Abstract
Hepatocellular carcinoma (HCC) is the fourth-leading cause of cancer-related mortality worldwide and the fastest-rising cause of cancer-related death in the United States. Given the strong association between tumor stage and prognosis, HCC surveillance is recommended in high-risk patients, including patients with cirrhosis from any etiology. The diagnosis can be made based on characteristic imaging findings, with histologic confirmation primarily reserved for patients with atypical imaging findings. Over the last 2 decades, the treatment landscape for HCC has experienced significant advances. Curative therapies, including liver transplantation and surgical resection, are available to patients with early-stage HCC; however, recent data have expanded the potentially eligible patient population. Locoregional therapies, including transarterial chemoembolization and transarterial radio-embolization, continue to be standard therapies for patients with intermediate-stage disease. The greatest advances have been observed for patients with advanced HCC, where there are now multiple first- and second-line options that can prolong survival by up to 2 years when used sequentially. The increasing complexity of HCC treatment options underlies the necessity for multidisciplinary care, which has been associated with increased survival. This article reviews data on best practices for early detection and diagnosis of HCC and the current status of treatment options.
Keywords: Hepatocellular carcinoma, screening, diagnosis, LI-RADS, treatment, immunotherapy
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and the fourth-leading cause of cancer- related mortality worldwide.1,2 HCC has been increasing in incidence since the 1980s,3 and is now the fastest-rising cause of cancer-related death in the United States, with an estimated 1,284,252 deaths predicted between 2018 and 2040 worldwide.1,4 The majority of HCC occurs in the setting of chronic liver disease, with the most common risk factors being chronic hepatitis B virus (HBV) worldwide and hepatitis C virus–related cirrhosis and nonalcoholic steatohepatitis in the Western world. The estimated 5-year survival rate for HCC is only 18%,5 which is driven by a large proportion of patients being diagnosed at advanced stages when curative options are not feasible, as well as underuse of curative therapies among patients detected at early stages.6,7 This article reviews best practices for early detection and diagnosis of HCC and the current status of treatment options that can afford improved survival when applied in clinical practice.
Hepatocellular Carcinoma Surveillance
Given the connection between tumor stage and prognosis, the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Asian Pacific Association for the Study of the Liver recommend surveillance in patients with cirrhosis and in subsets of patients with chronic HBV infection.8-10 Two large randomized, controlled trials (RCTs) found that semiannual screening reduced HCC-related mortality among patients with HBV infection.11,12 Although no RCTs have evaluated HCC surveillance in patients with cirrhosis, several cohort studies have reported earlier detection and improved survival in patients with cirrhosis who undergo surveillance.6,13 Major guidelines recommend surveillance testing with semiannual abdominal ultrasound, and many experts also recommend serologic testing for alpha-fetoprotein (AFP), a serum biomarker.8,9 At best, semiannual ultrasound with AFP achieves a sensitivity of 63% for early-stage HCC, missing over one-third of tumors at that stage, and is associated with screening-related harms from false-positive results in 10% to 20% of patients.14,15 Ongoing studies are evaluating novel blood-based biomarker panels and imaging techniques to optimize surveillance outcomes, although none has sufficient data to be implemented into routine practice at this time.16,17 Despite improved utilization over time, surveillance is implemented in less than 50% of patients with cirrhosis, with few receiving consistent semiannual surveillance.18 These data highlight the need for interventions to improve surveillance effectiveness.19
Patients with abnormal surveillance tests should undergo follow-up testing to confirm their HCC diagnosis. Patients with small ultrasound nodules (<10 mm) have a low risk of HCC and should undergo repeat ultra-sound imaging in 3 to 6 months.8,20 In contrast, patients with nodules 10 mm or larger or abnormal AFP should undergo diagnostic imaging with multiphase computed tomography (CT) or magnetic resonance imaging (MRI) to establish a diagnosis.8,20
Hepatocellular Carcinoma Diagnosis
Unlike most solid malignancies, HCC can be diagnosed with imaging alone in high-risk individuals.9,20 The American College of Radiology has proposed a nomenclature called the Liver Imaging Reporting and Data System for the standardization of interpreting and reporting multiphase CT scan and MRI.21 Lesions are classified into 5 main categories ranging from definite benign (LR-1) to definite HCC (LR-5) based on a combination of major criteria, including arterial hyper enhancement, delayed washout, and an enhancing capsule, as well as several minor criteria (Table).21 The sensitivity of LR-3, LR-4, and LR-5 for HCC is 38%, 74%, and 94%, respectively.22 Therefore, patients with characteristic imaging (ie, LR-5) can be treated for HCC without histologic confirmation.
Table.
LI-RADS Major and Minor Imaging Features on Contrast-Enhanced CT/MRI That Favor Hepatocellular Carcinomaa
| Major Features | Minor Features |
|---|---|
| Nonrim-like arterial phase hyperenhancement | Nodule-in-nodule architecture |
| Enhancing capsule | Nonenhancing capsule |
| Nonperipheral washout | Mosaic architecture |
| Threshold growth (≥50% increase in size of a lesion in ≤6 months) | Blood products in the lesion |
| Size ≥20 mm | Fat in the lesion (more than adjacent liver) |
CT, computed tomography; LI-RADS, Liver Imaging Reporting and Data System; MRI, magnetic resonance imaging.
aBased on LI-RADS version 2018.
Serum tumor markers play a minor role in the diagnosis of HCC. AFP has limited sensitivity and specificity for HCC, and therefore was removed from the diagnostic criteria for HCC. AFP can be elevated in a variety of other gastrointestinal malignancies, including cholangiocarcinoma, and is often normal in patients with early-stage HCC.23-25
Biopsy is primarily reserved for select patients with atypical imaging, which can be observed in 10% of HCC patients. Some HCC lesions can have enhancement without washout or washout with enhancement (often classified as LR-4), whereas other lesions have atypical features worrisome for malignancy but not definite for HCC (classified as LR-M), such as rim arterial phase enhancement or peripheral washout.21 There are well- defined histopathologic criteria for classifying and grading HCC, with classic histologic features including wide trabeculae, prominent acinar pattern, cytologic atypia, vascular invasion, and vascularization.26,27 Although most HCC diagnoses can be established using histology alone, stains such as glypican-3, glutamine synthetase, and heat shock protein 70 can be helpful in some cases.26,27
Tumor Staging
Staging is necessary for prognostication and selection of therapy, and should take into account the degree of underlying liver dysfunction. Multiple staging systems have been proposed for HCC, and there is no universally recommended system. The Barcelona Clinic Liver Cancer (BCLC) system classifies patients based on tumor burden (number of lesions, maximum tumor diameter, and presence of vascular invasion or metastasis), degree of liver dysfunction (Child-Pugh class), and cancer-related symptoms (Eastern Cooperative Oncology Group performance status).28 A study comparing 7 staging systems reported that the BCLC system had the best independent predictive power for estimating survival in a US cohort.29 The BCLC system has also been validated in several cohorts from North America, Europe, and Asia, and is, therefore, recommended by both the AASLD and EASL for HCC staging.20 The BCLC classification ranges from very-early–stage HCC (BCLC 0), with a 5-year survival rate exceeding 70%, to terminal-stage HCC (BCLC D), with a median survival below 6 months.
Hepatocellular Carcinoma Treatment
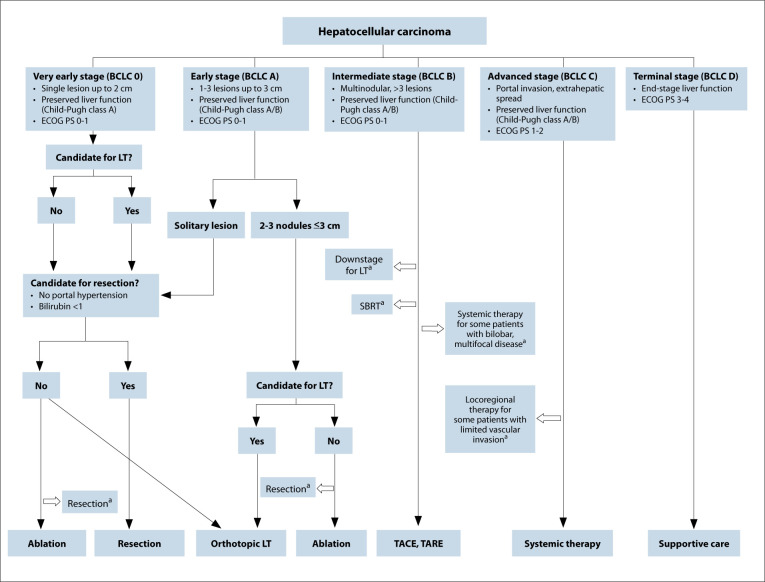
The BCLC system is linked to a treatment algorithm (Figure 1) that includes curative options for early-stage HCC and palliative options for intermediate- and advanced-stage HCC. Given an increasing number of treatment options, a multidisciplinary approach is recommended and has been shown to improve appropriate treatment receipt and overall survival (OS).
Figure 1.
BCLC treatment recommendations according to BCLC stage, including additional evidence-based therapies.
BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; LT, liver transplantation; PS, performance status; SBRT, stereotactic body radiation therapy; TACE, transarterial chemoembolization; TARE, transarterial radioembolization.
aThese therapies have not yet been incorporated into guidelines but are evidence-based.
Surgical Resection
Surgical resection is the therapy of choice in HCC patients without cirrhosis and those with Child-Pugh class A cirrhosis without portal hypertension; however, careful patient selection is critical. Although resection is widely used in Asia because many patients have HBV- related HCC and compensated liver function, only a small proportion of patients in the United States and Europe are eligible for resection given underlying liver dysfunction. Patients with advanced liver dysfunction are at high risk of postoperative liver failure. The best outcomes are observed in patients with Child-Pugh class A cirrhosis, bilirubin 1 mg/dL or less, and no portal hypertension.30,31 A Model for End-Stage Liver Disease (MELD) score greater than 9 has been associated with an increased risk of perioperative mortality and postoperative liver failure in retrospective studies.32-34 In addition to the degree of underlying liver dysfunction, the risk of postoperative liver failure is driven by the quantity of future liver remnant (FLR).35 In patients without cirrhosis, the risk of postoperative liver failure is low if the FLR exceeds 20%; however, an FLR of 40% is typically required in patients with cirrhosis.36 In patients with insufficient FLR, portal vein embolization or neoadjuvant transarterial radioembolization (TARE) promotes hypertrophy of the contralateral lobe and allows for resection in select cases.36-38
Observational studies suggest that surgical resection can be expanded to select patients with portal hypertension, multifocal tumors, and vascular invasion.39,40 Retrospective studies have reported acceptable 5-year survival rates in patients with Child-Pugh class A cirrhosis with portal hypertension.41-44 The BRIDGE study, a large multicenter, retrospective study, demonstrated no significant differences in survival among patients with or without portal hypertension undergoing liver resection.45 The use of surgical resection in patients with portal hypertension may be further facilitated by adoption of laparoscopic resection. Although multifocality may be a risk factor for HCC recurrence, retrospective studies have reported improved survival with surgical resection compared to ablation, transarterial chemoembolization (TACE), and supportive care for select patients with multifocal HCC.46,47 These findings were confirmed in an RCT in which 230 patients with HCC within the Milan criteria (unifocal lesion ≤5 cm or 2-3 lesions each ≤3 cm, without large-vessel invasion or metastases) were randomized to surgical resection or radiofrequency ablation (RFA); the study reported 5-year survival rates of 76% and 55%, respectively.48 In patients with limited vascular invasion, a case-control study of 603 patients with resectable HCC and portal vein tumor thrombus found that resection improved survival compared to TACE.49 Taken together, these data suggest that survival may be increased with surgical resection compared to palliative treatment in select patients in high-volume, expert centers.
Although resection is considered curative, with a 5-year survival rate greater than 60%,50 it is associated with a high rate of tumor recurrence; thus, close surveillance is critical.51 The risk of recurrence increases with tumor size, number of lesions, and presence of microvascular invasion with up to a 50% risk of tumor recurrence within 5 years for larger or multinodular HCC.51 The STORM trial failed to find any benefit of adjuvant sorafenib (Nexavar, Bayer) in improving time to recurrence or survival.52 There are several ongoing studies evaluating the role of neoadjuvant and adjuvant checkpoint inhibitors to reduce the risk of recurrence.53-56
Liver Transplantation
Liver transplantation (LT) provides the best chance for long-term survival, as it offers a cure for both HCC and the underlying cirrhosis. When LT was initially offered to all patients with HCC in the late 1980s and early 1990s, it was associated with a 5-year survival rate of only 30% to 40% and, thus, a moratorium was placed for this indication.57 In a landmark study published in 1996, Mazzaferro and colleagues identified the Milan criteria, which were associated with excellent posttransplant outcomes.58 Subsequently, the Milan criteria defined the standard eligibility of HCC patients for LT, resulting in improved 5-year survival rates of more than 70% and recurrence rates of approximately 10%.58,59
In 2002, the Milan criteria were adopted for the MELD exception pathway in the United States, providing additional points for HCC patients. Initially, HCC patients within the Milan criteria were provided a MELD score of 24 to 29 based on tumor size and a 10% increase every 3 months until LT or removal from the waiting list.60 However, this over-advantaged patients with HCC, raising concern for disparities in LT access compared to non-HCC patients.61 Over time, changes have been made to the allocation system, involving decreases in allocated MELD exception points. As of May 2019, HCC patients are required to wait 6 months from listing before being granted MELD exception points, at which time they receive a score of the median MELD score for the region minus 3 points.62
Given the success of LT for HCC, there is substantial interest in expanding LT to additional populations. In 2001, the University of California San Francisco (UCSF) derived the UCSF criteria (single tumor ≤6.5 cm or 2-3 tumors with largest lesion ≤4.5 cm and total tumor volume ≤8 cm, without vascular invasion or metastases) from explant pathology, and reported similar 1- and 5-year posttransplant survival rates compared to patients who underwent LT within the Milan criteria.63 Subsequent single-center observational studies reported similar posttransplant survival rates among patients undergoing LT within the Milan criteria and UCSF criteria.64-66 The up-to-7 criteria is an additional proposal to expand HCC size limits, which has been associated with a 5-year post-transplant survival rate of 71%.67
At the forefront of LT is tumor downstaging, which involves the application of locoregional therapy to reduce tumor burden to meet LT eligibility criteria and is associated with encouraging results.68,69 An intention-to-treat analysis from UCSF reported similar 5-year posttrans-plant survival rates among patients transplanted after successful downstaging and patients who initially fulfilled the Milan criteria (77.8% vs 81.0%; P=.69).70 The first multicenter study using the UCSF downstaging protocol evaluated outcomes of 187 HCC patients who underwent downstaging over a 10-year period and reported a 5-year posttransplant survival rate of 80% in patients transplanted after successful downstaging to within the Milan criteria.71 In an effort to standardize downstaging criteria, the United Network for Organ Sharing (UNOS) adopted the UCSF criteria for downstaging in 2017 and allows patients to receive MELD exception points if the tumor is successfully downstaged.70 A validation study used the UNOS database to compare outcomes among patients transplanted with HCC always within the Milan criteria, patients successfully downstaged to within the Milan criteria using the UCSF protocol, and patients successfully downstaged using other downstaging protocols nationwide.72 The study reported similar 3-year posttransplant survival rates among patients always within the Milan criteria (83%) and patients successfully downstaged using the UCSF protocol (79%), but lower survival rates were reported in patients downstaged for HCC exceeding UCSF criteria (71%).72
The risk of HCC recurrence post-LT is estimated to be 12% to 19%.73-75 AFP is predictive of post-LT survival and HCC recurrence, and has been incorporated into several models for predicting post-LT recurrence. The Risk Estimation of Tumor Recurrence After Transplant score is an externally validated model that uses the AFP at transplant and explant pathology to predict 5-year recurrence.76 Similarly, the Model of Recurrence After Liver Transplant score incorporates preoperative factors to predict 5-year recurrence-free survival.77 Currently, UNOS restricts patients with AFP levels higher than 1000 ng/mL from receiving MELD exception points regardless of tumor size unless successfully downstaged to AFP levels lower than 500 ng/mL.
Local Ablative Therapy
Ablative therapy is a potentially curative treatment for early-stage HCC. It destroys tumor cells via chemical injection or thermal destruction,78 either percutaneously or surgically, and is recommended for patients with very-early–stage or early-stage HCC (BCLC 0-A) who are ineligible for surgical resection.8 RFA generates heat via the application of high-frequency electric current and is most effective in lesions 2 cm or smaller, achieving a complete response of 97.2% over a median follow-up of 31 months.79 Its efficacy is limited by large tumor size and proximity to large vessels and bile ducts, which results in the dissipation of heat (called the heat-sink effect).80 Multiple RCTs have compared resection to RFA and have suggested that resection is likely associated with improved survival at 1, 3, and 5 years.48,81,82 However, differences in outcomes between resection and ablation are mitigated in lesions 2 cm or smaller, as demonstrated by a retrospective study from Italy reporting similar rates of 4-year survival (74.4% for resection vs 66.2% for RFA), recurrence, and complications.83-85 In a cost-effectiveness analysis, RFA offered similar quality-adjusted life-years at a lower cost than resection for very-early–stage HCC (≤2 cm).86 In lesions that were 3 to 5 cm, resection offered better life expectancy and was more cost-effective compared to RFA.86 Microwave ablation (MWA) is a newer technique that generates heat by creating an electromagnetic field, resulting in higher temperatures, larger ablation volumes over fewer sessions, and less heat-sink effect than RFA. A 2017 RCT reported similar rates of 1-, 3-, and 5-year local tumor progression, OS, and disease-free survival between RFA and MWA.87 However, a meta-analysis reported no difference in tumor response, recurrence, 3-year survival rate, or major complications between the 2 modalities.88 When only studies evaluating patients with larger tumor size were analyzed, MWA was superior to RFA in terms of tumor response and recurrence rate.88
Transarterial Chemoembolization
TACE involves the intra-arterial administration of chemotherapy followed by embolization, and it has been the traditional standard-of-care therapy for patients with intermediate-stage HCC (BCLC B) per AASLD and EASL guidelines. Although the procedure is noncurative in most cases, robust data show TACE can produce objective responses in 16% to 70% of patients and significantly prolong survival compared to supportive management, providing a median survival of approximately 26 months.89,90 Substantial center-to-center variation exists in terms of the number of treatments and chemotherapeutic agents used for conventional TACE. Adoption of drug-eluting beads with TACE (DEB-TACE), in which beads slowly release chemotherapy over time,91 may help reduce some of the heterogeneity between centers, although there still remain differences in the degree of selectiveness when treating tumors. There have been no demonstrated differences in tumor response or OS between TACE and DEB-TACE; however, DEB-TACE is believed to be better tolerated with a lower incidence of postembolization syndrome.92-94
Transarterial Radioembolization
TARE, which is the intra-arterial administration of radioactive microemboli, has emerged as an alternative to TACE. Unlike TACE, TARE maintains patency of the hepatic artery and can, therefore, be used in patients with portal vein thrombosis. A prospective cohort study including 1000 patients with HCC stage BCLC A, B, or C reported an OS of 47.3, 25.0, and 15.0 months, respectively, in patients with Child-Pugh class A cirrhosis and of 27.0, 15.0, and 8.0 months in Child-Pugh class B cirrhosis patients.95 Other nonrandomized studies have reported OS rates of approximately 17 months in patients with intermediate-stage HCC.96,97 A small phase 2 RCT comparing TARE and conventional TACE demonstrated a significant improvement in time to progression (>26.0 months vs 6.8 months; P=.0012) with fewer adverse effects, but no significant difference in OS (18.6 months vs 17.7 months; P=.99).98 A meta-analysis that included 2 RCTs and 8 retrospective studies found no significant difference in 1-year survival rates but significant improvements in 2- and 3-year survival rates with TARE.99 Lastly, observational studies have reported that TARE is better tolerated and associated with shorter hospital stays.100 Although there are growing data supporting TARE and increased adoption in clinical practice, the lack of large phase 3 data have precluded its inclusion in clinical practice guidelines.
Stereotactic Body Radiation Therapy
HCC was historically thought to be radioresistant because sufficient doses of external beam radiation were limited by high rates of radiation-induced liver injury. Stereotactic body radiation therapy (SBRT) is an emerging therapy that uses overlapping beams of radiation to safely deliver sufficient radiation doses to HCC lesions while limiting radiation exposure to the background liver. There are increasing single-arm data demonstrating promising outcomes, as well as retrospective analyses comparing outcomes following SBRT to other therapies. In a retrospective analysis of 224 patients who underwent RFA vs SBRT, the latter was associated with improved 1- and 2-year local control rates (83.6% and 80.2%, respectively, with RFA vs 97.4% and 83.8%, respectively, with SBRT) and similar 1- and 2-year survival rates.101 Similarly, analyses comparing SBRT vs TACE and RFA demonstrate similar OS rates, although these studies continue to be limited by risk of selection bias and residual confounding.102,103
Systemic Therapy
Systemic therapy is recommended for advanced HCC (BCLC C), that is, patients with vascular invasion, extra-hepatic metastasis, or tumor-related symptoms (Eastern Cooperative Oncology Group 1-2), as well as patients who progress after locoregional therapy.8,9 Over the last 10 years, the landscape of systemic therapy has rapidly expanded. Despite the introduction of sorafenib, the prognosis of advanced HCC remained poor over time.104 New first- and second-line therapies have recently become available and are expected to improve survival.
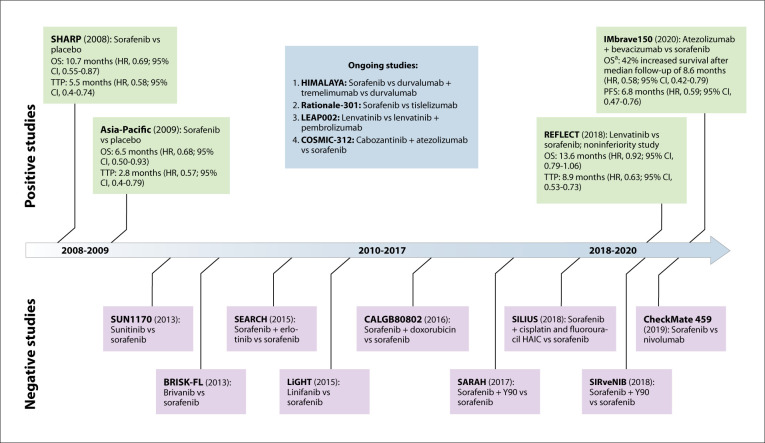
First-line systemic options for advanced HCC include sorafenib, lenvatinib (Lenvima, Eisai), and combination atezolizumab (Tecentriq, Genentech) plus bevacizumab (Avastin, Genentech). The first agent approved was sorafenib, which is a multitargeted tyrosine kinase inhibitor with antiangiogenic effects through inhibition of vascular endothelial growth factor (VEGF) receptors.105 Sorafenib was approved based on 2 large phase 3 RCTs, the SHARP trial106 and the Asia-Pacific trial (Figure 2).107 The SHARP trial randomized 602 patients to receive sorafenib or placebo, and was stopped early when the second interim analysis reported improved median OS of 10.7 vs 7.9 months (hazard ratio [HR], 0.69; 95% CI, 0.55-0.87).106 The Asia-Pacific trial similarly reported improved OS of 6.5 vs 4.2 months with sorafenib (HR, 0.68; 95% CI, 0.50-0.93).107 Notably, both studies predominantly included patients with Child-Pugh class A cirrhosis. No RCTs have evaluated sorafenib in patients with Child-Pugh class B cirrhosis, who represent a significant proportion of advanced HCC patients. The GIDEON study, a prospective observational database evaluating treatment practices, found that sorafenib in patients with Child-Pugh class B cirrhosis had acceptable tolerability with a median survival of 5.2 months.108
Figure 2.
Timeline of major phase 3 clinical trials of first-line treatments for advanced hepatocellular carcinoma by year of publication or presentation at a national meeting.
HAIC, hepatic arterial infusion chemotherapy; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
aMedian survival not yet reached. Results reported are after a median follow-up of 8.6 months.
Lenvatinib was the second agent approved for first-line therapy. Like sorafenib, it is a multitargeted tyrosine kinase inhibitor with antiangiogenic effects through the inhibition of VEGF receptors as well as increased activity against other growth factor receptors, such as fibroblast growth factor receptors.109 Lenvatinib was approved in 2018 based on the results of the REFLECT study, a phase 3 noninferiority trial that compared lenvatinib to sorafenib.109 The trial included 954 patients with Child-Pugh class A cirrhosis and advanced HCC without significant (>50%) liver involvement or main portal vein invasion, and reported noninferiority of lenvatinib to sorafenib with an OS of 13.6 vs 12.3 months (HR, 0.92; 95% CI, 0.79-1.06). Lenvatinib also demonstrated superiority for several secondary outcomes, including increased objective response rate (24.1% vs 9.2%) and prolonged progression-free survival (7.4 vs 3.7 months).109
The combination of immune checkpoint inhibitor atezolizumab, a programmed death-ligand 1 (PD-L1) inhibitor, and bevacizumab, a VEGF inhibitor, was the most recent treatment approved for first-line therapy of advanced HCC and is the first treatment in more than 10 years to be associated with improved OS and progression-free survival compared to sorafenib. The combination therapy was first evaluated in a phase 1b study of patients with advanced HCC, which reported an objective response rate of 34% and a 6-month progression-free survival rate of 71%.110 The treatment received breakthrough therapy designation by the US Food and Drug Administration (FDA) in April 2020 based on results of the IMbrave150 study, a multicenter phase 3 study that randomized 501 patients with Child-Pugh class A cirrhosis and advanced HCC in a 2:1 ratio to atezolizumab plus bevacizumab vs sorafenib.111 Atezolizumab plus bevacizumab was associated with a 42% reduction in mortality (HR, 0.58; 95% CI, 0.42-0.79) after a median follow-up of 8.6 months and improved progression-free survival (HR, 0.59; 95% CI, 0.47-0.76) compared to sorafenib. At the interim analysis, median OS had not yet been reached for the atezolizumab plus bevacizumab arm but was 13.2 months for the sorafenib arm. The combination therapy was associated with an increased response rate (33.2% vs 13.2% per modified Response Evaluation Criteria in Solid Tumors; P<.0001) and was well tolerated, with minimal adverse events.111 Of specific note, incident gastrointestinal bleeding was low in the atezolizumab plus bevacizumab arm, likely due to patient selection (Child-Pugh class A cirrhosis without significant portal hypertension) and patients being required to have an upper endoscopy with control of varices prior to entering the trial.
Second-line options have become available since 2017 and include 2 tyrosine kinase inhibitors (regorafenib [Stivarga, Bayer] and cabozantinib [Cabometyx, Exelixis]), a monoclonal VEGF inhibitor (ramucirumab [Cyramza, Eli Lilly]), 2 programmed cell death protein 1 (PD-1) checkpoint inhibitors (nivolumab [Opdivo, Bristol Myers Squibb] and pembrolizumab [Keytruda, Merck]), and a combination regimen targeting PD-1 and cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) (nivolumab and ipilimumab [Yervoy, Bristol Myers Squibb]). Regorafenib was the first agent approved based on the results of the phase 3 RESORCE study.112 Patients who tolerated sorafenib but had radiologic progression were randomized to receive regorafenib vs placebo, and regorafenib provided a survival benefit of 10.7 vs 7.8 months (HR, 0.63; 95% CI, 0.50-0.79).112 In a post hoc analysis, median survival from the start of sorafenib was 26.0 vs 19.2 months in the regorafenib vs placebo groups, highlighting the potential for second-line therapy to provide meaningful survival of approximately 2 years in select patients.113 Cabozantinib was evaluated in the phase 3 CELESTIAL trial, which included patients who failed sorafenib due to intolerance or radiologic progression, and which reported improved median survival of 10.2 vs 8.0 months (HR, 0.76; 95% CI, 0.63-0.92).114 Ramucirumab was initially evaluated in the REACH trial, which failed to show a survival benefit, but a post hoc analysis suggested it may be beneficial in patients with AFP levels higher than 400 ng/dL. In the subsequent REACH-II trial, among patients who failed sorafenib and had an AFP higher than 400 ng/dL, ramucirumab demonstrated a modest improvement in median survival of 8.5 vs 7.3 months compared to placebo (HR, 0.71; 95% CI, 0.50-0.95).115,116
Immune checkpoint inhibitors targeting CTLA-4, PD-1, and PD-L1 are being evaluated for advanced HCC. Nivolumab and pembrolizumab are anti–PD-1 monoclonal antibodies that received accelerated approval based on phase 2 studies demonstrating long-lasting response rates in approximately 15% to 20% of patients, but failed to improve OS in subsequent phase 3 studies. Nivolumab was evaluated in the phase 3 CheckMate 459 trial, which did not significantly improve OS compared to sorafenib (Figure 2).117 Pembrolizumab was evaluated in the phase 3 KEYNOTE-240 trial, which did not improve OS compared to supportive care.118 Recently, the FDA granted accelerated approval for the combination of nivolumab and ipilimumab based on phase 2 single-arm data showing durable responses in 33% of patients.116 Preclinical studies suggest a potential synergistic effect between checkpoint inhibitors and VEGF inhibitors, as well as with dual immune checkpoint blockade. Excitement for combination therapies has been further bolstered by results observed in the phase 3 IMbrave150 trial.111 As a result, several ongoing trials are evaluating combination therapy with checkpoint inhibitors, tyrosine kinase inhibitors, and VEGF inhibitors.119-122
Conclusion
HCC surveillance is associated with earlier diagnosis of HCC and the best long-term survival given potentially curative treatment options such as surgical resection, local ablation, and LT. There are several new treatment options for patients with larger tumor burden, particularly for patients requiring systemic therapy. The availability of combination therapies, such as atezolizumab plus bevacizumab, and options for sequential therapy herald an opportunity to achieve survival approaching 2 years in patients with advanced HCC. With evidence-based application of surveillance, recall, and treatment principles, notable improvements in HCC survival may be seen.
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Choo SP, Tan WL, Goh BKP, Tai WM, Zhu AX. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016;122(22):3430–3446. doi: 10.1002/cncr.30237. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340(10):745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer; World Health Organization. Cancer tomorrow. https://gco.iarc.fr/tomorrow Accessed September 17, 2020.
- Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012;55(2):476–482. doi: 10.1002/hep.24710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DT, Kum HC, Park S et al. Hepatocellular carcinoma screening is associated with increased survival of patients with cirrhosis. Clin Gastroenterol Hepatol. 2019;17(5):976–987.e4. doi: 10.1016/j.cgh.2018.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38(7):703–712. doi: 10.1111/apt.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach JK, Kulik LM, Finn RS et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- Omata M, Cheng AL, Kokudo N et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11(4):317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130(7):417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- Yang B, Zhang B, Xu Y et al. Prospective study of early detection for primary liver cancer. J Cancer Res Clin Oncol. 1997;123(6):357–360. doi: 10.1007/BF01438313. [DOI] [PubMed] [Google Scholar]
- Singal AG, Mittal S, Yerokun OA et al. Hepatocellular carcinoma screening associated with early tumor detection and improved survival among patients with cirrhosis in the US. Am J Med. 2017;130(9):1099–1106.e1. doi: 10.1016/j.amjmed.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Tzartzeva K, Obi J, Rich NE et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology. 2018;154(6):1706–1718.e1. doi: 10.1053/j.gastro.2018.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atiq O, Tiro J, Yopp AC et al. An assessment of benefits and harms of hepatocellular carcinoma surveillance in patients with cirrhosis. Hepatology. 2017;65(4):1196–1205. doi: 10.1002/hep.28895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, An J, Lim YS et al. MRI with liver-specific contrast for surveillance of patients with cirrhosis at high risk of hepatocellular carcinoma. JAMA Oncol. 2017;3(4):456–463. doi: 10.1001/jamaoncol.2016.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berhane S, Toyoda H, Tada T et al. Role of the GALAD and BALAD-2 sero-logic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14(6):875–886.e6. doi: 10.1016/j.cgh.2015.12.042. [DOI] [PubMed] [Google Scholar]
- Singal AG, Yopp A, Skinner CS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singal AG, Tiro JA, Murphy CC et al. Mailed outreach invitations significantly improve HCC surveillance rates in patients with cirrhosis: a randomized clinical trial. Hepatology. 2019;69(1):121–130. doi: 10.1002/hep.30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero JA, Kulik LM, Sirlin CB et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- Elsayes KM, Kielar AZ, Chernyak V et al. LI-RADS: a conceptual and historical review from its beginning to its recent integration into AASLD clinical practice guidance. J Hepatocell Carcinoma. 2019;6:49–69. doi: 10.2147/JHC.S186239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol CB, Lim CS, Sirlin CB et al. Accuracy of the Liver Imaging Reporting and Data System in computed tomography and magnetic resonance image analysis of hepatocellular carcinoma or overall malignancy—a systematic review. Gastroenterology. 2019;156(4):976–986. doi: 10.1053/j.gastro.2018.11.020. [DOI] [PubMed] [Google Scholar]
- Chan SL, Mo F, Johnson PJ et al. Performance of serum α -fetoprotein levels in the diagnosis of hepatocellular carcinoma in patients with a hepatic mass. HPB (Oxford). 2014;16(4):366–372. doi: 10.1111/hpb.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65(2):95–101. doi: 10.1159/000072332. [DOI] [PubMed] [Google Scholar]
- Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211(3):277–287. [PMC free article] [PubMed] [Google Scholar]
- Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol. 2014;20(43):15955–15964. doi: 10.3748/wjg.v20.i43.15955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Consensus Group for Hepatocellular Neoplasia; The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the International Consensus Group for Hepatocellular Neoplasia. Hepatology. 2009;49(2):658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- Marrero JA, Fontana RJ, Barrat A et al. Prognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohort. Hepatology. 2005;41(4):707–716. doi: 10.1002/hep.20636. [DOI] [PubMed] [Google Scholar]
- Berzigotti A, Reig M, Abraldes JG, Bosch J, Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: a systematic review and meta-analysis. Hepatology. 2015;61(2):526–536. doi: 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Kanematsu T, Arii S et al. Liver Cancer Study Group of Japan. Recurrence-free survival more than 10 years after liver resection for hepatocellular carcinoma. Br J Surg. 2011;98(4):552–557. doi: 10.1002/bjs.7393. [DOI] [PubMed] [Google Scholar]
- Cucchetti A, Ercolani G, Vivarelli M et al. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12(6):966–971. doi: 10.1002/lt.20761. [DOI] [PubMed] [Google Scholar]
- Teh SH, Christein J, Donohue J et al. Hepatic resection of hepatocellular carcinoma in patients with cirrhosis: model of end-stage liver disease (MELD) score predicts perioperative mortality. J Gastrointest Surg. 2005;9(9):1207–1215. doi: 10.1016/j.gassur.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Delis SG, Bakoyiannis A, Dervenis C, Tassopoulos N. Perioperative risk assessment for hepatocellular carcinoma by using the MELD score. J Gastrointest Surg. 2009;13(12):2268–2275. doi: 10.1007/s11605-009-0977-5. [DOI] [PubMed] [Google Scholar]
- Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29(1):6–17. doi: 10.1159/000335713. [DOI] [PubMed] [Google Scholar]
- Vauthey JN, Chaoui A, Do KA et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127(5):512–519. doi: 10.1067/msy.2000.105294. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Castaing D, Krissat J et al. Percutaneous portal vein embolization increases the feasibility and safety of major liver resection for hepatocellular carcinoma in injured liver. Ann Surg. 2000;232(5):665–672. doi: 10.1097/00000658-200011000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vouche M, Lewandowski RJ, Atassi R et al. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59(5):1029–1036. doi: 10.1016/j.jhep.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa T, Hasegawa K, Aoki T et al. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134(7):1908–1916. doi: 10.1053/j.gastro.2008.02.091. [DOI] [PubMed] [Google Scholar]
- Zhong JH, Ke Y, Gong WF et al. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260(2):329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]
- Santambrogio R, Kluger MD, Costa M et al. Hepatic resection for hepato-cellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford). 2013;15(1):78–84. doi: 10.1111/j.1477-2574.2012.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchetti A, Ercolani G, Vivarelli M et al. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250(6):922–928. doi: 10.1097/SLA.0b013e3181b977a5. [DOI] [PubMed] [Google Scholar]
- Ruzzenente A, Valdegamberi A, Campagnaro T et al. Hepatocellular carcinoma in cirrhotic patients with portal hypertension: is liver resection always contraindicated? World J Gastroenterol. 2011;17(46):5083–5088. doi: 10.3748/wjg.v17.i46.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capussotti L, Ferrero A, Viganò L, Muratore A, Polastri R, Bouzari H. Portal hypertension: contraindication to liver surgery? World J Surg. 2006;30(6):992–999. doi: 10.1007/s00268-005-0524-9. [DOI] [PubMed] [Google Scholar]
- Roayaie S, Jibara G, Tabrizian P et al. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62(2):440–451. doi: 10.1002/hep.27745. [DOI] [PubMed] [Google Scholar]
- Zhong JH, Xiang BD, Gong WF et al. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8(7):e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MC, Huang GT, Tsang YM et al. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009;16(4):848–855. doi: 10.1245/s10434-008-0282-7. [DOI] [PubMed] [Google Scholar]
- Huang J, Yan L, Cheng Z et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903–912. doi: 10.1097/SLA.0b013e3181efc656. [DOI] [PubMed] [Google Scholar]
- Peng ZW, Guo RP, Zhang YJ, Lin XJ, Chen MS, Lau WY. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118(19):4725–4736. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KK, Vauthey JN, Pawlik TM et al. International Cooperative Study Group on Hepatocellular Carcinoma. Is hepatic resection for large or multinodular hepatocellular carcinoma justified? Results from a multi-institutional database. Ann Surg Oncol. 2005;12(5):364–373. doi: 10.1245/ASO.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Bruix J, Takayama T, Mazzaferro V et al. STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. A study of atezolizumab plus bevacizumab versus active surveillance as adjuvant therapy in patients with hepatocellular carcinoma at high risk of recurrence after surgical resection or ablation (IMbrave050) https://www. clinicaltrials.gov/ct2/show/NCT04102098. Identifier: NCT04102098 Accessed September 16, 2020.
- ClinicalTrials.gov. A study of nivolumab in participants with hepatocellular carcinoma who are at high risk of recurrence after curative hepatic resection or ablation (CheckMate 9DX). https://clinicaltrials.gov/ct2/show/NCT03383458. Identifier: NCT03383458 Accessed September 16, 2020.
- ClinicalTrials.gov. Safety and efficacy of pembrolizumab (MK-3475) versus placebo as adjuvant therapy in participants with hepatocellular carcinoma (HCC) and complete radiological response after surgical resection or local ablation (MK-3475-937 / KEYNOTE-937). https://clinicaltrials.gov/ct2/show/NCT03867084 Identifier: NCT3867084. Accessed September 16, 2020.
- ClinicalTrials.gov. Assess efficacy and safety of durvalumab alone or combined with bevacizumab in high risk of recurrence HCC patients after curative treatment (EMERALD-2). https://clinicaltrials.gov/ct2/show/NCT03847428. Identifier: NCT03847428 Accessed September 16, 2020.
- Olthoff KM, Millis JM, Rosove MH, Goldstein LI, Ramming KP, Busuttil RW. Is liver transplantation justified for the treatment of hepatic malignancies? Arch Surg. 1990;125(10):1261–1266. doi: 10.1001/archsurg.1990.01410220045007. [DOI] [PubMed] [Google Scholar]
- Mazzaferro V, Regalia E, Doci R et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- Doyle MB, Vachharajani N, Maynard E et al. Liver transplantation for hepatocellular carcinoma: long-term results suggest excellent outcomes. J Am Coll Surg. 2012;215(1):19–28. doi: 10.1016/j.jamcollsurg.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(5):S261–S267. doi: 10.1053/j.gastro.2004.09.040. suppl 1. [DOI] [PubMed] [Google Scholar]
- Washburn K, Edwards E, Harper A, Freeman R. Hepatocellular carcinoma patients are advantaged in the current liver transplant allocation system. Am J Transplant. 2010;10(7):1643–1648. doi: 10.1111/j.1600-6143.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- Heimbach JK, Hirose R, Stock PG et al. Delayed hepatocellular carcinoma model for end-stage liver disease exception score improves disparity in access to liver transplant in the United States. Hepatology. 2015;61(5):1643–1650. doi: 10.1002/hep.27704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao FY, Ferrell L, Bass NM et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- Yao FY, Ferrell L, Bass NM, Bacchetti P, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: comparison of the proposed UCSF criteria with the Milan criteria and the Pittsburgh modified TNM criteria. Liver Transpl. 2002;8(9):765–774. doi: 10.1053/jlts.2002.34892. [DOI] [PubMed] [Google Scholar]
- Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- Duffy JP, Vardanian A, Benjamin E et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg. 2007;246(3):502–509. doi: 10.1097/SLA.0b013e318148c704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferro V, Llovet JM, Miceli R et al. Metroticket Investigator Study Group. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- Yao FY, Fidelman N. Reassessing the boundaries of liver transplantation for hepatocellular carcinoma: where do we stand with tumor down-staging? Hepatology. 2016;63(3):1014–1025. doi: 10.1002/hep.28139. [DOI] [PubMed] [Google Scholar]
- Parikh ND, Waljee AK, Singal AG. Downstaging hepatocellular carcinoma: a systematic review and pooled analysis. Liver Transpl. 2015;21(9):1142–1152. doi: 10.1002/lt.24169. [DOI] [PubMed] [Google Scholar]
- Yao FY, Mehta N, Flemming J et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumors within Milan criteria. Hepatology. 2015;61(6):1968–1977. doi: 10.1002/hep.27752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Guy J, Frenette CT et al. Excellent outcomes of liver transplantation following down-staging of hepatocellular carcinoma to within Milan criteria: a multicenter study. Clin Gastroenterol Hepatol. 2018;16(6):955–964. doi: 10.1016/j.cgh.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Dodge JL, Grab JD, Yao FY. National experience on down-staging of hepatocellular carcinoma before liver transplant: influence of tumor burden, alpha-fetoprotein, and wait time. Hepatology. 2020;71(3):943–954. doi: 10.1002/hep.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodzin AS, Lunsford KE, Markovic D, Harlander-Locke MP, Busuttil RW, Agopian VG. Predicting mortality in patients developing recurrent hepatocellular carcinoma after liver transplantation: impact of treatment modality and recurrence characteristics. Ann Surg. 2017;266(1):118–125. doi: 10.1097/SLA.0000000000001894. [DOI] [PubMed] [Google Scholar]
- Nagai S, Mangus RS, Kubal CA et al. Prognosis after recurrence of hepato-cellular carcinoma in liver transplantation: predictors for successful treatment and survival. Clin Transplant. 2015;29(12):1156–1163. doi: 10.1111/ctr.12644. [DOI] [PubMed] [Google Scholar]
- de’Angelis N, Landi F, Carra MC, Azoulay D. Managements of recurrent hepatocellular carcinoma after liver transplantation: a systematic review. World J Gastroenterol. 2015;21(39):11185–11198. doi: 10.3748/wjg.v21.i39.11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta N, Heimbach J, Harnois DM et al. Validation of a risk estimation of tumor recurrence after transplant (RETREAT) score for hepatocellular carcinoma recurrence after liver transplant. JAMA Oncol. 2017;3(4):493–500. doi: 10.1001/jamaoncol.2016.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazun KJ, Najjar M, Abdelmessih RM et al. Recurrence after liver transplantation for hepatocellular carcinoma: a new MORAL to the story. Ann Surg. 2017;265(3):557–564. doi: 10.1097/SLA.0000000000001966. [DOI] [PubMed] [Google Scholar]
- Dodd GD, 3rd, Soulen MC, Kane RA et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20(1):9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- Livraghi T, Meloni F, Di Stasi M et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47(1):82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- Pillai K, Akhter J, Chua TC et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore). 2015;94(9):e580. doi: 10.1097/MD.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng K, Yan J, Li X et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794–802. doi: 10.1016/j.jhep.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang ZG, Fu SY et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103(4):348–356. doi: 10.1002/bjs.10061. [DOI] [PubMed] [Google Scholar]
- Pompili M, Saviano A, de Matthaeis N et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59(1):89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Lencioni R, Cioni D, Crocetti L et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234(3):961–967. doi: 10.1148/radiol.2343040350. [DOI] [PubMed] [Google Scholar]
- Curley SA, Marra P, Beaty K et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239(4):450–458. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucchetti A, Piscaglia F, Cescon M et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300–307. doi: 10.1016/j.jhep.2013.04.009. [DOI] [PubMed] [Google Scholar]
- Yu J, Yu XL, Han ZY et al. Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut. 2017;66(6):1172–1173. doi: 10.1136/gutjnl-2016-312629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciorusso A, Di Maso M, Muscatiello N. Microwave ablation versus radio-frequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2016;32(3):339–344. doi: 10.3109/02656736.2015.1127434. [DOI] [PubMed] [Google Scholar]
- Llovet JM, Real MI, Montaña X et al. Barcelona Liver Cancer Group. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- Lo CM, Ngan H, Tso WK et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- Lewis AL, Gonzalez MV, Leppard SW et al. Doxorubicin eluting beads – 1: effects of drug loading on bead characteristics and drug distribution. J Mater Sci Mater Med. 2007;18(9):1691–1699. doi: 10.1007/s10856-007-3068-8. [DOI] [PubMed] [Google Scholar]
- Lammer J, Malagari K, Vogl T et al. PRECISION V Investigators. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogl TJ, Lammer J, Lencioni R et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197(4):W562–W570. doi: 10.2214/AJR.10.4379. [DOI] [PubMed] [Google Scholar]
- Golfieri R, Giampalma E, Renzulli M et al. PRECISION ITALIA STUDY GROUP. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264. doi: 10.1038/bjc.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem R, Gabr A, Riaz A et al. Institutional decision to adopt Y90 as primary treatment for hepatocellular carcinoma informed by a 1,000-patient 15-year experience. Hepatology. 2018;68(4):1429–1440. doi: 10.1002/hep.29691. [DOI] [PubMed] [Google Scholar]
- Salem R, Lewandowski RJ, Kulik L et al. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140(2):497–507.e2. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Luna LE, Yang JD, Sanchez W et al. Efficacy and safety of transarterial radioembolization versus chemoembolization in patients with hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2013;36(3):714–723. doi: 10.1007/s00270-012-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem R, Gordon AC, Mouli S et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2016;151(6):1155–1163.e2. doi: 10.1053/j.gastro.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: a systematic review and meta-analysis. World J Hepatol. 2016;8(18):770–778. doi: 10.4254/wjh.v8.i18.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fouly A, Ertle J, El Dorry A et al. In intermediate stage hepatocellular carcinoma: radioembolization with yttrium 90 or chemoembolization? Liver Int. 2015;35(2):627–635. doi: 10.1111/liv.12637. [DOI] [PubMed] [Google Scholar]
- Wahl DR, Stenmark MH, Tao Y et al. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol. 2016;34(5):452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapisochin G, Barry A, Doherty M et al. Stereotactic body radiotherapy vs. TACE or RFA as a bridge to transplant in patients with hepatocellular carcinoma. An intention-to-treat analysis. J Hepatol. 2017;67(1):92–99. doi: 10.1016/j.jhep.2017.02.022. [DOI] [PubMed] [Google Scholar]
- Bush DA, Smith JC, Slater JD et al. Randomized clinical trial comparing proton beam radiation therapy with transarterial chemoembolization for hepato-cellular carcinoma: results of an interim analysis. Int J Radiat Oncol Biol Phys. 2016;95(1):477–482. doi: 10.1016/j.ijrobp.2016.02.027. [DOI] [PubMed] [Google Scholar]
- Shah C, Mramba LK, Bishnoi R, Bejjanki H, Chhatrala HS, Chandana SR. Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: pre and post sorafenib era. J Gastrointest Oncol. 2017;8(5):789–798. doi: 10.21037/jgo.2017.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48(4):1312–1327. doi: 10.1002/hep.22506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V et al. SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Chen Z et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- Marrero JA, Kudo M, Venook AP et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: the GIDEON study. J Hepatol. 2016;65(6):1140–1147. doi: 10.1016/j.jhep.2016.07.020. [DOI] [PubMed] [Google Scholar]
- Kudo M, Finn RS, Qin S et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- Pishvaian MJ, Lee MS, Ryoo B et al. Updated safety and clinical activity results from a phase Ib study of atezolizumab + bevacizumab in hepatocellular carcinoma (HCC). Ann Oncol. 2018;29(suppl 8):viii718–viii719. [Google Scholar]
- Cheng AL, Qin S, Ikeda M et al. IMbrave 150: efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Ann Oncol. 2019;30(suppl 9):ix186–ix187. [Google Scholar]
- Bruix J, Qin S, Merle P et al. RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- Finn RS, Merle P, Granito A et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353–358. doi: 10.1016/j.jhep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Abou-Alfa GK, Meyer T, Cheng AL et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AX, Park JO, Ryoo BY et al. REACH Trial Investigators. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859–870. doi: 10.1016/S1470-2045(15)00050-9. [DOI] [PubMed] [Google Scholar]
- Zhu AX, Kang YK, Yen CJ et al. REACH-2 study investigators. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282–296. doi: 10.1016/S1470-2045(18)30937-9. [DOI] [PubMed] [Google Scholar]
- Yau T, Park JW, Finn RS et al. CheckMate 459: a randomized, multi-center phase 3 study of nivolumab (nivo) vs sorafenib (sor) as first-line (1L) treatment in patients (pts) with advanced hepatocellular carcinoma (aHCC). Ann Oncol. 2019;30(suppl 5):v874–v875. [Google Scholar]
- Finn RS, Ryoo BY, Merle P et al. KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307. [DOI] [PubMed] [Google Scholar]
- ClinicalTrials.gov. Safety and efficacy of lenvatinib (E7080/MK-7902) in combination with pembrolizumab (MK-3475) versus lenvatinib as first-line therapy in participants with advanced hepatocellular carcinoma (MK-7902-002/ E7080-G000-311/LEAP-002). https://clinicaltrials.gov/ct2/show/NCT03713593 Identifier: NCT03713593. Accessed September 16, 2020.
- ClinicalTrials.gov. Study of cabozantinib in combination with atezolizumab versus sorafenib in subjects with advanced HCC who have not received previous systemic anticancer therapy (COSMIC-312). https://clinicaltrials.gov/ct2/show/ NCT03755791 Identifier: NCT03755791. Accessed September 16, 2020.
- ClinicalTrials.gov. Study of durvalumab and tremelimumab as first-line treatment in patients with advanced hepatocellular carcinoma (HIMALAYA). https://clinicaltrials.gov/ct2/show/NCT03298451 Identifier: NCT03298451. Accessed September 16, 2020.
- ClinicalTrials.gov. A study of nivolumab in combination with ipilimumab in participants with advanced hepatocellular carcinoma (CheckMate 9DW). https://clinicaltrials.gov/ct2/show/NCT4039607 Identifier: NCT4039607. Accessed September 16, 2020.