Abstract
Context:
Advanced cancer patients have unrecognized gaps in their understanding about palliative radiation therapy (PRT).
Objectives:
To build a video decision aid for hospitalized patients with advanced cancer referred for PRT and prospectively test its efficacy in reducing decisional uncertainty, improving knowledge, increasing treatment readiness and readiness for palliative care consultation, and its acceptability among patients.
Methods:
Forty patients with advanced cancer hospitalized at Memorial Sloan Kettering Cancer Center watched a video decision aid about PRT and palliative care. Patients’ conceptual and logistical knowledge of PRT, decisional uncertainty, treatment readiness, and readiness for palliative care consultation were assessed before and after watching the video with a 6-item knowledge survey, the decisional uncertainty subscale of the Decisional Conflict Scale, and Likert instruments to assess readiness to accept radiation treatment and/or palliative care consultation, respectively. A post-video survey assessed the video’s acceptability among patients.
Results:
After watching the video, decisional uncertainty was reduced (28.3 vs. 21.7, p=0.02); knowledge of PRT improved (60.4 vs. 88.3, p<0.001); and PRT readiness increased (2.0 vs. 1.3, p=0.04). Readiness for palliative care consultation was unchanged (p=0.58). Patients felt very comfortable (70%) watching the video and would highly recommend it (75%) to others.
Conclusion:
Among hospitalized patients with advanced cancer, a video decision aid reduced decisional uncertainty, improved knowledge of PRT, increased readiness for PRT, and was well-received by patient viewers.
Keywords: palliative radiation therapy, advanced cancer, video tool, decision aid
Introduction
Palliative radiation treatment (PRT) is a cancer-directed therapy delivered by radiation oncologists for patients with advanced or incurable cancer. Its purpose is to ameliorate or prevent pain and other symptoms from tumor masses pressing or invading into organs, nerves, and tissues. PRT can successfully alleviate cancer-related symptoms and improve quality of life. On the other hand, it is often associated with temporary but significant adverse effects that precede clinical benefits. It generally does not extend survival. Moreover, PRT can involve lengthy hospital stays for patients with significant functional impairment or inability to travel to/from a radiation facility.1 In many cases, benefits and quality of life improvements may not manifest until weeks after treatment completion.2 Thus, PRT can result in both benefits and harms, and treatment decision-making can be complex for patients weighing risks and trade-offs.
The decision to undergo PRT is often made quickly and sometimes without sufficient information to make a fully informed choice, especially during hospitalization. This is due to the shortened interval between an inpatient radiation oncology consultation and actual treatment. Also, the often abrupt nature by which metastatic lesions are found prompting an urgent palliative radiation referral can leave patients little time to process implications of the results. Hence, patients receiving PRT may hold inaccurate expectations of treatment, believing it to be potentially curative when it is in fact not.3 Clinical decision aids may help, yet few have been created for PRT.
A comprehensive review of patient decision aids in radiation oncology describes tools providing written information (e.g. pamphlets, decision boards, and workbooks), audiotaped consultations, online materials, and videos. However, the studied populations included primarily breast and prostate cancer, and the decisions studied were related to curative treatment options in early stage disease.4 A decision board for ambulatory care patients undergoing PRT for bone metastases demonstrated that the majority of patients preferred a longer course of radiation and expressed positive opinions about being involved in the decision-making process.5 Ultimately, the choice of decision aid format depends upon the decision in question, the type of information being provided, and the patient population being studied.6 Video aids can provide visual representations of radiation therapy, often a challenging concept to grasp given its inherent abstractness. Videos allow the possibility of explaining a concept like PRT, which can often be difficult to fully describe using text or static images.
No decision aid currently exists for hospitalized patients considering PRT. Yet, PRT is common, comprising up to 35–50% of patients in a radiation oncology practice and patients may spend significant portions of their remaining lives undergoing PRT.7–10 We chose a video format for our decision aid because the information being presented was highly visual in nature and benefited from animated graphics and footage of radiation treatment processes that could not have been equivalently portrayed through other formats.
Our primary objective was to test the ability of a newly created video decision aid for hospitalized patients considering PRT to reduce decisional uncertainty. Our secondary objectives were to assess the video’s ability to improve patients’ knowledge of PRT and palliative care, increase patients’ readiness to accept PRT, readiness to see a palliative care specialist, and to ascertain its acceptability among patients.
Methods
Design and setting
Patients at Memorial Sloan Kettering Cancer Center (MSKCC) with advanced cancer who were referred for PRT consultation during a hospitalization between August 2012 and April 2013 were eligible for recruitment onto an IRB-approved, prospective, single-arm cohort study (MSKCC IRB #12–172; NCT01667965) in which patients were surveyed before and after viewing a video decision aid about PRT.
Intervention
The study intervention was a video tool (accessible at https://www.mskcc.org/videos/palliativeradiation-therapy) that explained PRT and introduced key concepts of palliative care using patient actors. The video was built according to International Patient Decision Aids Standards criteria to ensure the information conveyed was balanced, unbiased, provided sufficient detail for patients to make their decisions and employed plain language and visual representations to improve patient understanding.11,12 It contained four segments explaining: 1) the process of radiation simulation including making a face mask; 2) what to expect at the time of treatment; 3) commonly encountered treatment PRT side effects; 4) the purpose of palliative care. Content related to the palliative care specialty included a 2-minute segment narrated by a palliative care physician (Roma Tickoo) describing palliative care’s role in addressing physical as well as non-physical symptoms, the possibility of its concurrent delivery with ongoing cancer treatments in order to add an extra layer of support; and its distinctness from hospice care. The video storyboard was approved by the director of patient and caregiver engagement (CBW), a cancer communication expert (TL), a palliative care specialist (RT), and two radiation oncologists (KD, BM).
Participants
Patients with metastatic cancer who were candidates for PRT, >18 years old, English-speaking, and had capacity to provide informed consent and answer questions were eligible. Those with malignant spinal cord compression were ineligible since enrollment procedures could have delayed urgent treatment.
Assessments
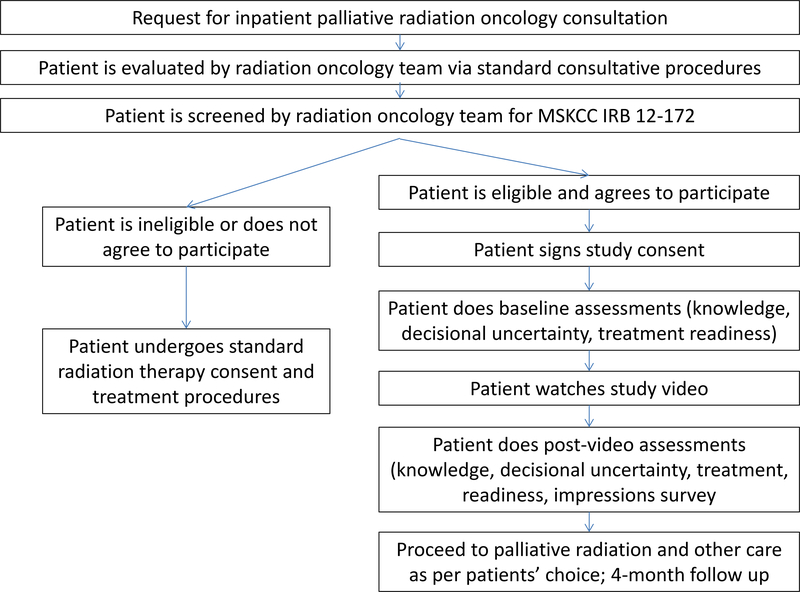
Upon enrollment, patients answered baseline paper-based assessments about decisional uncertainty, PRT knowledge, and their readiness to accept PRT and/or a consultation with a palliative care specialist. Patients then watched the video decision aid about PRT in their hospital room on an iPad (Apple, Cupertino, CA) and answered the same questions within 24 hours post-viewing, also in their hospital room. We chose the 24-hour period to minimize the possibility that a patient may have been discharged prior to completing the study. Patients were given one additional assessment post-viewing to rate the video’s acceptability. The study process is shown in Figure 1. All assessments were captured with pen and paper. The assessment items, scoring, and time-points of administration are further detailed in the supplementary index.
Figure 1.
Study flow chart
The primary outcome, uncertainty regarding the decision to undergo PRT, was assessed by the 3-item decisional uncertainty subscale of the validated Decisional Conflict Scale developed at the Ottawa Hospital Research Institute and rated on a 5-point scale from 0 indicating “strongly agree” meaning feeling certain about decision-making to 4 indicating “strongly disagree“ meaning feeling least certain. 13,14 Basic knowledge of PRT and palliative care was assessed using a 6-item questionnaire (one multiple-choice and five true/false/unsure). Questions were created by the investigators based on a study of video education used in advance care planning, given the absence of a validated tool in radiation.15 Readiness to accept PRT and to see a palliative care specialist were assessed separately by investigator-created Likert instruments with an ordinal 1 (most ready) to 10 (not ready at all) scale, with a text box allowing explanation if > 5. Acceptability of the video aid was rated via a 5-item tool with a 4-point scale from 0 indicating “very helpful,” to 4 indicating “not helpful,” and included perceptions regarding usefulness, comfort of seeing information in the video-based format, and likelihood of recommending it to others facing a similar treatment choice. The knowledge, readiness, and impressions scales have not been previously validated and were developed for use in this study due to the lack of available tools relevant to this particular patient population and study question.
Follow-up
Patients were followed for four months after study enrollment. Patient characteristics, occurrence of palliative care consultations, and death were ascertained by chart reviews.
Case study
One case example of the video’s impact upon a patient was highlighted and described separately as a case study. The case was included because it provides a more detailed qualitative description of how viewing the video specifically affected a patient’s outcome by stimulating discussion of expectations of PRT and of the trade-offs that the particular patient was willing to make to undergo PRT in light of these expectations.
Statistical analysis
Assuming a desired power of 80%, type I error of 5%, and a lowest expected decisional conflict scale effect size of 0.4, we calculated a target sample size of 40 patients. Patient characteristics were examined using descriptive statistics. Paired t-tests and Wilcoxon signed-rank tests compared pre- and post-video assessments. A planned subgroup analysis was conducted between patients who had prior RT vs. patients who did not. Analyses were performed using SPSS Statistics 20 (IBM, Chicago, USA).
Results
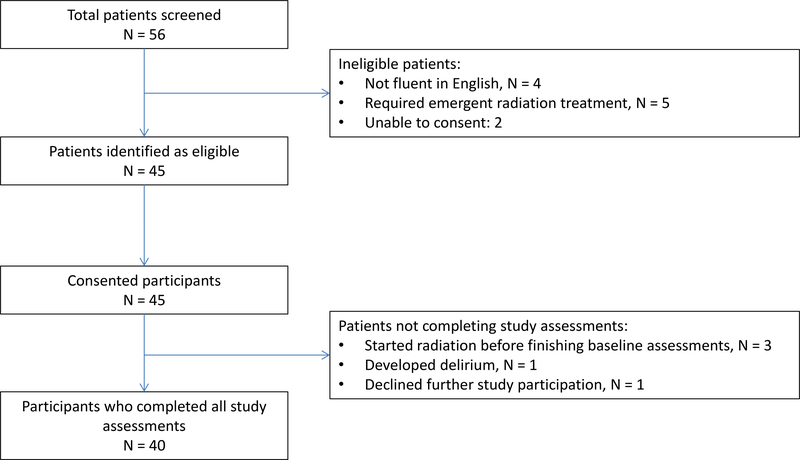
Of 45 patients (n=56 screened) who consented, 40 completed all questionnaires and were included in the analysis. Three patients did not complete timely baseline questionnaires and were excluded. One patient developed delirium, precluding further participation. One patient withdrew from the study after hearing the word ‘palliative’ and did not wish to hear more. Patient enrollment is described in Figure 2. Patient and treatment characteristics are described in Table 1. Nineteen patients (48%) had prior radiation therapy. Most patients received a radiation dose of 30 Gray in 10 once-daily treatments, one of the most commonly used palliative radiation regimen by radiation oncologists.
Figure 2.
Recruitment and enrollment
Table 1.
Baseline patient characteristics
| Variable | N | Proportion |
|---|---|---|
| Median age (years) | 59 (range 31–76) | |
| Male sex | 17 | 43% |
| Race | ||
| White | 30 | 75% |
| Black | 6 | 15% |
| Hispanic | 3 | 8% |
| Asian | 1 | 2% |
| Primary cancer diagnosis | ||
| Prostate | 9 | 23% |
| Lung | 7 | 18% |
| Ovarian | 6 | 15% |
| Breast | 5 | 13% |
| Gastrointestinal/hepatobiliary | 3 | 8% |
| Renal/adrenal | 2 | 5% |
| Lymphoma | 2 | 5% |
| Central nervous system | 1 | 2% |
| Uterine | 1 | 2% |
| Thyroid | 1 | 2% |
| Bladder | 1 | 2% |
| Melanoma | 1 | 2% |
| Sarcoma | 1 | 2% |
| Extent of prior cancer treatment | ||
| Patients with at least one prior course of radiation | 19 | 48% |
| Average number of prior lines of chemotherapy per patient | 2 (range, 0–7) | |
| Prior palliative care | ||
| Patients with prior palliative care consultations | 2 | 5% |
| Reasons for radiation oncology consultation | ||
| Pain | 24 | 60% |
| Neurologic symptoms (e.g, weakness, numbness) | 11 | 28% |
| Bleeding (e.g. vaginal, gastrointestinal) | 4 | 10% |
| Obstruction (e.g. of blood/lymph flow by mass compression) | 1 | 2% |
| Treatment site | ||
| Spine | 15 | 38% |
| Bone | 12 | 30% |
| Soft tissue | 10 | 25% |
| Brain | 3 | 8% |
Assessment scores are detailed in Table 2. The mean baseline decisional uncertainty score was 28.3, which declined to 21.7 (p=0.02) in the post-video setting, meaning patients were more certain about their decision. The effect was greater among patients with no prior radiation therapy, in whom the mean baseline score of 30.2 declined to 21.4 (p=0.02). No significant change in decisional uncertainty was seen among patients with prior RT (p=0.28).
Table 2.
Mean baseline and post-video scores of decisional uncertainty, knowledge, and treatment readiness assessments
| Entire cohort (n=40) | Subgroup with NO prior radiation (n=21) | Subgroup WITH prior radiation (n=19) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (range) | Post-video (range) | p-value | Baseline (range) | Post-video (range) | p-value | Baseline (range) | Post-video (range) | p-value | |
| Decisional uncertainty | 28.3 (0–66.67) | 21.7 (0–66.67) | 0.02 | 30.2 (0–66.67) | 21.4 (0–58.33)) | 0.02 | 26.3 (0–66.67) | 21.9 (0–66.67) | 0.28 |
| Knowledge | 60.4 (16.67–100) | 88.3 (33.33–100) | <0.001 | 61.1 (16.67–100) | 89.7 (66.67–100) | <0.001 | 59.6 (16.67–100) | 86.8 (33.33–100) | <0.001 |
| Readiness to consent for palliative radiation | 2.0 (1–10) | 1.3 (1–5) | 0.04 | 2.0 (1–7) | 1.3 (1–3) | 0.06 | 2.0 (1–10) | 1.4 (1–5) | 0.31 |
| Readiness to consult with palliative care specialist | 3.5 (1–10) | 3.1 (1–10) | 0.58 | 3.8 (1–10) | 3.6 (1–10) | 0.82 | 3.2 (1–10) | 2.5 (1–10) | 0.35 |
Patients demonstrated improved knowledge of PRT and palliative care after seeing the video. Mean knowledge increased from 60.4 to 88.3, p<0.001. In the subgroup analyses of patients with no prior RT and those with prior RT, score increases were also seen after viewing the video. Scores of patients with no prior RT increased from 61.1 to 89.7 (p<0.001) after the video, and those of patients with prior RT increased from 59.6 to 86.8 (p<0.001) after the video. Readiness to accept PRT improved from a mean of 2.0 to 1.3 (p=0.04), meaning patients felt more ready for PRT after the video. Post-video, no patient chose a number > 5. Among patients with no prior radiation, a trend was observed in the direction of feeling more ready for PRT (baseline mean score 2.0 vs. post-video mean score 1.3, p=0.06). No difference was seen among those with prior RT. In terms of readiness to see a palliative care specialist, the mean score was 3.5 (baseline) and 3.1 (post-video), p=0.58. No significant differences were seen in the subgroups of patients who had or did not have prior radiation. Several free-texted reasons cited by patients for their lack of readiness to see a palliative care specialist included needing approval from their primary oncologist beforehand; being satisfied with their current level of care; feeling the video supplied enough information to decide that seeing a palliative care specialist was not necessary at that time; and being potentially interested in a consultation in the future. Overall, patients indicated relatively less readiness to see a palliative care specialist in comparison to readiness to undergo PRT.
The video was well-received by patients (Table 3). Overall, 55% of patients felt it was very helpful, 75% felt very comfortable watching it, and 70% would highly recommend it to others considering similar treatment decisions. In addition, patients liked the video’s step-by-step structure, felt that information was clearly and non-condescendingly presented, it could be viewed in a private setting, and the description of palliative treatment was not overwhelming. Two patients did not like the emphasis on palliative treatment citing that it took away hope. No difference was observed between the subgroup of patients who had prior RT vs. that of those who did not.
Table 3.
Impressions survey of acceptability
| Entire cohort | Subgroup with no prior RT | Subgroup with prior RT | p-value | |
|---|---|---|---|---|
| Impression – total score Mean frange) | 11.67 (0–44.44) | 8.99 (0–33.33) | 14.61 (0–44.44) | 0.093 |
| Impression – tally (frequency) by question item | Yes, very n (%) | Yes, somewhat n (%) | A little n (%) | Not at all n (%) |
| “Was the video helpful?” | 22 (55%) | 17 (43%) | 1 (2%) | 0 |
| “Did you feel comfortable watching the video?” | 30 (75%) | 10 (25%) | 0 | 0 |
| “Would you recommend the video to others?” | 28 (70%) | 11 (28%) | 1 (2%) | 0 |
Patients were followed for 3.9 (median) months (range 0.1–7.0 months). Five patients (13%) did not complete their prescribed radiation course due to clinical deterioration or a change in their choice. The remainder of patients all underwent the prescribed course of PRT. Ten patients (25%) ultimately underwent consultation with a palliative care specialist within one month of study enrollment. Of these, nine indicated the highest level of readiness, a score of 1, on their post-video assessment. At study completion, twenty-five (62.5%) of patients had died.
In one notable case of a patient with leptomeningeal carcinomatosis for whom whole brain radiation therapy was being considered, the video prompted a discussion of the bigger picture and is presented in Table 4 to illustrate the video’s impact upon decision-making and end-of-life planning.
Table 4:
Case presentation
| A patient was diagnosed with leptomeningeal carcinomatosis from ovarian cancer and was contemplating palliative whole brain radiation therapy. Upon viewing the video, the patient wished to understand differences between “palliative” and “curative” treatments and prognosis. After this discussion, the patient opted to try radiation but had significant difficulty tolerating the face mask due to claustrophobia and pain. The patient decided to forego further attempts at whole brain radiation citing that the hassle, discomfort, and life-altering side effects were not worth the potential benefit of slightly increased longevity but no chance of cure. |
| The patient accepted the referral to palliative care and was seen five days after hospital discharge. At that time, the patient told the palliative care specialist she had decided to stop all cancer-directed therapies. Goals of care were discussed; do-not-resuscitate orders were signed, a health care proxy was chosen (previously none was listed), and the patient initiated home hospice. The patient noted a wish for more openness about the goals of treatment from the beginning of the diagnosis. |
| The patient died six weeks later without any subsequent emergency room visits or hospitalizations. |
Discussion
The primary goal of the study was to determine whether a video intervention could improve hospitalized advanced-stage cancer patients’ understanding of the process and implications of PRT and alleviate uncertainty around decision-making. Our study is the first to employ an educational video decision aid that serves as an adjunct to standard radiation oncology consultation for a treatment that may improve quality of life but will likely not extend survival, may be burdensome, and may harbor potential adverse side effects. A video format was chosen based upon previous research demonstrating that video is superior to standard verbal and text-based methods in improving understanding of complex health information. 16–19
We saw an improvement in patients’ decisional uncertainty after watching the video. The magnitude of the effect was greater in patients who had no prior experiences with radiation therapy. One potential explanation for this finding is that patients who were wholly unfamiliar with radiation treatment were less certain about how a course of PRT might impact their lives. For patients with prior RT, adding more detailed information about the specific differences between curative radiation and PRT may further help in reducing decisional uncertainty and should be studied in future trials.
Patients had improved knowledge of PRT after watching the video. At baseline, we discovered that patients had an incomplete understanding of the risks, benefits, and expected outcomes of radiation treatment even after speaking with a radiation oncologist. This knowledge gap may hinder patients’ abilities to align decisions about therapy with overall treatment goals. A similar magnitude of improvement was also seen in the subgroup of patients who had prior RT, suggesting that gaps in knowledge about the palliative nature of treatment are present despite patients’ familiarity with RT. Our results are in line with a Cochrane review of decision aids which found they facilitated informed decision-making by improving patients’ knowledge of options, conferring more accurate expectations regarding side effects and benefits, and helping them reach choices that were more consistent with their informed values.16 Van Oorschot and colleagues in fact posited that certain conditions are important to take into account when considering PRT aside from the simple probability of treatment success, for example the practical requirements and impact of unwanted side effects.20
Patients had a small improvement in readiness to accept PRT but not in their readiness to see a palliative care physician. All study patients had a high symptom burden at the time of study enrollment, which would have warranted a palliative care referral according to National Comprehensive Cancer Network guidelines.21 However, despite the information presented on the supportive role of palliative care, individuals remained relatively hesitant to accept this initial consultation perhaps due to misperceptions that accepting palliative care means giving up hope, a fear that two patients expressed in this study. Previous interventions have also struggled to break down the stigma toward palliative care among cancer patients.22,23 Several studies have shown that a name change to “supportive oncology” led to a higher number of earlier referrals.24,25 Further work is needed to better clarify whether patients’ reluctance stems from persistent misconceptions of palliative care’s role or from other causes. Nonetheless, we found the video became a useful conversation starter about palliative care, prompting many questions about the scope and breadth of services offered within palliative and supportive oncology and also about goals of care, as illustrated by the case presentation, and as such deserves further study. Caution is warranted in interpreting the outcome of the singular case presentation as it may not, nor should it, be representative of the expected outcome from all hospitalized PRT cases.
Our results should be interpreted in the context of several limitations. First, the study was conducted using a pre-post design rather than a randomization. Post-video responses may thus be biased by patients’ familiarity with question sets or an inclination to provide positive answers in order not to offend. Nonetheless, we found significant knowledge gains by asking objective data-driven questions in the knowledge portion of our assessment (rather than eliciting subjective responses in this area). Second, there was no significant improvement in readiness for a palliative care specialist. These points argue that respondents tended toward more socially neutral responses. Third, our study employed non-validated measurement tools for assessing secondary outcomes of knowledge, readiness, and acceptability due to the lack of availability of validated tools relevant to our study population and question. Fourth, our study was limited to hospitalized patients with high symptom burden and may not be generalizable to ambulatory patients considering PRT. Finally, the video was approved by the Director of Patient and Caregiver Engagement (CBW), who had years of experience developing tools for engagement and education for patients and caregivers. However, we did not explicitly seek approval from a single patient or caregiver advocate prior to administration of the video. Rather, we intended to incorporate the qualitative feedback solicited from all patients enrolled in the study when filming subsequent versions of the video in the future.
Many hospitalized cancer patients with advanced stage disease face complex treatment choices with regard to PRT as they approach end of life. Standard consultative procedures are not sufficient for patients to optimally comprehend necessary information about the risks and benefits of treatment. These knowledge gaps are associated with decisional uncertainty and less readiness to undergo PRT. We have shown that a clear and simple video tool can lead to reduced decisional uncertainty, improved knowledge, and increased readiness to receive PRT in these seriously ill individuals. This approach could stimulate patient-provider discussions around PRT and palliative treatments in general, and may help clinicians better align treatment goals with patients’ informed values and priorities.
Supplementary Material
Acknowledgements
This work was supported by the American Medical Association Foundation (KVD), the Memorial Sloan Kettering Patient & Caregiver Education Program (KVD, CBW), and the National Palliative Care Research Center (KVD).
Footnotes
Conflicts: None
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Timmermans LM, van der Maazen RW, Verhaak CM, van Roosmalen MS, van Daal WA, Kraaimaat FW. Patient participation in discussing palliative radiotherapy. Patient Educ Couns. 2005;57(1):53–61. [DOI] [PubMed] [Google Scholar]
- 2.Lutz S, Korytko T, Nguyen J, Khan L, Chow E, Corn B. Palliative radiotherapy: when is it worth it and when is it not? Cancer J. 2010;16(5):473–482. [DOI] [PubMed] [Google Scholar]
- 3.Chen AB, Cronin A, Weeks JC, et al. Expectations about the effectiveness of radiation therapy among patients with incurable lung cancer. J Clin Oncol. 2013;31(21):2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodhouse KD, Tremont K, Vachani A, et al. A Review of Shared Decision-Making and Patient Decision Aids in Radiation Oncology. J Cancer Educ. 2017;32(2):238–245. [DOI] [PubMed] [Google Scholar]
- 5.Shakespeare TP, Lu JJ, Back MF, Liang S, Mukherjee RK, Wynne CJ. Patient preference for radiotherapy fractionation schedule in the palliation of painful bone metastases. J Clin Oncol. 2003;21(11):2156–2162. [DOI] [PubMed] [Google Scholar]
- 6.Fagerlin A. Getting down to details in the design and use of decision aids. Med Decis Making. 2009;29(4):409–411. [DOI] [PubMed] [Google Scholar]
- 7.Addington-Hall J, McCarthy M. Dying from cancer: results of a national population-based investigation. Palliat Med. 1995;9(4):295–305. [DOI] [PubMed] [Google Scholar]
- 8.van Daal WA, Bos MA. Infrastructure for radiotherapy in The Netherlands: development from 1970 to 2010. Int J Radiat Oncol Biol Phys. 1997;37(2):411–415. [DOI] [PubMed] [Google Scholar]
- 9.van Leer EM, Coebergh JW, van Leeuwen FE. [Trends in cancer incidence and cancer mortality in Netherlands: good and bad news]. Ned Tijdschr Geneeskd. 1999;143(29):1502–1506. [PubMed] [Google Scholar]
- 10.Gripp S, Mjartan S, Boelke E, Willers R. Palliative radiotherapy tailored to life expectancy in endstage cancer patients: reality or myth? Cancer. 2010;116(13):3251–3256. [DOI] [PubMed] [Google Scholar]
- 11.Collaboration IPDAS. http://ipdas.ohri.ca. Accessed February 15, 2012.
- 12.Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103(19):1436–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15(1):25–30. [DOI] [PubMed] [Google Scholar]
- 14.O’Connor AM. User Manual - Decisional Conflict Scale (10-item question format). https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decisional_Conflict.pdf. Accessed July 8, 2019.
- 15.El-Jawahri A, Podgurski LM, Eichler AF, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28(2):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011(10):CD001431. [DOI] [PubMed] [Google Scholar]
- 17.Volandes AE, Lehmann LS, Cook EF, Shaykevich S, Abbo ED, Gillick MR. Using video images of dementia in advance care planning. Arch Intern Med. 2007;167(8):828–833. [DOI] [PubMed] [Google Scholar]
- 18.Volandes AE, Barry MJ, Chang Y, Paasche-Orlow MK. Improving decision making at the end of life with video images. Med Decis Making. 2010;30(1):29–34. [DOI] [PubMed] [Google Scholar]
- 19.Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: randomised controlled trial. BMJ. 2009;338:b2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Oorschot B, Rades D, Schulze W, Beckmann G, Feyer P. Palliative radiotherapy-new approaches. Semin Oncol. 2011;38(3):443–449. [DOI] [PubMed] [Google Scholar]
- 21.Levy M, Smith T, Alvarez-Perez A, Back A, et al. NCCN Guidelines Version 1.2016 Palliative Care. January 2016. https://jnccn.Org/abstract/journals/jnccn/14/l/article-p82.xml. Accesssed July 8, 2019.
- 22.Morstad Boldt A, Yusuf F, Himelstein BP. Perceptions of the term palliative care. J Palliat Med. 2006;9(5): 1128–1136. [DOI] [PubMed] [Google Scholar]
- 23.Miyashita M, Hirai K, Morita T, Sanjo M, Uchitomi Y. Barriers to referral to inpatient palliative care units in Japan: a qualitative survey with content analysis. Support Care Cancer. 2008; 16(3):217–222. [DOI] [PubMed] [Google Scholar]
- 24.Dalai S, Palla S, Hui D, et al. Association between a name change from palliative to supportive care and the timing of patient referrals at a comprehensive cancer center. Oncologist. 2011; 16(1): 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadul N, Elsayem A, Palmer JL, et al. Supportive versus palliative care: whaťs in a name?: a survey of medical oncologists and midlevel providers at a comprehensive cancer center. Cancer. 2009;115(9):2013–2021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.