Abstract
Background
Delay to breast cancer diagnosis following an abnormal screening result is associated with anxiety and personal disruption. We assessed the patterns and timeliness of diagnostic follow-up after breast cancer screening for women with abnormal results who attended organized screening programs in 7 provinces.
Methods
Using data from the Canadian Breast Cancer Screening Database, we identified 203 141 women aged 50–69 years who underwent screening in 1996 through provincially organized breast cancer screening programs in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Nova Scotia and Newfoundland. We prospectively followed women with an abnormal screening result through to the completion of the assessment process. We evaluated the waiting times from screening examination to first assessment, from screening examination to first imaging, from screening examination to diagnosis and from first assessment to diagnosis for 13 958 women, stratified according to screening program, mode of detection, whether a biopsy was performed and whether cancer was diagnosed.
Results
We observed considerable variations between and within programs in the time to diagnosis. The median time from screening examination to first assessment was 2.6 weeks. The median time from screening examination to diagnosis was 3.7 weeks; this time increased to 6.9 weeks for women undergoing biopsy. Even when no biopsy was performed, 10% of the women waited 9.6 weeks or longer for a diagnosis, as compared with 15.0 weeks or longer for 10% of the women undergoing biopsy. Among the women who had a biopsy, the use of core biopsy was associated with a shorter median time to diagnosis than was open biopsy, and those found to have cancer had shorter waiting times than women with benign biopsy findings.
Interpretation
Women undergoing assessment of an abnormal breast cancer screening result waited many weeks for a diagnosis, especially when a biopsy was performed. To ensure that targets for timeliness, adopted nationally in 1999, are realized, improved models of care or dissemination of existing efficient techniques to reach a diagnosis will be needed.
Since the late 1980s organized breast cancer screening programs have been developed in many countries with the goal of reducing breast cancer mortality.1,2,3,4,5 By the end of 1999 all provinces and 2 territories in Canada had, or were implementing, organized screening programs.6
Part of breast cancer screening is the assessment of abnormal screening results. The assessment may involve physical examination, imaging with magnified or other special mammographic studies, ultrasonography, imaging-directed biopsy or surgical biopsy.7,8 In some countries assessment occurs within the screening program.1,9 In Canada the woman and her family physician are usually notified about the abnormal result, and the family physician organizes subsequent referrals. In Nova Scotia, the screening program regularly navigates women through the diagnostic process on behalf of the family physician.10
About 3%–10% of women with abnormal screening results will be found to have breast cancer following assessment.6 For the women without cancer, being informed of an abnormal screening result and the need to undergo subsequent investigations may cause morbidity that includes, but is not limited to, an acute increase in anxiety and the discomfort, time and expense of additional tests.11,12,13,14,15,16 Because screening is offered to well women and breast cancer is absent in most who have abnormal screening results, the morbidity associated with an abnormal result should be reduced by providing timely follow-up that assures a firm diagnosis with the minimum number of interventions.
In April 1997 the Workshop on Organized Breast Cancer Screening, held in Ottawa, identified delays during the assessment process and poor integration of screening and diagnosis as areas of significant concern requiring action.17 To address this issue, a working group, whose members included a consumer (R.McG.), was established in November 1997 by Health Canada's Canadian Breast Cancer Screening Initiative. An initial step for the working group was to assess the patterns and timeliness of diagnosis after an abnormal screening result. We describe the patterns and timeliness of diagnostic follow-up for women attending 7 provincially organized screening programs in 1996. These findings in part formed the basis for timeliness targets adopted nationally in November 1999.
Methods
Data were abstracted from records in the Canadian Breast Cancer Screening Database, a database established in 1993 and maintained by Health Canada to facilitate the monitoring and evaluation of organized breast cancer screening in Canada. Data are submitted by organized screening programs to the national database every 2 years and are validated for appropriate ranges, frequencies and completeness. When our study was initiated, the most recent data available in the database were for the 1996 calendar year.
We analyzed data that were available from organized screening programs in British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, Nova Scotia and Newfoundland. These programs were established between 1988 and 1996 and, in 1996, provided data on 3119 to 166 744 screens. The variation in annual volume was related to the program's duration, provincial population, program eligibility criteria and the proportion of all provincial mammograms provided within the organized screening program.6,18,19 Program-specific and cumulative screening outcomes are described elsewhere.6,10,18,20,21,22 Most of the programs invited women for screening every 2 years, and all provided a bilateral, 2-view mammogram.6 In 4 of the programs, a nurse or technologist at the screening centre also provided a clinical breast examination. All of the programs included women aged 50–69 years, and some included other age groups. To improve comparability between programs, eligibility for this study was restricted to women aged 50–69 years. For women who underwent more than 1 screen in 1996, only the first was evaluated.
Women with an abnormal screening result (mammogram or clinical breast examination, or both) were followed prospectively to completion of the assessment. Fig. 1 shows, for a woman proceeding all the way to open biopsy, the potential steps in the diagnostic process after the screening examination and the assessment intervals we studied. We evaluated the number of weeks required to complete 4 assessment intervals: from screening examination to first assessment, from screening examination to first imaging assessment, from screening examination to diagnosis and from first assessment to diagnosis. The time from screening to first assessment was the number of weeks from the index screening to the first diagnostic mammogram, breast ultrasound or, when reported, physician visit. The time from screening to first imaging assessment was the interval between the index screening and the first diagnostic mammogram or breast ultrasound. The time from screening to diagnosis was the number of weeks from the index screening to the first pathological diagnosis of cancer, the last biopsy with benign findings or the last intervention before a recommendation to return to screening or return for early recall. The time from first assessment to diagnosis was the number of weeks from the first diagnostic mammogram, breast ultrasound or physician visit to the first pathological diagnosis of cancer, the last biopsy with benign findings or the last intervention before a recommendation to return to screening or return for early recall. The recommendation to return for early recall was considered an appropriate end point because, according to current clinical practice guidelines,7 it is an accepted management decision for women with low-risk lesions found on mammography. Exploration of data identified 22 weeks as the point at which the initial sequence of investigations was complete before the 6-month recall. Women who were recalled to undergo diagnostic imaging at 22 weeks or beyond were considered to have completed the follow-up at the last diagnostic test before the recall image.
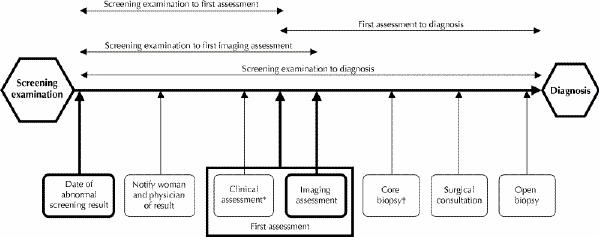
Fig. 1: Potential steps in the assessment of an abnormal breast cancer screening result for a woman undergoing open biopsy. Not all steps are required to arrive at a diagnosis. [Intervals are not drawn to scale.] Bold boxes indicate the start and end points of assessment intervals studied. *May include physical breast examination, fine-needle aspiration or referral, or a combination. †Depending on practice patterns, core biopsy may be performed before, after or without surgical consultation.
Patterns of diagnostic tests were evaluated through simple cross-tabulations. The durations of the 4 assessment intervals (Fig. 1) were calculated for women with valid dates reported for the start and end points of interest and who underwent the assessment procedures studied. Because of the skewness of waiting times, the median was used as the descriptive measure of central tendency, and the variability observed for the population was described using the 25th, 75th and 90th percentiles. Because the study population consisted of all women aged 50–69 who underwent breast cancer screening in the 7 programs in 1996, our calculations represent actual population parameters, and confidence intervals were thus not calculated. Waiting times were evaluated for all study subjects and were stratified according to screening program, mode of detection, whether a biopsy was performed (core or open biopsy [fine-needle aspiration alone was not considered a biopsy]) and whether cancer was diagnosed. Pathological diagnosis of primary invasive carcinoma of the breast of any histologic type or ductal carcinoma in situ (DCIS) were considered cancer; lobular carcinoma in situ, atypia or borderline lesions were not considered cancer.
Results
In 1996, 203 141 women aged 50–69 years underwent screening in the 7 provincial programs studied (Table 1). Overall, 15 342 (7.6%) were recalled for assessment because of an abnormality detected by the radiologist or the clinical examiner, or both; 991 cancers were detected (4.9 cancers per 1000 women screened or 6.8% of those with abnormal screening results). Of the cancers detected, 17.3% were DCIS. Mammographic abnormalities (with or without an abnormal result on clinical examination) accounted for 81.3% of the abnormal screening results and 97.8% of the program-detected cancers. Complete follow-up information was available for 95.0% of the women; assessment was still being finalized for 1.5% of the women, and 3.5% were lost to follow-up (patient refused follow-up testing or relocated, or the attending physician declined to provide follow-up information). Of the 14 575 women who completed the follow-up, 448 lacked diagnostic test information or their 6-month recall imaging examination was not preceded by other diagnostic tests, and a further 169 women had invalid information regarding test dates. In all, data for 13 958 women were available for analysis.
Table 1
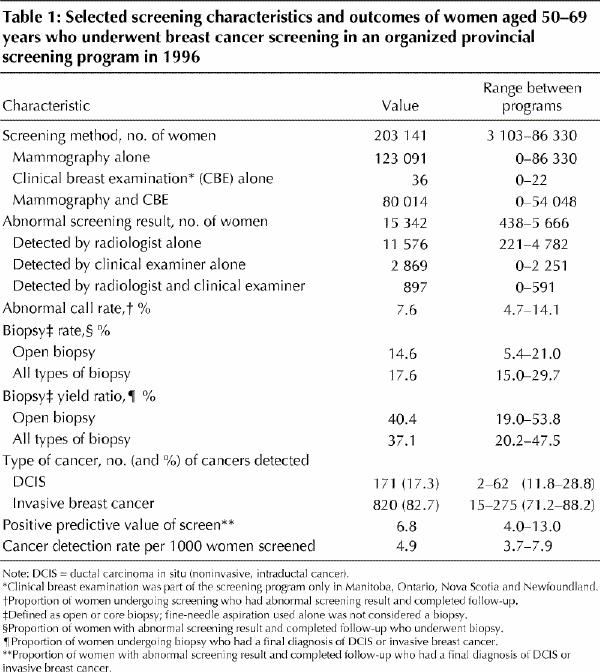
As part of their assessment follow-up, 11 329 (81.2%) of the women underwent an imaging procedure (diagnostic mammography or ultrasonography, or both). Of the 13 958 women, 9065 (64.9%) underwent diagnostic imaging procedures alone, 573 (4.1%) underwent fine-needle aspiration, 531 (3.8%) had core biopsy, and 2136 (15.3%) had open biopsy. The use of core biopsy directed by ultrasonography or stereotactic mammography varied considerably across programs, from 3% to 90% of women receiving a tissue diagnosis.
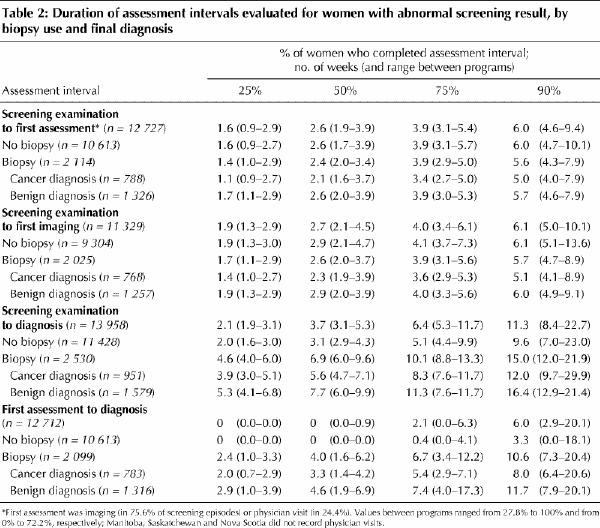
Table 2 summarizes the durations of the 4 intervals in the assessment process overall and by biopsy use and final diagnosis. The cumulative experience across all the programs and the range of values between the programs for each interval are presented. The median time from screening examination to the first assessment was 2.6 weeks. The duration was similar whether a biopsy was performed (2.4 weeks) or the assessment concluded without a biopsy (2.6 weeks). The median time from screening examination to diagnosis was 3.7 weeks; however, 10% of the women waited 11.3 weeks or longer for a diagnosis. Having a tissue diagnosis lengthened the time to diagnosis in all programs evaluated. The median waiting time was 3.1 weeks for women who completed the assessment process without a biopsy, as compared with 6.9 weeks for those who received a biopsy. Even without a biopsy, 10% of the women waited 9.6 weeks or longer for a final diagnosis. With a biopsy, 10% waited 15.0 weeks or longer.
Table 2
The median waiting times to diagnosis without biopsy were comparable between the 2 programs that used core biopsy most frequently and the other 5 programs; however, when biopsy was required, the median waiting times to diagnosis in the 2 programs were among the shortest. Fewer women in these 2 programs experienced exceedingly long waits; in both programs, assessment was completed within 13 weeks for 90% of the women, as compared with 12.4–21.9 weeks for 90% in the other 5 programs.
The waiting times varied greatly within and between programs (Table 2). The interquartile range of times for each interval evaluated was as large as or greater than the range of median times between programs for that interval. For example, for the interval from screening examination to diagnosis, 25% of the women who underwent a biopsy had a diagnosis within 4.6 weeks, but a further 25% of women waited 10.1 weeks or longer for a diagnosis; for women who did not have a biopsy, the corresponding 25th and 75th percentile times to diagnosis were 2.0 and 5.1 weeks. The range of median times between programs for this interval was 6.0–9.6 weeks with biopsy and 2.9–4.3 weeks without biopsy.
For women who had a biopsy, we compared the time to diagnosis according to final diagnosis. Overall and within each program, for all intervals evaluated, investigations were initiated and completed more promptly for women with cancer than for those with a benign lesion. In addition, there was less variation in waiting times for women with a biopsy showing cancer than for those whose biopsy showed a benign lesion.
Interpretation
The ability of screening programs to reduce breast cancer mortality depends on the adequacy of follow-up of women with abnormal screening results.23 Women with abnormalities detected through organized breast cancer screening programs in Canada in 1996 waited many weeks to receive a diagnosis, especially when a biopsy was performed. The interval to diagnosis varied considerably within and between programs. In each program, the 25% of women with the longest times to diagnosis waited about twice as long as the 25% of women with the shortest times to diagnosis (2 to 8 weeks longer without biopsy and 5 to 7 weeks longer with biopsy).
There is considerable evidence that an abnormal breast cancer screening result precipitates acute anxiety.10,15,24,25,26,27 Anxiety may persist for several months, even after a woman has been informed that she does not have cancer.2,24,26,27 Completing investigations promptly will reduce the time that women live in fear and may also reduce their overall level of anxiety.
Our analysis revealed that women found to have breast cancer had shorter times to diagnosis than did women whose biopsy showed a benign lesion. This suggests that physicians expedited investigations depending on the degree of suspicion of cancer. Despite this informal process of prioritization, it still took 12 weeks or longer for 10% of the women to receive a diagnosis of cancer, and additional time to complete definitive treatment. In a recent Manitoba study the median waiting time for lumpectomy or mastectomy of breast lesions was 2.3 weeks from the preoperative visit.28 It is unknown whether cumulative delays to treatment of 3 to 4 months from a screen-detected abnormality will affect a woman's chance of cancer progression or cure. However, it is worrisome that a 1999 meta-analysis suggested that delay to treatment as short as 3 to 6 months among women with symptomatic breast cancer was associated with poorer survival.29
In our study, the type of investigations reported, in particular the use of core biopsy, varied by program. Core biopsies are usually performed without the need for surgical consultation, hospital beds or day-care facilities.30,31,32 Although we looked at 1996 data, 1997/98 data from the Canadian Breast Cancer Screening Database indicate that patterns of biopsy use have changed little.33 In 1996, 2 of the programs used core biopsy more often than the other programs did (51% and 90% of patients having a tissue diagnosis v. 3% to 27%). These 2 programs had among the shortest median and 90th percentile times to diagnosis when a biopsy was performed and the highest cancer yield ratios with open biopsy, despite demonstrating average times to diagnosis when biopsy was not performed. The relatively shorter times to diagnosis in the 2 programs could be attributed to better access to core biopsy technology. Not all jurisdictions have access to this technology.30,31,32 Increased use of core biopsy in Canada might facilitate diagnosis and reduce delay for the minority of women who require a tissue diagnosis; however, not all abnormalities detected at screening are suitable for imaging-directed core biopsy, and wider dissemination of this technique should be monitored to ensure that the rate of unnecessary biopsies remains appropriately low.30,31,32 It is also possible that other differences in the provincial health care systems could explain the shorter times to diagnosis in the 2 programs.10
The United Kingdom and Australian national breast screening programs, supported by legislation, have mandated the development of interdisciplinary assessment clinics affiliated with screening centres. It has been suggested that Canadian programs are at a disadvantage compared with those where the diagnostic process is undertaken at specialized centres.23 Although similar programs have developed in some jurisdictions in Canada, in general women and their family physicians are notified by the breast screening program of an abnormality, and the family physician then initiates and coordinates the assessment process.6,18 Advantages of this system are that the family physician remains an integral participant in the diagnostic sequence and that existing referral and practice patterns are not disrupted. However, this often means multiple visits to different health care providers and facilities, which increases the time to diagnosis and the inconvenience and anxiety for the woman.10,34,35 The absence of integrated information systems to support health services can contribute to the delays.
One of 3 priorities for action identified at the Workshop on Organized Breast Cancer Screening in April 1997 was to improve the integration of screening and diagnosis.17 To further these efforts, it is necessary to achieve consensus on standards for reasonable timeliness for the different phases of the diagnostic process. Following a literature review as well as consideration of existing time standards established by Canadian and international programs and the distribution of times to diagnosis reported herein, timeliness targets were proposed (Table 3) and subsequently adopted in November 1999 by the Canadian Breast Cancer Screening Initiative.36
Table 3
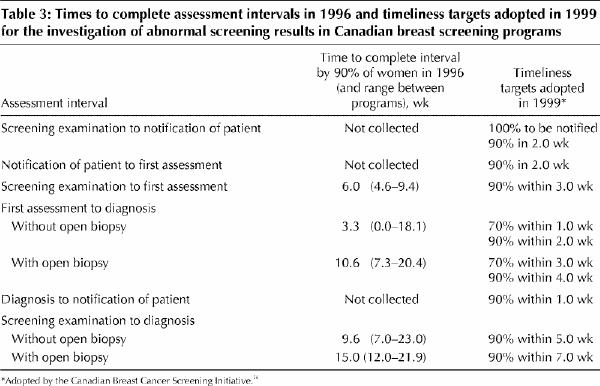
The proposed targets are still lengthy. If women were investigated within the timelines proposed, the time from screening examination to diagnosis could still be as long as 5 weeks without an open biopsy and 7 weeks with an open biopsy (Table 3). In 1996 these targets were achieved for more than 50% of the women in most of the 7 programs. However, the goal is to achieve the targets for more than 90% of women. In 1996, 90% of the women with an abnormal screening result received a diagnosis within 9.6 weeks without a biopsy and 15.0 weeks with a biopsy. Achieving the timeliness targets adopted in 1999 would represent a substantial improvement over usual practice in 1996.
A limitation of our study is that follow-up information was unavailable for 9% of the eligible women. These losses likely occurred randomly, and the inclusion of the missing data would probably not have affected the distribution of waiting times appreciably. Women without any test information recorded may have completed follow-up with clinical assessment only, which is less well-documented in the national database. This may have led to overestimated median times to diagnosis but would have affected few of the eligible women.
There may be many explanations for the observed distribution of waiting times to diagnosis. Experience with an appropriate investigation sequence after an abnormal screening result was a factor that influenced waiting times. The most recently implemented program had the smallest volume of screens and the longest times to diagnosis. However, even in the provinces with the shortest times between screening and diagnosis, the waits were long. In all regions of Canada evaluated, the interquartile variation in times was broad. In each province, the diagnostic system was working smoothly for some women and not for others.
We were unable to distinguish between patient, physician and system delays.37 Waiting times can be affected by women who postpone follow-up until a time that is appropriate for them, by physicians waiting to obtain comparison films to reduce unnecessary invasive procedures, and by varying degrees of integration of the organized screening programs within their province's health care system.
Many options to reduce waiting times are available, including reorganization, incorporation of new care routines,10 use of new technology, improved handling of referrals, formalization of continuity of care, and prioritization by severity of condition.38 Such measures need not improve health care for select groups of patients at the expense of others. However, in Canada, as in other countries, implementation of policies to reduce waiting times will be a challenge.38 Some practitioners may be concerned about a crowding-out effect on the health care needs of other patients (e.g., women with symptomatic breast cancer or patients with health problems other than breast cancer). Others may prefer to follow their practice policies and have greater flexibility in assessing different care needs.37 To improve services to their clients, programs should learn from each other's experiences in addressing these concerns when developing strategies and examining innovative approaches to integrate breast cancer screening and diagnosis.
Footnotes
This article has been peer reviewed.
Acknowledgements: We thank the provincially organized breast screening programs that contributed data and members of the Health Canada working group whose work contributed to the analysis.
This study was made possible by access to data submitted by provincially organized breast screening programs to the Canadian Breast Cancer Screening Database, a collaboration of the Cancer Bureau, Centre for Chronic Disease Prevention and Control, Health Canada, and the Canadian Breast Cancer Screening Initiative Data Management Committee.
Competing interests: None declared.
Correspondence to: Dr. Ivo A. Olivotto, BC Cancer Agency – Vancouver Island, 2410 Lee Ave., Victoria BC V8R 6V5
References
- 1.Shapiro S, Coleman EA, Broeders M, Codd M, de Koning H, Fracheboud J, et al. Breast cancer screening programmes in 22 countries: current policies, administration and guidelines. International Breast Cancer Screening Network (IBSN) and the European Network of Pilot Projects for Breast Cancer Screening. Int J Epidemiol 1998;27(5):735-42. [DOI] [PubMed]
- 2.Fletcher SW, Black W, Harris R, Rimer BK, Shapiro S. Report of the International Workshop on Screening for Breast Cancer. J Natl Cancer Inst 1993;85(20):1644-56. [DOI] [PubMed]
- 3.Nystrom L, Rutqvist LE, Wall S, Lindgren A, Lindqvist M, Ryden S, et al. Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet 1993;341(8851):973-8. [DOI] [PubMed]
- 4.Kerlikowske K, Grady D, Rubin SM, Sandrock C, Ernster VL. Efficacy of screening mammography. A meta-analysis. JAMA 1995;273(2):149-54. [PubMed]
- 5.Ballard-Barbash R, Klabunde C, Paci E, Broeders M, Coleman EA, Fracheboud J, et al. Breast cancer screening in 21 countries: delivery of services, notification of results and outcomes ascertainment. Eur J Cancer Prev 1999;8(5):417-26. [DOI] [PubMed]
- 6.Canadian Breast Cancer Screening Initiative. Organized breast cancer screening programs in Canada: 1996 report. Ottawa: Health Canada; 1999. Cat no H1-9/13-1999.
- 7.Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 2. Investigation of lesions detected by mammography. CMAJ 1998;158(3 Suppl):9S-14S. Available: www.cma.ca/cmaj/vol-158/issue-3/breastcpg/0009.htm [PubMed]
- 8.Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. Clinical practice guidelines for the care and treatment of breast cancer: 1. The palpable breast lump: information and recommendations to assist decision-making when a breast lump is detected. CMAJ 1998;158(3 Suppl):3S-8S. Available: www.cma.ca/cmaj/vol-158/issue-3/breastcpg/0003.htm [PubMed]
- 9.Organizing assessment. Sheffield (UK): National Health Service Breast Screening Programme Publications; 1989.
- 10.Caines JS, Chantziantoniou K, Wright BA, Konok GP, Iles SE, Bodurtha A, et al. Nova Scotia Breast Screening Program experience: use of needle core biopsy in the diagnosis of screening-detected abnormalities. Radiology 1996;198(1):125-30. [DOI] [PubMed]
- 11.Fentiman IS. Pensive women, painful vigils: consequences of delay in assessment of mammographic abnormalities. Lancet 1988;1:1041-2. [DOI] [PubMed]
- 12.Ellman R, Angeli N, Christians A, Moss S, Chamberlain J, Maguire P. Psychiatric morbidity associated with screening for breast cancer. Br J Cancer 1989;60(5):781-4. [DOI] [PMC free article] [PubMed]
- 13.Bull AR, Campbell MJ. Assessment of the psychological impact of a breast screening programme. Br J Radiol 1991;64(762):510-5. [DOI] [PubMed]
- 14.Ong G, Austoker J, Brett J. Breast screening: adverse psychological consequences one month after placing women on early recall because of a diagnostic uncertainty. A multicentre study. J Med Screen 1997;4(3):158-68. [DOI] [PubMed]
- 15.Brett J, Austoker J, Ong G. Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last breast screening appointment. J Public Health Med 1998;20(4):396-403. [DOI] [PubMed]
- 16.Thorne SE, Harris SR, Hislop TG, Vestrup JA. The experience of waiting for a diagnosis after an abnormal mammogram. Breast J 1999;5:42-51. [DOI] [PubMed]
- 17.Steering Committee for the National Workshop on Organized Breast Cancer Screening Programs. Workshop report: report of the theme discussion groups. The Committee; 1997. p. 25-8. Available on request from: cancer_division@hc-sc.gc.ca
- 18.Paquette D, Snider J, Bouchard F, Olivotto I, Bryant H, Decker K, et al, for the Database Management Subcommittee to the National Committee for the Canadian Breast Cancer Screening Initiative. Performance of screening mammography in organized programs in Canada in 1996. CMAJ 2000;163(9): 1133-8. Available: www.cma.ca/cmaj/vol-163/issue-9/1133.htm [PMC free article] [PubMed]
- 19.Gaudette LA, Altmayer CA, Nobrega KM, Lee J. Trends in mammography utilization, 1981 to 1994. Health Rep 1996;8(3):17-27. [PubMed]
- 20.Olivotto IA, Kan L, d'Yachova Y, Warren Burhenne LJ, Hayes M, Hislop TG, et al. Ten years of breast screening in the Screening Mammography Program of British Columbia, 1988-97. J Med Screen 2000;7:152-9. [DOI] [PubMed]
- 21.Bryant HE, Desautels JE, Castor WR, Horeczko N, Jackson F, Mah Z. Quality assurance and cancer detection rates in a provincial screening mammography program. Work in progress. Radiology 1993;188(3):811-6. [DOI] [PubMed]
- 22.Libstug AR, Moravan V, Aitken SE. Results from the Ontario breast screening program, 1990-1995. J Med Screen 1998;5(2):73-80. [DOI] [PubMed]
- 23.Miller AB. Organized breast cancer screening programs in Canada. CMAJ 2000;163(9):1150-1. Available: www.cma.ca/cmaj/vol-163/issue-9/1150.htm [PMC free article] [PubMed]
- 24.Cockburn J, Staples M, Hurley SF, De Luise T. Psychological consequences of screening mammography. J Med Screen 1994;1(1):7-12. [DOI] [PubMed]
- 25.Rimer B, Blumann L. The psychological consequences of mammography. Monogr J Natl Cancer Inst 1997;22:131-8. [DOI] [PubMed]
- 26.Lerman C, Trock B, Rimer BK, Boyce A, Jepson C, Engstrom PF. Psychological and behavioral implications of abnormal mammograms. Ann Intern Med 1991;114(8):657-61. [DOI] [PubMed]
- 27.Ong G, Austoker J. Recalling women for further investigation of breast screening: women's experiences at the clinic and afterwards. J Public Health Med 1997;19(1):29-36. [DOI] [PubMed]
- 28.DeCoster C, Carriere KC, Peterson S, Walld R, MacWilliam L. Waiting times for surgical procedures. Med Care 1999;37(6 Suppl):187S-205S. [DOI] [PubMed]
- 29.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 1999;353(9159):1119-26. [DOI] [PubMed]
- 30.Bear HD. Image-guided breast biopsy: How, when, and by whom? J Surg Oncol 1998;67(1):1-5. [DOI] [PubMed]
- 31.Britton PD, Flower CD, Freeman AH, Sinnatamby R, Warren R, Goddard MJ, et al. Changing to core biopsy in an NHS breast screening unit. Clin Radiol 1997;52(10):764-7. [DOI] [PubMed]
- 32.Lind DS, Minter R, Steinbach B, Abbitt P, Lanier L, Haigh L, et al. Stereotactic core biopsy reduces the reexcision rate and the cost of mammographically detected cancer. J Surg Res 1998;78(1):23-6. [DOI] [PubMed]
- 33.Canadian Breast Cancer Screening Initiative. Organized breast cancer screening programs in Canada: 1997 and 1998 report. Ottawa: Health Canada; 2001. Cat no H1-9/13-1998.
- 34.Burhenne LJ, Hislop TG, Burhenne HJ. The British Columbia Mammography Screening Program: evaluation of the first 15 months. Am J Roentgenol 1992;158(1):45-9. [DOI] [PubMed]
- 35.Katz SJ, Hislop TG, Thomas DB, Larson EB. Delay from symptom to diagnosis and treatment of breast cancer in Washington State and British Columbia. Med Care 1993;31(3):264-8. [DOI] [PubMed]
- 36.Waiting for a diagnosis after an abnormal breast screen in Canada. Report of the Working Group on the Integration of Screening and Diagnosis for the Canadian Breast Cancer Screening Initiative. Ottawa: Canadian Breast Cancer Screening Initiative Working Group on the Integration of Screening and Diagnosis; 2000. Cat no H39-526/2000E. Available (in pdf): www.hc-sc.gc.ca/hppb/ahi/breastcancer/pubs/diagnosis-report_eng.pdf (accessed 2001 July 3).
- 37.Kerlikowske K. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat 1996;40:53-64. [DOI] [PubMed]
- 38.Hanning M, Spangberg UW. Maximum waiting time: A threat to clinical freedom? Implementation of a policy to reduce waiting times. Health Policy 2000;52(1):15-32. [DOI] [PubMed]


