Abstract
Background
Paediatric Multisystem Inflammatory Syndrome temporally associated with SARS-CoV-2 (PIMS-TS), first identified in April 2020, shares features of both Kawasaki disease (KD) and toxic shock syndrome (TSS). The surveillance describes the epidemiology and clinical characteristics of PIMS-TS in the United Kingdom and Ireland.
Methods
Public Health England initiated prospective national surveillance of PIMS-TS through the British Paediatric Surveillance Unit. Paediatricians were contacted monthly to report PIMS-TS, KD and TSS cases electronically and complete a detailed clinical questionnaire. Cases with symptom onset between 01 March and 15 June 2020 were included.
Findings
There were 216 cases with features of PIMS-TS alone, 13 with features of both PIMS-TS and KD, 28 with features of PIMS-TS and TSS and 11 with features of PIMS-TS, KD and TSS, with differences in age, ethnicity, clinical presentation and disease severity between the phenotypic groups. There was a strong geographical and temporal association between SARS-CoV-2 infection rates and PIMS-TS cases. Of those tested, 14.8% (39/264) children had a positive SARS-CoV-2 RT-PCR, and 63.6% (75/118) were positive for SARS-CoV-2 antibodies. In total 44·0% (118/268) required intensive care, which was more common in cases with a TSS phenotype. Three of five children with cardiac arrest had TSS phenotype. Three children (1·1%) died.
Interpretation
The strong association between SARS-CoV-2 infection and PIMS-TS emphasises the importance of maintaining low community infection rates to reduce the risk of this rare but severe complication in children and adolescents. Close follow-up will be important to monitor long-term complications in children with PIMS-TS.
Funding
PHE.
Research in context.
Evidence before this study
We searched Embase, Medline, Scopus, medRxiv, bioRxiv and Google for articles on PIMS-TS or Multisystem Inflammatory Syndrome in Children (MIS-C) during the COVID-19 pandemic. The search was for papers published from 01 Jan 2020, and no language restrictions were applied. A full list of search terms are listed in Supplementary Material 1, but in brief, search terms were (“SARS” OR “COVID” OR “coronavirus” or “nCoV”) AND (“kawasaki” OR “hyper inflammatory” OR “cytokine” OR “lymph node syndrome” OR “toxic shock” OR “PIMS-TS” OR “MIS-C” OR “TSS”) AND (“infant” OR “child” OR “adolescent” OR “minors” OR “puberty” OR “paediatrics” OR “pediatrics”). We identified 10 relevant studies (combined N = 847), in which: 12–100% cases had cardiovascular involvement, 42–100% had gastrointestinal involvement, and 13–56% had neurological involvement. In these studies, 9–62% required mechanical ventilation, 21–100% required admission to intensive care, and 0–4% died. Where reported, 20–75% cases were SARS-CoV-2 PCR positive, and 33–90% were SARS-CoV-2 serology positive. Criteria for KD were fulfilled in 4–100% of cases in reported studies, although this was sometimes an inclusion criteria.
Added value of this study
This study provides information on a large prospective cohort of children with PIMS-TS. There was a regional association between trends in SARS-CoV-2 incidence and PIMS-TS reports by lower tier Local Authorities, providing additional evidence of an epidemiological link between the syndrome and SARS-CoV-2. While signs and symptoms are comparable to other studies, we also characterise different phenotypes of case presentations using Latent Class Analysis, which broadly agreed with findings reported by United States Centers for Disease Prevention and Control (CDC).
Implications of all the available evidence
The strong temporal association between SARS-CoV-2 infection and PIMS-TS emphasises the importance of maintaining low community infection rates to reduce the risk of this rare but severe complication in children and adolescents. Based on the reported cases that did not fulfil the PIMS-TS criteria, we propose that an elevated C-Reactive Protein is not a sensitive criterion for syndromic diagnosis.
Alt-text: Unlabelled box
1. Introduction
Children make up only a small proportion of coronavirus disease 2019 (COVID-19) cases, hospitalisations or deaths. [1] Several countries in Europe, [2], [3], [4], [5], [6], [7], [8] the Americas [9], [10], [11], [12] and Asia [13], [14], [15] have, however, reported cases of a rare Paediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2 (PIMS-TS). The symptoms and signs are similar to both Kawasaki disease (KD) and toxic shock syndrome (TSS), and are typically characterised by single- or multi-organ dysfunction, although less serious presentations, such as abdominal pain, vomiting and diarrhoea have also been reported. [12] The first reports of KD-/TSS-like presentations with COVID-19 first emerged in April 2020, [16, 17] and PIMS-TS cases have since been reported globally. [18] In the United States, where PIMS-TS is termed Multisystem Inflammatory Syndrome in Children (MIS-C), 1659 cases and 26 deaths were reported by 08 January 2021. [12] In Europe and elsewhere, most PIMS-TS reports have been limited to small case series with varying case definitions. Following the first reports of PIMS-TS cases in London, England, [16] Public Health England (PHE) initiated enhanced prospective national surveillance of PIMS-TS, along with KD and TSS, in the United Kingdom (UK) and the Republic of Ireland through the British Paediatric Surveillance Unit team (BPSU). Here we present the epidemiology, temporal and geographical association with SARS-CoV-2 incidence, clinical and analytical findings, and outcomes of the first tranche of cases reported up until 24 July 2020.
2. Methods
2.1. Literature review
A literature search was conducted to compare the BPSU data with other similar scale studies of PIMS-TS cases published to date (Supplementary Material 1).
2.2. Data collection
This study was conducted through the BPSU of the Royal College of Paediatrics and Child Health (RCPCH), a unit established three decades ago to study rare paediatric diseases. Paediatricians across the UK and Ireland receive monthly email requests to report cases of rare conditions listed on the “Orange Card”, including nil returns. Around 90% of paediatricians complete the Orange Card each month. [19] PIMS-TS was added to the Orange Card from 04 May 2020 onwards, and paediatricians reporting cases were contacted to complete an electronic questionnaire regarding demographics, concurrent medical conditions, symptoms, signs, investigations, laboratory results and treatments.
2.3. Case definitions
The BPSU reporting case definition was purposely broad to capture all potential cases and included children aged <16 years, with illness onset from 01 March 2020, who had PIMS-TS, [20] typical/atypical KD, [21] or typical/atypical TSS, [22] regardless of SARS-CoV-2 status (Table 1, Supplementary Material 2).
Table 1.
Definitions of PIMS-TS, KD, TSS used for analysis of cases.
As per the RCPCH definition, [20] PIMS-TS cases were defined as patients with:
|
KD was defined according to the criteria set out in McCrindle et al. (2017): [21] having fever duration >4 days and at least four of the following signs:
|
TSS cases were defined by the presence of at least four of the following:
|
Current or past infection with SARS-CoV-2 was explicitly not an inclusion requirement
ALT Alanine transaminase; CRP C-reactive Protein; KD Kawasaki disease; LFT Liver function test; PIMS-TS Paediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2; RCPCH Royal College of Paediatrics and Child Health; TSS Toxic shock syndrome.
2.4. Data analysis
Reported cases were categorised according to criteria for individual syndromes. Descriptive analysis was undertaken in STATA v.15.0 (StataCorp, Tx). Since case numbers in most categories were small, P-values were not calculated in comparisons between categories. The geographic distribution of PIMS-TS cases was compared to SARS-CoV-2 infection rates per 10,000 population by lower tier local authority. Only qualitative analysis was performed because PIMS-TS rates at local authority level were too low to make meaningful quantitative comparisons. Geographical analysis was performed in Python using the GeoPandas (v.0.8.1) package alongside ArcMap software (ESRI). Temporal analysis was performed using published national and regional SARS-CoV-2 infection rates in England. [23] Lag time was defined as the number of days between peak regional SARS-CoV-2 incidence and peak PIMS-TS cases. Mean weight by age and sex data [24] were used to calculate Z-scores [=(value-population mean)/population SD] and identify children who were overweight (2 standard deviations (SDs) above their mean weight for age and sex).
2.5. Latent class analysis
Latent Class Analysis (LCA) aims to define unobserved groups of cases within the data, based on indicator (yes/no) variables. The method identifies possible clustering models which group cases into distinct latent classes. The fit of each model is assessed using a Bayesian Information Criterion score, with the best fitting model being the one which minimises this score. The selected model provides a conditional probability for membership of the identified latent classes, given the ‘yes/no’ value of each indicator variable. The R package poLCA (v.1.4.1) was used to identify latent classes based on the 23 indicator variables corresponding to the PIMS-TS, KD and TSS definitions, along with variables confirming SARS-CoV-2 infection by RT-PCR or serology (Supplementary Material 3).
3. Results
3.1. Literature review
Data from ten published studies are summarised alongside the BPSU dataset in Tables S1.2 and S1.3.
3.2. BPSU surveillance
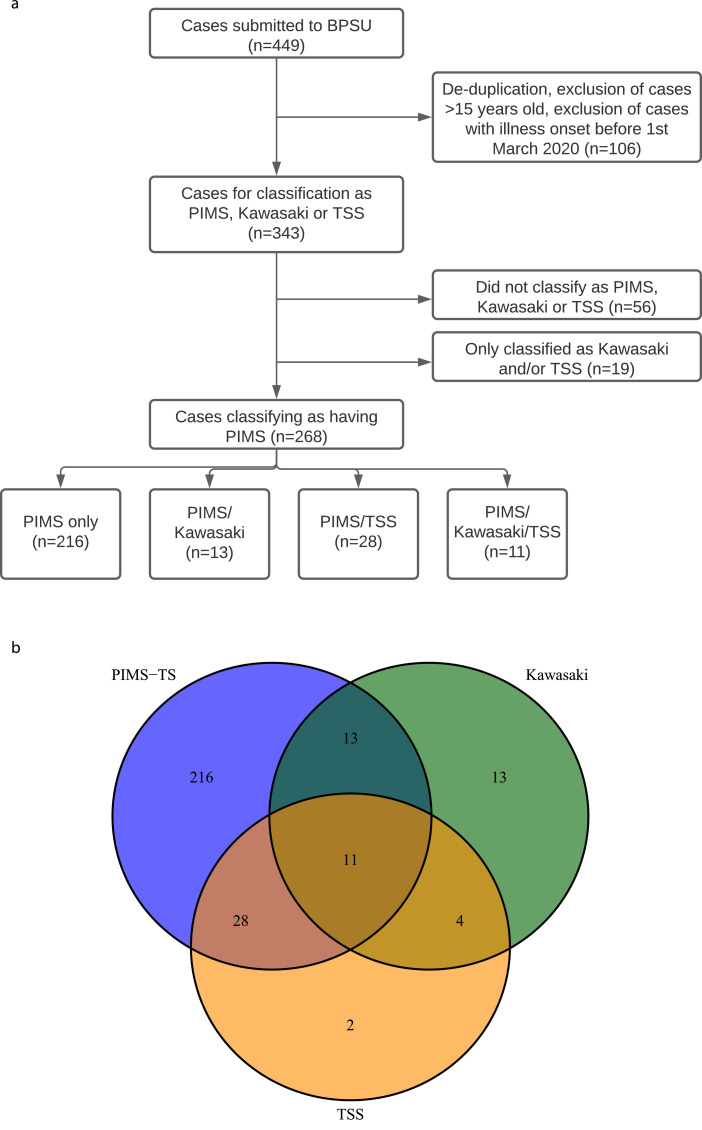
There were 449 cases reported to BPSU. Eighty-nine duplicates were removed, 12 were ≥16 years old and five had onset dates pre-01 March 2020. This left 343 cases with illness onset between 01 March and 15 June 2020; 268 were classified as PIMS-TS and included in the analysis (Fig. 1a). The remaining 75 cases included 56 cases that did not fulfil the PIMS-TS, KD or TSS criteria (35 because of C-reactive protein (CRP) <100 mg/L, eight because CRP level was missing; 13 lacked other criteria), 13 with KD only, two with TSS only and four with KD/TSS.
Fig. 1a.
(a) Diagram showing cases submitted to BPSU through to those used in the final analysis. (b) Venn diagram showing overlap of PIMS-TS/Kawasaki/TSS case definitions.
Of the PIMS-TS cases, 246 were in England, six in Scotland, 11 in Wales, two in Northern Ireland, and three in the Republic of Ireland. Cases were categorised based on pre-defined syndromic criteria (Fig. 1b) and included 216 cases classified as PIMS-TS only without features of KD or TSS, 13 with PIMS-TS and complete/typical KD (PIMS-TS/KD), 28 cases with PIMS-TS and TSS (PIMS-TS/TSS) and 11 with all three phenotypes (PIMS-TS/KD/TSS). Data are presented for all 343 children by phenotype (Supplementary Material 4). Two cases were classified as TSS only, and four as KD and TSS; these were included in total cases only.
3.3. Demographics
The median age of cases was 8·2 (IQR 4·0–12·1) years, with PIMS-TS/KD cases being younger (5.2 years) and PIMS-TS/TSS cases older (8·8 years) than PIMS-TS only cases (7·8 years; Table 2). Males were over-represented in all categories, 19·0% (51/268) had co-morbidities, and 13·4% (36/268) were overweight. Although the numbers of children with multiple syndromes were small, a higher proportion of PIMS-TS/KD and PIMS-TS/TSS cases were Asian (46·2% and 42·9%, respectively) or Black (23·1% and 35·7%, respectively), while White children were underrepresented (15·4% and 14·3%, respectively) compared to PIMS-TS only cases where 48·6% were White, 20·4% Asian and 19·4% Black. Of the 63 cases in Asian children, 50 were from the Indian subcontinent (Bangladeshi/Indian/Pakistani/Nepali/Sri Lankan/Afghani), one was East Asian and ten did not have additional information. Parental occupation was reported for 105 cases and 77·1% had a healthcare (39·0%; n = 41) or non-healthcare keyworker (38·1%; n = 40) parent.
Table 2.
Demographic features of cases presenting with PIMS-TS, with illness onset after 1st March 2020.
| Total with any PIMS | PIMS-TS only | PIMS-TS/KD | PIMS-TS/TSS | PIMS-TS/ KD/ TSS | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 268 (100·0%) | 216 (100·0%) | 13 (100·0%) | 28 (100·0%) | 11 (100·0%) |
| Sex | |||||
| Male | 161 (60·1%) | 124 (57·4%) | 11 (84·6%) | 21 (75·0%) | 5 (45·5%) |
| Female | 90 (33·6%) | 79 (36·6%) | 2 (15·4%) | 5 (17·9%) | 4 (36·4%) |
| Age group | |||||
| <1 year | 23 (8·6%) | 19 (8·8%) | 2 (15·4%) | 2 (7·1%) | 0 (0·0%) |
| 1–4 years | 65 (24·3%) | 54 (25·0%) | 4 (30·8%) | 4 (14·3%) | 3 (27·3%) |
| 5–9 years | 80 (29·9%) | 61 (28·2%) | 4 (30·8%) | 10 (35·7%) | 5 (45·5%) |
| 10–15 years | 100 (37·3%) | 82 (38·0%) | 3 (23·1%) | 12 (42·9%) | 3 (27·3%) |
| Median age (IQR) | 8·21 years (4·03–12·09) | 7·84 years (4·03–12·60) | 5·19 years (1·85–9·75) | 8·83 years (6·15–12·32) | 7·09 years (4·32–10·07) |
| Weight 2 SDs above mean for age/sex | 36 (13·4%) | 32 (14·8%) | 0 (0·0%) | 1 (3·6%) | 3 (27·3%) |
| Underlying medical conditions | |||||
| Any | 51 (19·0%) | 43 (19·9%) | 2 (15·4%) | 2 (7·1%) | 4 (36·4%) |
| Neurodevelopmental | 12 (4·5%) | 10 (4·6%) | 2 (15·4%) | 0 (0·0%) | 0 (0·0%) |
| Gastrointestinal | 7 (2·6%) | 6 (2·8%) | 0 (0·0%) | 0 (0·0%) | 1 (9·1%) |
| Haematological | 7 (2·6%) | 4 (1·9%) | 0 (0·0%) | 0 (0·0%) | 3 (27·3%) |
| Asthma requiring regular medication | 6 (2·2%) | 6 (2·8%) | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
| Ethnicity | |||||
| White | 114 (42·5%) | 105 (48·6%) | 2 (15·4%) | 4 (14·3%) | 3 (27·3%) |
| Black/ African/ Caribbean/ Black British | 60 (22·4%) | 42 (19·4%) | 3 (23·1%) | 10 (35·7%) | 5 (45·5%) |
| Asian/ Asian British | 63 (23·5%) | 44 (20·4%) | 6 (46·2%) | 12 (42·9%) | 1 (9·1%) |
| Mixed/ multiple ethnic groups | 11 (4·1%) | 9 (4·2%) | 1 (7·7%) | 0 (0·0%) | 1 (9·1%) |
| Other ethnic groups | 10 (3·7%) | 8 (3·7%) | 0 (0·0%) | 1 (3·6%) | 1 (9·1%) |
| Outcome | |||||
| Admitted to PICU | 118 (44·0%) | 89 (41·2%) | 4 (30·8%) | 19 (67·9%) | 6 (54·5%) |
| Required conventional ventilation/ High Frequency Oscillation | 44 (16·4%) | 28 (13·0%) | 1 (7·7%) | 10 (35·7%) | 5 (45·5%) |
| Required peritoneal dialysis | 1 (0·4%) | 1 (0·5%) | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
| Required inotropes | 80 (29·9%) | 56 (25·9%) | 3 (23·1%) | 15 (53·6%) | 6 (54·5%) |
| Died | 3 (1·1%) | 2 (0·9%) | 0 (0·0%) | 1 (3·6%) | 0 (0·0%) |
| Median duration of hospital stay (IQR) | 8 days (5–11) |
7 days (5–10) |
5 days (4–6) |
13 days (10·5–17) |
9 days (5–12) |
| Median duration of PICU stay (IQR) | 3 days (2–7) |
3 days (2–6) |
2·5 days (1·5–3·5) |
6 days (2–8) |
3·5 days (2–5) |
IQR Interquartile Range; n Number of cases; PICU Paediatric Intensive Care Unit; SD Standard Deviation.
3.4. Clinical characteristics
Presenting symptoms by category are presented in Supplementary Material 4. PIMS-TS/KD/TSS cases had the highest proportion with cough, sore throat, pale/mottled skin and neurological involvement (Table S4.2), while >80% of PIMS-TS/TSS cases had hypotension or required fluid boluses – higher than other phenotypes. Cases meeting the TSS criteria had higher rates of: abdominal pain, vomiting, diarrhoea, respiratory involvement, cold hands/feet, age-specific tachycardia and urinary output <2 ml/kg/hr. Additionally, a widespread, polymorphous (not vesicular) rash (82·1%) and conjunctivitis (82·1%) were frequently reported for PIMS-TS/TSS cases but were less common among PIMS-TS only cases (32·9% and 40·7%, respectively).
Where tested, ST or T wave changes were the most common ECG findings (14·7%; 24/163) (Table S4.4). None of the children had a myocardial infarction but five had a cardiac arrest: 10·7% (3/28) PIMS-TS/TSS cases and 0·9% (2/216) PIMS-TS only cases. Of the cases that had an echocardiogram, 38·4% (78/203) had decreased myocardial contractility, 26·6% (54/203) had coronary artery abnormalities (dilation/aneurysm), 20·2% (41/203) had pericardial effusion and 16·3% (33/203) had mitral or aortic valve insufficiency (Table S4.4). Pericardial effusion and mitral or aortic valve insufficiency were more common among PIMS-TS cases associated with another syndrome.
3.5. SARS-CoV-2 testing
Nearly all cases (264/268, 98.5%) had an RT-PCR test for SARS-CoV2 infection but only 44·0% (118/268) had SARS-CoV-2 serology. Of those tested, 14·8% (39/264) children were RT-PCR positive and 63·6% (75/118) were seropositive. Overall, 35·1% (94/268) had evidence of current or previous SARS-CoV-2 infection (Table S4.5). Where reported, 46·6% (125/268) had a positive SARS-CoV-2 test or a household contact with confirmed SARS-CoV-2 or COVID-19-like symptoms in the 4 weeks prior to illness (Table S4.5).
3.6. Laboratory findings
PIMS-TS/TSS cases had higher values of CRP, d-dimers, ferritin, INR, NT-proB-type Natriuretic Peptide and fibrinogen, and PIMS-TS/TSS and PIMS-TS/KD/TSS cases had higher values of triglycerides, amylase, creatine kinase, urea, creatinine, neutrophils, and ALT (Table S4.7). Most cases had low albumin (86·9%), with PIMS-TS/TSS and PIMS-TS/KD/TSS cases having lower values than other presentations. For all PIMS-TS cases, where reported, the median (IQR) CRP was 223 (162–289) mg/L, ferritin 543 (284–1049) ug/L, and d-dimers 3400 (1757–6921) ug/L.
3.7. Treatment
92·9% (249/268) cases were reported to have received antibiotics, 73·1% (196/268) aspirin/other anticoagulant, 55·6% (149/268) steroids, 70·5% (189/268) immunoglobulins, and 6·7% (18/268) antivirals. Ten (3·7%) children each received monoclonal antibodies against TNF-α and an interleukin-1 receptor antagonist, respectively, one other child received both (0·4%), and four others (1·5%) received monoclonal antibodies against the interleukin-6 receptor.
3.8. Outcomes
In total, 44·0% (118/268) children were admitted to a Paediatric Intensive Care Unit (PICU) (Table 2), which was proportionately more common among cases meeting TSS criteria (67·9% [19/28] with PIMS-TS/TSS, 54·5% [6/11] with PIMS-TS/KD/TSS). This group was also more likely to require conventional ventilation or high frequency oscillation (35·7% PIMS-TS/TSS, 45.5% PIMS-TS/KD/TSS) than PIMS-TS only (13·0%) or PIMS-TS/KD (7·7%), as well as inotropes – just over half of cases, compared to around a quarter of PIMS-TS only or PIMS-TS/KD cases. Three children died (all male), accounting for 3·6% (1/28) with PIMS-TS/TSS and 0·9% (2/216) with PIMS-TS only. Two had underlying comorbidities (anorexia and obesity, respectively). One was an infant and the other two were aged 10–15 years. The cause of death included stroke, ruptured coronary aneurysm and invasive fungal infection secondary to bone marrow suppression.
3.9. Agreement with clinical classification
In terms of clinician-reported syndromes, 68·1% (147/216) PIMS-TS only cases were reported as suspected PIMS-TS only, with 29 remaining cases reported as suspected PIMS-TS plus TSS or KD (Supplementary Material 5).
3.10. Time and place
Overall, 46·3% (124/268) of cases were reported from Greater London (Table S4.10). The most notable difference was ethnicity distribution: 35·3% of Greater London cases were Black and 27·6% White compared to 13·4% and 57·7% cases, respectively, outside London. Cases within London were also older with 5·6% under 1 year and 46·0% 10–15 years, compared to 11·1% and 29·9%, respectively, outside London.
3.10.1. Geographic analysis
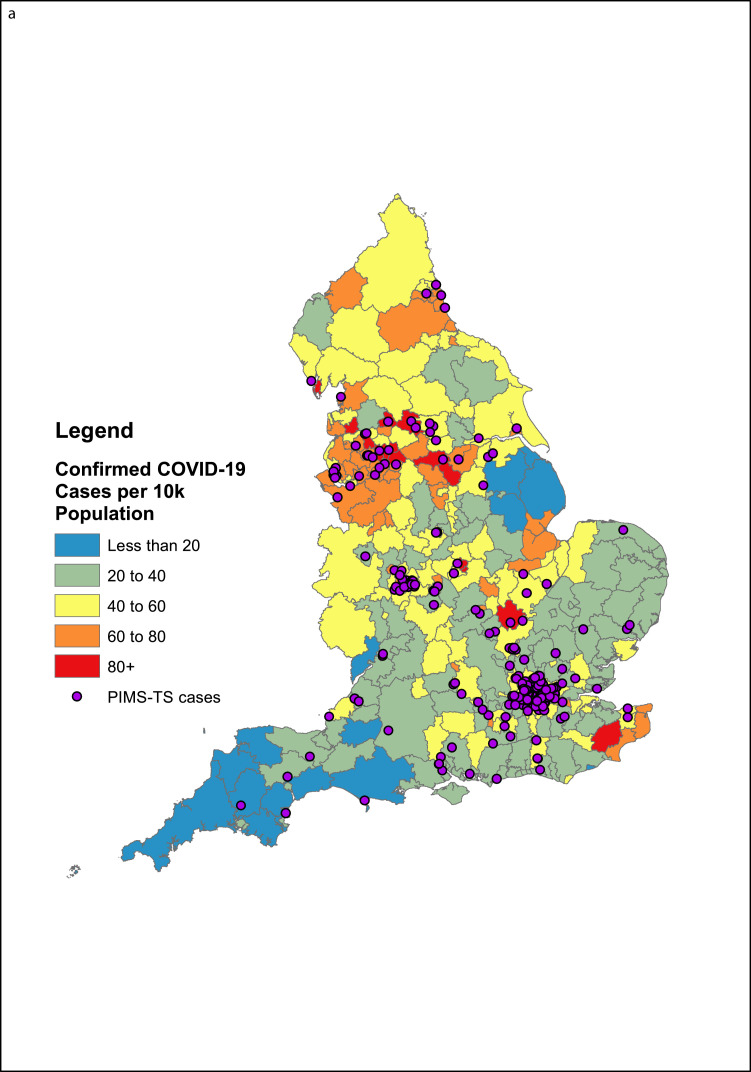
Geographic analysis identified clustering with Lower Tier Local Authorities (LTLAs) reporting higher numbers of confirmed COVID-19 cases per 10,000 population up to 01 July 2020 (Fig. 2a). There was no association with population size, or regions with the highest COVID-19 prevalence (London, the West Midlands and the North-West). Smaller urban areas with high COVID-19 prevalence (Leicester, Luton) reported more cases than other cities of similar size with COVID-19 rates (Nottingham, York). Notably, too, the South West, a region with low COVID-19 incidence, reported relatively more cases during the surveillance period.
Fig. 2a.
A map of all BPSU reports of PIMS-TS cases in England with postcode information (n = 246), alongside the rate of COVID-19 cases in LTLAs in England up to the 1st of July.
3.10.2. Temporal analysis
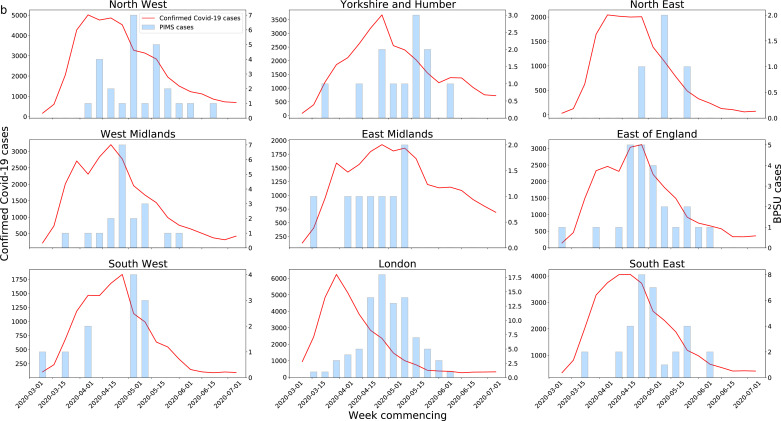
Temporal analysis found the 246 PIMS-TS cases in England followed the epidemic curve of COVID-19 in the English regions, as defined by the Public Health England Centres (PHECs), between March and July 2020 with a median lag of approximately 16·3 (IQR 9–23) days, although this varied between PHECs (Fig. 2b, Fig. 2c). Notable were reports of cases from the East of England, East Midlands and the South West during periods of low regional COVID-19 prevalence.
Fig. 2b.
Confirmed COVID-19 cases by PHECs alongside PIMS-TS cases by week of onset. Note different y-axes used. Left axes for confirmed COVID-19 cases, right axes for PIMS-TS cases.
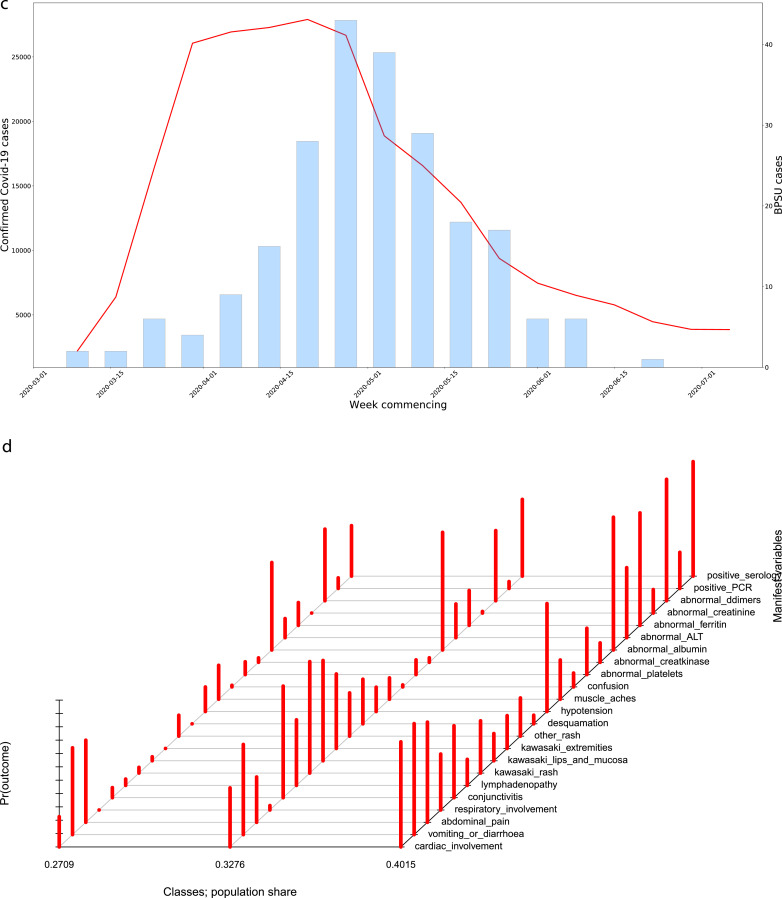
Fig. 2c.
(c) Confirmed COVID-19 cases in England alongside PIMS-TS cases by week of onset. Note different y-axes used. Left axes for confirmed COVID-19 cases, right axes for PIMS-TS cases. (d) Graphical representation of the latent class model identified from the PIMS-TS data. The red bars represent the conditional probability, by class, that a case will have a given organ system involvement or a positive PCR/serological test. The proportion of total cases falling into each class are given at the bottom of the figure. Bars l-R represent: Class 1, Class 2, Class 3.
3.11. Latent class analysis
Fig. 2d shows a graphical representation of the different classes identified by LCA, and the probability of membership conditioned on each indicator variable. Class 1 (n = 75) consisted solely of PIMS-TS only cases; Class 2 (n = 88) contained 66·7% (16/24) of cases that met KD criteria; Class 3 (n = 105) contained 61·5% (24/39) of cases that met TSS criteria (Table 3). Ethnicity distributions varied between the groups, with Black, Asian, Mixed and Other ethnicities accounting for only 40·0% cases in Class 1 (13·3% Black, 17·3% Asian), 53·4% in Class 2 (15·9% Black, 26·1% Asian), and 73·7% in Class 3 (34·3% Black, 25·7% Asian). Additionally, the median age of cases in Class 2 was 4·5 (IQR 1·5–7·5) years, compared to 7·8 (IQR 3·1–11·2) years in Class 1 and 11·2 (IQR 8·4–13·9) years in Class 3.
Table 3.
Characteristics of classes identified in the latent class analysis.
| Class 1 (N = 75) | Class 2 (N = 88) | Class 3 (N = 105) | P value | |
|---|---|---|---|---|
| Positive SARS-CoV-2 PCR test (N = 39) | 6 (8·0%) | 4 (4·5%) | 29 (27·6%) | <0·01 |
| Positive SARS-CoV-2 serology test (N = 75) | 8 (10·7%) | 22 (25·0%) | 45 (42·9%) | <0·01 |
| Median Age (IQR) in years | 7·8 (3·1–11·2) | 4·5 (1·5–7·5) | 11·2 (8·4–13·9) | |
| Median Hospital Stay (IQR) in days | 5 (4–8) | 6 (4–11) | 9·5 (8–13) | |
| Weight 2 SDs above mean for age/sex | 11 (14·7%) | 4 (4·5%) | 21 (20·0%) | <0·01 |
| Ethnicity | ||||
| White | 45 (60·0%) | 41 (46·6%) | 28 (26·7%) | <0·01 |
| Black/ African/ Caribbean/ Black British | 10 (13·3%) | 14 (15·9%) | 36 (34·3%) | <0·01 |
| Asian/ Asian British | 13 (17·3%) | 23 (26·1%) | 27 (25·7%) | <0·01 |
| Mixed/ multiple ethnic groups | 3 (4·0%) | 5 (5·7%) | 3 (2·9%) | <0·01 |
| Other ethnic groups | 1 (1·3%) | 3 (3·4%) | 6 (5·7%) | <0·01 |
| Cardiovascular Involvement | ||||
| Any cardiac arrest or findings on ECG/Echo | 18 (24·0%) | 39 (44·3%) | 83 (79·1%) | <0·01 |
| Hypotension | 14 (18·7%) | 16 (18·2%) | 84 (80·0%) | <0·01 |
| Dermatological Involvement | ||||
| Kawasaki-type rash (widespread, polymorphous, not vesicular) | 3 (4·0%) | 74 (84·1%) | 40 (38·1%) | <0·01 |
| Other rash | 6 (8·0%) | 21 (23·9%) | 22 (21·0%) | 0·06 |
| Conjunctivitis (bilateral, bulbar, non-suppurative) | 5 (6·7%) | 74 (84·1%) | 56 (53·3%) | <0·01 |
| Kawasaki lips and mucosa (red cracked lips, strawberry tongue, erythematous oral cavity) | 2 (2·7%) | 67 (76·1%) | 20 (19·0%) | <0·01 |
| Gastrointestinal Involvement | ||||
| Vomiting/diarrhoea | 49 (65·3%) | 60 (68·2%) | 87 (82·9%) | 0·01 |
| Abdominal pain | 45 (60·0%) | 29 (33·0%) | 78 (74·3%) | <0·01 |
| Low albumin | 50 (66·7%) | 78 (88·6%) | 104 (99·1%) | <0·01 |
| Raised ALT | 10 (13·3%) | 22 (25·0%) | 57 (54·3%) | <0·01 |
| Raised d-dimers | 41 (54·7%) | 46 (52·3%) | 96 (91·4%) | <0·01 |
| Haematological Involvement | ||||
| Low platelets (<100 × 109/L) | 7 (9·3%) | 9 (10·2%) | 38 (36·2%) | <0·01 |
| Neurological Involvement | ||||
| Headache | 16 (21·3%) | 13 (15·3%) | 32 (30·5%) | 0·03 |
| Seizure | 1 (1·3%) | 0 | 2 (1·9%) | 0·45 |
| Confusion | 1 (1·3%) | 1 (1·1%) | 11 (10·5%) | <0·01 |
| Drowsiness | 7 (9·3%) | 10 (11·4%) | 19 (18·1%) | 0·10 |
| Renal involvement | ||||
| Raised creatinine | 0 | 1 (1·1%) | 19 (18·1%) | <0·01 |
| Acute Kidney Disease | 0 | 3 (3·4%) | 25 (23·8%) | <0·01 |
| Respiratory Involvement | ||||
| Cough | 11 (14·7%) | 30 (34·1%) | 28 (26·7%) | 0·02 |
| Tachypnoea | 19 (25·3%) | 21 (23·9%) | 48 (45·7%) | <0·01 |
| CPAP /ventilation / high frequency oscillation | 0 | 3 (3·4%) | 45 (42·9%) | <0·01 |
| Phenotype class | ||||
| TSS (N = 39) | 0 | 15 (17·0%) | 24 (22·9%) | <0·01 |
| KD (N = 24) | 0 | 16 (18·2%) | 8 (7·6%) | <0·01 |
| PIMS-TS only (N = 216) | 75 (100·0%) | 63 (71·6%) | 78 (74·3%) | <0·01 |
| PIMS-TS/KD (N = 13) | 0 | 10 (11·4%) | 3 (2·9%) | <0·01 |
| PIMS-TS/TSS (N = 28) | 0 | 9 (10·2%) | 19 (18·1%) | <0·01 |
| PIMS-TS/Kawasaki/TSS (N = 11) | 0 | 6 (6·8%) | 5 (4·8%) | <0·01 |
ALT Alanine transaminase; CPAP Continuous positive airway pressure; IQR Interquartile Range; KD Kawasaki disease; PCR Polymerase chain reaction; PIMS-TS Paediatric Inflammatory Multisystem Syndrome temporally associated with SARS-CoV-2; TSS Toxic shock syndrome.
Class 1 cases had the least severe presentations, with a lower proportion reporting dermatological/cardiac/haematological/neurological involvement, compared to other syndromes. This group did have high proportions with vomiting/diarrhoea (65·3%), abdominal pain (60·0%), hypoalbuminaemia (66·7%), and raised d-dimers (54·7%), although these were all more prevalent in Class 3 cases. Only 14·7% (11/75) reported a cough and, while 25·3% (19/75) reported tachypnoea, none required respiratory support.
Class 2 cases had the highest proportions with dermatological involvement, with >75% having KD features of rash, conjunctivitis or lips/mucosa signs. This group also had 68·2% (60/88) presenting with vomiting/diarrhoea, and 88·6% (78/88) had hypoalbuminaemia. Cough (34·1%; 30/88) was relatively more common, while abdominal pain (33·0%; 29/88) less common. One case died.
Class 3 cases had the most severe disease, with the highest proportions having multi-organ involvement. Their median duration of hospitalisation was 9·5 days (IQR 8–13) compared to 5 days (IQR 4–8) and 6 days (IQR 4–11) in Classes 1 and 2, respectively. There were two deaths in Class 3.
4. Discussion
Enhanced prospective national surveillance identified 268 cases that fulfilled a broad case definition for PIMS-TS since the start of the COVID-19 pandemic in the UK and Ireland. These included 216 with PIMS-TS only, 13 PIMS-TS/KD, 28 PIMS-TS/TSS and 11 PIMS-TS/KD/TSS. Cases differed in age, ethnicity, clinical presentation, cardiovascular involvement and disease severity. Children classified as PIMS-TS and another syndrome were more likely to require intensive care support and longer hospitalisation stay than those with PIMS-TS only. There was a temporal and geographical association between community SARS-CoV-2 prevalence and PIMS-TS cases, both nationally and regionally, with a median lag of 16 days between COVID-19 and PIMS-TS cases in England. LCA identified three different classes which broadly divided into PIMS-TS only (Class 1), PIMS-TS/KD (Class 2) and PIMS-TS/TSS (Class 3), with Class 2 cases tending to be younger, and Class 3 older.
The age distribution of cases (median 8.2 years; IQR 4.0–12.1 years) was similar to that described in other large [9, 10] and smaller [[3], [4], [5], 14, 15] case series, and this included the 570 cases described by Godfred-Cato et al. [10] (median 8 years; IQR 4–12 years) that included cases up to 20 years of age. In common with most other cohorts, males were overrepresented. [3, 7, [9], [10], [11]] The percentage of patients with comorbidities and/or overweight was largely similar to reported studies (Table S1.2), although a smaller percentage had comorbidities in Latin America [9] and India, [15] and a greater proportion were obese in the US. [10] The low case fatality rate was also consistent with the international literature (Table S1.2).
The percentage of white children with PIMS-TS in our cohort (42·5%) was higher than in the US (13·2%) [10] or France (Paris, 25·0%), [8] where higher proportions of Hispanic and/or Black ethnicities were reported, likely reflecting the ethnic diversity of their respective populations. Nonetheless, Black and Asian ethnicities in our cohort were hugely over-represented relative to the UK population, [25] and accounted for a disproportionate proportion with the most severe presentations. Although this may in part be due to London having the highest COVID-19 infection rate in the first wave, it may also be due to overrepresentation of these ethnicities among key workers and lower socioeconomic groups: both at increased risk of COVID-19.
Notably, Asian children other than from the Indian Subcontinent, who are known to be increased risk of KD, appeared to be under-represented among PIMS-TS cases. Whether the observed ethnic disparities among children with PIMS-TS, as observed with COVID-19 cases too, are due to genetic or socio-economic risk factors – or both – remains to be established. [26]
SARS-CoV-2 infection was confirmed by RT-PCR in 14·8% of PIMS-TS cases. Although some studies reported similarly low PCR-positivity rates, [4, 9, 15] most reported higher positivity rates (38·1–75·0%). [3, 5, 7, 8, 10, 11, 14] The large variation is likely due to test availability in addition to case definitions, since the US MIS-C definition, used by several studies, required confirmed SARS-CoV-2 infection or known exposure. [3, [9], [10], [11], 14] However, SARS-CoV-2 antibody-positivity (63·6%) was similar to the reported range (50·0–90·5%), [[3], [4], [5], [7], [8], [9], [10], [11]] although only 44·0% in our cohort were tested for SARS-CoV-2 antibodies.
The following rates of system involvement were within the ranges reported in the literature: (a) 52% with cardiovascular involvement (vs. 12–100% in the literature) (b) 99% with gastrointestinal involvement (vs. 42–100%), and (c) 32% with neurological involvement (vs. 13–56%). [[3], [4], [5], [7], [8], [9], [10], [11], 14, 15] Despite the BPSU case definition not being limited to those with confirmed SARS-CoV-2 infection or known COVID-19 exposure, the distribution of symptoms was broadly similar to the US report: [10] 53% (UK) vs. 55% (USA) with rash, 50% vs. 48% conjunctival injection, 99% vs. 91% gastrointestinal involvement, 32% vs. 38% neurological involvement, 26% vs. 29% cough, 16% vs. 13% requiring mechanical ventilation, 9% vs. 5% with complete KD; the main differences being a lower proportion with cardiovascular involvement and ICU admission in our cohort (Table S1.3).
LCA validated the findings of the different phenotypes in our cohort and identified three separate classes which broadly defined patients with PIMS-TS only, those with KD features and a third group with TSS manifestations. Such characterisations are likely to be useful for clinical management of children presenting with such a wide syndrome. The Class 3 patients, for example, had very high proportions with cardiac involvement, vomiting/diarrhoea, abdominal pain, hypotension, low albumin, and raised d-dimers and ferritin, were older, as well as the highest proportions with positive SARS-CoV-2 PCR or antibody, and were most likely to present with multiorgan failure requiring inotropic support and assisted ventilation. In contrast, Class 2 patients had more typical KD features, while Class 1 patients had features of PIMS-TS only and a milder course of illness. These findings are similar to the LCA analysis performed in US children, where Classes 2 and 3 corresponded to their Classes 3 and 1, respectively. [10] [27, 28]
Cardiovascular assessment was performed at the discretion of the clinician and was, therefore, more likely to be undertaken for more severe cases. Whilst most children had favourable cardiovascular outcomes, the clinical presentation of five children with cardiac arrest and the need for inotropes in a significant proportion of cases suggests that such children need careful cardiovascular assessment, [27, 28] even after recovery to assess their risk of long-term complications as has been reported for KD. [21, 29]
Current epidemiological evidence strongly indicates an association between SARS-CoV-2 infection and PIMS-TS 2–4 weeks later. [2, 12] In our cohort, a substantial proportion of tested children were SARS-CoV-2 RT-PCR and antibody negative. This could be because PIMS-TS occurred in the cusp between elimination of the virus and antibody development. The mechanism by which SARS-CoV-2 infection leads to PIMS-TS is unknown but would provide useful insight into the pathogenesis of both KD and TSS which have remained elusive so far. It also remains unclear whether PIMS-TS and KD represent a continuum of illness or are completely distinct syndromes. [30] A recent study reported similar immune and plasma protein profiles but different autoantibody targets among KD and PIMS-TS cases, although the sample size was small. [31]
20,26In our surveillance, 35 children had PIMS-TS features but did not meet the case definition only because their CRP level was below the arbitrary 100 mg/L cut-off. It is likely that these were milder cases along the PIMS-TS spectrum, which needs to be taken into account in future case definitions. As the number of reported cases continue to increase worldwide, it is important to reach a consensus in naming and case definitions so that the data are more comparable between countries and across continents. We purposely opted for a broad reporting case definition to capture as many cases as possible but needed to define boundaries to reduce the reporting of non-PIMS-TS cases. We also asked clinicians to report TSS and KD cases that were not associated with SARS-CoV-2 for comparison, but there were very few such cases reported for comparison. As ours was a paediatric study, we also limited our surveillance to <16 year-olds who would be managed by paediatricians since, in the UK and Ireland, older children and adults would typically present to adult services. [32] Additionally, the BPSU methodology requires paediatricians to report cases to the investigators. Mild cases and those that were not hospitalised or had short hospital stays may, therefore, be missed. Also, since so many reported cases were SARS-CoV-2 negative, it possible that some KD and TSS cases might have been misclassified as PIMS-TS. Moreover, the BPSU was also the only source of reporting; we therefore, cannot comment on the completeness of case ascertainment, especially in nations that reported very few cases. After reporting a case, paediatricians completed a questionnaire using the information documented in the medical records, so some data, such as the child's height or parental occupation, for example, may not be documented. Finally, the investigation and treatment of cases was at the discretion of the clinician and, therefore, variable both geographically and over time with increasing recognition, knowledge and experience with managing PIMS-TS cases.
5. Conclusions
The strong association between SARS-CoV-2 and PIMS-TS emphasises the importance of maintaining low community infection rates to reduce the risk of PIMS-TS. Understanding the relationship between SARS-CoV-2 infection and PIMS-TS could provide useful insight into the pathogenesis of both KD and TSS. Close follow-up will be important to monitor rapidly changing epidemiology as well as the short- to long-term complications in children with PIMS-TS.
Author Contributions
SNL, GO and RL conceived the study. SNL, GO, PD, TB, CEP, DJ, EW, BD, RW, CW, OS, MGS, MR, CEJ, AVR, NG, JA and RL were responsible for study design/methodology. RL, JA, ZA-C and JF contributed to project administration. SNL, JF, EB, BW, JS, NG contributed to the original draft and JF and JS conducted the formal analyses. JF, JS, EB, BW were responsible for data validation. EB, BW and JF conducted the literature review. All authors contributed to reviewing and editing the manuscripts.
Data sharing statement
Applications for relevant anonymised data should be submitted to the PHE Office for Data Release.
Ethics approval
PHE has legal permission under Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to conduct national surveillance of communicable diseases in England and, as such, individual patient consent is not required. Public Health Wales, through the established order legislation, is required to conduct surveillance of communicable diseases in Wales and, as such, individual patient consent is not required. The surveillance protocol was approved by the Public Benefit and Privacy Panel for Health and Social Care in Scotland (Ref: 2021–0041, 19th May 2020).
Declaration of Interests
Prof. Semple reports grants from DHSC National Institute of Health Research UK, grants from Medical Research Council UK, grants from Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool, during the conduct of the study; other from Integrum Scientific LLC, Greensboro, NC, USA, outside the submitted work. Dr. Ramanan reports personal fees from EliLilly, personal fees from Roche, personal fees from SOBI, personal fees from UCB, personal fees from Abbvie, personal fees from Novartis, outside the submitted work.
Acknowledgments
Acknowledgements
PHE, BPSU and the authors would like to thank all the paediatricians across the UK and the Republic of Ireland for their unending support with this and all other BPSU studies.
Funding
This surveillance was internally funded by PHE and did not receive any specific grant funding from agencies in the public, commercial or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2021.100075.
Appendix. Supplementary materials
References
- 1.European Centre for Disease Prevention and Control (ECDC). COVID-19 in Children and the Role of School Settings in Transmission - First Update. January 14, 2021 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-children-and-the-role-of-school-settings-in-transmission-first-update_1.pdf.
- 2.Belot A., Antona D., Renolleau S. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25(22):06. doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moraleda C., Serna-Pascual M., Soriano-Arandes A. Multi-inflammatory syndrome in children related to SARS-CoV-2 in Spain. Clin Infect Dis. 2020;25:25. doi: 10.1093/cid/ciaa1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L., Mazza A., Gervasoni A. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toubiana J., Poirault C., Corsia A. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. Bmj. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.6 Dallan C., Romano F., Siebert J., Politi S., Lacroix L., Sahyoun C. Septic shock presentation in adolescents with COVID-19. Lancet Child Adolesc Health. 2020;4(7):e21–e23. doi: 10.1016/S2352-4642(20)30164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belhadjer Z., Méot M., Bajolle F. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 8.Pouletty M., Borocco C., Ouldali N. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antunez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F. COVID-19 and multisystem inflammatory syndrome in Latin American children: a multinational study. medRxiv. 2020 doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- 10.Godfred-Cato S., Bryant B., Leung J. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres J.P., Izquierdo G., Acuna M. Multisystem inflammatory syndrome in children (MIS-C): report of the clinical and epidemiological characteristics of cases in Santiago de Chile during the SARS-CoV-2 pandemic. Int J Infect Dis. 2020;27:27. doi: 10.1016/j.ijid.2020.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. 2020. https://www.cdc.gov/mis-c/cases/index.html. Accessed January 14, 2021.
- 13.Bahrami A., Vafapour M., Moazzami B., Rezaei N. Hyperinflammatory shock related to COVID-19 in a patient presenting with multisystem inflammatory syndrome in children: first case from Iran. J Paediatr Child Health. 2020;08:08. doi: 10.1111/jpc.15048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S., Sen S., Lakshmivenkateshiah S. Multisystem inflammatory syndrome in children with COVID-19 in Mumbai, India. Indian Pediatr. 2020;11:11. doi: 10.1007/s13312-020-2026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhanalakshmi K., Venkataraman A., Balasubramanian S. Epidemiological and clinical profile of pediatric inflammatory multisystem syndrome - temporally associated with SARS-CoV-2 (PIMS-TS) in Indian children. Indian Pediatr. 2020;06:06. doi: 10.1007/s13312-020-2025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toubiana J., Poirault C., Corsia A. Outbreak of Kawasaki disease in children during COVID-19 pandemic: a prospective observational study in Paris, France. medRxiv. 2020 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO). Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19. 2020.https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed November 4, 2020.
- 19.Lynn R., Ross E. The British paediatric surveillance unit: the first 20 years. Arch Dis Child. 2007;92(9):744–745. doi: 10.1136/adc.2006.105908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Royal College of Paediatrics and Child Health. Guidance: Paediatric Multisystem Inflammatory Syndrome Temporally Associated with COVID-19. 2020. https://www.rcpch.ac.uk/sites/default/files/2020-05/COVID-19-Paediatric-multisystem-%20inflammatory%20syndrome-20200501.pdf. Accessed October 22, 2020.
- 21.McCrindle B.W., Rowley A.H., Newburger J.W. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the american heart association. Circulation. 2017;135(17):e927–ee99. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). Toxic Shock Syndrome (Other Than Streptococcal) (TSS) 2011 Case Definition. https://wwwn.cdc.gov/nndss/conditions/toxic-shock-syndrome-other-than-streptococcal/case-definition/2011/. Accessed January 8, 2021.
- 23.Birrell P., Blake J., van Leeuwen E., MRC Biostatistics Unit COVID-19 Working Group, De Angelis D. COVID-19: Nowcast and Forecast. 2020.https://www.mrc-bsu.cam.ac.uk/nowcasting-and-forecasting-21st-december-2020/. Accessed December 15, 2020.
- 24.NHS Digital. Health survey for England 2018: adult and child overweight and obesity. 2019. https://digital.nhs.uk/data-and-information/publications/statistical/health-survey-for-england/2018. Accessed March 13, 2021.
- 25.Office for National Statistics. UK Population by Ethnicity: Age Groups. 2020.https://www.ethnicity-facts-figures.service.gov.uk/uk-population-by-ethnicity/demographics/age-groups/latest#black-ethnic-groups-age-profile. Accessed December 21, 2020.
- 26.Kwak J.H., Lee S.Y., Choi J.W. Clinical features, diagnosis, and outcomes of multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Clin Exp Pediatr. 2021;64(2):68–75. doi: 10.3345/cep.2020.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alsaied T., Tremoulet A.H., Burns J.C. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 28.Valverde I., Singh Y., Sanchez-de-Toledo J. Acute cardiovascular manifestations in 286 children with multisystem inflammatory syndrome associated with COVID-19 infection in Europe. Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.050065. [DOI] [PubMed] [Google Scholar]
- 29.Tulloh R.M.R., Mayon-White R., Harnden A. Kawasaki disease: a prospective population survey in the UK and Ireland from 2013 to 2015. Arch Dis Child. 2019;104(7):640–646. doi: 10.1136/archdischild-2018-315087. [DOI] [PubMed] [Google Scholar]
- 30.Henderson L.A., Yeung R.S.M. MIS-C: early lessons from immune profiling. Nat Rev Rheumatol. 2021;17(2):75–76. doi: 10.1038/s41584-020-00566-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consiglio C.R., Cotugno N., Sardh F. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183(4):968–981. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morris S., Schwartz N., Patel P. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection — United Kingdom and United States, March–August 2020. Morb Mortal Wkly Rep. 2020;69(40):1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.