Abstract
The recent outbreak of coronavirus pandemic (COVID-19) introduced by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has greatly affected the global public health. This pandemic disease became particularly threatening after the start of a new wave. Vaccines of tested efficacy to stop COVID-19 infection are being investigated vigorously worldwide. Currently, some specific drugs have been authorized for COVID-19, but the improvement of antivirals requires time. Hence, a faster way of treatment is done by drug repurposing. Repurposing of drugs is promising for treating and reducing the symptoms of the disease, and it a fast, easy, and safe method to address the crisis, because of their previously known applications. Some antimalarial drugs, especially chloroquine and hydroxychloroquine, have been repurposed, as they exhibited promising results in vitro and in vivo. This article investigates repurposed antimalarial drugs, focusing on their antiviral mechanisms of action, effects in combinations, trial results, and their side effects.
Keywords: COIVD-19, Anti-malarial drugs, Drug repurposing, Combination therapy
Graphical abstract
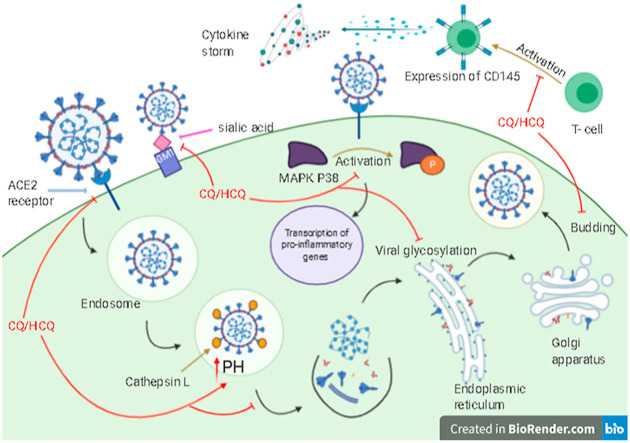
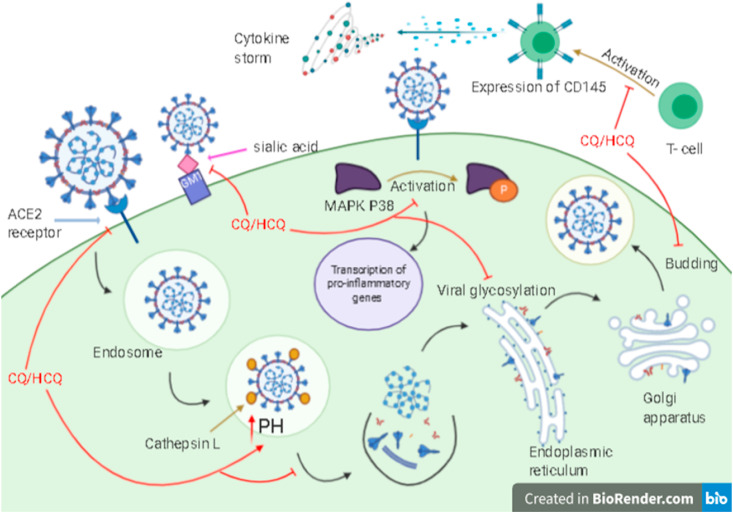
COVID-19 virus lifecycle and several inhibitory mechanisms of CQ/HCQ.
1. Introduction
In Wuhan city (China), December 2019, COVID-19 was identified as the reason for numerous and severe pneumonia-like cases [1,2]. SARS-CoV-2 is the cause of mild to severe pneumonia accompanied by some symptoms like cough, fever, and difficulty in breathing, which constitute the first stage of infection [3]. In the second stage, a cytotoxic effect is produced, causing respiratory failure and probably respiratory distress syndrome. The third stage of the disease is activated by a hyper-inflammatory reaction leading to systemic effects such as fatal cardiovascular effects [4]. It causes alveolar damage and respiratory failure followed by death in severe conditions [5]. Also, a cytokine storm can be produced as a result of the persistent and strong immune response, causing viral progression and death [6]. Therefore, COVID-19 has been identified as a pandemic disease [5]. It has a high morbidity rate and this has led to quarantine, causing massive economic loss [6] (see Fig. 1 )
Fig. 1.
SARS-CoV-2 lifecycle and several inhibitory mechanisms of CQ/HCQ.
The transmission of the virus occurs through social communication with COVID-19 patients, affected by sneezing, coughing, and contacting with infected elements, then touching the mouth, nose, eyes, or by the possible oral-fecal route [[7], [8], [9]]. It was found that the virus is stable for hours in aerosols (half-life is about 1 hour) and for 72 hours on metal and plastic surfaces (half-life is about 7 hours) [10].
It has similarities in phylogenic characters with severe acute respiratory syndrome (SARS-CoV-1) and Middle East respiratory syndrome (MERS-CoV). Thus, SARS-CoV-2 is the third coronavirus showing the progression of viral transmission's capability [11].
In 2002, 8000 individuals were reported as SARS-CoV-1 cases, about 30% of them needed mechanical ventilation, and about 10% of the patients suffered from fatal symptoms. In 2012, more than 2500 cases were reported as MERS-CoV patients, and the first place that reported its existence was Saudi Arabia with a 35% mortality rate. SARS-CoV-2 is thought to be transferred from bats -its natural reservoir-to humans via an intermediate host [12]. The transmission by humans greatly occurs through the symptomatic patient in SARS-CoV-1 and MERS-CoV, unlike SARS-CoV-2, which transmission can occur through the asymptomatic case [4,13].
SARS-CoV-2 is an enveloped single-strand RNA virus [4,11]. It carries a 96% genome in common with bat coronavirus. Its essential target is type-II alveolar epithelial cells in the airway, which contain angiotensin-converting enzyme 2 receptors (ACE2) on their surface. This receptor is utilized by the virus for internalization like SARS-CoV-1. The SARS-CoV-2 is quickly replicating in the host cell and then producing massive signals leading to a response of pro-inflammation of the body. Consequently, the virus spreads fast through the blood to other organs such as the heart, liver, spleen, and kidney. However, SARS-CoV-2 has low pathogenic effect than MERS-CoV and SARS-CoV-1, it is greatly transmitted from one case to another leading to global lock-down and affecting adversely global health [14].
A new wave of coronavirus is envisioned by several researchers. The occurrence of the new wave is not accurate, but it may infect people who have been cured of COVID-19 or uninfected people. All predictions concerning herd immunity remain a matter of conjecture [15].
The in vitro and in vivo studies are still defined, effective treatments and their recovery approaches are urgently needed to overcome this pandemic disease. Moreover, developing a new effective drug takes time to examine its biotherapeutics, and this is unacceptable especially for the current difficult situation. So, repurposing of medication is needed because of its known side effects, pharmacokinetics, safety, and exact dosage; as a result, their usage will be faster with low cost of clinical trials [[16], [17], [18]]. However, many antiviral and anti-inflammatory drugs have been used, no drug has been confirmed as an effective treatment [5]. Besides, the development process of the new effective drug needs more than a billion dollars taking from 10 to 15 years to be produced with only a 2.01% of success rate [19].
Drug development includes five steps: discovering, in vitro studies, in vivo studies, FDA reviewing, and finally FDA post-market monitoring of the safety. While drug repurposing needs only four steps: compound identification, compound acquisition, clinical research, and FDA post-market monitoring of the safety [20]. There is global pressure to discover a cheaper and faster solution for this pandemic disease. Also, there is a global agreement to use repurposed drugs as a faster solution [21].
Repurposing, re-tasking, or re-profiling drugs refers to utilizing pre-approved, shelved, discontinued, and investigational drugs to cover the need for a fast cure to terminate pandemic disease problems [[22], [23], [24], [25], [26], [27], [28]]. Repurposing has two concepts, the first one is that multiple targets are interacted by a single drug, which helps discover new targets for that acknowledged drug. The second concept is that targets correlated with a disease are usually pertinent to some pathogenesis biological processes, that help in designing a new indication [19]. So, repurposing provides fast, riskless, efficient, and good economic treatment [11,22]. There are three steps in the repurposing process: the first one is the recognition of the candidate drug for a specific indication, the second one is the mechanistic application of the effectiveness of the drug in the preclinical stage, the third and final step is the efficacy estimation in phase II clinical trials. This can be done by several methods: mainly by computational approaches like molecular docking, signature matching, genetic association method, retrospective clinical analysis, novel data sources screening, and pathway mapping, and the second approach is an experimental one including phenotypic screening and binding assays for recognizing the target interactions [29].
Many clinical trials were initiated to examine the repurposed drugs that can be used for inhibiting SARS-CoV-2 in vitro or even for holding therapeutic benefits in the treatment [6]. The repurposed drugs should be accessible, cheap and at least one of protocoled drugs has an antiviral property for off-label usage [30]. By skipping the first steps, it goes straight to preclinical and clinical testing, thus minimizing the risks and the cost [11].
However, there are some challenges in the repurposing process: like big data problems that include expanding the gap between the capability of producing huge biomedical data and the capability of interpreting, analyzing, and integrating data. Besides, these data are heterogeneous and dissimilar, which produce extreme difficulties in data integration [29]. Besides, selecting the optimal dose in a different indication. However, the knowledge of drug previous data like preclinical and toxicologic data, may not be suitable for the new indication. These challenges raise the total cost and make the project less attractive in some situations [31].
Antimalarial drugs are potent candidates for repurposing because they were broadly examined in the prophylaxis and therapy frame, and also are widely used with extent groups of age [13,32]. It has immunomodulatory and anti-inflammatory effects [7]. Therefore, the authors explore the overall analysis of candidates of antimalarial drugs that were repositioned against SARS-CoV-2.
1.1. Antimalarial drugs fighting against SARS-CoV-2
1.1.1. Chloroquine
Chloroquine (CQ) is a form of quinine that is used for treating malaria [8]. It was examined in Beijing hospitals and according to the results, it was recommended to be the first drug used for COVID-19 treatment in China especially chloroquine phosphate [10,12]. Its antiviral effect has been shown through in vitro studies in 1960 [12,13]. It showed improvement in the radiological examination of lung more than lopinavir and ritonavir, and also decreased the hospitalization period [33]. Some studies showed the efficacy of CQ and hydroxychloroquine (HCQ) in the cell culture but HCQ was lower in toxicity compared to CQ [34]. CQ and HCQ share nearly the same chemical structure and mechanism of action as well [35]. CQ was tested in vitro and on an animal model showing a promising result but the animal model for HCQ is not confirmed yet [36]. CQ has been used in treating greater than 100 COVID-19 patients exhibited promising outcomes [37].
It was found that COVID-19 patients with mild, moderate, or severe pneumonia cases without CQ contraindication can be treated by it for ten days, 500 mg two times in the day showing high efficacy in recovery time and body temperature [10,21]. Also, these two drugs treat asymptomatic cases, and they can be used for prophylactic treatment [38]. In vitro studies showed that CQ can block coronavirus infection with EC50 of 1.13 μm and with CC50 of more than 100 μm [39].
1.1.1.1. Clinical trials of chloroquine
Patients who do not suffer any complications with chloroquine took oral CQ phosphate 500 mg (300 mg of CQ) twice a day for a maximum of 10 days. This duration was reduced to avoid serious side effects to 7 days for patients with more than 50 kg and an age range of 18–65 years old. Also, for patients below 50 kg with the same age range, the duration decreased to 2 days, and then they took the drug once daily for the next 5 days. The adverse effects of CQ were observed like nausea, vomiting, diarrhea, abdominal pain, cough, itching, and shortening in breathing but it was not excluded during the treatment duration [10,15]. Some trials indicated that greater than 100 cases of COVID-19 who took CQ phosphate showed recovery in lung imaging and a decrease in the treatment course duration despite its narrow margin between the toxic and the therapeutic doses [12,30].
1.1.2. Hydroxychloroquine
Hydroxychloroquine (HCQ) differs from CQ by the hydroxyl group. This group makes the compound more water-soluble, which may cause a large field of conformations and fewer side effects [29,30]. Its intramolecular hydrophobic activity shows that CQ is more compacted than HCQ [40]. It was used for malaria and autoimmune diseases like rheumatoid arthritis and lupus, and currently, in vitro studies showed that it is capable to hamper the COVID-19 virus [12,33].
It was found that HCQ and CQ were effective with coronavirus patients in China [41]. Over the long term usage, HCQ has a safety profile and efficacy higher than that of CQ, and so low drug interactions and a high daily dose can be obtained with good tolerance [11,21,42]. Its greatest tolerable dose is 1200 mg, because of its low accumulation level in tissues so 400 mg daily is considered a safe dose and consequently minor side effects can be expected in long-term therapy. It has a large lung concentration and distribution volume [43]. Also, it has a safe profile for pregnant women [13,44].
Coronavirus cases that carry the nasopharyngeal virus could be cleared within three to six days by indication of HCQ which has fast renal elimination and rapid absorption from the gastrointestinal tract [11,21]. Results from a Chinese team indicated that HCQ inhibits coronavirus in vitro with EC50 of 0.72 μm and with a higher potency than CQ with EC50 of 5.47 μm [41].
1.1.2.1. Clinical trials of hydroxychloroquine
Some solidarity trials which assessed the effectiveness of HCQ, remdesivir, interferon, and lopinavir/ritonavir against SARS-CoV-2 showed no or small impact on total mortality, hospitalization period, and ventilation initiation. Their temporal trial outcomes showed that the effect of HCQ and lopinavir/ritonavir on hospitalized cases mortality have a small or no impact compared to the standard record. So, their investigator disrupted the trials with quick effect. Besides, the temporary outcomes did not give a strong proof of mortality rate increasing; so, this decision covered only the behavior of solidarity trials in hospitalized cases. Moreover, it does not influence the other studies estimation of HCQ or lopinavir/ritonavir in pre and post-exposure prophylaxis and non-hospitalized patients [45].
1.1.2.1.1. Clinical trials of hydroxychloroquine alone and with azithromycin
A French study showed that the administration of 200 mg HCQ three times per day by 26 COVID-19 patients resulted in decreasing the viral carriage on the sixth day of treatment in comparison to the control group, which did not take HCQ [10,46]. By adding azithromycin (ATM) in the treatment protocol of 80 patients, a fast reduction in the viral load of the nasopharynx with 93% on the eighth day was recorded. A Chinese study divided 62 cases by ratio 1:1, the first group took 200 mg of HCQ-sulfate twice daily for 5 days with standard treatment and the control group took antibacterial, antiviral drugs, and immunoglobulin with corticosteroid or without it and with oxygen therapy. The study results showed that HCQ reduced the cough duration, the time required to normal body temperature return, and improved the lung image [33].
1.1.2.1.2. Hydroxychloroquine trials with a zinc supplement
Zinc is important for the physiological antiviral effect. It is an important micronutrient for our body and its deficiency can occur in elderly patients with chronic pulmonary issues, cardiovascular issues, or diabetes. There is a hypothesis that indicates that zinc with CQ or HCQ will be more efficient than CQ/HCQ alone. In vitro studies showed that zinc inhibits RdRp of coronavirus and stops its replication besides its antiviral effect against some viruses like Hepatitis E. Adding zinc supplement as ionophore to CQ or HCQ can produce a synergistic or additive effect [47,48]. Moreover, zinc activates antiviral immunity, which is so important especially for older cases. Increasing zinc level intracellularly especially in lysosomes can decrease the viral RdRp activity; thus, decreasing virus replication. It can be taken with 45 mg daily for a year as a supplement to reduce infection incidence in the elderly, and it is well tolerated and safe taking dietary allowances into account.
According to some treatment experiments, it is important to take an antibiotic in combination, so taking HCQ 200 mg twice daily, azithromycin 500 mg once daily and zinc-sulfate 220 mg once daily for 5 days is recommended [48].
1.1.2.1.3. Hydroxychloroquine combination with nitazoxanide, lopinavir, or ritonavir
A synergistic combination of HCQ and nitazoxanide was reported by Padmanabhan, through inhibiting the viral replication by HCQ and regulating innate immunity by nitazoxanide. There is another combination in India that indicated the promising activity of HCQ with lopinavir or ritonavir as lopinavir and ritonavir act on viral 3CLpro protease, which causes proteolysis in the viral replication cycle [49].
1.1.2.1.4. Hydroxychloroquine combination with ivermectin
Ivermectin is an antiparasitic drug with broad-spectrum activity, represents the second drug line in COVID-19 treatment. It was approved by the FDA by having broad-spectrum antiviral effects using in vitro studies. It was found that it can decrease SARS-CoV-2 replication by 99.98% after 48 h of indication [50]. Although its mechanism of action is not well-known, it is thought to have the same mechanism against other viruses by inhibiting the host protein and viral nuclear import. Besides, it shows no toxic side effects at any time [51]. It is also safe for the COVID-19 pregnant cases, and there is only one clinical study concerning ivermectin and HCQ combination to prove its efficacy [50].
1.1.2.1.5. Postexposure hydroxychloroquine trail
The postexposure prophylaxis from COVID-19 using HCQ has a narrow capacity [52]. There is a study that registered 821 patients with no symptoms, who had occupational exposure to a confirmed case of COVID-19 with lesser than 6 ft in distance and within greater than 10 min without wearing an eye shield or face mask (high risk) or with face mask-wearing but without eye shield (moderate risk), receiving HCQ or placebo 800 mg once then 600 mg through 6–8 h followed by 600 mg every day for additional 4 days. After the patients' exposure to moderate to high risk of coronavirus infection HCQ did not block the infection [53].
1.1.2.1.6. The failure of hydroxychloroquine and some other drugs in clinical trials
The solidarity trials in 30 countries, at 405 hospitals, assigned 11,330 adults to randomized trials, 2750 patients received remdesivir, 954 received HCQ, 1411 received lopinavir without interferon, and 2063 with interferon, and finally 4088 without trial drug. The adherence was about 94–96% and the crossover was about 2–6%, this is all with total deaths of 1253 cases, and the maximum number occurred on day 8. 301 deaths were recorded of 2743 remdesivir treated cases, 303 deaths of 2708 cases that received its control, 104 deaths of 947 HCQ treated cases, 84 deaths of 906 cases who received its control, 148 deaths of 1399 lopinavir treated cases,146 deaths of 1372 who received its control, 243 deaths of 2050 interferon treated patients, and 216 deaths of 2050 cases who received its control. This indicated that no drug has approved its ability to reduce the mortality rate [54].
1.1.3. Chloroquine and hydroxychloroquine pharmacokinetics
Bioavailability of oral CQ and HCQ can be raised to 80% in plasma peak through 2–4 h. Thus, the parenteral route may be notably better than the oral route that represents a big interpatient changeability. Their half-life between 30 and 60 days and their distribution volume is 200–800 L/kg. More than 60% of these drugs are excreted unchanged in the urine and the other 40% is cleared via liver, skin, and stool [55]. CQ has 51% unchanged drug in renal excretion and 21% for HCQ [43].
1.1.4. Chloroquine and hydroxychloroquine mechanisms of action
1.1.4.1. Acting on ACE2 receptor
They can inhibit the virus pre-entry stage by occupying the cell receptors (ACE2), where the S (spike) virus protein can bind, and prevent endocytosis [34,46,55]. In-silico study supported that CQ strongly blocked S-ganglioside. The N-terminal domain (NTD) of SARS-CoV-2 S protein was observed as an attachment interface, its tip acts as a connection interface. Besides, the S protein contains a receptor-binding domain (RBD) that binds to the ACE2 receptor. This study supported the approach of dual receptor binding: RBD has a role in the recognition of the ACE2 receptor, and NTD has a role in finding lipid raft (ganglioside-rich landing place) on the cell surface. The human ganglioside (GM1) cannot bind to NTD and CQ or HCQ at the same time, because of the same method of interaction (CH-π stacking interaction and H-bonding). This study represented the competitive inhibition activity of CQ/HCQ to bind ACE2 [40].
1.1.4.2. Increasing the cellular PH
These drugs are weak diprotic bases, so they can disrupt viral replication like uncoating and fusion by rapidly increasing the endosomal PH (alkalization). This procedure can be made through their accumulation in lysosomes and endosomes, as they act like weak bases, interfere with viral protein modification (post-translation), and impair viral antigen recognition, which needs acidification of the endosome for the proper function of the virus [56,57].
1.1.4.3. Interacting with sialic acid
Interaction of CQ with monosaccharides sialic acids which takes place at the end of the sugar chains on the proteins of cell transmembrane (ganglioside of the cell) could increase the antiviral spectrum of the drug since some viruses like orthomyxoviruses and human coronavirus O43 (HCoV-O43) bind to sialic acids as receptors and recently SARS-CoV-2 was proved for its ability to bind to sialic acid [3,40,42].
Through an in-silico study, it was exhibited that sialic acid usually is presented in gangliosides and glycoprotein taking human ganglioside GM1 in this study as an example. By the formation of H-bond, OH group can force HCQ to bind to sialic acid, two molecules of CQ or two molecules of HCQ can bind to GM1 polar head at the same time, and this clarified that HCQ binding is stronger than CQ. There are two binding sites that GM1 holds, the first in the saccharide moiety tip of GM1, and the second one is large that includes saccharide moiety and the junction of the ceramide-sugar. This in-silico study supported that CQ and HCQ have good binding energy to the isolated sialic acid, and also have a good affinity to GM; so, binding of SARS-CoV-2 S protein to the ganglioside occupied by the drug is blocked. Also, these drugs have a great affinity to 9-O-SIA (9-O-acetyl sialic acid) -a subtype of sialic acid-which the SARS-CoV-2 can bind. There is a good interaction between 9-O-SIA and CQ having an energy of −45 kJ/mol. In this situation, the cationic group in the nitrogen-containing ring of CQ bound to the sialic acid carboxylate group, and OH-π and van der Waals interactions stabilized the complex [40].
1.1.4.4. Interfering with the glycosylation
CQ has an antiretroviral activity through inhibiting gp120 envelope glycoprotein glycosylation and the newly synthesized particles of the virus are non-infective [42,55]. In vitro studies showed that they hamper the N-glycosylation of viral receptors [58]. CQ and HCQ deactivate different enzymes like glycosyltransferases, resulting in glycosylation inhibition. This inhibition can also be a result of developing the response of host adaptive immunity and blocking the fusion and ACE2 receptor [5].
1.1.4.5. Chloroquine and hydroxychloroquine as anti-inflammatory drugs
It was suggested that CQ is an immunomodulatory agent, indirectly reduces proinflammatory signals production, inhibits interleukin-beta (IL-β) mRNA expression and release, it also inhibits interleukin-6 (IL-6), interleukin-1 (IL-1), and tumor necrosis factor-alpha (TNF-α). As a result, it blocks the event cascade which causes acute respiratory distress syndrome (ARDS) [59]. It can increase soluble antigen exportation to dendritic cells cytosol and improve the response of human cytotoxic T lymphocytes (CD8 + T-cell) against the antigen of the virus [40,46,56]. HCQ showed its ability to hinder the cytokine storm by reducing the expression of CD154 (CD40 ligand) [35]. It also decreased T-cell differentiation, activation, expression, and prevented the presentation of major histocompatibility complex (MHC) to T-cells. It can also disrupt the interaction between DNA/RNA, toll-like receptor, and the nucleic acid sensor cyclic GMP-AMP (cGAMP) synthase (cGAS) therefore pro-inflammatory gene transcription cannot be activated [35,60].
1.1.4.6. Inhibition of mitogen-activated protein kinase and the budding
It was recommended that CQ can inhibit kinases like p38 mitogen-activated protein kinase (p38 MAPK), alter the budding of the virus occurring in the endoplasmic reticulum Golgi intermediate compartment membrane (ERGIC), where the protein of the viral envelope can be infused by creating mature virions [58], interfere with the M protein proteolytic process and probably inhibit cathepsins which is essential for S protein cleavage [41,46].
1.1.5. General side effects of chloroquine and hydroxychloroquine
CQ is a human ether a-go-go related gene (hERG) inhibitor, and it has a proarrhythmic effect because it inhibits the inward cardiac potassium current; so it can cause lethal-ventricular arrhythmias and lengthen the QT interval [60,61]. It can also bind to melanin pigment and irreversible retinopathy can be produced with prolonged use [43]. Other adverse effects can be indicated like vomiting, nausea, dizziness, vertigo, and myopathy. These toxicities raised fears about self-administration, severe toxicity, overdose, and death presented in popular journalism [62].
1.1.5.1. QTc interval prolongation
Through HCQ accumulation and protonation, it hampers the lysosomal phospholipase effect leading to skeletal and cardiac muscle vacuolization [56], QTc interval prolongation, and raises the incidence of torsades de-pointes (TdP). Other medications like macrolides can raise that risk [43], this depends on the dose. Studies showed that volunteers who took 600 mg their QTc increased by 6.1 ms, and those who took 1200 mg their QTc increased by 28 ms [63]. Some studies showed that the HCQ and ATM combination has more QTc interval variation than HCQ alone and increased cardiovascular problems [64]. Cases treated with HCQ or CQ and ATM have no reported arrhythmogenic or TdP deaths [65], but patients with cardiac issues should be monitored with QTc interval assessment [63].
1.1.5.2. Hyper/hypo-glycemia
The mortality of coronavirus cases with diabetes is 7.6% despite it is 0.9% with non-comorbidities corona cases. It is claimed that hyperglycemia is produced by pancreatic islet cells transitory inflammation over SARS-CoV-2 binding to ACE2 in islet cells, so transitory insulin-dependent diabetes is produced, which recover with the resolution of COVID-19. The high ACE2 glycosylation in the nasal airway, oropharynx, tongue, and lung with no control of hyperglycemia may increase the infection and the severity of the disease. CQ and HCQ act as hypoglycemic agents, taking one of these drugs for treating rheumatologic diseases in diabetic patients that are producing hemoglobin A1c reduction in the comparison of methotrexate; so, there is a reduction of COVID-19 hyperglycemia, the disease risk factor. An in-silico study suggested that these drugs bind to the UDP-N-acetylglucosamine-2-epimerase active site, which activates the step of rate-determine within the pathway of sialic acid biosynthesis, so these drugs interfere with the protein glycosylation within the Golgi apparatus [66].
1.1.5.3. Neuropsychiatric effect
CQ and HCQ can cause mania, insomnia, confusion, agitation, depression, paranoia, psychosis, catatonic, and suicidal thoughts. It can happen to different ages through acute or chronic treatment with or without mental illness history. The recovery is thought to happen when the drug is stopped, despite the recovery of the symptoms that may not happen quickly [63].
1.1.5.4. Drug-drug interactions
Both CQ and HCQ inhibit CYP2D6 activity through competitive mechanisms. For example, these drugs raise the peak concentration of oral metoprolol to 75% and systemic exposure by 65%. These drugs also potentiate more CYP2D6 substrates like carvedilol and sap the efficacy of prodrugs, which depends on the activation of CYP2D6 like tramadol and codeine. Unlike azithromycin showing a bit decreasing in CyP450 or P-glycoprotein (drug-transport protein) [63]. Other drugs that should not be taken or should be taken with highly monitoring include antiepileptics, digoxin, cyclosporine, antacids, moxifloxacin, amiodarone, insulin, azithromycin, praziquantel, tamoxifen, and antibiotics [67].
1.1.5.5. Overdose side effects
Both CQ and HCQ are very toxic especially when taken with overdose showing coma and seizures due to toxicity of CNS, prolongation of QT interval and QRS widening due to cardiovascular collapse, and hypokalemia. Treating overdose administration can be done by taking activated charcoal, vasopressors, intravenous benzodiazepine, hypertonic saline, sodium bicarbonate for QRS widening and correlated arrhythmias, and wise hypokalemia management to avoid overcorrection [63].
1.1.6. Safety interests
Side effects are vacuolar myopathy, retinopathy, restrictive cardiomyopathy, and neuropathy. These side effects are negligible in COVID-19 treatment, but they may appear when we use them as prophylactic drugs [63]. The appearance of these side effects vary from one case to another, as myopathy can appear at the minimum in the first 6 months after CQ or HCQ therapy initiation [68], retinopathy can appear after 5 years of CQ or HCQ treatment, and HCQ shows less toxicity than CQ in retinopathy [69,70]. Also, cardiac problems appear in median 7 years of these drugs treatment [70], and 6 months after HCQ treatment can induce neuromyotoxicity [71]. These side effects and dose adjustment are considered big challenges for these drugs [72].
1.2. Other antimalarial drugs used in treating COVID-19
There is a newly approved antimalarial drug-like halofantrine HCL with SARS-CoV-2 EC50 equals 0.33 μm and oral approximately plasma peak concentration (Approx. Cmax) equals 1 μm [2].
Mefloquine was used in COVID-19 management [1], but the danger of convulsions increased with cases treated with it [56].
N1-(7-chloroquinolin-4-yl) (AQ-13) is another antimalarial drug in phase II clinical trials with SARS-CoV-2 EC50 equals 1.07 and oral Approx. Cmax equals 10 μm [2].
Also, pyronaridine is an antimalarial drug that has broad-spectrum antiviral activities, it could fit greatly to the main protease (Mpro) binding pocket; so it may be a good promising repurposed drug for COVID-19 [73].
Moreover, artemisinin is an antimalarial drug and holds an overlapping immunomodulatory effect against viral replication and inflammatory diseases. It is used to treat fever and can be a suitable drug for 83.3% of COVID-19 patients, who suffer from fever. It can inhibit TNF-α and IL-6, mediators of ARDS which deteriorate COVID-19 patients’ state. It can be a promising treatment, as it has a safe toxic profile; so, the high dose can be taken with lesser damage. Also, it can be an alternative to other drugs having higher side effects like CQ and HCQ. Furthermore, it can suppress the cytokine storm as well [74].
2. Conclusion
The large mortality rate makes COVID-19 a pandemic disease and a crucial target for researchers nowadays, especially after the new wave arrived. Currently, some complex drug regimens are available for treating chronic patients with SARS-CoV-2. The safety and efficacy of these potential drugs are most likely to be studied in the treatment of COVID-19. The repurposing of drugs that have antiviral activity, like antimalarial drugs, could be a suitable solution to overcome this pandemic crisis, specifically when manufacturing a new vaccine takes a long time to confirm efficacy and safety towards humans. CQ and HCQ hinder SARS-CoV-2 replication in symptomatic and asymptomatic cases by several mechanisms, such as interfering with the virus endocytosis, budding, and reproduction, which were tested by preclinical and clinical studies. Clinical studies showed the speedy recovery of more than 100 patients within a maximium10 days by exhibiting recovery in lung image. These two drugs can be taken with supplements to increase their efficacy, like azithromycin, nitazoxanide, ivermectin, lopinavir or ritonavir, and zinc supplement. Azithromycin with HCQ reduces the nasopharynx viral load by 93% in only 8 days, and this represents a good combination efficacy. Ivermectin is an effective combination because it was represented as the second drug line in the treatment protocols. Also, zinc is an important supplement especially with elderly patients as it hinders RdRp activity in host tissues. However, HCQ showed greater efficacy and low toxicity than CQ, because it has better activity, an improved safety profile, and can be tolerated with a high daily dose. Also, it is safe to administer to pregnant women and its accumulation in the lung is greater than its accumulation in other host tissues; hence, it exhibits good lung activity and the side effects are low. Other antimalarial drugs that have anti-SARS-CoV-2 activities can be promising alternatives to CQ and HCQ because they have lower toxicity. These findings suggest that antimalarial drugs are an important class for treating COVID-19.
Ethical approval
Not applicable.
Sources of funding
This study received no funding at any stage.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The authors are very thankful to Ass. Prof. Dr. Shimaa Hassanin, Associate Professor at the Department of English Language, Faculty of Physical Therapy, Horus University-Egypt, Damietta, Egypt, for her great effort in reviewing and editing the final manuscript.
References
- 1.Rodrigues‐Diez R.R., et al. Statins: could an old friend help in the fight against COVID‐19? Br J Pharmacol. 2020;177(21):4873–4886. doi: 10.1111/bph.15166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakowski M.A., et al. Oral drug repositioning candidates and synergistic remdesivir combinations for the prophylaxis and treatment of COVID-19. BioRxiv. 2020 [Google Scholar]
- 3.Ciliberto G., Mancini R., Paggi M.G. Drug repurposing against COVID-19: focus on anticancer agents. J Exp Clin Canc Res. 2020;39:1–9. doi: 10.1186/s13046-020-01590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lang J.P., et al. A current review of COVID-19 for the cardiovascular specialist. Am Heart J. 2020 doi: 10.1016/j.ahj.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathy S., et al. A review on possible modes of action of chloroquine/hydroxychloroquine: repurposing against SAR-CoV-2 (COVID-19) pandemic. Int J Antimicrob Agents. 2020;56(2):106028. doi: 10.1016/j.ijantimicag.2020.106028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez M.A. Clinical trials of repurposed antivirals for SARS-CoV-2. Antimicrob Agents Chemother. 2020;64(9) doi: 10.1128/AAC.01101-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altay O., et al. Current status of COVID-19 therapies and drug repositioning applications. Iscience. 2020:101303. doi: 10.1016/j.isci.2020.101303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojkovic‐Filipovic J., Bosic M. Treatment of COVID 19—repurposing drugs commonly used in dermatology. Dermatol Ther. 2020;33(5) doi: 10.1111/dth.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan W., Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol. 2020;309:70–77. doi: 10.1016/j.ijcard.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Doremalen N., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanty S., et al. Application of artificial intelligence in COVID-19 drug repurposing. Diabetes, Metab Syndrome: Clin Res Rev. 2020 doi: 10.1016/j.dsx.2020.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atri D., et al. COVID-19 for the cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. Basic Transl Med. 2020;5(5):518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollard C.A., Morran M.P., Nestor-Kalinoski A.L. The COVID-19 pandemic: a global health crisis. Physiol Genom. 2020;52(11):549–557. doi: 10.1152/physiolgenomics.00089.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rastogi M., et al. SARS coronavirus 2: from genome to infectome. Respir Res. 2020;21(1):1–15. doi: 10.1186/s12931-020-01581-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlagenhauf P., et al. Repurposing antimalarials and other drugs for COVID-19. Trav Med Infect Dis. 2020;34:101658. doi: 10.1016/j.tmaid.2020.101658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Karmalawy A.A., Khattab M. Molecular modelling of mebendazole polymorphs as a potential colchicine binding site inhibitor. New Journal of Chemistry. 2020;44:13990–13996. [Google Scholar]
- 17.Eliaa S.G., et al. vol. 3. 2020. pp. 1330–1338. (Empagliflozin and doxorubicin synergistically inhibit the survival of triple-negative breast cancer cells via interfering with the mTOR pathway and inhibition of calmodulin: in vitro and molecular docking studies). 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khattab M., Al‐Karmalawy A.A. Revisiting activity of some nocodazole analogues as a potential anticancer drugs using molecular docking and DFT calculations. Front. Chem. 2021;9:92. doi: 10.3389/fchem.2021.628398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh T.U., et al. Pharmacological Reports; 2020. Drug repurposing approach to fight COVID-19; pp. 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xue H., et al. Review of drug repositioning approaches and resources. Int J Biol Sci. 2018;14(10):1232. doi: 10.7150/ijbs.24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glebov O.O. Understanding SARS‐CoV‐2 endocytosis for COVID‐19 drug repurposing. FEBS J. 2020;287(17):3664–3671. doi: 10.1111/febs.15369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al‐Karmalawy A.A., Eissa I.H.J.P.S. 2021. Molecular docking and dynamics simulations reveal the potential of anti-HCV drugs to inhibit COVID-19 main protease. [Google Scholar]
- 23.Alnajjar R., et al. vol. 6. 2020. (Molecular docking, molecular dynamics, and in vitro studies reveal the potential of angiotensin II receptor blockers to inhibit the COVID-19 main protease). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eissa I., et al. Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting hACE2 receptor. Front. Chem. 2021;9:227. doi: 10.3389/fchem.2021.661230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghanem A., et al. vol. 44. 2020. pp. 17374–17381. (Tanshinone IIA synergistically enhances the antitumor activity of doxorubicin by interfering with the PI3K/AKT/mTOR pathway and inhibition of topoisomerase II: in vitro and molecular docking studies). 40. [Google Scholar]
- 26.Zaki A.A., et al. vol. 44. 2020. pp. 16752–16758. (Molecular docking reveals the potential of Cleome amblyocarpa isolated compounds to inhibit COVID-19 virus main protease). 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elmaaty A.A., et al. Revisiting activity of some glucocorticoids as a potential inhibitor of SARS-CoV-2 main protease: theoretical study. RSC Adv. 2021;11(17):10027–10042. doi: 10.1039/d0ra10674g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elmaaty A.A., et al. In a search for potential drug candidates for combating COVID-19: computational study revealed salvianolic acid B as a potential therapeutic targeting 3CLpro and spike proteins. J Biomol Struct Dyn. 2021:1–28. doi: 10.1080/07391102.2021.1918256. [DOI] [PubMed] [Google Scholar]
- 29.Pushpakom S., et al. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov. 2019;18(1):41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigo C., Fernando S.D., Rajapakse S. Clinical evidence for repurposing chloroquine and hydroxychloroquine as antiviral agents: a systematic review. Clin Microbiol Infect. 2020 doi: 10.1016/j.cmi.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breckenridge A., Jacob R. Overcoming the legal and regulatory barriers to drug repurposing. Nat Rev Drug Discov. 2019;18(1):1–2. doi: 10.1038/nrd.2018.92. [DOI] [PubMed] [Google Scholar]
- 32.Krishna S., et al. Repurposing antimalarials to tackle the COVID-19 pandemic. Trends Parasitol. 2020 doi: 10.1016/j.pt.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao J., Hu S. Update on use of chloroquine/hydroxychloroquine to treat coronavirus disease 2019 (COVID-19) Biosci Trends. 2020 doi: 10.5582/bst.2020.03072. [DOI] [PubMed] [Google Scholar]
- 34.Ulm J.W., Nelson S.F. COVID‐19 drug repurposing: summary statistics on current clinical trials and promising untested candidates. Transboundary Emerg Dis. 2020 doi: 10.1111/tbed.13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meo S., Klonoff D., Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–4547. doi: 10.26355/eurrev_202004_21038. [DOI] [PubMed] [Google Scholar]
- 36.Thomas D.S., Yesodharan D.K., Arulappan J. Emerging pharmacotherapy for COVID-19 treatment: an integrative review. Int J Nutr Pharmacol Neurol Dis. 2020;10(4):171. [Google Scholar]
- 37.Spinelli F.R., et al. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann Rheum Dis. 2020;79(5):666–667. doi: 10.1136/annrheumdis-2020-217367. [DOI] [PubMed] [Google Scholar]
- 38.Sinha N., Balayla G. Hydroxychloroquine and covid-19. Postgrad Med. 2020;96(1139):550–555. doi: 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao J., Tian Z., Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020 doi: 10.5582/bst.2020.01047. [DOI] [PubMed] [Google Scholar]
- 40.Fantini J., et al. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int J Antimicrob Agents. 2020;55(5):105960. doi: 10.1016/j.ijantimicag.2020.105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gautret P., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Devaux C.A., et al. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saha B.K., Bonnier A., Chong W. Antimalarials as antivirals for COVID-19: believe it or not! Am J Med Sci. 2020 doi: 10.1016/j.amjms.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir Res. 2020;177:104762. doi: 10.1016/j.antiviral.2020.104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO “Solidarity” clinical trial for COVID-19 treatments. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov/solidarity-clinical-trial-for-covid-19-treatments Available from.
- 46.Sun X., Ni Y., Zhang M. Rheumotologitsts' view on the use of hydroxychloroquine to treat COVID-19. Emerg Microb Infect. 2020;9(1):830–832. doi: 10.1080/22221751.2020.1760145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xue J., et al. Chloroquine is a zinc ionophore. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derwand R., Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19? Med Hypotheses. 2020;142:109815. doi: 10.1016/j.mehy.2020.109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pawar A.Y. Elsevier; 2020. Combating devastating COVID-19 by drug repurposing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drożdżal S., et al. FDA approved drugs with pharmacotherapeutic potential for SARS-CoV-2 (COVID-19) therapy. Drug Resist Updates. 2020:100719. doi: 10.1016/j.drup.2020.100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Choudhary R., Sharma A.K. New microbes and new infections; 2020. Potential use of hydroxychloroquine, ivermectin and azithromycin drugs in fighting COVID-19: trends, scope and relevance; p. 100684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Avidan M.S., Dehbi H.-M., Delany-Moretlwe S. Hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020:383. doi: 10.1056/NEJMc2023617. [DOI] [PubMed] [Google Scholar]
- 53.Boulware D.R., et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383(6):517–525. doi: 10.1056/NEJMoa2016638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Consortium W.S.T. Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N Engl J Med. 2021;384(6):497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashem A.M., et al. Travel medicine and infectious disease; 2020. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: a narrative review; p. 101735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pereira B.B. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J Toxicol Environ Health, Part B. 2020;23(4):177–181. doi: 10.1080/10937404.2020.1752340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C.-C., Chen M.-Y., Chang Y.-L. Potential therapeutic agents against COVID-19: what we know so far. J Chin Med Assoc. 2020 doi: 10.1097/JCMA.0000000000000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roldan E.Q., et al. The possible mechanisms of action of 4-aminoquinolines (chloroquine/hydroxychloroquine) against Sars-Cov-2 infection (COVID-19): a role for iron homeostasis? Pharmacol Res. 2020:104904. doi: 10.1016/j.phrs.2020.104904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giri A., et al. Mutagenic, Genotoxic and Immunomodulatory effects of Hydroxychloroquine and Chloroquine: a review to evaluate its potential to use as a prophylactic drug against COVID-19. Gene Environ. 2020;42(1):1–14. doi: 10.1186/s41021-020-00164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kamp T.J., Hamdan M.H., January C.T. Chloroquine or hydroxychloroquine for COVID‐19: is cardiotoxicity a concern? J Am Heart Assoc. 2020;9(12) doi: 10.1161/JAHA.120.016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lentini G., Cavalluzzi M.M., Habtemariam S. COVID-19, chloroquine repurposing, and cardiac safety concern: chirality might help. Molecules. 2020;25(8):1834. doi: 10.3390/molecules25081834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keshtkar-Jahromi M., Bavari S. A call for randomized controlled trials to test the efficacy of chloroquine and hydroxychloroquine as therapeutics against novel coronavirus disease (COVID-19) Am J Trop Med Hyg. 2020;102(5):932. doi: 10.4269/ajtmh.20-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Juurlink D.N. Safety considerations with chloroquine, hydroxychloroquine and azithromycin in the management of SARS-CoV-2 infection. CMAJ (Can Med Assoc J) 2020;192(17):E450–E453. doi: 10.1503/cmaj.200528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiolet T., et al. Effect of hydroxychloroquine with or without azithromycin on the mortality of coronavirus disease 2019 (COVID-19) patients: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(1):19–27. doi: 10.1016/j.cmi.2020.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Saleh M., et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circulation: Arrhythmia and Electrophysiology. 2020;13(6) doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID‐19 pandemic. J Med Virol. 2020;92(7):770–775. doi: 10.1002/jmv.25887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pastick K., et al. 2020. Review: hydroxychloroquine and chloroquine for treatment of SARS-CoV-2 (COVID-19) open forum infect. Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwon J.-B., et al. Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16(1):28–31. doi: 10.1097/RHU.0b013e3181c47ec8. [DOI] [PubMed] [Google Scholar]
- 69.Yusuf I., et al. Hydroxychloroquine retinopathy. Eye. 2017;31(6):828–845. doi: 10.1038/eye.2016.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatre C., et al. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41(10):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 71.Vinciguerra C., et al. Hydroxychloroquine neuromyotoxicity: a case with rapid course and complete recovery. Neurol Sci. 2015;36(12):2293–2294. doi: 10.1007/s10072-015-2355-2. [DOI] [PubMed] [Google Scholar]
- 72.Chyu K.-Y., Dimayuga P.C., Shah P.K. Vaccine against arteriosclerosis: an update. Ther Adv Vaccine. 2017;5(2):39–47. doi: 10.1177/2051013617693753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hosseini F.S., Amanlou M. Anti-HCV and anti-malaria agent, potential candidates to repurpose for coronavirus infection: virtual screening, molecular docking, and molecular dynamics simulation study. Life Sci. 2020;258:118205. doi: 10.1016/j.lfs.2020.118205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voss A., et al. Publishing in face of the COVID-19 pandemic. Int J Antimicrob Agents. 2020;56(1):106081. doi: 10.1016/j.ijantimicag.2020.106081. [DOI] [PMC free article] [PubMed] [Google Scholar]