Graphical abstract
Keywords: SARS-CoV-2, Spillover, Outdoor environment
Abstract
Facing the ongoing coronavirus infectious disease-2019 (COVID-19) pandemic, many studies focus on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in indoor environment, on solid surface or in wastewater. It remains unclear whether SARS-CoV-2 can spill over into outdoor environments and impose transmission risks to surrounding people and communities. In this study, we investigated the presence of SARS-CoV-2 by measuring viral RNA in 118 samples from outdoor environment of three hospitals in Wuhan. We detected SARS-CoV-2 in soils (205–550 copies/g), aerosols (285−1,130 copies/m3) and wastewaters (255−18,744 copies/L) in locations close to hospital departments receiving COVID-19 patients or in wastewater treatment sectors. These findings revealed a significant viral spillover in hospital outdoor environments that was possibly caused by respiratory droplets from patients or aerosolized particles from wastewater containing SARS-CoV-2. In contrast, SARS-CoV-2 was not detected in other areas or on surfaces with regular implemented disinfection. Soils may behave as viral warehouse through deposition and serve as a secondary source spreading SARS-CoV-2 for a prolonged time. For the first time, our findings demonstrate that there are high-risk areas out of expectation in hospital outdoor environments to spread SARS-CoV-2, calling for sealing of wastewater treatment unit and complete sanitation to prevent COVID-19 transmission risks.
Introduction
The outbreak of coronavirus infectious disease-2019 (COVID-19) pandemic has rapidly spread throughout over 200 countries, posing a global threat to human health. Till 10th April 2021, there are 130 million confirmed cases and 2.9 million deaths. SARS-CoV-2 is an enveloped, positively-stranded RNA virus belonging to the beta coronavirus genus that causes COVID-19 (Lai et al., 2020, Li et al., 2020b, Ralph et al., 2020). It can transmit among people (Chan et al., 2020; Chang et al., 2020, Li et al., 2020b, Poon and Peiris, 2020) via direct contact and respiratory droplet routes (Carlos et al., 2020; Lai et al., 2020; Wu et al., 2020), while aerosol or faecal transmission route is also possible (Holshue et al., 2020; Tian et al., 2020). Many studies have analyzed SARS-CoV-2 in hospital indoor environment to assess its transmission dynamics and develop strategies to protect medical staffs or drop-in visitors (Liu et al., 2020;), or in wastewater for disease surveillance as wastewater-based epidemiology (WBE) (Medema et al., 2020). In contrast, there is no knowledge about the viral presence in outdoor environment, which might pose risks for secondary spread and infection (Zhang et al., 2021a). As a result, it is intractable to evaluate the potential spillover into open space and distribution in outdoor environmental matrices of SARS-CoV-2, that potentially survives for a prolonged time and threatens the surrounding communities and public health.
In this study, we collected water, aerosol, soil and surface (road, outside wall, and waste bag) samples from the outdoor environment of three specialized hospitals (Jinyintan Hospital, Huoshenshan Hospital and Wuchang Cabin Hospital) in Wuhan dedicated for COVID-19 treatments in March and April, 2020. By analyzing the presence of SARS-CoV-2 viral RNA in these outdoor samples, we aimed to uncover the occurrence of SARS-CoV-2 viral RNA in hospital outdoor environment, evaluate the potential risks to surrounding staffs and patients, and reveal the potential mechanisms of SARS-CoV-2 spillover in hospitals.
Materials and methods
Hospitals
Jinyintan Hospital is the first hospital in Wuhan receiving COVID-19 patients, with outpatient and inpatient departments. Its medical wastewater treatment sector is comprised of an adjusting tank, a bio-aeration tank using biological contact oxidation process, a coagulation-sedimentation tank and a disinfection tank (Fig. S1A). Huoshenshan hospital is a newly-constructed hospital designated for COVID-19 patients and confirmed patents were transferred directly into wards. There is no inpatient or outpatient department, and the wastewater treatment sector consists of a process integrated storage sector with two adjusting tanks, one septic tank, a moving-bed biofilm reactor (MBBR), a coagulation-sedimentation tank and a disinfection tank (Fig. S1B). Medical staff area is located south-east to ward area. Wuchang Cabin Hospital is a temporary shielding hospital open from 5th February to 10th March 2020, receiving 1,124 COVID-19 patients. Wastewater from eight outdoor toilets were pumped in 4 preliminary disinfection tanks, transferred into three septic tanks outside, following a final disinfection. After 24-h, the effluent was pumped and discharged into pipe network and wastewater treatment plants. Chlorine-based disinfectants are supplemented in wards and the disinfection tank only.
Sample collection
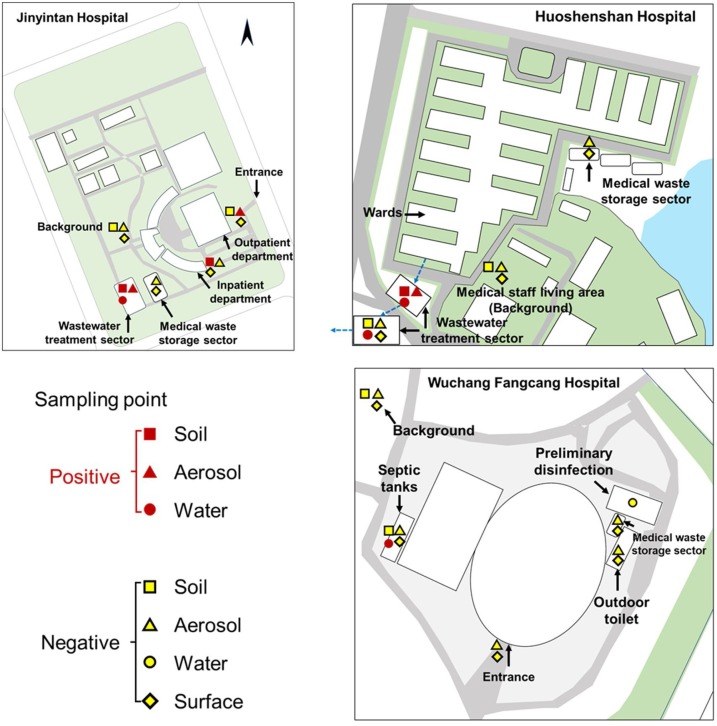
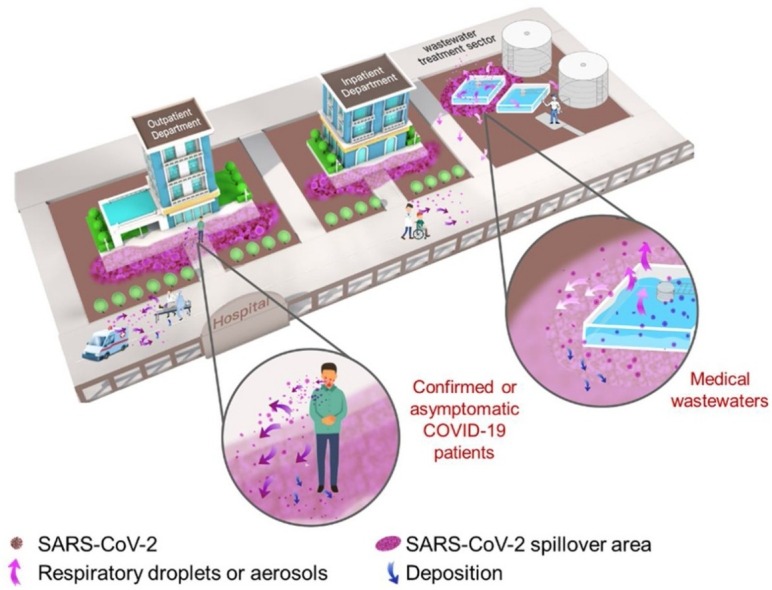
Sampling sites are located in the outdoor environment of Jinyintan, Huoshenshan and Wuchang Cabin Hospitals, including wastewater treatment sectors, medical waste storage sectors, inpatient departments, outpatient departments, outdoor toilets and temperate septic tanks (Fig. 1 and Table S1). Around 2.0 L of top layer water in different wastewater treatment sectors (0−20 cm) was directly collected in a plexiglass sampler. Aerosol samples were collected using bioaerosol liquid impingers (WA-15, Beijing Dinglan Tech. Ltd., China) at a height of 1.5 m above the water surface and a flow rate of 14.0 L/min for 30 min (details see Supplementary Material), which were widely used for studies on SARS-CoV-2 in aerosols in China (Ding et al., 2021; Ma et al., 2021; Zhou et al., 2021). About 20 g of soils were sampled at the unplanted ground surface (< 5 cm) using a sterile plastic shovel. Surface samples were collected by wiping in an “S” pattern in 2 directions to cover 20 × 20 cm area of roads, walls or medical waste bags using swabs wetted with phosphate buffer saline (pH = 7.4). All samples were immediately placed in 4 °C ice-boxes and transferred into laboratory for RNA extraction on the same day.
Fig. 1.
Outdoor environment sampling sites in Jinyintan Hospital, Huoshenshan Hospital and Wuchang Cabin Hospital.
RNA extraction and RT-qPCR
RNA extraction from all environmental samples used PEG-6000 settlement and RNeasy® PowerSoil® Total RNA Kit (MOBIO, Carlsbad, CA, USA) following our previously reported protocol (details see supplementary materials) (Zhang et al., 2020). SARS-CoV-2 RNA was quantified by RT-qPCR using AgPath-ID™ One-Step RT-PCR Kit (Life Technologies, Carlsbad, CA, USA) on a LightCycler 480 Real-time PCR platform (Roche, Indianapolis, IN, USA) in duplicates with two sets of primers targeting open reading frame lab (CCDC-ORF1) and nucleocapsid protein (CCDC-N), respectively. RT-qPCR amplification for CCDC-ORF1 and CCDC-N was performed in 25 μL reaction mixtures containing 12.5 μL of 2×RT-PCR Buffer, 1 μL of 25×RT-PCR Enzyme Mix, 4 μL mixtures of forward primer (400 nM), reverse primer (400 nM) and probe (120 nM), and 5 μL of template RNA. The details of primers and thermocycling program are listed in Supplementary Material. For each RT-qPCR run, both positive and negative controls were included. The copy numbers of SARS-CoV-2 were obtained from a standard calibration curve by a 10-fold serial dilution of genes encoding nucleocapsid protein with an amplification efficiency of 102.6 %, calculated as copies = 10[−(Cq−39.086)/3.262] (R2 = 0.991). For quality control, a reagent blank and extraction blank were included for RNA extraction procedure and no contamination was observed.
Results and discussions
We collected 28 water, 20 aerosol, 15 soil, and 55 surface samples of roads, outside walls and medical waste bags from 17 sites in three specialized hospitals in Wuhan dedicated for COVID-19 treatments, i.e., Jinyintan Hospital, Huoshenshan Hospital and Wuchang Cabin Hospital, during their operation receiving COVID-19 patients in March and April (Fig. 1 and Table S1, see Methods for details). These sites covered major concerned locations in hospital outdoor environment, including wastewater treatment sector, medical waste storage sector and patient department. Three out of 15 soil samples (20 %) exhibited positive results for SARS-CoV-2 viral RNA, which were collected near wastewater treatment sectors and outside patient departments (Table 1 ). Among them, the soil sample with 2-m distance to the adjusting tank in wastewater treatment sector of Jinyintan Hospital contained 253 copies/g of SARS-CoV-2 (Table S1), whereas the other sample 2-m away from the disinfection tank was negative in SARS-CoV-2. In other areas, there was 205 copies/g of SARS-CoV-2 in soil with 5 m distance to the outpatient department. In Huoshenshan Hospital, only soil with 2 m distance to the first adjusting tank contained SARS-CoV-2 viral RNA at a level of 550 copies/g, while soils in other wastewater treatment units or medical staff living area exhibited negative signals. None of soils in Wuchang Cabin Hospital surrounding the septic tanks and in background areas was positive for virus. To our knowledge, this is the first report showing the presence of SARS-CoV-2 viral RNA in soils. Since wastewater and soils are environmental matrices rich in organic matters that can protect and shield viruses, SARS-CoV-2 might not be sanitized by disinfectants as evidenced by previous studies on other viruses (Hurst et al., 1980; Vettori et al., 2000), and possibly survive for a prolonged time in hospital outdoor environment, like over 12 h in aerosols, over 7 days in wastewater (Bivins et al., 2020), up to 28 days on surfaces (Riddell et al., 2020) and >10 weeks in soils or groundwater (Li et al., 2020a). Thus, the presence of SARS-CoV-2 RNA on surface and in soils might pose long-term risks to surrounding residents.
Table 1.
Presence of SARS-CoV-2 in hospital outdoor environments.
| Hospital | Site | Sample typea |
|||
|---|---|---|---|---|---|
| Soil (copies/g) | Aerosol (copies/m3) | Water (copies/L) | Surface (copies/m2) | ||
| Jinyintan | Wastewater treatment sector | NDb-253 (1/2) | 285 (1/1) | ND-255 (1/5) | – |
| Out- and In-patient department | ND-205 (1/2) | ND-1,130(1/2) | – | ND (0/4) | |
| Medical waste storage sector | – | ND (0/1) | – | ND (0/13) | |
| Background | ND (0/1) | ND (0/1) | – | ND (0/1) | |
| Huoshenshan | Wastewater treatment sector | ND-550 (1/2) | ND-603 (1/3) | ND-2,208(3/10) | ND (0/2) |
| Medical waste storage sector | – | ND (0/1) | – | ND (0/14) | |
| Background (Medical staff living area) | ND (0/2) | ND (0/2) | – | ND (0/2) | |
| Wuchang Cabin | Wastewater treatment sector | ND (0/4) | ND (0/2) | ND-18,744 (7/13) | – |
| Medical waste storage sector | – | ND (0/2) | – | ND (0/11) | |
| Entrance | – | ND (0/2) | – | ND (0/2) | |
| Outdoor toilet | – | ND (0/1) | – | ND (0/2) | |
| Background | ND (0/2) | ND (0/2) | – | ND (0/2) | |
Fraction in bracket is number of positive samples to number of total samples.
ND, non-detected.
All road- and wall-surfaces in outdoor environment of the three studied hospitals were negative in viral signals (Table 1). Surface contamination of SARS-CoV-2 (Ong et al., 2020), Middle East Respiratory Syndrome (MERS) coronavirus (Kim et al., 2016; Weber et al., 2019) and norovirus (Morter et al., 2011) has been observed in hospital indoor environment. All medical waste bags also exhibited negative qPCR results (Table 1), as they were all sanitated before transferring from wards to waste storage sector. Our results documented that frequent disinfection, like three times per day in hospital outdoor environment and careful sanitation of medical waste bags, effectively removed the virus of SARS-CoV-2.
To trace the source of SARS-CoV-2 in soils, we collected the surrounding aerosol samples. Inside the adjusting tank of Jinyintan Hospital and Huoshenshan Hospital, SARS-CoV-2 in aerosols was found at a level of 285 copies/m3 and 603 copies/m3, respectively (Tables 1 and S1). They were of comparable levels to SARS-CoV-2 detected in intensive care units (Guo et al., 2020; Liu et al., 2020) and exhibited high transmission potential via aerosol deposition. On the contrary, aerosol SARS-CoV-2 was not detected in downstream wastewater treatment units of the second adjusting tank and the moving-bed biofilm reactor (MBBR). Outside patient departments of Jinyintan Hospital, SARS-CoV-2 in aerosols collected 5 m away from outpatient building were 1130 copies/m3, whereas undetected in aerosols collected 5 m away from inpatient building. Since aerosols are highly dynamic, SARS-CoV-2 in aerosols depends on the sources of respiratory droplets or airborne viruses in a short period, which is largely attributed to the aerosolized droplets from the tidal breathing of COVID-19 patients (Qian et al., 2020). In contrast, no SARS-CoV-2 was detected in aerosols above soils exhibiting negative results, e.g., background area of Jinyintan Hospital, medical staff living area of Huoshenshan Hospital, and the entrance and outdoor toilet of Wuchang Cabin Hospital. Our results imply that hospitals receiving COVID-19 patients have high-risk outdoor areas (patient departments and wastewater treatment sector). SARS-CoV-2 contamination on ground surface was only previously reported in indoor studies, explained by deposition of airflow-displaced virus-laden droplets in COVID-19 patient living room (Ong et al., 2020). The co-existence of SARS-CoV-2 in both soils and aerosols in the high-risk areas hints viral spillover, deposition and accumulation in soils from airborne SARS-CoV-2.
To further examine whether the aerosol and soil SARS-CoV-2 was derived from wastewater treatment sector, we analyzed SARS-CoV-2 viral RNA in waters from different treatment tanks. SARS-CoV-2 in Jinyintan Hospital was only detected in water from the adjusting tank (255 copies/L, Table S1), but undetected in other tanks and effluents. Raw medical wastewater in the adjusting tank of Huoshenshan Hospital contained 633 copies/L of SARS-CoV-2, which was only occasionally found in MBBR (505 copies/L) and sedimentation tank (2,208 copies/L) (Table S1). No SARS-CoV-2 was detected in effluents after disinfection. Our results were comparable to previously reported SARS-CoV-2 RNA level in wastewaters from the septic tanks in Wuchang Cabin Hospital before disinfection (557 to 18,744 copies/L) (Zhang et al., 2020), crude wastewater from wards of Jinyintan Hospital (255 to 3,010 copies/L) (Zhang et al., 2021b) and wastewater in pipelines near Huanan Seafood market (28,800 copies/L) (Zhang et al., 2021b). Till now, there was no routine equations for the emission flux of viral aerosolization in wastewater treatments, and our calculation followed the equations describing the aerosolization of Ebola viruses in an aeration basin model (Lin and Marr, 2017). The estimated emission rates of SARS-CoV-2 ranged from 468 to 990 copies/(m3·hr), comparable to 27 copies/(m3·hr) for Rotavirus and 3,099 copies/(m3·hr) for Norovirus in wastewater treatment plants (Pasalari et al., 2019). Our findings prove apparent presence of SARS-CoV-2 viral RNA in raw water from wards, signifying the need for complete disinfection. As it decayed rapidly in medical wastewater treatment process and a complete disinfection was applied for all effluents before discharge, there was negligible risk of SARS-CoV-2 spread through pipe network receiving treated wastewater from hospitals.
Considering the different mechanisms of SARS-CoV-2 spillover into hospital outdoor environment, we carefully compare the presence of SARS-CoV-2 RNA in different environmental media at the same site and evaluate the possibility by reviewing the management strategies in hospitals (Table 2 ). During the COVID-19 pandemics, all the patient wards were strictly isolated and sanitated, and thus it is extremely unlikely that aerosols and soils in outdoor environment could receive viruses directly from leakage. In contrast, medical wastewater was not disinfected until reaching the wastewater treatment sectors, most probably explaining the occurrence of SARS-CoV-2 in wastewater. As all medical waste bags were sanitated before transport from wards to waste storage sector, they were extremely unlikely to contaminate aerosols and soils outside inpatient department, and the most probable source of SARS-CoV-2 in aerosols and soils were respiratory droplets from drop-in or asymptomatic COVID-19 patients. It is worthy of noting that SARS-CoV-2 was observed in all waters, aerosols and surrounding soils at the adjusting tank of wastewater treatment sector in Jinyintan Hospital and Huoshenshan Hospital (Table 1). The access of COVID-19 patients and medical staffs to these areas were denied by hospital regulations during the COVID-19 pandemics, and it was most probably that SARS-CoV-2 arose from viral RNA-containing medical wastewater via aerosolization in the uplifting process, forming airborne virus-containing aerosols, and eventually depositing on soils. The wastewater treatment sector in Wuchang Cabin Hospital is a temporary enclosed system effectively preventing the spillover of airborne SARS-CoV-2 from wastewater, resulting in negative viral signals in surrounding aerosols and soils. This implies appropriate sealing of the adjusting tank and other treatment units might block the potential viral transport from wastewater into aerosols and deposition on soils.
Table 2.
Possible sources of SARS-CoV-2 spillover in hospital outdoor environments.
| Media | Source | Possibility |
|---|---|---|
| Aerosol | Leakage from patient room | Extremely unlikely |
| Respiratory droplet from drop-in patients | Most probablya | |
| Respiratory droplet from asymptomatic COVID-19 patients | Most probablya | |
| Contamination from medical wastes | Unlikely | |
| Aerosolization and emission from wastewater | Most probablyb | |
| Wastewater | Leakage from patient room | Most probably |
| Respiratory droplet from drop-in patients | Extremely unlikely | |
| Respiratory droplet from asymptomatic COVID-19 patients | Extremely unlikely | |
| Contamination from medical wastes | Extremely unlikely | |
| Soil | Leakage from patient room | Extremely unlikely |
| Respiratory droplet from drop-in patients | Most probablyc | |
| Respiratory droplet from asymptomatic COVID-19 patients | Most probablyc | |
| Contamination from medical wastes | Unlikely | |
| Deposition from aerosols | Most probablyc,d |
Aerosols in outpatient and inpatient departments.
Aerosols in wastewater treatment sector.
Soils around outpatient and inpatient departments.
Soils around in wastewater treatment sector.
For the first time, we demonstrate the presence of SARS-CoV-2 viral RNA in hospital outdoor environments of three specialized hospitals dedicated for COVID-19 treatments. SARS-CoV-2 existed in all environmental matrices at hospital departments receiving confirmed or suspected COVID-19 patients (aerosols and soils) and wastewater treatment sector (waters, aerosols and soils), revealing high-risk areas for potential SARS-CoV-2 transmission as illustrated by Fig. 2 . High-risk areas located outside patient departments are exposed to respiratory droplets containing SARS-CoV-2 by receiving confirmed or asymptomatic COVID-19 patients. Alternatively, undisinfected medical wastewater in the adjusting tank of wastewater treatment sector might spill airborne viruses through uplifting or aeration and then deposit SARS-CoV-2 on surrounding soils and solid-surfaces. Traditional outdoor disinfection strategies mainly focus on walls, roads or facilities and can deactivate viruses on solid-surfaces with high efficiency (Brady et al., 1990; Hota, 2004), explaining the negative results on all road- and wall-surfaces in this work. Viral presence and survival in soils are seldom examined and there is limited work addressing the potential risks of soil viruses (Kuzyakov and Mason-Jones, 2018). Soils can receive the viruses from aerosols and waters, and potentially become a new source for SARS-CoV-2 transmission in outdoor environment. Non-point outdoor spillover of SARS-CoV-2 from individual houses of confirmed or asymptomatic COVID-19 patients and environmental viral residue in crowded areas need attentions and further investigations.
Fig. 2.
Spillover and potential transmission of SARS-CoV-2 in high-risk areas of hospital outdoor environments.
Although we do not address SARS-CoV-2 infectivity by viral culture and only collected limited numbers of samples owing to the strict control during the COVID-19 outbreak in Wuhan, China, our study unravels the distributions of SARS-CoV-2 in soils, aerosols, waters, and surfaces, covering the major outdoor environments of hospitals. Although viruses might decay rapidly from these high-risk areas and regular disinfection can effectively eliminate SARS-CoV-2, the overall risks of hospital outdoor environments are significant, particularly in those high-risk areas.
Author contribution
D.Z., G.L., Y.L. and J.Q. conceptualized the study design; D.Z., H.L., W.L. and C.Y. collected samples; D.Z., J.L., X.Z., T.Z., Y.J., Y.X., Z.H. and X.W. did the laboratory test; D.Z., Y.Y., X.H., J.J., M.L., G.L., S.D. and P.Z. interpreted the results; D.Z., Y.Y., J.J., X.H., M.L., S.D. and J.Q. drafted the manuscript. All authors read and approved the final manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was financially supported by the Major Program of National Natural Science Foundation of China (52091543) and the Chinese Academy of Engineering (2020-ZD-15). DZ also acknowledges the support of Chinese Government’s Thousand Talents Plan for Young Professionals.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.hazl.2021.100027.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Bivins A., Greaves J., Fischer R., Yinda K.C., Ahmed W., Kitajima M., Munster V.J., Bibby K. Persistence of SARS-CoV-2 in water and wastewater. Environ. Sci. Technol. Lett. 2020;7:937–942. doi: 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady M.T., Evans J., Cuartas J. Survival and disinfection of parainfluenza viruses on environmental surfaces. Am. J. Infect. Control. 1990;18:18–23. doi: 10.1016/0196-6553(90)90206-8. [DOI] [PubMed] [Google Scholar]
- Carlos W.G., Dela Cruz C.S., Cao B., Pasnick S., Jamil S. Novel Wuhan (2019-nCoV) coronavirus. Am. J. Respir. Crit. Care Med. 2020;201:P7–P8. doi: 10.1164/rccm.2014P7. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S.F., Kok K.H., To K.K.W., Chu H., Yang J., Xing F.F., Liu J.L., Yip C.C.Y., Poon R.W.S., Tsoi H.W., Lo S.K.F., Chan K.H., Poon V.K.M., Chan W.M., Ip J.D., Cai J.P., Cheng V.C.C., Chen H.L., Hui C.K.M., Yuen K.Y. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D., Lin M., Wei L., Xie L., Zhu G., Dela Cruz C.S., Sharma L. Epidemiologic and clinical characteristics of novel coronavirus infections involving 13 patients outside Wuhan, China. JAMA. 2020;323:1092–1093. doi: 10.1001/jama.2020.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z., Qian H., Xu B., Huang Y., Miao T., Yen H.L., Xiao S.L., Cui L.B., Wu X.S., Shao W., Song Y., Sha L., Zhou L., Xu Y., Zhu B.L., Li Y.G. Toilets dominate environmental detection of severe acute respiratory syndrome coronavirus 2 in a hospital. Sci. Total Environ. 2021;753 doi: 10.1016/j.scitotenv.2020.141710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z.-D., Wang Z.-Y., Zhang S.-F., Li X., Li L., Li C., Cui Y., Fu R.-B., Dong Y.-Z., Chi X.-Y., Zhang M.-Y., Liu K., Cao C., Liu B., Zhang K., Gao Y.-W., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerging Infect. Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S.X., Lu X.Y., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K., Washington State-nCo, V.C.I First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hota B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin. Infect. Dis. 2004;39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C.J., Gerba C.P., Cech I. Effects of environmental variables and soil characteristics on virus survival in soil. Appl. Environ. Microbiol. 1980;40:1067–1079. doi: 10.1128/aem.40.6.1067-1079.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.-H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H., Choi J.-P., Choi W.S., Min J.-Y. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin. Infect. Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyakov Y., Mason-Jones K. Viruses in soil: nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018;127:305–317. [Google Scholar]
- Lai C.-C., Shih T.-P., Ko W.-C., Tang H.-J., Hsueh P.-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Yang Y., Lu Y., Zhang D., Liu Y., Cui X., Yang L., Liu R., Liu J., Li G., Qu J. Natural host-environmental media-human: a new potential pathway of COVID-19 outbreak. Engineering. 2020;6:1085–1098. doi: 10.1016/j.eng.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B., Leung G.M., Feng Z. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K., Marr L.C. Aerosolization of ebola virus surrogates in wastewater systems. Environ. Sci. Technol. 2017;51:2669–2675. doi: 10.1021/acs.est.6b04846. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ning Z., Chen Y., Guo M., Liu Y., Gali N.K., Sun L., Duan Y., Cai J., Westerdahl D., Liu X., Xu K., Ho K.-F., Kan H., Fu Q., Lan K. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–561. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- Ma J., Qi X., Chen H., Li X., Zhang Z., Wang H., Sun L., Zhang L., Guo J., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Yao M. COVID-19 patients in earlier stages exhaled millions of SARS-CoV-2 per hour. Clin. Infectious Diseases. 2021;72:e652–e654. doi: 10.1093/cid/ciaa1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G., Heijnen L., Elsinga G., Italiaander R., Brouwer A. Presence of SARS-Coronavirus-2 RNA in sewage and correlation with reported COVID-19 prevalence in the early stage of the epidemic in the Netherlands. Environ. Sci. Technol. Lett. 2020;7:511–516. doi: 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Morter S., Bennet G., Fish J., Richards J., Allen D.J., Nawaz S., Iturriza-Gomara M., Brolly S., Gray J. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J. Hosp. Infect. 2011;77:106–112. doi: 10.1016/j.jhin.2010.09.035. [DOI] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasalari H., Ataei-Pirkooh A., Aminikhah M., Jafari A.J., Farzadkia M. Assessment of airborne enteric viruses emitted from wastewater treatment plant: atmospheric dispersion model, quantitative microbial risk assessment, disease burden. Environ. Pollut. 2019;253:464–473. doi: 10.1016/j.envpol.2019.07.010. [DOI] [PubMed] [Google Scholar]
- Poon L.L.M., Peiris M. Emergence of a novel human coronavirus threatening human health. Nat. Med. 2020;26:317–319. doi: 10.1038/s41591-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Miao T., Liu L., Zheng X.H., Luo D.T., Li Y.G. Indoor transmission of SARS-CoV-2. Indoor Air. 2020;31:639–645. doi: 10.1111/ina.12766. [DOI] [PubMed] [Google Scholar]
- Ralph R., Lew J., Zeng T.S., Francis M., Xue B., Roux M., Ostadgavahi A.T., Rubino S., Dawe N.J., Al-Ahdal M.N., Kelvin D.J., Richardson C.D., Kindrachuk J., Falzarano D., Kelvin A.A. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J. Infect. Dev. 2020;14:3–17. doi: 10.3855/jidc.12425. [DOI] [PubMed] [Google Scholar]
- Riddell S., Goldie S., Hill A., Eagles D., Drew T.W. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol. J. 2020;17:145. doi: 10.1186/s12985-020-01418-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 2020;51:843–851. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettori C., Gallori E., Stotzky G. Clay minerals protect bacteriophage PBS1 of Bacillus subtilis against inactivation and loss of transducing ability by UV radiation. Can. J. Microbiol. 2000;46:770–773. [PubMed] [Google Scholar]
- Weber D.J., Sickbert-Bennett E.E., Kanamori H., Rutala W.A. New and emerging infectious diseases (Ebola, Middle Eastern respiratory syndrome coronavirus, carbapenem-resistant Enterobacteriaceae, Candida auris): Focus on environmental survival and germicide susceptibility. Am. J. Infect. Control. 2019;47:A29–A38. doi: 10.1016/j.ajic.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Liu J., Zhao X., Liu C., Wang W., Wang D., Xu W., Zhang C., Yu J., Jiang B., Cao H., Li L. Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study. Clin. Infect. Dis. 2020:ciaa199. doi: 10.1093/cid/ciaa199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Ling H., Huang X., Li J., Li W., Yi C., Zhang T., Jiang Y., He Y., Deng S., Zhang X., Wang X., Liu Y., Li G., Qu J. Potential spreading risks and disinfection challenges of medical wastewater by the presence of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) viral RNA in septic tanks of Fangcang Hospital. Sci. Total Environ. 2020;741 doi: 10.1016/j.scitotenv.2020.140445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Yang Y., Li M., Lu Y., Liu Y., Jiang J., Liu R., Liu J., Huang X., Li G., Qu J. Ecological barrier deterioration driven by human activities poses fatal threats to public health due to emerging infectious diseases. Engineering. 2021 doi: 10.1016/j.eng.2020.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Zhang X., Ma R., Deng S., Wang X., Wang X., Zhang X., Huang X., Liu Y., Li G., Qu J., Zhu Y., Li J. Ultra-fast and onsite interrogation of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in waters via surface enhanced Raman scattering (SERS) Water Res. 2021;200:117243. doi: 10.1016/j.watres.2021.117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Yao M.S., Zhang X., Hu B.C., Li X.Y., Chen H.X., Zhang L., Liu Y., Du M., Sun B.C., Jiang Y.Y., Zhou K., Hong J., Yu N., Ding Z., Xu Y., Hu M., Morawska L., Grinshpun S.A., Biswas P., Flagan R.C., Zhu B.L., Liu W.Q., Zhang Y.H. Breath-, air- and surface-borne SARS-CoV-2 in hospitals. J. Aerosol Sci. 2021;152 doi: 10.1016/j.jaerosci.2020.105693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.