Abstract
Objectives
To measure SARS-CoV-2 neutralizing antibody (NtAb) titres in previously infected or uninfected health care workers who received one or two doses of BNT162b2 mRNA COVID-19 vaccine.
Methods
NtAbs were titrated as dose-inhibiting 50% virus replication (ID50) by live virus microneutralization. We evaluated 41 health care workers recovering from mild or asymptomatic infection at first vaccination dose (T1_inf) and 21 days later (T2_inf). Sixteen uninfected health care workers were evaluated 20 days after first dose (T2_uninf) and 20 days after second vaccine dose (T3_uninf).
Results
At T2_inf, but not at T1_inf, there was a significant correlation between days from diagnosis (median 313, interquartile range 285–322) and NtAb levels (P = 0.011). NtAb titres increased at T2_inf with respect to T1_inf (1544 (732–2232) vs 26 (10–88), P < 0.001). Similarly, there was a significant increase in NtAb titres at T3_uninf compared with T2_uninf (183 (111–301) vs 5 (5–15), P < 0001). However, NtAb levels at T2_inf were significantly higher than those at T2_uninf and T3_uninf (P < 0.0001 for both analyses).
Conclusions
A single vaccination in people with mild or asymptomatic previous infection further boosts SARS-CoV-2 humoral immunity to levels higher than those obtained by complete two-vaccination in uninfected subjects.
Keywords: Health care workers, BNT162b2 mRNA COVID-19 vaccine, Neutralizing antibody response
Introduction
While there is consensus that mass vaccination is the key strategy to keep the current SARS-CoV-2 pandemic under control, the need to vaccinate those who have recovered from natural infection is still under debate. Several countries have been vaccinating previously infected people, and this group has not been excluded from the main vaccine field trials (Krammer et al., 2021, Saadat et al., 2021, Manisty et al., 2021), although placing them lower on the vaccination priority list has been suggested (www.has-sante.fr accessed on March 31, 2021). For this group, short-lasting humoral protection after infection has been described (Marot et al., 2021), but a robust T-cell response after a single dose of vaccine has also been reported (Prendecki et al., 2021).
Methods
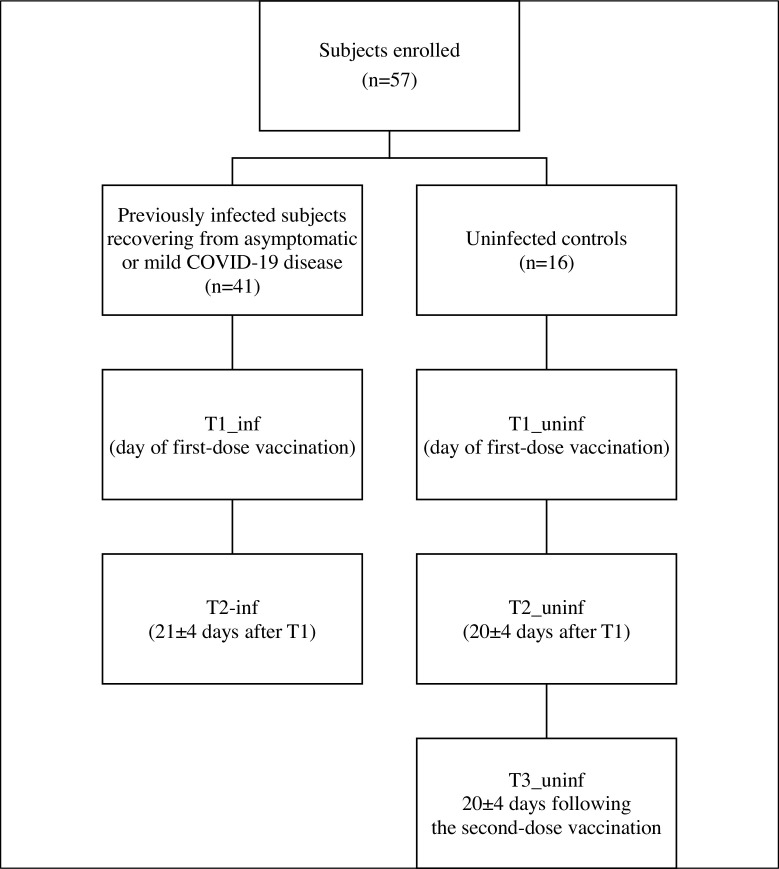
To investigate the role of post-infection vaccination (BNT162b2 mRNA COVID-19 vaccine), we enrolled 57 Italian health care workers (HCW), including 41 SARS-CoV-2 infected individuals (median age 45 (34–51) years, male 34.1%) recovering from asymptomatic infection or mild disease and 16 uninfected controls (median age 49 (34–59), male 31.2%). Written informed consent was obtained from all the HCWs to be enrolled in a neutralizing antibody (NtAb) follow-up study, as approved by the local ethics committee. The previously infected HCWs were tested on the day of their first-dose vaccination (T1_inf) and 21 ± 4 days after that (T2_inf). The uninfected HCW controls were tested on the day of their first-dose vaccination (T1_uninf, all confirmed to be negative), 20 ± 4 days after that (T2_uninf) and 20 ± 4 days following their second-dose vaccination (T3_uninf) (Figure 1 ). For SARS-CoV-2 virus neutralization, two-fold serial dilutions (starting at 1:10 dilution) of heat-inactivated sera were incubated with 100 TCID50 of SARS-CoV-2 virus (lineage B.1) at 37 °C, 5% CO2 for 1 h in 96-well plates. At the end of incubation, 10,000 pre-seeded Vero E6 cells per well (ATCC catalog no. CRL-1586) were treated with serum-virus mixtures and incubated at 37 °C, 5% CO2. Each run included an uninfected control, a virus back titration to confirm the virus inoculum, and a known SARS-CoV-2 neutralizing serum yielding a median (interquartile range [IQR]) titre of 69 (59.3–69.9) in 5 independent runs. After 72 h, cell viability was determined through the commercial kit Cell-titer Glo2.0 (Promega, Wisconsin, USA) following the manufacturer’s instructions. The serum neutralization titre (ID50) was defined as the reciprocal value of the sample dilution that showed a 50% protection of virus cytopathic effect. Antibodies with ID50 titres ≥10 were defined as SARS-CoV-2 seropositive and neutralizing; sera with ID50 <10 were defined as negative and scored as 5 for statistical analysis. NtAb titres were expressed as median (IQR) and the non-parametric Wilcoxon Signed Rank Sum test and Mann–Whitney test were used to analyse changes in paired and unpaired data, respectively. Pearson analysis was used to measure the correlation between NtAb titres before vaccination and the time from diagnosis. Analyses were run by IBM SPSS Statistics, version 20, and all P values were 2-sided.
Figure 1.
Flowchart describing study groups and study time points.
Results
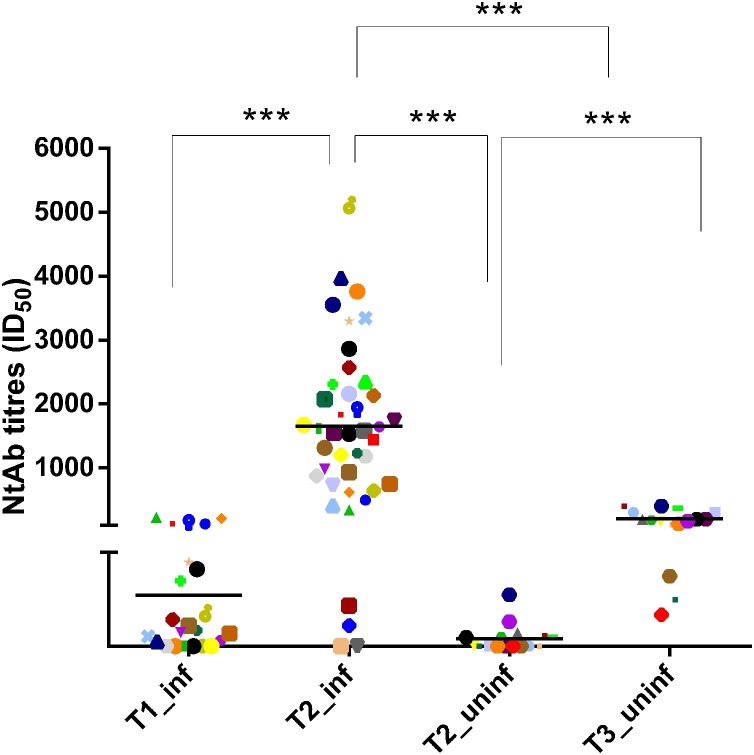
Previously infected HCWs were vaccinated after a median (IQR) of 313.0 (285.5–322.5) days since diagnosis. Mild disease was experienced by 17 subjects, with symptoms of fever (15, 88.2%), cough (11, 64.7%), and loss of smell or taste (3, 17.6%). A detailed description of patients’ disease course is provided in Supplementary Table 1. There was a significant correlation between days from diagnosis and NtAb levels at T2_inf (Pearson R2 = 0.395; P = 0.011) but not at T1_inf (Pearson R2 = 0.119; P = 0.502). NtAb titres increased significantly at T2_inf with respect to T1_inf (median values 1544 (732–2232) vs 26 (10–88), P < 0.001). Similarly, in the previously uninfected control group, there was a significant increase in NtAb titres at T3_uninf with respect to T2_uninf (median values 183 (111–301) vs 5 (5–15), P < 0.001). To investigate differences in post-vaccination NtAb response in previously infected vs uninfected HCWs, T2_inf NtAb titres were compared with T2_uninf and T3_uninf NtAb titres. NtAb levels in the previously infected group were significantly higher than NtAb titres in the uninfected group, when measured both at T2_uninf and at T3_uninf (P < 0.0001 for both analyses) (Figure 2 ).
Figure 2.
Neutralizing antibodies (NtAb) titres in previously infected and uninfected health care workers (HCW) tested at T1, T2 and T3. Data are reported as individual ID50 values and as median value at each study time. The same symbols indicate the same subjects at different time points. Asterisks indicate significance levels: ***, P < 0.001.
T1_inf: NtAb titres on the day of first-dose vaccination in infected HCWs.
T2_inf and T2_uninf: NtAb titres after first-dose vaccination in infected and uninfected HCWs.
T3_uninf: NtAb titres after second-dose vaccine.
ID50: reciprocal value of the sample dilution that showed a 50% protection of virus-induced cytopathic effect.
Discussion
Our data supports vaccination of people with mild or asymptomatic previous infection to boost SARS-CoV-2 immunity further. However, it is not yet known whether this translates into increased protection from reinfection for immunity driven by natural infection only. Response to vaccination appeared to be variable and correlate with time from diagnosis in this selected population, indicating that different patterns of response are obtained following vaccination and suggesting that maturation of immunity improves the efficacy of the vaccination booster. This data, together with the wide range of NtAb levels following natural infection, could suggest reserving vaccinations for those with lower response and/or more remote infection, although there is currently limited evidence that protection from reinfection correlates with NtAb titres (Cohen and Burbelo, 2020, Selhorst et al., 2020, Harvey et al., 2021). It must be noted that variability in pre-vaccination NtAb levels could be partially explained by additional, asymptomatic exposure to SARS-CoV-2 following recovery from primary infection, further complicating our understanding of the kinetics of NtAb response. Finally, the relatively low NtAb titres detected in the uninfected, vaccinated group is apparently of concern; however, importantly, the observation that SARS-CoV-2 infections and morbidity are consistently declining in countries with high vaccination rates (Dagan et al., 2021) is reassuring.
Authors’ contributions
IV: performed the laboratory experiments, helped to interpret the findings and to write the paper.
AB: performed the laboratory experiments.
FG: managed the patients and collected the samples.
RS: managed the patients and collected the samples.
AB: performed the laboratory experiments.
DZ: helped to interpret the findings.
MB: helped to interpret the findings.
FD: performed the laboratory experiments.
MZ: supervised the laboratory experiments, helped to interpret the findings, performed the statistical analysis and wrote the paper.
SGP: designed and coordinated the study, collected and managed the samples and the data, interpreted the findings, and wrote the paper.
Funding source
This work was supported by University of Padova, grant numbers DOR-2019 and DOR-2020to SGP and MB.
Ethical approval
A written informed consent was obtained from all the HCW to be enrolled in a neutralizing antibody (NtAb) follow-up study, as approved by the local Ethics Committee.
Conflict of interests
The authors declare that they have no conflict of interest.
Acknowledgments
We would like to thank Alessia Lai for making the SARS-CoV-2 lineage B strain available for this study.
Preliminary data from this study were presented as Science Spotlight™ presentation at virtual CROI 2021.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2021.05.033.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Cohen J.I., Burbelo P.D. Reinfection with SARS-CoV-2: implications for vaccines. Clin Infect Dis. 2021 doi: 10.1093/cid/ciaa1866. ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021 doi: 10.1056/NEJMoa2101765. NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R.A., Rassen J.A., Kabelac C.A., Turenne W., Leonard S., Klesh R. Association of SARS-CoV-2 seropositive antibody test with risk of future infection. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.has-sante.fr/jcms/p_3237271/fr/strategie-de-vaccination-contre-le-sars-cov-2-vaccination-des-personnes-ayant-un-antecedent-de-covid-19. [Accessed on 31 March 2021].
- Krammer F., Srivastava K., Alshammary H., Amoako A.A., Awawda M.H., Beach K.F. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021 doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C., Otter A.D., Treibel T.A., McKnight Á, Altmann D.M., Brooks T. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marot S., Malet I., Leducq V., Zafilaza K., Sterlin D., Planas D. Rapid decline of neutralizing antibodies against SARS-CoV-2 among infected healthcare workers. Nat Commun. 2021;12:844. doi: 10.1038/s41467-021-21111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendecki M., Clarke C., Brown J., Cox A., Gleeson S., Guckian M. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadat S., Tehrani Z.R., Logue J., Newman M., Frieman M.B., Harris A.D. Binding and neutralization antibody titers after a single vaccine dose in health care workers previously infected with SARS-CoV-2. JAMA. 2021 doi: 10.1001/jama.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selhorst P., Van Ierssel S., Michiels J., Mariën J., Bartholomeeusen K., Dirinck E. Symptomatic SARS-CoV-2 reinfection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1850. ciaa1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.