:2012–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khanna A, English SW, Wang XS, et al. ; ATHOS-3 Investigators. Angiotensin II for the treatment of vasodilatory shock. N Engl J Med. 2017; 377:419–430. [DOI] [PubMed] [Google Scholar]
- 5.Chow JH, Mazzeffi MA, McCurdy MT. Angiotensin II for the treatment of COVID-19-related vasodilatory shock. Anesth Analg. 2020; 131:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ham KR, Boldt DW, McCurdy MT, et al. Sensitivity to angiotensin II dose in patients with vasodilatory shock: a prespecified analysis of the ATHOS-3 trial. Ann Intensive Care. 2019; 9:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020; 382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Das S, Wieruszewski PM, et al. Unexpected BP sensitivity to angiotensin II in a patient with Coronavirus disease 2019, ARDS, and septic shock. Chest. 2020; 158:e55–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kai H, Kai M. Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res. 2020; 43:648–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler C, Cao Z, Wu Y, et al. Counter-regulatory ‘renin-angiotensin’ system-based candidate drugs to treat COVID-19 diseases in SARS-CoV-2-infected patients. Infect Disord Drug Targets. 2020. [DOI] [PubMed] [Google Scholar]


