Abstract
Cigarette smoking is the leading cause of preventable disease and death in the United States, causing approximately 480,000 deaths per year, which is equivalent to 1 in 5 deaths being attributable to tobacco use. The adverse effects of cigarette smoking on the lungs and cardiovascular system are well described; however, the detrimental effects of smoking on the liver are not as well defined. Smoking affects the liver via 3 separate mechanisms: toxic (both direct and indirect), immunologic, and oncogenic. There is an emerging body of evidence of an association between cigarette smoking and progression of fibrosis in chronic liver diseases such as nonalcoholic fatty liver disease and primary biliary cholangitis. Smoking is associated with accelerated development of hepatocellular carcinoma in patients with chronic hepatitis B or C virus infection. Tobacco smoking adversely affects lung function, which increases physical limitations and may preclude liver transplantation. Following liver transplantation, smoking is associated with several adverse outcomes, including increased risk of de novo malignancy, vascular complications, and nongraft-associated mortality. The respiratory illness caused by the novel coronavirus disease 2019 serves as a good example of the complex interplay between the lungs and the liver. It is evident that cigarette smoking has important negative effects on a multitude of liver diseases and that patients’ smoking cessation must be prioritized. The data are limited, and more research is needed to better understand how smoking affects the liver. This article summarizes what is known about the pathologic effects of cigarette smoking on common liver diseases.
Keywords: Cigarettes, smoking, liver disease, cirrhosis, toxins, hepatocellular carcinoma
Cigarettes contain over 4000 toxic chemicals, including nicotine, which contributes significantly to smoking’s addictive and stimulant properties. Smoking is the leading cause of preventable disease and death in the United States, causing approximately 480,000 deaths per year, which equates to 1 in 5 deaths being attributable to tobacco use.1 Approximately 1 billion people around the world smoke, making smoking a major cause of preventable morbidity and mortality globally.2 The key detrimental effects of smoking on health can be divided into 3 main categories: lung diseases (eg, chronic obstructive pulmonary disease [COPD], pulmonary fibrosis, and respiratory infections),3 malignancies (specifically lung, oral, esophageal, bladder, pancreatic, and colon cancers),4 and thrombotic or cardiovascular events (eg, myocardial infarctions and cerebrovascular accidents).5 Over the past 20 years, substantial progress has been made with regard to reducing rates of smoking in developed countries. However, rates of smoking are on the rise in developing countries, where smoking-related diseases are emerging as a significant cause of morbidity and mortality for the first time in those countries’ histories.6
Historically, little attention has been paid to the adverse effects of smoking on the liver. Cigarettes were not thought to cause direct liver injury or to increase the risk of developing a chronic liver disease such as non alcoholic fatty liver disease (NAFLD) or alcohol-associated cirrhosis.7 However, there is a growing body of evidence that demonstrates that cigarette smoking is associated with increased progression and severity of liver disease, particularly fibrosis and liver cancer. This is logical, given what is known about the carcinogenic and profibrogenic effects of smoking on the human body. This article summarizes the effects of cigarette smoking on liver disease by describing the pathophysiology and collating the data that demonstrate these effects.
Mechanism of the Effects of Smoking on the Liver
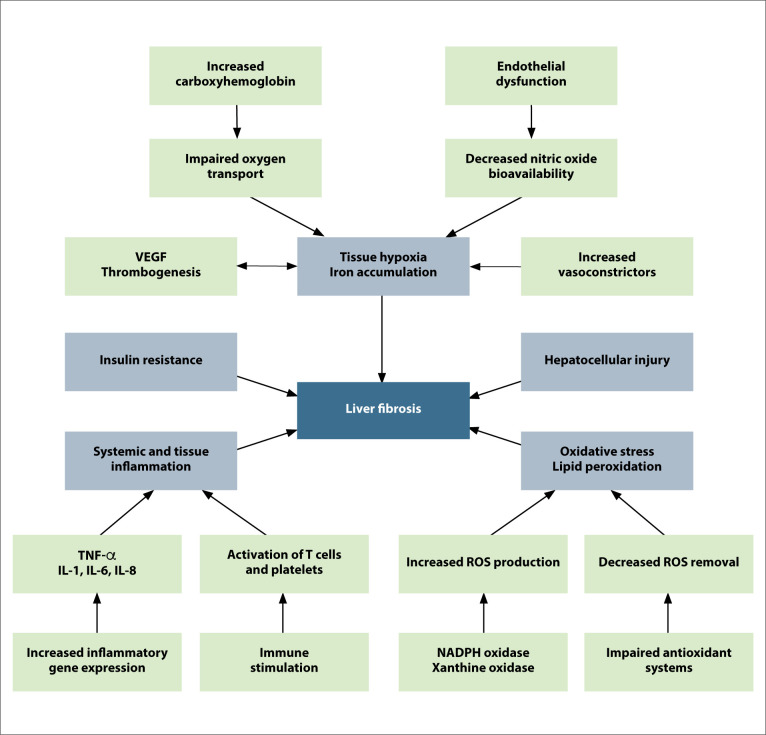
Smoking appears to have adverse effects on the liver via 3 separate mechanisms: toxic (both direct and indirect), immunologic, and oncogenic (Figure).8 The direct toxic effects include oxidative stress caused by substances in cigarettes with cytotoxic properties, which result in the activation of stellate cells, leading to fibrosis.9-11 Smoking also increases proinflammatory cytokines (interleukin [IL] 1, IL-6, IL-8, and tumor necrosis factor α), which leads to liver cell injury.12 One of the indirect toxic effects of smoking is secondary polycythemia; increased carboxyhemoglobin due to smoking results in decreased oxygen-carrying capacity of tissues, leading to increased erythropoietin and resultant increased red cell mass. There is subsequently a rise in catabolic iron due to increased red cell destruction, and a rise in erythropoietin, which stimulates more absorption of iron from the intestines.13 Iron is scavenged by macrophages and eventually accumulates in hepatocytes, where it promotes oxidative stress, which, in turn, can lead to liver injury.14
Figure.
The various pathways by which cigarette smoking results in liver fibrosis. Adapted from Altamirano and Bataller.93
IL, interleukin; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
The immunologic effects of smoking are both cell-mediated and humoral. Nicotine inhibits lymphocyte proliferation and differentiation, which results in suppression of antibody formation.12,15 Smoking induces apoptosis of lymphocytes,16 increases CD8+ cytotoxic T cells,10 decreases CD4+ cells, and impairs natural killer cell activity.17 Reversal of these changes can be detected as soon as 1 month after smoking cessation.18
The oncologic effects of smoking are multifold, including carcinogens found in cigarettes such as hydrocarbons, nitrosamine, tar, and vinyl chloride.19 Cigarettes are a source of 4-Aminobiphenyl, a substance that has been shown to increase the risk of hepatocellular carcinoma (HCC).20 Tobacco smoking has also been implicated in the reduction of p53, a tumor-suppressing gene and a common pathway of oncogenesis for many neoplasms.21,22
An interesting connection between cigarette smoking and the liver has been the identification of an independent protective effect of serum bilirubin on lung function in COPD.23 This is believed to be mediated through bilirubin’s inhibition of lipid peroxidation, a mechanism associated with improved lung function that has been utilized in experimental models for the prevention and treatment of chronic respiratory diseases.24,25
Smoking and Viral Hepatitides
The data relating to the effects of smoking on chronic hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are somewhat scarce. One study of more than 6000 Taiwanese individuals showed that cigarette smoking (specifically, smoking >1 pack/day) was associated with increased alanine aminotransferase (ALT) levels in patients who are HCV antibody–positive.26 Smoking was an independent risk factor for elevated ALT levels, with an odds ratio (OR) of 1.8 (95% CI, 1.1-2.7). There was a synergistic effect of smoking and alcohol consumption on the likelihood of having elevated ALT levels, with an OR of 7.0 (95% CI, 2.7-18.8) for HCV antibody– positive patients who smoked at least 1 pack per day and frequently drank alcohol compared to HCV antibody– positive patients who did not engage in smoking or drinking alcohol. There was no such effect of cigarettes or alcohol found on patients who were hepatitis B surface antigen (HBsAg)-seropositive.26
In the era of interferon treatment for HCV infection, it was found that individuals who smoked had a lower response rate to interferon,27 and that therapeutic phlebotomy to reduce iron overload could help improve response rates.28,29 Smoking has been associated with increased fibrosis and inflammation activity scores on liver biopsy, as one study of 310 patients with HCV infection demonstrated.30 A study of 1506 asymptomatic carriers of HBV (HBsAg-seropositive) in Taiwan demonstrated an increased risk of cirrhosis in patients who smoked 20 (1 pack) or more cigarettes per day.31 This effect was particularly pronounced in patients who drank alcohol habitually compared to those who did not drink, again suggestive of a possible additive effect of cigarettes and alcohol on the rate of progression to cirrhosis.31
Even the daily smoking of cannabis has been implicated in increasing the risk of fibrosis in patients with chronic HCV infection due to the proinflammatory effects of the cannabinoid receptor type 1 in the liver.32 However, a 2014 study contradicted these findings and did not demonstrate increased fibrosis, inflammation, or steatosis in individuals who used marijuana daily after adjusting for genotype, alcohol use, sex, and race.33 The authors note that the discrepant results could be due to the variation in the cutoffs used for hepatic steatosis and excess alcohol consumption.33
Cigarette Smoking and Liver Cancer
Liver cancer is the fifth most common cancer and third-highest cause of cancer-related deaths worldwide.34 Unlike many other types of cancer, the incidence of HCC has been increasing, with a tripling of rates since 1980, and is predicted to continue to rise until 2030.35 Although the link between the hepatitides (namely HBV and HCV) and HCC is well established, the association between cigarette smoking and HCC may be less frequently acknowledged by clinicians.
There are several cigarette constituents associated with the development of liver cancer. N-Nitrosodimethylamine promotes liver fibrosis and cancer, and 4-Aminobiphenyl is metabolized by hepatic CYP1A2 and causes DNA adducts in liver cancer cells.36 Vinyl chloride is directly associated with hepatic angiosarcoma and its precursor, and chloroethene has been linked with increased liver cancer risk in occupational exposure (typically through welding pipes, wires, cable coatings, or other polyvinylchloride-containing materials).36 Finally, cadmium elevates liver cancer risk via proto-oncogenic activation and the activation of proinflammatory cytokines.37
Lee and colleagues conducted a meta-analysis of 38 cohort studies and 58 case-control studies.38 After adjusting for HBV infection, HCV infection, and alcohol consumption, they found that current cigarette smoking increased the risk of HCC with a relative risk ratio of 1.51 (95% CI, 1.37-1.67). In the same study, former smokers had a nonstatistically significant relative risk ratio of 1.12 (95% CI, 0.78-1.60) for the development of HCC. Another meta-analysis of 9 studies found that smoking alone nearly doubled the risk of HCC, and in patients with HBV infection, smoking further increased the risk 1.4-fold.39 The relative risk of HCC increased from 15.8 (95% CI, 9.69-25.7) for HBV-infected nonsmokers to 21.6 (95% CI, 15.2-30.5) for HBV-infected individuals who had ever smoked. More notably, in individuals with HCV infection, the risk of HCC was increased 2.9-fold if there was a history of cigarette smoking, from a relative risk of 7.94 (95% CI, 4.40-14.3) to 23.1 (95% CI, 9.43-56.8).39
There is a specific tobacco signature seen in liver tumors. People who smoke cigarettes have multiple cyto-sine-to-adenine base substitutions and dinucleotide substitutions. Guanine adducts are associated with smoking in cancers of the lung and head-and-neck area.40 Exome sequencing analysis reveals the same signature in liver tumors of European and Asian smokers.41 HCC with telomerase reverse transcriptase promoter mutations is also associated with smoking.41,42 It has been suggested that alcohol consumption and cigarette smoking increase the risk of HCC synergistically in patients with chronic HBV and/or HCV infections.43
Cigarette Smoking and Nonalcoholic Fatty Liver Disease
NAFLD is the most common liver disorder in industrialized countries,44 where rates of obesity, type 2 diabetes mellitus, dyslipidemia, and metabolic syndrome are highest.45 The prevalence of NAFLD in the United States is forecasted to increase to 100.9 million by 2030, representing 33.5% of the adult population.46 NAFLD is defined as hepatic steatosis on imaging and/or histology in the absence of significant alcohol use or steatogenic medications. Nonalcoholic steatohepatitis (NASH), an aggressive subtype of NAFLD, is defined histologically as more than 5% hepatic steatosis and inflammation with hepatocyte injury (hepatocyte ballooning) with or without liver fibrosis.47 While hepatic steatosis is present in 25% to 30% of the general population, approximately 6% of the population is believed to have hepatic steatosis with inflammation and injury (ie, NASH). Of people with NASH, 15% to 20% go on to develop cirrhosis. NASH cirrhosis portends an increased risk of cardiovascular death, carcinoma, and liver-related mortality.47
A commonly used pathologic scoring system for NAFLD is the NAFLD activity score, which scores liver biopsies on a scale of 0 to 8 based on degree of steatosis, inflammation, and ballooning.48 A widely used scoring system for degree of fibrosis has been characterized by Kleiner and colleagues, and ranges from F0 (no fibrosis) to F4 (cirrhosis).48 It has been shown that degree of fibrosis is directly related to liver-related mortality. Younossi and colleagues performed a study of 257 patients with NAFLD and demonstrated that advanced fibrosis had the best independent association with liver-related mortality, with an adjusted hazard ratio (HR) of 5.68 (95% CI, 1.50‐21.45).49 The link between degree of liver fibrosis and liver-related mortality has been further validated in other cohorts as well.50
More recently, it has become evident that smoking is associated with worse outcomes in patients with NAFLD. In a retrospective study of 619 patients from the United States, Europe, and Asia, all with liver biopsies and a median follow-up of 12.6 years, current cigarette smoking was an independent risk factor for death or liver transplantation (HR, 2.62; 95% CI, 1.67-4.10).51 The most common cause of death was cardiovascular (38% of deaths), followed by nonliver malignancy and complications of cirrhosis. Stage 4 fibrosis was associated with increased risk of death or liver transplantation when compared with stage 0 (HR, 10.9; 95% CI, 6.06-19.62).51
A Korean cohort study reviewed the medical records of 228,497 asymptomatic individuals with a liver ultra-sound and urinary cotinine measurement.52 Patients were excluded if they had excessive alcohol consumption (>140 g/week for men and >70 g/week for women), positive HBsAg, positive HCV antibody, a history of HCC, or incomplete data on smoking status. A total of 160,862 patients were included in the study, 18% of whom were smokers. The authors found that 25.5% of all patients (approximately 40,000) had hepatic steatosis on imaging. Of current smokers, former smokers, and nonsmokers, 42%, 39%, and 18%, respectively, had evidence of NAFLD on imaging. After multivariate analysis, it was found that a smoking history of more than 10 pack-years had an adjusted odds ratio (AOR) for NAFLD of 1.04 (95% CI, 1.01-1.08), compared to an AOR of 1.11 (95% CI, 1.05-1.16) in patients with a less than 10 pack-year smoking history. The authors concluded that current smoking (either cotinine‐verified or self‐reported) was an independent risk factor for NAFLD.52
Airborne Toxins and the Liver
Air pollution has been linked to increased insulin resistance, systemic inflammation, immune injury, and mortality. Insulin resistance and systemic inflammation are both associated with NAFLD, making air pollution particularly important in this population. Mechanistically, oxidative stress via cytochrome P450 pathways in hepatocytes has been implicated in the production of reactive oxygen species. Occupational exposure of certain airborne agents, such as aflatoxin and carbon tetrachloride, can lead to direct hepatoxic and hepato-carcinogenic effects. Airborne toxins can be both inhaled and ingested.53,54
Fine particulate matter (PM2.5) are particles smaller than 2.5 μm in diameter and are a component of air pollution. PM2.5 are linked to systemic inflammation and associated with increased levels of gamma-glutamyl transferase, aspartate aminotransferase, and ALT as well as increased rates of HCC.55 PM2.5 impair insulin sensitivity, leading to an insulin-resistant state and type 2 diabetes mellitus.56 Exposure to PM2.5 in mouse models was directly related to hepatic inflammation and liver fibrosis, suggesting that ambient PM2.5 exposure may be a significant risk factor for NAFLD progression.22,57
Nitrogen dioxide (NO2) is another component of air pollution, and has been associated with increased ALT and aspartate aminotransferase levels.58 Similar to PM2.5, NO2 has been shown to impair insulin sensitivity and lead to type 2 diabetes mellitus.56,59 This seems to occur through decreased insulin receptor substrate-1/protein kinase B signaling, and through p38 mitogen-activated protein kinase (MAPK) and/or WT-Jun NH2-terminal kinase (JNK) activation.60 Both p38 MAPK and JNK activation cause metabolic dysfunction, are associated with hepatic stellate cell activation and resultant liver fibrosis, lead to increased liver fat content, contribute to liver cancer pathogenesis and growth, and are linked to endoplasmic reticulum stress–induced cell death pathways in the liver.61 Exposure to NO2 has been associated with higher body mass index (BMI) and obesity.56
In a study of World Trade Center responders, advanced liver fibrosis (Fibrosis-4 score ≥2.67) was associated with male sex, being a former smoker, COPD, ratio of the forced expiratory volume in the first 1 second to the forced vital capacity (FEV1/FVC) below normal range, and a lower BMI. Liver fibrosis was positively associated with male sex, being a former smoker, FEV1/FVC below normal range, and COPD. Liver fibrosis was negatively associated with BMI. These factors fit the picture of toxi-cant-associated liver injury.62
Cigarette Smoking and Primary Biliary Cholangitis
Primary biliary cholangitis (PBC) is a cholestatic liver disease characterized by progressive destruction of intrahepatic bile ducts, resulting in fibrosis that can lead to cirrhosis. Molecules found in cigarettes have both proinflammatory (increase in IL-1, IL-6, IL-13, and tumor necrosis factor α) and immunosuppressive effects. It is believed that smoking may result in an adaptive Th1 immune response to cellular antigens and disruption of regulatory T-cell function.63-65 PBC is characterized histologically by the infiltration of portal tracts by mostly Th1 lymphocytes; thus, it has been postulated that cigarette smoking could be a risk factor for PBC, but studies have not yet conclusively proven this.66,67
Cigarette smoking has been implicated in the progression of fibrogenesis, as reactive oxygen species promote the transformation of liver stellate cells into myofibroblasts.68 A study by Zein and colleagues showed a correlation between smoking and stage of liver fibrosis in PBC.69 A smoking history of 10 pack-years or more was the only significant predictor of advanced liver disease at presentation, after correcting for age, sex, and alcohol use.69 Another study demonstrated that smoking is directly correlated with the risk of liver fibrosis in PBC in a dose-dependent fashion.70 For each pack-year increase in smoking, there was a 5.0% (95% CI, 1.3-8.7%) increased likelihood of advanced fibrosis. Finally, a study of 171 patients with PBC (148 with liver biopsy) showed an association between smoking and severe steatosis (AOR, 5.3; 95% CI, 2.019-9.919) as well as fibrosis stage F3 to F4 (AOR, 1.21; 95% CI, 1.015-3.031).71 As in the previously described study, for every pack-year increase in smoking intensity, there was a 3.2-times higher likelihood of advanced fibrosis (95% CI, 2.018-6.294). In patients with advanced fibrosis, increased mortality rates were found in patients with a smoking history compared to those without (P=.04).71
Smoking and Hepatopulmonary Syndrome
Hepatopulmonary syndrome (HPS) is a syndrome of hypoxemia (a partial pressure of oxygen <80 mm Hg on room air) with a widened alveolar-arterial oxygen gradient in patients with cirrhosis and portal hypertension.72 It is thought to occur due to failure of the liver to clear pulmonary vasodilators and increased bacterial gut translocation in portal hypertension, which stimulates release of the vasodilator nitric oxide.73 The result is dilated pulmonic vessels at the precapillary and capillary levels and direct arteriovenous communications.74 This causes right-to-left shunting of blood flow, mismatch between ventilation and perfusion, and diffusion limitation.75 The reported prevalence of HPS in cirrhosis is estimated to be 4% to 32%, although 40% to 60% of cirrhotic patients have detectable intrapulmonary vascular dilatations, even in the absence of symptoms.72,76,77 Classic symptoms include dyspnea, orthodeoxia, and platypnea with clinical signs including digital clubbing, cyanosis, and spider angiomata.72 Liver transplantation is the only treatment for HPS, and, for unclear reasons, there is a variable delay in oxygenation improvement posttransplant that ranges from 3 months to 3 years.77
Cigarette smoking further worsens ventilation/perfusion mismatch in the already compromised and shunting respiratory system of HPS. However, the relationship between smoking and HPS appears to be even more complex. Cigarette smoking seems to inhibit respiratory tract nitric oxide production through downregulation of inducible nitric oxide synthase, in addition to impairing endothelium-dependent vasodilatation.78,79 Interestingly, a case report showed that resumption of smoking actually expedited the resolution of HPS after liver transplant (LT), and postulated that it was the aforementioned 2 mechanisms related to cigarette smoking that counteracted the pathophysiology of HPS.80
Cigarette Smoking and Liver Transplantation
Tobacco smoking has been shown to delay wound healing post-LT81 and to significantly increase post-LT vascular complications, particularly arterial events such as hepatic artery thrombosis.82 Patients who stopped smoking at least 2 months prior to LT had a significant reduction in the risk of vascular complications, with a number needed to treat of 7 (ie, 7 patients needed to quit smoking to prevent 1 arterial complication).82
Patients with active smoking following LT have increased nongraft-related mortality rates, which seem to be driven by cardiovascular events, sepsis, and malignancy.83 Cigarette smoking is an independent risk factor for de novo neoplasms posttransplant, including lung and head-and-neck tumors.84,85 This is particularly relevant in transplant recipients who are prescribed immunosuppressants, which are associated with increased malignancy risk, particularly for skin cancers and posttransplant lymphoproliferative disease (smoking is an independent risk factor for both of these malignancies).86 The overall rate of new neoplasms post-LT is approximately 5% to 15%, and malignancy is one of the leading causes of late post-LT death.87
A study of 301 patients who underwent ortho-topic LT found that approximately 17% of transplanted patients were active smokers.88 There were similar rates of smoking pre- and posttransplant, with the number of active smokers who quit following orthotopic LT offset by the number of former smokers who restarted smoking post–orthotopic LT.88 These rates are comparable to the active smoking rate of 15% post-LT found in a telephone survey by Ehlers and colleagues.89 The former study demonstrated that at 10 years, the cumulative malignancy rate was 12.7% in smokers vs 2.1% in nonsmokers.88 Smoking is also associated with COPD (present in 18% of patients undergoing LT evaluation in the United States), which was linked to increased physical limitations and lower quality of life but was not found to adversely affect survival post-LT.90
It is well known that alcohol use and cigarette smoking are closely correlated. A Veterans Administration study demonstrated that 90% of patients enrolled in a substance abuse program with alcohol use disorder also smoked cigarettes.91 Patients with alcohol-associated liver disease often resume smoking early post-LT and increase intensity of smoking over time. Alcohol and smoking have a synergistic effect on the risk of oropharyngeal cancers in particular. A study by Duvoux and colleagues showed that the incidence of tumors was higher in patients receiving a transplant for alcohol-associated cirrhosis than nonalcohol-associated cirrhosis (26.7% vs 5.0%; P<.01), specifically oropharyngeal and esophageal squamous cell carcinoma and posttransplant lymphoproliferative disease.92 An important ethical question is whether smoking should be a contraindication to liver transplantation, as is the case for alcohol in many transplant centers. Currently, many transplant centers do not offer liver transplantation to active smokers, but may offer behavioral and medical assistance to augment smoking and alcohol cessation prior to listing the patient for LT.93
Coronavirus Disease 2019 and the Liver
Although not directly related to smoking, coronavirus disease 2019 (COVID-19) infection provides a good example of the intricate interplay between the lungs and the liver in a systemic viral illness.
In December 2019, a novel RNA betacoronavirus, named severe acute respiratory syndrome coronavirus 2 or COVID-19, was discovered in Wuhan, China.94 Since this time, the highly contagious virus with variable clinical symptoms (ranging from asymptomatic to acute respiratory distress syndrome and death) has resulted in a pandemic and over 1.3 million deaths to date.95
Elevated liver enzymes (typically in a hepatocellular pattern, ranging from mild to severe) are commonly seen in patients presenting with COVID-19, present in up to 76% of hospitalized patients, and potentially exacerbated by the use of lopinavir/ritonavir.96 The prevalence of elevated aminotransferases in individuals with severe COVID-19 or whose disease resulted in admission to an intensive care unit, mechanical ventilation, or death was approximately double that of individuals with non-severe disease or who did not reach the aforementioned endpoints.97 A retrospective study showed that elevation of ALT levels to greater than 40 U/L was associated with higher mortality rates (univariable OR, 2.87 [1.48-5.57]; P=.0018).98
Clinically significant liver injury appears to be uncommon, even in the most severely ill patients, but there have been case reports of acute liver failure, possibly related to multiorgan failure in the setting of severe inflammatory response syndrome.99 Postulated mechanisms for the effect of COVID-19 on liver enzymes include direct viral toxicity, liver ischemia due to hypoxia, or a dysregulated immune response or cytokine storm phenomenon with coagulation activation and changes in iron metabolism due to macrophage activation.100,101
Although patients with cirrhosis and end-stage liver disease are thought to be at higher risk for complications of COVID-19, worse outcomes were not seen in an analysis of COVID-19 studies that included patients with chronic liver disease and COVID-19 infection (42 patients in total from various studies).100
Conclusion
While the adverse effects of cigarette smoking on the lungs and cardiovascular system are well described, the detrimental effects of smoking on the liver are less well understood. There is an emerging body of evidence that supports an association between cigarette smoking and progression of fibrosis in chronic liver disease (eg, NAFLD52 and PBC70). Smoking also appears to accelerate the development of HCC in patients with chronic HBV and/or HCV infections.43 Finally, tobacco smoking has a negative impact on lung function, which increases physical limitations and may preclude liver transplantation.90 Following liver transplantation, smoking specifically increases the risk of de novo malignancy,92 vascular complications,82 and nongraft-associated mortality.83 Cigarette smoking has an important negative effect on a multitude of liver diseases via various mechanisms, and smoking cessation must be prioritized. The data are limited, and more research is needed to better understand how smoking affects the liver and how these effects can be mitigated. Currently, it is recommended that all patients with liver disease be offered pharmacologic and psychologic smoking cessation resources, particularly patients being considered for liver transplantation.93
References
- Warren GW, Alberg AJ, Kraft AS, Cummings KM. The 2014 Surgeon General’s report: “The health consequences of smoking—50 years of progress”: a paradigm shift in cancer care. Cancer. 2014;120(13):1914–1916. doi: 10.1002/cncr.28695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Gu D, Wu X et al. Major causes of death among men and women in China. N Engl J Med. 2005;353(11):1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- Patel RR, Ryu JH, Vassallo R. Cigarette smoking and diffuse lung disease. Drugs. 2008;68(11):1511–1527. doi: 10.2165/00003495-200868110-00004. [DOI] [PubMed] [Google Scholar]
- Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(suppl 2):S3–S9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- Wilson PWF. Smoking, smoking cessation, and risk of cardiovascular disease. Curr Treat Options Cardiovasc Med. 2006;8(4):276–281. doi: 10.1007/s11936-006-0048-0. [DOI] [PubMed] [Google Scholar]
- Abdullah AS, Husten CG. Promotion of smoking cessation in developing countries: a framework for urgent public health interventions. Thorax. 2004;59(7):623–630. doi: 10.1136/thx.2003.018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead TP, Robinson D, Allaway SL. The effects of cigarette smoking and alcohol consumption on serum liver enzyme activities: a dose-related study in men. Ann Clin Biochem. 1996;33(Pt 6):530–535. doi: 10.1177/000456329603300607. [DOI] [PubMed] [Google Scholar]
- El-Zayadi A-R. Heavy smoking and liver. World J Gastroenterol. 2006;12(38):6098–6101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain K, Scott BR, Reddy SK, Somani SM. Chronic ethanol and nicotine interaction on rat tissue antioxidant defense system. Alcohol. 2001;25(2):89–97. doi: 10.1016/s0741-8329(01)00176-8. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Eto K, Furuno K, Mori T, Kawasaki H, Gomita Y. Effect of cigarette smoke on lipid peroxidation and liver function tests in rats. Acta Med Okayama. 1995;49(5):271–274. doi: 10.18926/AMO/30402. [DOI] [PubMed] [Google Scholar]
- Yu MW, Yang SY, Chiu YH, Chiang YC, Liaw YF, Chen CJ. A p53 genetic polymorphism as a modulator of hepatocellular carcinoma risk in relation to chronic liver disease, familial tendency, and cigarette smoking in hepatitis B carriers. Hepatology. 1999;29(3):697–702. doi: 10.1002/hep.510290330. [DOI] [PubMed] [Google Scholar]
- Moszczyński P, Zabiński Z, Moszczyński P, Jr, Rutowski J, Słowiński S, Tabarowski Z. Immunological findings in cigarette smokers. Toxicol Lett. 2001;118(3):121–127. doi: 10.1016/s0378-4274(00)00270-8. [DOI] [PubMed] [Google Scholar]
- Young CJ, Moss J. Smoke inhalation: diagnosis and treatment. J Clin Anesth. 1989;1(5):377–386. doi: 10.1016/0952-8180(89)90079-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge JM, Halliwell B. Iron toxicity and oxygen radicals. Baillieres Clin Haematol. 1989;2(2):195–256. doi: 10.1016/s0950-3536(89)80017-4. [DOI] [PubMed] [Google Scholar]
- Geng Y, Savage SM, Razani-Boroujerdi S, Sopori ML. Effects of nicotine on the immune response. II. Chronic nicotine treatment induces T cell anergy. J Immunol. 1996;156(7):2384–2390. [PubMed] [Google Scholar]
- Suzuki N, Wakisaka S, Takeba Y, Mihara S, Sakane T. Effects of cigarette smoking on Fas/Fas ligand expression of human lymphocytes. Cell Immunol. 1999;192(1):48–53. doi: 10.1006/cimm.1998.1432. [DOI] [PubMed] [Google Scholar]
- Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46(8):959–964. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Stunkard ME, Gilbert DG, Jensen RA, Martinko JM. Immune function in cigarette smokers who quit smoking for 31 days. J Allergy Clin Immunol. 1995;95(4):901–910. doi: 10.1016/s0091-6749(95)70135-4. [DOI] [PubMed] [Google Scholar]
- Helen A, Vijayammal P. Vitamin C supplementation on hepatic oxidative stress induced by cigarette smoke. Paper presented at: Journal of Applied Toxicology: An International Forum Devoted to Research and Methods Emphasizing Direct Clinical, Industrial and Environmental Applications. 1997. [DOI] [PubMed]
- Wang L-Y, Chen C-J, Zhang Y-J et al. 4-Aminobiphenyl DNA damage in liver tissue of hepatocellular carcinoma patients and controls. Am J Epidemiol. 1998;147(3):315–323. doi: 10.1093/oxfordjournals.aje.a009452. [DOI] [PubMed] [Google Scholar]
- Wang B, Zhang Y, Xu D et al. Meta-analysis on the relationship between tobacco smoking, alcohol drinking and p53 alteration in cases with esophageal carcinoma [in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2004;25(9):775–778. [PubMed] [Google Scholar]
- Xu X, Yavar Z, Verdin M et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010;30(12):2518–2527. doi: 10.1161/ATVBAHA.110.215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Roisin R, Bartolome SD, Huchon G, Krowka MJ. Inflammatory bowel diseases, chronic liver diseases and the lung. Eur Respir J. 2016;47(2):638–650. doi: 10.1183/13993003.00647-2015. [DOI] [PubMed] [Google Scholar]
- Nowak D, Kasielski M, Antczak A, Pietras T, Bialasiewicz P. Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: no significant effect of cigarette smoking. Respir Med. 1999;93(6):389–396. doi: 10.1053/rmed.1999.0574. [DOI] [PubMed] [Google Scholar]
- Kluchová Z, Petrásová D, Joppa P, Dorková Z, Tkácová R. The association between oxidative stress and obstructive lung impairment in patients with COPD. Physiol Res. 2007;56(1):51–56. doi: 10.33549/physiolres.930884. [DOI] [PubMed] [Google Scholar]
- Wang C-S, Wang S-T, Chang T-T, Yao W-J, Chou P. Smoking and alanine aminotransferase levels in hepatitis C virus infection: implications for prevention of hepatitis C virus progression. Arch Intern Med. 2002;162(7):811–815. doi: 10.1001/archinte.162.7.811. [DOI] [PubMed] [Google Scholar]
- El-Zayadi A, Selim O, Hamdy H et al. Impact of cigarette smoking on response to interferon therapy in chronic hepatitis C Egyptian patients. World J Gastroenterol. 2004;10(20):2963–2966. doi: 10.3748/wjg.v10.i20.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana RJ, Israel J, LeClair P et al. Iron reduction before and during inter-feron therapy of chronic hepatitis C: results of a multicenter, randomized, controlled trial. Hepatology. 2000;31(3):730–736. doi: 10.1002/hep.510310325. [DOI] [PubMed] [Google Scholar]
- Fargion S, Fracanzani AL, Rossini A et al. Iron reduction and sustained response to interferon-α therapy in patients with chronic hepatitis C: results of an Italian multicenter randomized study. Am J Gastroenterol. 2002;97(5):1204–1210. doi: 10.1111/j.1572-0241.2002.05705.x. [DOI] [PubMed] [Google Scholar]
- Pessione F, Ramond MJ, Njapoum C et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001;34(1):121–125. doi: 10.1053/jhep.2001.25385. [DOI] [PubMed] [Google Scholar]
- Yu M-W, Hsu F-C, Sheen I-S et al. Prospective study of hepatocellular carcinoma and liver cirrhosis in asymptomatic chronic hepatitis B virus carriers. Am J Epidemiol. 1997;145(11):1039–1047. doi: 10.1093/oxfordjournals.aje.a009060. [DOI] [PubMed] [Google Scholar]
- Hézode C, Roudot-Thoraval F, Nguyen S et al. Daily cannabis smoking as a risk factor for progression of fibrosis in chronic hepatitis C. Hepatology. 2005;42(1):63–71. doi: 10.1002/hep.20733. [DOI] [PubMed] [Google Scholar]
- Liu T, Howell GT, Turner L, Corace K, Garber G, Cooper C. Marijuana use in hepatitis C infection does not affect liver biopsy histology or treatment outcomes. Can J Gastroenterol Hepatol. 2014;28(7):381–384. doi: 10.1155/2014/804969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization; International Agency for Research on Cancer. Liver. http://gco.iarc.fr/today/data/factsheets/cancers/11-Liver-fact-sheet.pdf Published March 2019. Accessed November 13, 2020.
- Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman M. Vinyl chloride and the liver. J Hepatol. 2009;51(6):1074–1081. doi: 10.1016/j.jhep.2009.09.012. [DOI] [PubMed] [Google Scholar]
- Ledda C, Loreto C, Zammit C et al. Non-infective occupational risk factors for hepatocellular carcinoma: a review (review). Mol Med Rep. 2017;15(2):511–533. doi: 10.3892/mmr.2016.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-CA, Cohet C, Yang Y-C, Stayner L, Hashibe M, Straif K. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38(6):1497–1511. doi: 10.1093/ije/dyp280. [DOI] [PubMed] [Google Scholar]
- Chuang S-C, Lee Y-CA, Hashibe M, Dai M, Zheng T, Boffetta P. Interaction between cigarette smoking and hepatitis B and C virus infection on the risk of liver cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1261–1268. doi: 10.1158/1055-9965.EPI-09-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrov LB, Stratton MR. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev. 2014;24(100):52–60. doi: 10.1016/j.gde.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze K, Imbeaud S, Letouzé E et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47(5):505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Furuta M, Totoki Y et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48(5):500–509. doi: 10.1038/ng.3547. [DOI] [PubMed] [Google Scholar]
- Mukaiya M, Nishi M, Miyake H, Hirata K. Chronic liver diseases for the risk of hepatocellular carcinoma: a case-control study in Japan. Etiologic association of alcohol consumption, cigarette smoking and the development of chronic liver diseases. Hepatogastroenterology. 1998;45(24):2328–2332. [PubMed] [Google Scholar]
- Estes C, Anstee QM, Arias-Loste MT et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Afendy M et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524–530.e1. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–133. doi: 10.1002/hep.29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbach JK, Kulik LM, Finn RS et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M et al. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Rafiq N et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver-related mortality. Hepatology. 2011;53(6):1874–1882. doi: 10.1002/hep.24268. [DOI] [PubMed] [Google Scholar]
- Ekstedt M, Hagström H, Nasr P et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- Angulo P, Kleiner DE, Dam-Larsen S et al. Liver fibrosis, but no other histo-logic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Jung YS, Hong HP et al. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. 2018;38(8):1487–1494. doi: 10.1111/liv.13701. [DOI] [PubMed] [Google Scholar]
- Hectors TL, Vanparys C, Van Gaal LF, Jorens PG, Covaci A, Blust R. Insulin resistance and environmental pollutants: experimental evidence and future perspectives. Environ Health Perspect. 2013;121(11-12):1273–1281. doi: 10.1289/ehp.1307082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Kabir E, Kabir S. A review on the human health impact of airborne particulate matter. Environ Int. 2015;74:136–143. doi: 10.1016/j.envint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Markevych I, Wolf K, Hampel R et al. Air pollution and liver enzymes. Epidemiology. 2013;24(6):934–935. doi: 10.1097/EDE.0b013e3182a77600. [DOI] [PubMed] [Google Scholar]
- Alderete TL, Habre R, Toledo-Corral CM et al. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes. 2017;66(7):1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H-H, Fiel MI, Sun Q et al. Kupffer cell activation by ambient air particulate matter exposure may exacerbate non-alcoholic fatty liver disease. J Immunotoxicol. 2009;6(4):266–275. doi: 10.3109/15476910903241704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey T, Gogoi K, Unni B et al. Role of environmental pollutants in liver physiology: special references to peoples living in the oil drilling sites of Assam. PLoS One. 2015;10(4):e0123370. doi: 10.1371/journal.pone.0123370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E, Cyrys J, Kratzsch J et al. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–1704. doi: 10.1007/s00125-013-2925-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Ku T, Yue H, Li G, Sang N. NO2 inhalation causes tauopathy by disturbing the insulin signaling pathway. Chemosphere. 2016;165:248–256. doi: 10.1016/j.chemosphere.2016.09.063. [DOI] [PubMed] [Google Scholar]
- Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143(2):307–320. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch AD, Yip R, Vandromme M 339 Exposure to the World Trade Center attack is associated with increased risk of moderate-to-severe hepatic steatosis and liver injury [published online October 1, 2018]. Hepatology. doi:10.1002/ hep.30257.
- Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–J265. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Glossop JR, Dawes PT, Mattey DL. Association between cigarette smoking and release of tumour necrosis factor α and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology (Oxford). 2006;45(10):1223–1229. doi: 10.1093/rheumatology/kel094. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Goswami S, Grudo A et al. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- Mantaka A, Koulentaki M, Chlouverakis G et al. Primary biliary cirrhosis in a genetically homogeneous population: disease associations and familial occurrence rates. BMC Gastroenterol. 2012;12(1):110. doi: 10.1186/1471-230X-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra K, Kunst AE, Stadhouders PH et al. Epi PSC PBC study group. Rising incidence and prevalence of primary biliary cirrhosis: a large population-based study. Liver Int. 2014;34(6):e31–e38. doi: 10.1111/liv.12434. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (May-wood). 2008;233(2):109–122. doi: 10.3181/0707-MR-190. [DOI] [PubMed] [Google Scholar]
- Zein CO, Beatty K, Post AB, Logan L, Debanne S, McCullough AJ. Smoking and increased severity of hepatic fibrosis in primary biliary cirrhosis: a cross validated retrospective assessment. Hepatology. 2006;44(6):1564–1571. doi: 10.1002/hep.21423. [DOI] [PubMed] [Google Scholar]
- Corpechot C, Chrétien Y, Chazouillères O, Poupon R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J Hepatol. 2010;53(1):162–169. doi: 10.1016/j.jhep.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Mantaka A, Koulentaki M, Samonakis D et al. Association of smoking with liver fibrosis and mortality in primary biliary cholangitis. Eur J Gastroenterol Hepatol. 2018;30(12):1461–1469. doi: 10.1097/MEG.0000000000001234. [DOI] [PubMed] [Google Scholar]
- Schenk P, Fuhrmann V, Madl C et al. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51(6):853–859. doi: 10.1136/gut.51.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes H, Lebrec D, Mazmanian M et al. Role of nitric oxide in hepatopulmonary syndrome in cirrhotic rats. Am J Respir Crit Care Med. 2001;164(5):879–885. doi: 10.1164/ajrccm.164.5.2009008. [DOI] [PubMed] [Google Scholar]
- Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105(5):1528–1537. doi: 10.1378/chest.105.5.1528. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Roisin R, Agustí AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47(11):897–902. doi: 10.1136/thx.47.11.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin Z, Amin HZ, Tedyanto NM. Hepatopulmonary syndrome: a brief review. Rom J Intern Med. 2016;54(2):93–97. doi: 10.1515/rjim-2016-0015. [DOI] [PubMed] [Google Scholar]
- Than NN. Chapter 5: Pulmonary complications of liver cirrhosis: a concise review. In: Tsoulfas G, ed. Liver Cirrhosis: Update and Current Challenges. Intech Open. 2017 [Google Scholar]
- Robbins RA, Millatmal T, Lassi K, Rennard S, Daughton D. Smoking cessation is associated with an increase in exhaled nitric oxide. Chest. 1997;112(2):313–318. doi: 10.1378/chest.112.2.313. [DOI] [PubMed] [Google Scholar]
- Sabha M, Tanus-Santos JE, Toledo JCY, Cittadino M, Rocha JC, Moreno H., Jr Transdermal nicotine mimics the smoking-induced endothelial dysfunction. Clin Pharmacol Ther. 2000;68(2):167–174. doi: 10.1067/mcp.2000.108851. [DOI] [PubMed] [Google Scholar]
- Rolla G, Brussino L, Dutto L, Ottobrelli A, Bucca C. Smoking and hypoxemia caused by hepatopulmo-nary syndrome before and after liver transplantation. Hepatology. 2001;34(2):430–431. doi: 10.1053/jhep.2001.26791. [DOI] [PubMed] [Google Scholar]
- Silverstein P. Smoking and wound healing. Am J Med. 1992;93(1A):22S–24S. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]
- Pungpapong S, Manzarbeitia C, Ortiz J et al. Cigarette smoking is associated with an increased incidence of vascular complications after liver transplantation. Liver Transpl. 2002;8(7):582–587. doi: 10.1053/jlts.2002.34150. [DOI] [PubMed] [Google Scholar]
- Leithead JA, Ferguson JW, Hayes PC. Smoking-related morbidity and mortality following liver transplantation. Liver Transpl. 2008;14(8):1159–1164. doi: 10.1002/lt.21471. [DOI] [PubMed] [Google Scholar]
- Scheifele C, Reichart PA, Hippler-Benscheidt M, Neuhaus P, Neuhaus R. Incidence of oral, pharyngeal, and laryngeal squamous cell carcinomas among 1515 patients after liver transplantation. Oral Oncol. 2005;41(7):670–676. doi: 10.1016/j.oraloncology.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Jiménez C, Manrique A, Marqués E et al. Incidence and risk factors for the development of lung tumors after liver transplantation. Transpl Int. 2007;20(1):57–63. doi: 10.1111/j.1432-2277.2006.00397.x. [DOI] [PubMed] [Google Scholar]
- Rubio E, Moreno JM, Turrión VS, Jimenez M, Lucena JL, Cuervas-Mons V. De novo malignancies and liver transplantation. Transpl Proc. 2003;35(5):1896–1897. doi: 10.1016/s0041-1345(03)00645-6. [DOI] [PubMed] [Google Scholar]
- Vallejo GH, Romero CJ, de Vicente JC. Incidence and risk factors for cancer after liver transplantation. Crit Rev Oncol Hematol. 2005;56(1):87–99. doi: 10.1016/j.critrevonc.2004.12.011. [DOI] [PubMed] [Google Scholar]
- van der Heide F, Dijkstra G, Porte RJ, Kleibeuker JH, Haagsma EB. Smoking behavior in liver transplant recipients. Liver Transpl. 2009;15(6):648–655. doi: 10.1002/lt.21722. [DOI] [PubMed] [Google Scholar]
- Ehlers SL, Rodrigue JR, Widows MR, Reed AI, Nelson DR. Tobacco use before and after liver transplantation: a single center survey and implications for clinical practice and research. Liver Transpl. 2004;10(3):412–417. doi: 10.1002/lt.20087. [DOI] [PubMed] [Google Scholar]
- Rybak D, Fallon MB, Krowka MJ et al. Pulmonary Vascular Complications of Liver Disease Study Group. Risk factors and impact of chronic obstructive pulmonary disease in candidates for liver transplantation. Liver Transpl. 2008;14(9):1357–1365. doi: 10.1002/lt.21545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burling TA, Ziff DC. Tobacco smoking: a comparison between alcohol and drug abuse inpatients. Addict Behav. 1988;13(2):185–190. doi: 10.1016/0306-4603(88)90010-x. [DOI] [PubMed] [Google Scholar]
- Duvoux C, Delacroix I, Richardet J-P et al. Increased incidence of oropharyngeal squamous cell carcinomas after liver transplantation for alcoholic cirrhosis. Transplantation. 1999;67(3):418–421. doi: 10.1097/00007890-199902150-00014. [DOI] [PubMed] [Google Scholar]
- Altamirano J, Bataller R. Cigarette smoking and chronic liver diseases. Gut. 2010;59(9):1159–1162. doi: 10.1136/gut.2008.162453. [DOI] [PubMed] [Google Scholar]
- Lu R, Zhao X, Li J et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins University & Medicine; Corona-virus Resource Center. COVID-19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). https://coronavirus.jhu.edu/map.html Accessed November 17, 2020.
- Cai Q, Chen J. Reply to: Clinical characteristics of COVID-19 patients with abnormal liver tests. J Hepatol. 2020;73(3):713–714. doi: 10.1016/j.jhep.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber S, Mayerle J, Irlbeck M, Gerbes AL. Severe liver failure during SARS-CoV-2 infection. Gut. 2020;69(7):1365–1367. doi: 10.1136/gutjnl-2020-321350. [DOI] [PubMed] [Google Scholar]
- Bangash MN, Patel J, Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol Hepatol. 2020;5(6):529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X-W, Wu X-X, Jiang X-G et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]