Abstract
Esophagogastric junction outflow obstruction (EGJOO) is an abnormal topographic pattern seen on high-resolution manometry. EGJOO is characterized by an elevated median integrated relaxation pressure with intact or weak peristalsis, thus not meeting the criteria for achalasia. This diagnosis has a female predominance and is associated with varying presenting symptoms. EGJOO can be idiopathic or secondary. It is important to assess for secondary causes, including structural or medication-related ones. Cross-sectional imaging is recommended to rule out secondary causes; however, increasing evidence suggests that esophagogastroduodenoscopy and barium esophagram are usually sufficient. The disease course is variable, with up to three-quarters of patients experiencing spontaneous resolution of symptoms over 6 months. In patients who have mild symptoms, it is reasonable to observe and consider treatment if symptoms persist. Variable response has been seen in small studies with both medical treatment and botulinum toxin injection of the lower esophageal sphincter. For patients with significant symptoms and objective evidence of obstruction on imaging, targeted therapy of the lower esophageal sphincter should be considered via pneumatic dilation or myotomy.
Keywords: Esophagogastric junction outflow obstruction, esophageal manometry, dysphagia, pneumatic dilation, peroral endoscopic myotomy, laparoscopic Heller myotomy
The worldwide use of high-resolution manometry (HRM) of the esophagus continues on an upward trajectory. Intuitively, this leads to the continued need for refinement of diagnostic topographic patterns. Esophagogastric junction outflow obstruction (EGJOO) is a diagnosis of unclear etiology, defined solely by abnormal manometric parameters. According to the Chicago Classification of Esophageal Motility Disorders version 3.0 (CC v3.0), EGJOO is defined by an elevated median integrated relaxation pressure (IRP) with intact or weak peristalsis, thus not meeting the criteria for achalasia.1 EGJOO can be idiopathic or secondary to various causes, and there is heterogeneity in presenting symptoms, disease course, and response to treatment.1-6 EGJOO is a relatively new diagnosis, and further research will likely provide additional clarity to this finding. This article reviews the current literature and expert opinions on EGJOO.
Demographics
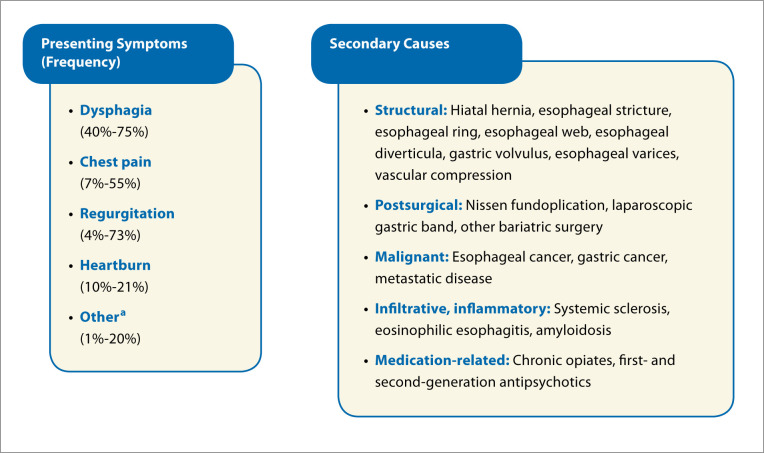
The finding of EGJOO has been reported in up to 14% of HRMs. This diagnosis is more commonly seen in females, with an average age range of 51 to 69 years.3,5-12 The most common presenting symptom is dysphagia. Other frequently seen symptoms include chest pain, regurgitation, heartburn, cough, and globus sensation (Figure 1). Often, patients present with a combination of symptoms.3,5,8,10-15 Regurgitation and chest pain have strikingly wide ranges of prevalence, spanning from 4% to 73%. The wide ranges may be related to the fact that these symptoms are more frequently seen in idiopathic rather than secondary EGJOO.10 Notably, EGJOO can also be an incidental finding, such as when routine manometry is performed prior to antireflux procedures.4 Thus, centers with high-volume foregut surgeries may report higher incidences of asymptomatic EGJOO.
Figure 1.
Presenting symptoms and secondary causes seen in esophagogastric junction outflow obstruction (EGJOO).
aCough, globus sensation, dyspepsia, belching, bloating, nausea, hoarseness, hiccups. EGJOO can be an incidental finding as well.
Diagnosis
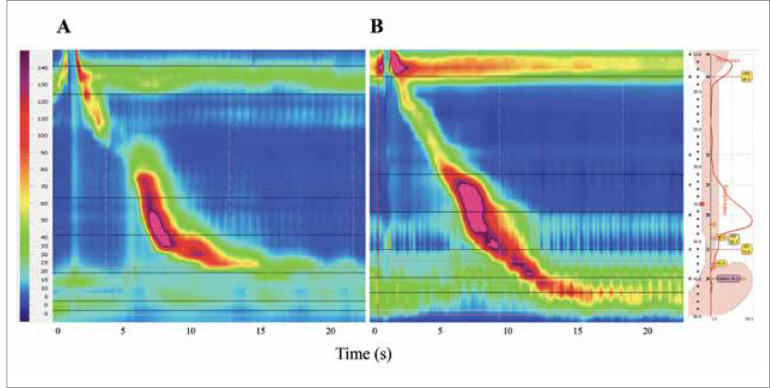
The diagnosis of EGJOO is made on HRM, defined by an elevated median IRP with intact or weak peristalsis (Figure 2).1 CC v3.0, which is the latest version of the classification, uses median IRP for criteria; notably, the prior version defined EGJOO via mean IRP.1,16 Currently, the threshold is a median IRP greater than 15 mm Hg, but it is important to make adjustments based on the transducer type. The upper limit of normal is 15 mm Hg for ManoScan ESO (Medtronic), whereas it has been reported to be as high as 28 mm Hg for other catheter types.1 Accuracy of the current cutoff has only been rigorously evaluated in ManoScan ESO.
Figure 2.
Normal high-resolution manometry (A) compared to esophagogastric junction outflow obstruction high-resolution manometry (B).
EGJOO is not always an exclusive diagnosis. Concurrent motility diagnoses have been reported in up to 62% of patients, including ineffective esophageal motility, diffuse esophageal spasm, and jackhammer esophagus.14,17 The clinical significance of these findings in the setting of an elevated median IRP is unclear and in need of further clarification.
EGJOO can also be secondary to various causes; once these causes are ruled out, it is considered to be idiopathic. Secondary causes can occur in 13% to 66% of EGJOO diagnoses, with potential causes listed in Figure 1.1,3,4,6-12,18-20 General categories include structural; infiltrative, inflammatory; and medication-related causes (eg, opiates). Numerous studies have revealed the association between chronic opioid therapy and elevated lower esophageal sphincter (LES) relaxation pressures.20-25 Unsurprisingly, a prompt decrease in LES pressure is seen following cessation of the medication.20,24,25 Given the potential for secondary causes, a thorough medication reconciliation should be performed and an esophagogastroduodenoscopy (EGD) and barium swallow should be considered if not already done.4,15 A barium swallow may help identify a hiatal hernia or stricture, whereas an EGD with biopsies may diagnose eosinophilic esophagitis or an infiltrative process. The CC v3.0 also states that the finding of EGJOO should prompt further investigation with cross-sectional imaging, such as a computed tomography scan or endoscopic ultrasound. However, several small studies have demonstrated minimal added yield of cross-sectional imaging.3,11 This was confirmed by 2 recent larger studies of over 100 patients with EGJOO.6,26 In one study, all secondary causes were found on EGD and barium esophagram with no additional causes revealed on computed tomography scan or endoscopic ultrasound.6 In fact, 2 patients had false-negative findings on computed tomography scan. As such, the use of routine cross-sectional imaging in these patients may not be merited but could be considered on a case-by-case basis.
Manometric Considerations
Pressure topography via HRM gives a plethora of information aside from peristaltic activity and sphincter tone. There are preliminary data on additional parameters that may help identify patients with EGJOO who will benefit from targeted LES treatment. These parameters include bolus transit, distal contractile integral, intrabolus pressure, upright swallows, rapid drink challenge, and assessment of LES response to pharmacotherapies.
Delayed bolus transit on intraluminal impedance of esophageal function may help better characterize patients with EGJOO. Delayed bolus transit is defined as fewer than 80% of liquid swallows having complete bolus transit. In a study of 169 patients with EGJOO, a combination of delayed bolus transit, dysphagia, and chest pain had the highest predictive value for identifying clinically relevant EGJOO (sensitivity, 90%; specificity, 92.5%; negative predictive value, 99.3%).27 EGJOO was deemed clinically relevant if there was delayed passage of contrast on timed barium esophagram, improvement in symptoms after pneumatic dilation, or the eventual development of achalasia. Regarding distal contractile integral, a study of 75 EGJOO patients demonstrated that a distal contrac-tile integral value greater than 884 mm Hg•s•cm had a positive predictive value of 98% and accuracy of 82% for complete bolus transit in each swallow.28 Thus, at higher distal contractile integral values, the LES was more likely to allow for esophageal clearance.
Intrabolus pressure has also been evaluated with regard to LES dysfunction. In a study of 11 patients with EGJOO, all patients who had delayed emptying on timed barium esophagram had an intrabolus pressure on manometry of greater than 24 mm Hg.29 These aforementioned measurements of bolus transit are easily evaluated on routine manometry and are a simple way to potentially stratify patients with EGJOO.
Additionally, upright swallows on esophageal manometry have been assessed retrospectively in a recent study of 155 EGJOO patients.5 This study found that a median upright IRP of greater than 12 mm Hg correlated well with an abnormal timed barium esophagram or a positive dysphagia symptom score on questionnaire (sensitivity of 98%).
Rapid drink challenge is a provocative esophageal test that has been used clinically to characterize the esophagogastric junction. This test involves rapidly swallowing 200 mL of liquid, enhancing deglutitive inhibition. A recent study of dysphagia patients evaluated the correlation of rapid drink challenge IRP with symptoms and timed barium esophagram. Ultimately, a threshold of an IRP 8 mm Hg or greater was proposed to identify non achalasia causes of obstruction at the esophagogastric junction.30 However, IRP characterization during rapid drink challenge, specifically in patients with the mano-metric diagnosis of EGJOO, has yet to be well established. Rapid drink challenge may also provide unique peristaltic information. Panesophageal pressurization during rapid drink challenge is frequently seen in patients with EGJOO that evolves to achalasia. Panesophageal pressurization has also been shown to correlate with more severe symptoms in these patients.31 Overall, rapid drink challenge shows much promise in recognizing clinically significant EGJOO patients.
The final manometric parameter that will be discussed is pharmacologic LES relaxation. Recent research by Babaei and colleagues evaluated the effects of amyl nitrite administration on the LES in EGJOO.32 Amyl nitrite is a potent nitric oxide donor that leads to rapid relaxation of smooth muscle; prior studies have revealed a decrease in IRP in achalasia patients after amyl nitrite administration.33-35 Babaei and colleagues theorized that patients with clinically significant idiopathic EGJOO similarly have impairment of deglutitive LES smooth muscle relaxation and, thus, would respond to amyl nitrite.32 Of the 49 patients with EGJOO, 27 patients were responsive to inhaled amyl nitrite administration (as measured by an IRP decrease of ≥10 mm Hg). Twenty-three of these patients underwent treatment, 12 with pneumatic dilation or laparoscopic Heller myotomy and 11 with medical therapy. The majority of these patients (78%) had resolution of their liquid dysphagia at a mean follow-up of 26 months.32 This is a potentially straightforward way to examine LES tone, and the clinical outcomes in this study were encouraging. However, limitations include a small number of patients, the logistics of obtaining and using the inhaled medication, and the lack of additional studies supporting these data.
Additional Investigations
Timed barium esophagram and the functional lumen imaging probe (FLIP) are 2 additional imaging modalities that provide complementary information regarding the LES in patients with EGJOO.
A barium esophagram is a widely employed and low-cost test to assess for secondary causes of EGJOO. Delay of barium tablet passage through the LES suggests functional or anatomic obstruction. A specific timed barium esophagram protocol requires patients to drink barium in the standing position and then for images to be taken at 1, 2, and 5 minutes to assess esophageal emptying. In a retrospective study of 309 patients who underwent timed barium esophagram and HRM, a column height of 6 cm or more at 1 minute and 2 cm or more at 5 minutes differentiated untreated achalasia from EGJOO with reasonable accuracy (at 1 minute, sensitivity of 91% and specificity of 56%; at 5 minutes, sensitivity and specificity of 84%). However, this study was unable to reliably differentiate EGJOO from nonachalasia motility disorders.36 Overall, timed barium esophagram is a simple and effective way to evaluate the esophagus and has potential for further LES characterization in EGJOO.
FLIP is a rapidly emerging catheter-based test that uses impedance planimetry to evaluate the pressure and cross-sectional area of hollow organs. These measurements are used to assess esophageal distensibility, compliance, and peristalsis. FLIP has been useful in assessing physiology and treatment response in patients with achalasia, eosinophilic esophagitis, gastroesophageal reflux disease, systemic sclerosis, and ineffective esophageal motility.37-42 In theory, given the precision with which the LES is assessed on impedance planimetry, it would make sense that FLIP can clarify characteristics of the esophagogastric junction in EGJOO. However, limited data exist on this topic. One study of 38 EGJOO patients noted that the majority of patients (87%) had an abnormal distensibility index of the esophagogastric junction on FLIP. The authors postulated that impedance planimetry can more accurately assess for true EGJOO, as HRM may be more prone to pressure artifact. However, outcome data were not assessed in this study, making definitive conclusions difficult.43 Overall, this device holds promise for further characterization of the esophagogastric junction and potentially in the management of patients with EGJOO.
Disease Course and Management
This section focuses on the disease course and management of idiopathic EGJOO, as management of secondary EGJOO is targeted toward the underlying cause. The disease course of idiopathic EGJOO is highly variable. In retrospective cohort studies, spontaneous resolution occurs in 15% to 74% of patients within 6 months.3,8,9,12 Among aggregate study data, the overall resolution rate is 43%. In stark contrast, there are reports of EGJOO progressing to achalasia.3,7,9,14,44,45 However, there is currently no way to predict which patients will have spontaneous resolution, which will have persistent symptoms, and which will progress to achalasia. Given the high rates of spontaneous resolution, treatment is not always warranted when this manometric finding is identified. Experts advise a trial of conservative management if symptoms are mild or atypical as well as consideration of targeted LES therapy for patients with dysphagia or objective evidence of obstruction.4,15,46 Such evidence includes liquid retention on timed barium esophagram, barium tablet delay on barium esophagram, and low distensibility on FLIP. Although this approach is reasonable, further outcome data are needed.
To date, studied treatment options include medications, botulinum toxin injection of the LES, standard endoscopic dilation, pneumatic dilation, peroral endoscopic myotomy, and surgical myotomy. Medications such as calcium channel blockers, nitrates, muscle relaxants, tricyclic antidepressants, and antispasmodics have been tried with a wide range of results. In a recent study by Lynch and colleagues, 3 out of 4 patients had an improvement in their symptoms at a median of 4 months (with a tricyclic antidepressant for chest pain, hyoscyamine for dysphagia, and a proton pump inhibitor for reflux).3 Among all studies, the aggregate symptom relief at 3 to 6 months is 41%, with a range of 0% to 75%.3,8,11 Large sample sizes are lacking with the aforementioned studies of medications, with a maximum of 8 patients described in a single study.11 Additionally, there is little standardization among studies in terms of differentiation between idiopathic and secondary EGJOO, as well as breakdown of the medication selection.
Concentrated peppermint oil has also been suggested. A major advantage is the unremarkable side-effect profile. In a retrospective study of 8 patients with EGJOO and dysphagia or chest pain, all patients reported symptom relief with no adverse events at a median follow-up of 6 months.47 There have also been preliminary data demonstrating that acotiamide, an acetylcholinesterase inhibitor, may decrease the IRP in EGJOO, but symptom follow-up has not yet been evaluated.48 It is apparent that current data on pharmacologic management in EGJOO are weak. Medications may be considered if side-effect profiles are favorable, but if the predominant symptom is dysphagia or chest pain, endoscopic or surgical therapies may be considered first.
Botulinum toxin injection of the LES is a low-risk intervention with a noteworthy response rate in the limited studies to date. In patients with dysphagia or chest pain, sustained symptomatic relief (≥6 months) was reported in up to two-thirds of patients and transient relief (<6 months) in up to 100% of patients.3,7,9,10,49 The largest study (N=11) found that 64% of patients with dysphagia and incomplete clearance on barium esophagram treated with botulinum toxin injection had persistent symptomatic relief at 2 years.7 There are several other studies that note similar clinical response rates to botulinum toxin injection; however, both idiopathic and secondary EGJOO patients were included.11,50,51 Botulinum toxin injection can be considered in EGJOO patients with dysphagia or chest pain with the understanding that response durability may be limited.
Traditional through-the-scope dilations have not demonstrated substantial symptomatic relief for EGJOO patients. Sample sizes are small (up to 9 patients), with an aggregate sustained symptomatic relief of 35% of patients.3,10,11,49
Pneumatic dilation has been increasingly utilized as targeted LES therapy in EGJOO. Several case series have noted sustained symptom relief for up to 18 months post–pneumatic dilation.9,12,49 The largest and most robust study to date has been by Clayton and colleagues, who observed 33 idiopathic EGJOO patients with dysphagia and abnormal timed barium esophagram.52 Almost 80% of patients who underwent pneumatic dilation had durable symptom improvement that lasted up to 5 years. Of note, 4 of these patients underwent a second dilation. No postprocedure complications were reported.
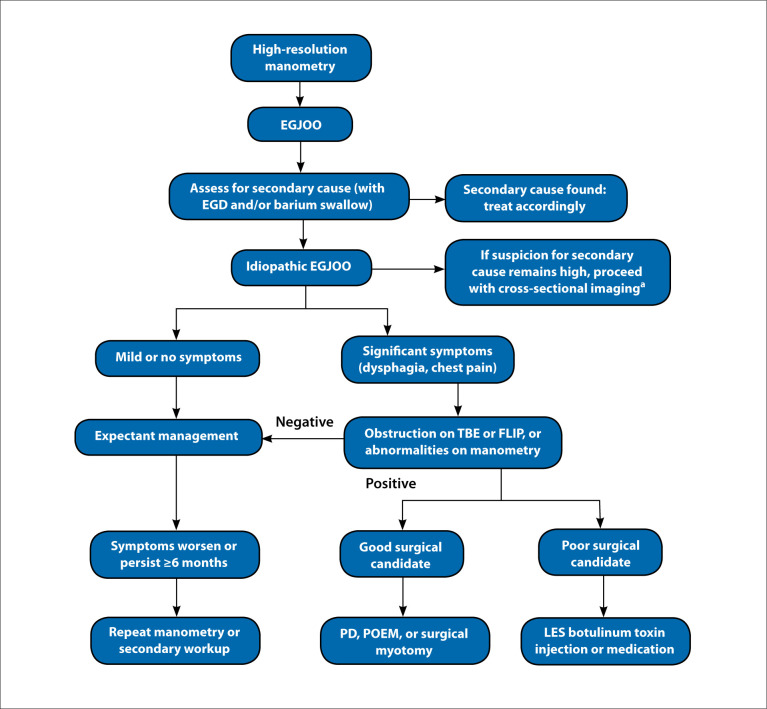
Surgical myotomies, including laparoscopic and robotic-assisted ones, have demonstrated excellent long-term symptomatic relief in multiple case series.3,49,53,54 Although sample sizes were small (range, 1-3), all studies noted that 100% of patients had symptomatic relief at 12 months or longer. All myotomies were performed for an indication of dysphagia or chest pain. Peroral endoscopic myotomy has similarly been successful in small studies. This procedure is done by way of endoscopic tunneling into the esophageal submucosal space, after which a myotomy of the circular muscle layer is performed and extended across the esophagogastric junction. After peroral endoscopic myotomy, 71% to 100% of EGJOO patients reported symptomatic improvement via the Eckardt score.11,55-57 A study by Filicori and colleagues noted that all 14 of their patients with EGJOO who underwent peroral endoscopic myotomy for chest pain or dysphagia had an improvement in both timed barium esophagram and symptoms via the Eckardt score at 6 months.55 The majority of patients (80%) had sustained improvement in symptoms at a median of 48 months. One consideration of peroral endoscopic myotomy is potential adverse events such as aspiration pneumonia, esophageal leak, and pneumothorax; however, these events are rare.56,58 More common is resultant gastroesophageal reflux disease, often reflected by esophagitis. This has been reported in 30% to 40% of patients, but can be reduced to 0% to 13% with proton pump inhibitor therapy.56,57 An overall suggested algorithm for workup and therapy is seen in Figure 3.
Figure 3.
Flowchart of suggested diagnosis and management.
EGD, esophagogastroduodenoscopy; EGJOO, esophagogastric junction outflow obstruction; FLIP, functional lumen imaging probe; LES, lower esophageal sphincter; PD, pneumatic dilation; POEM, peroral endoscopic myotomy; TBE, timed barium esophagram.
aComputed tomography scan or endoscopic ultrasound.
Conclusion
EGJOO is a manometric diagnosis with a wide variety in presenting symptoms, disease course, and response to treatment. Future studies investigating the characteristics of EGJOO may be helpful to better risk-stratify and treat patients with this condition. In practical terms, EGJOO may refer to any impediment at the esophago-gastric junction. Thus, alternatively, clarification of the manometric diagnosis of EGJOO itself may be worthwhile. There is potential in timed barium esophagram and distensibility measurements, as well as discriminating HRM parameters to further characterize the finding of EGJOO.
References
- Kahrilas PJ, Bredenoord AJ, Fox M et al. International High Resolution Manometry Working Group. The Chicago Classification of Esophageal Motility Disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino S, von Isenburg M, Fisher DA, Leiman DA. Management of functional esophagogastric junction outflow obstruction: a systematic review. J Clin Gastroenterol. 2020;54(1):35–42. doi: 10.1097/MCG.0000000000001156. [DOI] [PubMed] [Google Scholar]
- Lynch KL, Yang YX, Metz DC, Falk GW. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis Esophagus. 2017;30(6):1–6. doi: 10.1093/dote/dox004. [DOI] [PubMed] [Google Scholar]
- Samo S, Qayed E. Esophagogastric junction outflow obstruction: where are we now in diagnosis and management? World J Gastroenterol. 2019;25(4):411–417. doi: 10.3748/wjg.v25.i4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triggs JR, Carlson DA, Beveridge C et al. Upright integrated relaxation pressure facilitates characterization of esophagogastric junction outflow obstruction. Clin Gastroenterol Hepatol. 2019;17(11):2218–2226.e2. doi: 10.1016/j.cgh.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge CA, Falk GW, Ahuja NK, Yang YX, Metz DC, Lynch KL. Low yield of cross-sectional imaging in patients with esophagogastric junction out-flow obstruction [published online July 27, 2019]. Clin Gastroenterol Hepatol. doi:10.1016/j.cgh.2019.07.044. [DOI] [PubMed]
- Clayton SB, Patel R, Richter JE. Functional and anatomic esophagogastic junction outflow obstruction: manometry, timed barium esophagram findings, and treatment outcomes. Clin Gastroenterol Hepatol. 2016;14(6):907–911. doi: 10.1016/j.cgh.2015.12.041. [DOI] [PubMed] [Google Scholar]
- Pérez-Fernández MT, Santander C, Marinero A, Burgos-Santamaría D, Chavarría-Herbozo C. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil. 2016;28(1):116–126. doi: 10.1111/nmo.12708. [DOI] [PubMed] [Google Scholar]
- van Hoeij FB, Smout AJPM, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2015;27(9):1310–1316. doi: 10.1111/nmo.12625. [DOI] [PubMed] [Google Scholar]
- DeLay K, Austin GL, Menard-Katcher P. Anatomic abnormalities are common potential explanations of manometric esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2016;28(8):1166–1171. doi: 10.1111/nmo.12814. [DOI] [PubMed] [Google Scholar]
- Okeke FC, Raja S, Lynch KL et al. What is the clinical significance of esophagogastric junction outflow obstruction? Evaluation of 60 patients at a tertiary referral center. Neurogastroenterol Motil. 2017;29(6) doi: 10.1111/nmo.13061. [DOI] [PubMed] [Google Scholar]
- Ong AML, Namasivayam V, Wang YT. Evaluation of symptomatic esophago-gastric junction outflow obstruction. J Gastroenterol Hepatol. 2018;33(10):1745–1750. doi: 10.1111/jgh.14155. [DOI] [PubMed] [Google Scholar]
- Schupack D, Katzka DA, Geno DM, Ravi K. The clinical significance of esophagogastric junction outflow obstruction and hypercontractile esophagus in high resolution esophageal manometry. Neurogastroenterol Motil. 2017;29(10):1–9. doi: 10.1111/nmo.13105. [DOI] [PubMed] [Google Scholar]
- Triadafilopoulos G, Clarke JO. Clinical and manometric characteristics of patients with oesophagogastric outflow obstruction: towards a new classification. BMJ Open Gastroenterol. 2018;5(1):e000210. doi: 10.1136/bmjgast-2018-000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter JE, Clayton SB. Diagnosis and management of esophagogastric junction outflow obstruction. Am J Gastroenterol. 2019;114(4):544–547. doi: 10.14309/ajg.0000000000000100. [DOI] [PubMed] [Google Scholar]
- Bredenoord AJ, Fox M, Kahrilas PJ, Pandolfino JE, Schwizer W, Smout AJ. International High Resolution Manometry Working Group. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng E, Gideon RM, Sloan J, Katz PO. Esophagogastric junction outflow obstruction is often associated with coexistent abnormal esophageal body motility and abnormal bolus transit. Dis Esophagus. 2017;30(10):1–4. doi: 10.1093/dote/dox066. [DOI] [PubMed] [Google Scholar]
- Aggarwal N, Lopez R, Gabbard S, Wadhwa N, Devaki P, Thota PN. Spectrum of esophageal dysmotility in systemic sclerosis on high-resolution esophageal manometry as defined by Chicago classification. Dis Esophagus. 2017;30(12):1–6. doi: 10.1093/dote/dox067. [DOI] [PubMed] [Google Scholar]
- Crouse EL, Alastanos JN, Bozymski KM, Toscano RA. Dysphagia with second-generation antipsychotics: a case report and review of the literature. Ment Health Clin. 2018;7(2):56–64. doi: 10.9740/mhc.2017.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratuapli SK, Crowell MD, DiBaise JK et al. Opioid-induced esophageal dysfunction (OIED) in patients on chronic opioids. Am J Gastroenterol. 2015;110(7):979–984. doi: 10.1038/ajg.2015.154. [DOI] [PubMed] [Google Scholar]
- Sáez-González E, Díaz-Jaime FC, García-Morales N et al. Opioid-induced functional esophagogastric junction obstruction. Gastroenterol Hepatol. 2017;40(4):296–298. doi: 10.1016/j.gastrohep.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Dowlatshahi K, Evander A, Walther B, Skinner DB. Influence of morphine on the distal oesophagus and the lower oesophageal sphincter—a manometric study. Gut. 1985;26(8):802–806. doi: 10.1136/gut.26.8.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penagini R, Bianchi PA. Effect of morphine on gastroesophageal reflux and transient lower esophageal sphincter relaxation. Gastroenterology. 1997;113(2):409–414. doi: 10.1053/gast.1997.v113.pm9247457. [DOI] [PubMed] [Google Scholar]
- Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31(5):601–606. doi: 10.1111/j.1365-2036.2009.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González ES, Bellver VO, Jaime FC, Cortés JA, Gil VG. Opioid-induced lower esophageal sphincter dysfunction. J Neurogastroenterol Motil. 2015;21(4):618–620. doi: 10.5056/jnm15108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Woo M, Nasser Y et al. Esophagogastric junction outflow obstruction on manometry: outcomes and lack of benefit from CT and EUS. Neurogastroenterol Motil. 2019;31(12):e13712. doi: 10.1111/nmo.13712. [DOI] [PubMed] [Google Scholar]
- Song BG, Min YW, Lee H et al. Combined multichannel intraluminal impedance and high-resolution manometry improves detection of clinically relevant esophagogastric junction outflow obstruction. J Neurogastroenterol Motil. 2019;25(1):75–81. doi: 10.5056/jnm18148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Baker JR, Rubenstein JH, Chen JW. Bolus clearance in esophagogastric junction outflow obstruction is associated with strength of peristalsis. Neurogastroenterol Motil. 2017;29(9) doi: 10.1111/nmo.13093. [DOI] [PubMed] [Google Scholar]
- Hoscheit M, Gabbard S. Elevated intrabolus pressure predicts abnormal timed barium esophagram in esophagogastric junction outflow obstruction. J Neurogastroenterol Motil. 2019;25(4):521–524. doi: 10.5056/jnm19025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang D, Hollenstein M, Misselwitz B et al. Rapid drink challenge in high-resolution manometry: an adjunctive test for detection of esophageal motility disorders. Neurogastroenterol Motil. 2017;29(1) doi: 10.1111/nmo.12902. [DOI] [PubMed] [Google Scholar]
- Biasutto D, Mion F, Garros A, Roman S. Rapid drink challenge test during esophageal high resolution manometry in patients with esophago-gastric junction outflow obstruction. Neurogastroenterol Motil. 2018;30(6):e13293. doi: 10.1111/nmo.13293. [DOI] [PubMed] [Google Scholar]
- Babaei A, Shad S, Szabo A, Massey BT. Pharmacologic interrogation of patients with esophagogastric junction outflow obstruction using amyl nitrite. Neurogastroenterol Motil. 2019;31(9):e13668. doi: 10.1111/nmo.13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds WJ, Stewart ET, Kishk SM, Kahrilas PJ, Hogan WJ. Radiologic amyl nitrite test for distinguishing pseudoachalasia from idiopathic achalasia. AJR Am J Roentgenol. 1986;146(1):21–23. doi: 10.2214/ajr.146.1.21. [DOI] [PubMed] [Google Scholar]
- Mayrand S, Diamant NE. Measurement of human esophageal tone in vivo. Gastroenterology. 1993;105(5):1411–1420. doi: 10.1016/0016-5085(93)90146-4. [DOI] [PubMed] [Google Scholar]
- González M, Mearin F, Vasconez C, Armengol JR, Malagelada JR. Oesophageal tone in patients with achalasia. Gut. 1997;41(3):291–296. doi: 10.1136/gut.41.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonski W, Kumar A, Feldman J, Richter JE. Timed barium swallow: diagnostic role and predictive value in untreated achalasia, esophagogastric junction outflow obstruction, and non-achalasia dysphagia. Am J Gastroenterol. 2018;113(2):196–203. doi: 10.1038/ajg.2017.370. [DOI] [PubMed] [Google Scholar]
- Jain AS, Carlson DA, Triggs J et al. Esophagogastric junction distensibility on functional lumen imaging probe topography predicts treatment response in achalasia-anatomy matters! Am J Gastroenterol. 2019;114(9):1455–1463. doi: 10.14309/ajg.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandolfino JE, de Ruigh A, Nicodème F, Xiao Y, Boris L, Kahrilas PJ. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP™) in achalasia patients. Neurogastroenterol Motil. 2013;25(6):496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo IK, Choi SA, Kim WH, Hong SP, Cakir OO, Cho JY. Assessment of clinical outcomes after peroral endoscopic myotomy via esophageal distensibility measurements with the endoluminal functional lumen imaging probe. Gut Liver. 2019;13(1):32–39. doi: 10.5009/gnl18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DA, Hirano I, Zalewski A, Gonsalves N, Lin Z, Pandolfino JE. Improvement in esophageal distensibility in response to medical and diet therapy in eosinophilic esophagitis. Clin Transl Gastroenterol. 2017;8(10):e119. doi: 10.1038/ctg.2017.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano I. Future directions in eosinophilic esophagitis. Gastrointest Endosc Clin N Am. 2018;28(1):111–122. doi: 10.1016/j.giec.2017.07.010. [DOI] [PubMed] [Google Scholar]
- Fynne L, Liao D, Aksglaede K et al. Esophagogastric junction in systemic sclerosis: a study with the functional lumen imaging probe. Neurogastroenterol Motil. 2017;29(8) doi: 10.1111/nmo.13073. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Kahrilas PJ, Lin Z et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe. Am J Gastroenterol. 2016;111(12):1726–1735. doi: 10.1038/ajg.2016.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song BG, Min YW, Lee H et al. Clinicomanometric factors associated with clinically relevant esophagogastric junction outflow obstruction from the Sandhill high-resolution manometry system. Neurogastroenterol Motil. 2018;30(3) doi: 10.1111/nmo.13221. [DOI] [PubMed] [Google Scholar]
- Shin IS, Min YW, Rhee PL. Esophagogastric junction outflow obstruction transformed to type II achalasia. J Neurogastroenterol Motil. 2016;22(2):344–345. doi: 10.5056/jnm16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottmann F, Patti MG. Primary esophageal motility disorders: beyond achalasia. Int J Mol Sci. 2017;18(7):E1399. doi: 10.3390/ijms18071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf MHG, Chowdhary S, Elmunzer BJ, Elias PS, Castell D. Impact of peppermint therapy on dysphagia and non-cardiac chest pain: a pilot study. Dig Dis Sci. 2019;64(8):2214–2218. doi: 10.1007/s10620-019-05523-8. [DOI] [PubMed] [Google Scholar]
- Muta K, Ihara E, Fukaura K, Tsuchida O, Ochiai T, Nakamura K. Effects of acotiamide on the esophageal motility function in patients with esophageal motility disorders: a pilot study. Digestion. 2016;94(1):9–16. doi: 10.1159/000447010. [DOI] [PubMed] [Google Scholar]
- Scherer JR, Kwiatek MA, Soper NJ, Pandolfino JE, Kahrilas PJ. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13(12):2219–2225. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjoux S, Brochard C, Roman S et al. Botulinum toxin injection for hyper-contractile or spastic esophageal motility disorders: may high-resolution manometry help to select cases? Dis Esophagus. 2015;28(8):735–741. doi: 10.1111/dote.12282. [DOI] [PubMed] [Google Scholar]
- Porter RF, Gyawali CP. Botulinum toxin injection in dysphagia syndromes with preserved esophageal peristalsis and incomplete lower esophageal sphincter relaxation. Neurogastroenterol Motil. 2011;23(2):139–144. e27–e28. doi: 10.1111/j.1365-2982.2010.01604.x. [DOI] [PubMed] [Google Scholar]
- Clayton SB, Shin CM, Ewing A, Blonski W, Richter J. Pneumatic dilation improves esophageal emptying and symptoms in patients with idiopathic esophago-gastric junction outflow obstruction. Neurogastroenterol Motil. 2019;31(3):e13522. doi: 10.1111/nmo.13522. [DOI] [PubMed] [Google Scholar]
- Lin KH, Lee SC, Huang TW, Huang HK. Esophagogastric junction outflow obstruction-related functional chest pain treated using robotic-assisted thoracoscopic esophageal myotomy. J Thorac Dis. 2017;9(5):E432–E436. doi: 10.21037/jtd.2017.03.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira PF, Rosa AR, Mesquita LA et al. Esophagogastric junction outflow obstruction successfully treated with laparoscopic Heller myotomy and Dor fundoplication: first case report in the literature. World J Gastrointest Surg. 2019;11(2):112–116. doi: 10.4240/wjgs.v11.i2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filicori F, Dunst CM, Sharata A et al. Long-term outcomes following POEM for non-achalasia motility disorders of the esophagus. Surg Endosc. 2019;33(5):1632–1639. doi: 10.1007/s00464-018-6438-z. [DOI] [PubMed] [Google Scholar]
- Khashab MA, Familiari P, Draganov PV et al. Peroral endoscopic myotomy is effective and safe in non-achalasia esophageal motility disorders: an international multicenter study. Endosc Int Open. 2018;6(8):E1031–E1036. doi: 10.1055/a-0625-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum EN, Dunst CM, Reavis KM et al. Clinical outcomes five years after POEM for treatment of primary esophageal motility disorders. Surg Endosc. 2018;32(1):421–427. doi: 10.1007/s00464-017-5699-2. [DOI] [PubMed] [Google Scholar]
- Inoue H, Sato H, Ikeda H et al. Per-oral endoscopic myotomy: a series of 500 patients. J Am Coll Surg. 2015;221(2):256–264. doi: 10.1016/j.jamcollsurg.2015.03.057. [DOI] [PubMed] [Google Scholar]