Abstract
Background
Implementation of routinely collected patient-reported outcome measures (PROMs) ensures patients’ priorities are at the forefront of care planning and helps to standardize approaches to quality improvement. In palliative care, barriers to PROMs are widely known but what are not understood are the clinical and care settings in which patients are more likely to report and when proxy reporting is needed.
Objective
To examine the incidence of patient-reported symptom distress compared to the incidence of proxy reporting in palliative care and influencing factors.
Methods
A national observational study using routinely collected PROMs data with influencing factors investigated by logistic regression modelling. Participants were patients with an advanced life-limiting illness receiving palliative care in an inpatient or a community healthcare setting in Australia.
Results
Sixteen thousand one hundred and fifty-eight reports of symptom distress were collected from 1117 patients seen by 21 palliative care services. The majority of respondents were diagnosed with cancer (76%), were older (≥65 years, 72%) and had nominated English as their first language (88%). The majority of symptom distress reports were completed by patients (61%). The odds of a patient providing a self-report where grater when they were receiving community versus inpatient palliative care (odds ratio (OR): 3.0; 95% confidence interval (CI): 2.25–4.01), for patients diagnosed with malignant versus non-malignant disease (OR 1.7; 95% CI: 1.26–2.31), and for those who required an urgent change in their care plan versus those whose symptoms and problems were adequately managed (OR: 1.38; 95% CI: 1.04–1.83).
Conclusion
Three factors are associated with an increased likelihood of patient versus proxy reporting in palliative care: healthcare setting, diagnosis, and the acuity and urgency of the patient’s clinical needs. PROMs are feasible in most clinical scenarios in palliative care, including when an urgent clinical response is required.
Keywords: palliative care, patient-reported outcome measures, symptom assessment, caregivers, proxy
Introduction
Patient-reported outcome measures (PROMs) have become increasingly important in health care. Their value for quality improvement and their role in informing daily clinical care and in driving decision-making are particular advantages [1, 2]. Despite these well-recognized benefits, the national implementation of outcome measurement as part of routine care often remains elusive [3]. This gap potentially compromises the quality of health care and the health outcomes for populations and patients worldwide.
In palliative care, barriers related to the widespread use of PROMs have been identified. Commonly described barriers include the characteristics of palliative patients, staff beliefs, institutional barriers, and the psychometric and clinimetric properties of the measures available for use in palliative care [4]. It is not uncommon for patients requiring palliative care to be described as a vulnerable group who are unable to complete PROMs [4], with their decline in cognitive or physical functioning [5] and performance status [6] associated with an increased likelihood of proxy reporting.
These types of challenges to PROMs in palliative care have meant that clinicians may complete outcome measures on behalf of patients in their care, instead of asking patients. Whilst proxy reports are mostly accurate in relation to symptom distress [7, 8], clinicians tend to underestimate symptoms [6, 9] and unpaid carers [8, 10] have been found to overestimate symptoms in comparison to patient reports.
A number of demographic factors have been observed to influence the congruence between patient and proxy reports of symptom distress [7]. Unfortunately, setting of care is a rarely examined factor, meaning the influence of healthcare setting on patient versus proxy reports is largely unknown.
Whilst the available literature is useful in identifying challenges and discrepancies in PROMs, many studies have relatively small sample sizes and have been conducted with a particular focus on one patient or proxy group.
In Australia, the Palliative Care Outcomes Collaboration (PCOC) is a national palliative care initiative that focuses on patient- (and proxy-) reported outcomes. The collection and use of nationally standardized routine clinical assessment data are core to the PCOC model, which has been shown to be feasible and useful in improving outcomes and quality for patients receiving palliative care [11, 12].
The objective of our national study was to utilize the PCOC initiative and national data collection to identify and examine the incidence of patient-reported symptom distress in palliative care patients and to examine factors associated with patient versus proxy reporting for the purposes of identifying clinical scenarios in which patient reports of symptom distress are feasible. Informed by the literature, we hypothesized that both demographic (i.e. sex, age and preferred language) and clinical factors (i.e. diagnosis, the acuity and urgency of the patient’s clinical need, and performance status) would influence patient versus proxy reporting in palliative care. We also aimed to explore whether healthcare setting influenced patient-proxy reporting.
Methods
Study design
A national observational study involving analysis of routinely collected patient-reported and proxy-reported symptom distress data.
Participants and setting
Purposive sampling was used to identify eligible services that participate in PCOC and are representative of the type of inpatient and community palliative care service provision in Australia and their population catchment. This was important given that the Indigenous population constitutes approximately 3% of the population, some services have lower proportion of patients with non-malignant disease and there is variation in service models across the nation. To address this, 21 services were invited to participate.
Sample size
An analysis of throughput of patients was completed to estimate the number of palliative care assessments required. The G*power calculator [13] was used to obtain an estimate of the sample size required to detect a difference in patient reports of at least 10% points when comparing two groups. At power of 0.9 and level of significance of 0.05, a total sample size of 385 patients was identified as sufficient. To achieve the associated 95% confidence interval (CI) to be no wider than 10% points, the sample size was increased to 500 patients.
A minimum of 20 consecutive patients for each service was established to ensure meaningful results at a service level, as the primary purpose of PCOC is to drive improvements in outcomes and quality. This approach allowed for the possibility that 3–4 services might not complete the data submission and approximately 30% of data could be missing from patients that were either in a deteriorating or terminal palliative care phase. However, this approach also meant that for research purposes, we were likely to have a larger data set available for analysis. This was deemed appropriate given the purpose of PCOC.
Measures
Patient and proxy symptom distress reports were captured through the use of the PCOC Symptom Assessment Scale (SAS), which is a valid and reliable patient-reported measure able to be used by patients in a variety of palliative care settings as part of routine clinical care [14]. The scale assesses eight symptom dimensions: pain, insomnia, nausea, bowel problems, appetite problems, breathing problems and fatigue. An ‘other’ item may be added to the measure. Perceived distress is evaluated on a 0−10 numerical scale with zero representing absent distress, 1 being mild distress and 10 being the worst possible (supplementary file). In the Australian population, a moderate degree of internal consistency has been observed (Cronbach’s α of 0.62 (P < 0.001)). Test–retest reliability ranges from r = 0.84 for nausea and breathing to r = 0.94 for pain [15].
The urgency and acuity of patient need were derived through the use of the Palliative Care Phase (Phase) (See Figure 1). The Palliative Care Phase refers to a measure with moderate inter-rater reliability [16], which captures clinically relevant categories of palliative care, informed by the acuity and urgency of the patient’s need [16, 17]. Performance status was also measured at the point of care via the Australia-modified Karnofsky Performance Status (AKPS) scale, which is an 11-point scale validated to measure the patient’s performance across the dimensions of activity, work and self-care. A higher score equates to a better level of function (100 normal abilities to 0 death) [18].
Figure 1.
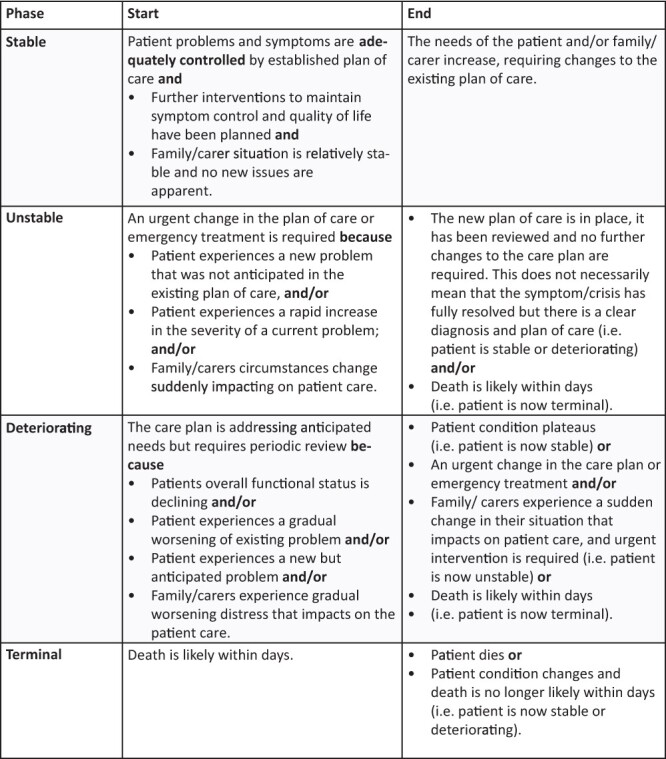
Diagnosis was captured at the point of clinical care and then collapsed into cancer versus non-malignant disease for analysis. Language and country of birth were captured using standardized questions published by the Australian Bureau of Statistics [19, 20].
Data collection
PCOC’s data set captures demographic, setting and clinical assessment information for palliative care patients. For patients in hospital, clinical assessments using the PCOC SAS, Phase and AKPS are routinely undertaken daily and at phase change. For patients at home, the assessments are collected at each encounter (e.g. each visit) and at phase change. Data are collected using paper forms that are stored with the patient’s medical record or captured directly at point-of-care using an electronic medical record (e.g. hand-held device).
At each assessment, clinicians record who rated the patient’s level of distress on the PCOC SAS (SAS rated by). A ‘patient-report’ is defined as an occasion where the patient reported on their own symptom distress. In this study, any report not classified as a patient report was treated as a report by a proxy, i.e. an occasion when an unpaid caregiver (e.g. family) or a palliative care clinician (e.g. physician, nurse and allied health) assessed and reported on the symptom distress on behalf of the patient with no input from the patient [21]. Data on patients’ socio-demographic characteristics and diagnosis are collected once at the commencement of each episode of care.
Analysis
Descriptive statistics were used to describe participants’ demographic characteristics (sex, country of birth, diagnosis and age), clinical characteristics (diagnoses and clinical assessments including the PCOC SAS, Phase and AKPS) and setting of care. This was followed by the calculation of counts and percentages of the variables of interest by patient versus proxy reporting. Logistic generalized linear modelling was used to evaluate associations between SAS rated by patient or proxy (the dependent variable) and demographic, clinical and healthcare setting variables.
Descriptive analyses were conducted using SAS 9.4 (the SAS Institute, Cary, NC, USA) for analysis. The model was developed using proc genmod [22] to accommodate the nested structure of the PCOC data set, accounting for patients having many assessments throughout their care and being treated by different palliative care services. Pre-established standards to define what was acceptable for the extent of missing items were used, with items with values of >4% deemed to be insufficient [23]. The regression analysis controlled for sex, country of birth, preferred language, Indigenous status, diagnosis, age, setting of care and phase. Records with missing data items were excluded from the logistic regression. No imputation of missing data occurred.
Results
All 21 services approached to participate in this study agreed to participate. A total of 16 158 symptom distress (PCOC SAS) assessments were collected for 1117 patients. Limited missing values were found for all items (i.e. ≤4%).
The majority of patients in this study had a cancer diagnosis (76%, n = 846), were ≥65 years (72%, n = 802) and nominated English as their preferred language (89%). A sizeable proportion of the patients were born outside of Australia (44%) (Table 1). A representative sample of the Australian palliative care population was achieved except in relation to place of birth. There was a slightly higher proportion of patients born in a country other than Australia in the sample analysed; however, approximately the same proportion preferred English as their first language (Table 1).
Table 1.
Characteristics of the patients involved in the study (N = 1117) compared to the entire PCOC cohort for the comparable time period (N = 5294)
| Study cohort (%) (N = 1117) |
PCOC cohort (%) (N = 5294) |
|
|---|---|---|
| Sex | ||
| Male | 52.9 | 51.8 |
| Female | 47.1 | 48.2 |
| Country of birth | ||
| Australia | 55.8 | 62.0 |
| Other | 44.2 | 38.0 |
| Preferred language | ||
| English | 88.8 | 90.5 |
| Other | 11.2 | 9.5 |
| Indigenous status | ||
| Aboriginal and/or Torres Strait Islander | 1.5 | 1.5 |
| Neither Aboriginal nor Torres Strait Islander | 98.5 | 98.5 |
| Life-limiting illness | ||
| Cancer diagnosis | 76.4 | 78.1 |
| Non-cancer diagnosis | 23.6 | 21.9 |
| Age | ||
| 0–34 | 1.5 | 1.1 |
| 35–44 | 3.1 | 2.2 |
| 45–54 | 7.7 | 6.9 |
| 55–64 | 15.5 | 16.5 |
| 65–74 | 25.0 | 25.9 |
| 75–84 | 26.4 | 27.1 |
| 85–94 | 18.2 | 18.2 |
| 95+ | 2.6 | 2.1 |
When comparing incidences of patient versus proxy reports, overall, 61% of symptom distress was reported by patients versus 39% reported by a proxy (Table 2). The proportion of patient reports varied by diagnosis, setting of care, performance status and palliative care phase (Table 2). Patient reports were more common for those with a malignant diagnosis (63%). Patient reports were substantially more common in the community setting (71%), compared to the proportion of patient reports completed in an inpatient setting (38%). The incidence of patient reports decreased with declining performance status and at the start of the terminal phase of care. Age-related trends were evident but were somewhat inconsistent (Table 2).
Table 2.
Characteristics of those reporting: All assessments by patient and proxy reports
| Rated by (%) | |||
|---|---|---|---|
| Assessments (N) | Patient | Proxy | |
| Type of assessments completed | 16 158 | 60.8 | 39.2 |
| Sex | |||
| Male | 8111 | 60.9 | 39.1 |
| Female | 7957 | 61.2 | 38.8 |
| Country of birth | |||
| Australia | 8770 | 62.2 | 37.8 |
| Not Australia | 7122 | 59.4 | 40.6 |
| Preferred language | |||
| English | 14 065 | 61.7 | 38.3 |
| Not English | 1814 | 54.9 | 45.1 |
| Diagnosis | |||
| Cancer diagnosis | 12 654 | 62.5 | 37.5 |
| Non-malignant diagnosis | 3396 | 44.1 | 55.9 |
| Age | |||
| 0–34 | 462 | 58.0 | 42.0 |
| 35–44 | 485 | 65.8 | 34.2 |
| 45–54 | 1318 | 66.5 | 33.5 |
| 55–64 | 2818 | 61.4 | 38.6 |
| 65–74 | 3765 | 64.0 | 36.0 |
| 75–84 | 4332 | 61.0 | 39.0 |
| 85–94 | 2699 | 53.9 | 46.1 |
| 95+ | 189 | 55.6 | 44.4 |
| Setting | |||
| Inpatient | 5101 | 38.3 | 61.7 |
| Community | 11 057 | 71.1 | 28.9 |
| Palliative care phase type | |||
| Stable | 7816 | 70.1 | 29.9 |
| Unstable | 692 | 65.3 | 34.7 |
| Deteriorating | 6271 | 58.5 | 41.5 |
| Terminal | 1368 | 15.4 | 84.6 |
| AKPS | |||
| 10 | 868 | 7.3 | 92.7 |
| 20–40 | 6270 | 45.2 | 54.8 |
| 50–60 | 6502 | 75.1 | 24.9 |
| 70+ | 2093 | 86.6 | 13.4 |
The logistic generalized linear model (Table 3) showed that diagnosis and setting of care were most strongly associated with patient reporting. Patients in the community were 3 times as likely to self-report as those in hospital (odds ratio (OR): 3.00; 95% CI: 2.25–4.01). Cancer patients were 1.7 times as likely to self-report as those with non-malignant disease (OR: 1.71; 95% CI: 1.26–2.31). Patients in an unstable phase were 1.38 times as likely to self-report than those in a stable phase (OR: 1.38; 95% CI: 1.04–1.83). Decreasing performance status was also related to decreasing odds of self-reports. Sex and preferred language were not associated with patient reporting in this study.
Table 3.
ORs and 95% CIs for factors associated with patient reports of symptom distress (versus proxy report)
| 95% CIs | ||||
|---|---|---|---|---|
| ORs | Lower | Upper | ||
| Sex | Male | 1 | ||
| Female | 1.033 | 0.816 | 1.308 | |
| Preferred language | Other | 1 | ||
| English | 0.957 | 0.583 | 1.571 | |
| Diagnosis | Non-cancer | 1 | ||
| Cancer | 1.707 | 1.264 | 2.306 | |
| Age group | 0–34 | 0.488 | 0.176 | 1.351 |
| 35–44 | 0.796 | 0.288 | 2.198 | |
| 45–54 | 0.962 | 0.386 | 2.401 | |
| 55–64 | 0.750 | 0.336 | 1.670 | |
| 65–74 | 0.880 | 0.400 | 1.934 | |
| 75–84 | 0.785 | 0.358 | 1.724 | |
| 85–94 | 0.700 | 0.321 | 1.527 | |
| 95+ | 1 | |||
| Setting of care | Inpatient | 1 | ||
| Community | 3.004 | 2.253 | 4.006 | |
| Phase | Stable | 1 | ||
| Unstable | 1.377 | 1.038 | 1.825 | |
| Deteriorating | 0.730 | 0.614 | 0.868 | |
| Terminal | 0.320 | 0.229 | 0.447 | |
| AKPS | 10 | 0.052 | 0.034 | 0.080 |
| 20–40 | 0.262 | 0.196 | 0.351 | |
| 50–60 | 0.639 | 0.507 | 0.804 | |
| 70+ | 1 | |||
Discussion
Statement of principal findings
This study is one of the first to examine patient versus proxy reporting of symptom distress using nation-wide routinely collected PROM data. We have identified three factors associated with an increased likelihood of patient versus proxy reporting in palliative care: healthcare setting, diagnosis, and the acuity and urgency of the patient’s clinical need. The implications of these findings for clinical practice means that PROMs are feasible in most clinical scenarios in palliative care, although proxy reporting is required in some scenarios, especially in inpatient settings. Importantly, PROMs are feasible across a range of clinically significant phases throughout a patient’s trajectory, although patient reporting is less likely to occur as a patient’s performance deteriorates, when a patient enters a phase of deterioration or when they begin to actively die.
Interpretation within the context of the wider literature
A major and novel finding from our study is that the acuity and urgency of patient need is associated with an increased likelihood of patient’s reporting their level of distress. This finding was surprising. We thought that patients whose symptoms were being adequately managed (i.e. patients in a stable phase) would be more likely to self-report compared to those who required urgent attention (i.e. unstable phase). However, patients assessed to be in an unstable phase were 38% more likely to self-report than those in the stable phase. This finding is contrary to the often-held belief in health care that palliative care patients are too unwell to use PROMs and that PROMs may be overly burdensome for patients to complete [4]. Our study helps debunk the myth that patients may not wish to self-report.
A second novel finding from our study is the difference in patient versus proxy reporting in inpatient and community settings, with a higher proportion of patients self-reporting in community settings. Many previous studies have been restricted by a focus on one particular healthcare setting, thus precluding comparisons across settings. Reasons for this observed difference are likely to be multi-factorial. Community patients have higher functional and performance statuses compared to inpatients [24] and patient report is associated with higher AKPS scores (i.e. higher performance status across the dimensions of activity, work and self-care). In contrast, inpatients in the Australian context tend to have poorer functional status and performance and are more likely to end their episode of palliative care in death (i.e. 54% of patients die in inpatient settings compared to 25% of patients receiving care in the community) [24].
A third key finding from our study is clarification of the characteristics of patients for whom proxy reporting may be more likely. Two key characteristics associated with increased proxy reporting is low performance status and deteriorating or terminal phase. This is when the patient is no longer able to complete all care tasks independently (AKPS ≤40) and when they have increased care needs or are actively dying. This finding aligns with results from other studies that have identified an association between performance status and proxy reporting [6] and may be used to help guide improvements in practice in palliative care settings. For example, auditing of the use of PROMs by patients’ performance status and palliative care phase may help identify areas to target to increase the use of PROMs in practice. Our study also showed that older old adults (95+ years) can use PROMs.
Finally, we found that patients with a primary cancer diagnosis, compared to those with a non-malignant diagnosis, were 1.7 times as likely to self-report. In advanced disease, cognitive impairment may limit the patient’s ability to self-report [25]. Neurological disease accounts for 28% of all non-malignant diagnoses in the national PCOC population [24], helping to substantiate this finding. Those with partial cognitive impairment may be able to self-report but patients who are unable to communicate verbally or using gestures will usually require reporting by proxy [26].
Implications for policy, practice and research
Our findings suggest patient-reported symptom distress measurement is feasible in most palliative care scenarios, especially in community healthcare settings. Along with other routine clinical assessment measures, the PCOC SAS is successfully implemented as a routine measure of patient-reported symptom distress throughout Australia. Their national implementation offers information about the symptoms that are most important to patients. This information at scale can be used to drive and inform policy, ensuring that what matters to patients is prioritized. Our study demonstrates that patient-reported symptom distress measures can be used in urgent palliative care clinical scenarios, regardless of setting of care and regardless of age. Further research is required to identify the particular characteristics and specific reasons for the need for proxy reporting in patients with non-malignant diagnoses.
Strengths and limitations
The embedding of PROMs into routine clinical care and quality improvement is core to PCOC. The integration of this national initiative into healthcare systems is likely to influence the feasibility of routinely collected PROMs, as well as the incidence of patient reporting. Although the PCOC model is currently being adopted in other national settings, including countries within the European and the Western Pacific regions, the findings may not be generalizable to all palliative care populations and countries. Nevertheless, our sample size is substantial and one of the larger applied health services’ research studies completed on this topic within palliative care. A sampling bias may have been introduced through the use of purposive sampling of the services. This was countered by the variation between the participating services, and the sample analysed was very similar to the broader PCOC population.
Secondary diagnosis data were not collected in our study. This information would have allowed analysis of the effect of neurological disorders which may affect the capacity of patients to self-report. The role of the caregiver on patient reports was not investigated, nor was the validity of the choice to complete a proxy report over a patient report. These limitations provide an indication of areas for future research.
Conclusions
In patients with advanced, life-limiting illness, patient reporting of symptom distress is feasible in most palliative care scenarios, including when an urgent clinical response is required. The urgency of the needs of patients should not limit opportunities to self-report, especially as these self-reports may be used as an early warning sign of the need for an urgent response. Proxy reporting is needed when patients are in the deteriorating and terminal phases. The reasons why patients in stable phases may have increased likelihood of proxy reports requires investigation, and further examination of the associations between diagnosis and PROMs may be useful.
Supplementary Material
Acknowledgements
The authors would like to thank the participating clinicians involved in assessing patients. The palliative care services in this study were: Clare Holland House (Calvary Canberra), Calvary Health Care Kogarah, Hammondcare—Greenwich Hospital, Hastings Macleay Com-munity Palliative Care Service, Mercy Health Service Albury, Port Kembla Hospital, Concord Centre for Palliative Care, Karuna Hospice Services, Wesley Private Hospital, Central Adelaide Palliative Service, Northern Adelaide Palliative Service, Melbourne Citymission Palliative Care, St Vincent’s Caritas Christi Hospice, St Vincent’s Hospital, Melbourne, Bethesda Hospital, Silver Chain Hospice Service, St John of God—Murdoch Community Hospice. The authors acknowledge Nesa Mossamet, BSc (Statistics), MSc (Statistics), PhD Honorary Research Fellow (Applied Statistics) for her assistance in preparation of the initial data set.
Contributor Information
Sabina Clapham, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Barbara A Daveson, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Samuel F Allingham, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Darcy Morris, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Pippa Blackburn, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Claire E Johnson, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Kathy Eagar, Palliative Care Outcomes Collaboration, Australian Health Services Research Institute, University of Wollongong, Building 234 (iC Enterprise 1), Wollongong, NSW 2522, Australia.
Supplementary material
Supplementary material is available at International Journal for Quality in Health Care online.
Funding
The project is funded by the Australian Government Department of Health. The views expressed in this paper do not necessarily reflect the views of the funder. The funder had no role in the design of the study, the analysis or preparation of the manuscript.
Data availability
PCOC is a mature and well-established national palliative care program. Researchers may apply for access to PCOC’s routinely collected, de-identified and aggregated outcome data.
Ethics
Ethical approval was granted by the University of Wollongong and Illawarra Shoalhaven Local Health District Health and Medical Human Research Ethics Committee (reference no. HE2006/045). This involved an amendment to the original PCOC ethics application to ensure approval of the analysis of patient and proxy data, which was not routinely submitted to PCOC although routinely documented by some services. As only routinely collected, de-identified, aggregated clinical data were used in this study, separate participant consent was not necessary.
References
- 1. Antunes B, Rodrigues PP, Higginson IJ et al. Outcome measurement—a scoping review of the literature and future developments in palliative care clinical practice. 2018;S196–206. [DOI] [PubMed] [Google Scholar]
- 2. Øvretveit J, Zubkoff L, Nelson EC et al. Using patient-reported outcome measurement to improve patient care. Int J Qual Health Care 2017;29:874–9. [DOI] [PubMed] [Google Scholar]
- 3. Morrison RS. A national palliative care strategy for Canada. J Palliat Med 2018;21:S63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bausewein C, Simon ST, Benalia H et al. Implementing patient reported outcome measures (PROMs) in palliative care—users’ cry for help. Health Qual Life Outcomes 2011;9:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack B, Ellershaw JE, Murphy D. A comparison of cancer patients and nurse specialist’s symptom-assessment scores, in an acute hospital. Eur J Oncol Nurs 2003;7:130–1. [DOI] [PubMed] [Google Scholar]
- 6. Laugsand EA, Sprangers MAG, Bjordal K et al. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes 2010;8:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kristjanson LJ, Nikoletti S, Porock D et al. Congruence between patients’ and family caregivers’ perceptions of symptom distress in patients with terminal cancer. J Palliat Care 1998;14:24. [PubMed] [Google Scholar]
- 8. Milne DJ, Mulder LL, Beelen HC et al. Patients’ self-report and family caregivers’ perception of quality of life in patients with advanced cancer: how do they compare? Eur J Cancer Care (Engl) 2006;15:125–32. [DOI] [PubMed] [Google Scholar]
- 9. Miyashita M, Yasuda M, Baba R et al. Inter-rater reliability of proxy simple symptom assessment scale between physician and nurse: a hospital-based palliative care team setting. Eur J Cancer Care (Engl) 2010;19:124–30. [DOI] [PubMed] [Google Scholar]
- 10. Sebring K, Shattuck J, Berk J et al. Assessing the validity of proxy caregiver reporting for potential palliative care outcome measures in Parkinson’s disease. Palliat Med 2018;32:1522–8. [DOI] [PubMed] [Google Scholar]
- 11. Currow DC, Allingham S, Yates P et al. Improving national hospice/palliative care service symptom outcomes systematically through point-of-care data collection, structured feedback and benchmarking. Support Care Cancer 2015;23:307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Currow DC, Eagar K, Aoun S et al. Is it feasible and desirable to collect voluntarily quality and outcome data nationally in palliative oncology care? J Clin Oncol Off J Am J Clin Oncol 2008;26:3853–9. [DOI] [PubMed] [Google Scholar]
- 13. Faul F, Erdfelder E, Lang A-G et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- 14. Daveson BA, Allingham S, Clapham S et al. The PCOC Symptom Assessment Scale (SAS): a valid measure for daily use at point of care and in palliative care programs. Technical report. PLoS ONE 2021;16:e0247250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aoun SM, Monterosso L, Kristjanson LJ et al. Measuring symptom distress in palliative care: psychometric properties of the Symptom Assessment Scale (SAS). J Palliat Med 2011;14:315–21. [DOI] [PubMed] [Google Scholar]
- 16. Masso M, Allingham SF, Banfield M et al. Palliative care phase: inter-rater reliability and acceptability in a national study. Palliat Med 2015;29:22–30. [DOI] [PubMed] [Google Scholar]
- 17. de Wolf-linder S, Dawkins M, Wicks F et al. Which outcome domains are important in palliative care and when? An international expert consensus workshop, using the nominal group technique. Palliat Med 2019;33:1058–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abernethy AP, Shelby-James T, Fazekas BS et al. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care 2005;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Australian Bureau of Statistics (ABS) Country of Birth Standard . 2011. https://www.abs.gov.au/statistics/standards/country-birth-standard (14 August 2020, date last accessed) .
- 20. Australian Bureau of Statistics (ABS) Australian Standard Classification of Languages . 2011. https://www.abs.gov.au/ausstats/abs@.nsf/Latestproducts/1267.0Main%20Features12016?opendocument&tabname=Summary&prodno=1267.0&issue=2016&num=&view=. (14 August 2020, date last accessed).
- 21. Clapham S, Connolly A. Clinical Manual Wollongong. Australia: Palliative Care Outcomes Collaboration, 2014. https://www.uow.edu.au/ahsri/pcoc/palliative-care/clinical-protocol/ (14 August 2020, date last accessed). [Google Scholar]
- 22. SAS Institute Inc. SAS/STAT® 14.2. User’s Guide High-Performance Procedures. Cary, NC: SAS Institute Inc, 2016. [Google Scholar]
- 23. Fayers PM, Machin D. Scores and measurements: validity, reliability, sensitivity. In: Quality of Life: The Assessment, Analysis and Interpretation of Patient Reported Outcomes. 2nd edn. Chichester: John Wiley & Sons, 2007:77–107. [Google Scholar]
- 24. Allingham S, Burns S, Foskett L et al. Outcomes in palliative care in Australia: national report for July – December 2019. University of Wollongong, 2020. [Google Scholar]
- 25. Howland M, Allan KC, Carlton CE et al. Patient-rated versus proxy-rated cognitive and functional measures in older adults. Patient Relat Outcome Meas 2017;8:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGuire DB, Reifsnyder J, Soeken K et al. Assessing pain in nonresponsive hospice patients: development and preliminary testing of the Multidimensional Objective Pain Assessment Tool (MOPAT). J Palliat Med 2011;14:287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
PCOC is a mature and well-established national palliative care program. Researchers may apply for access to PCOC’s routinely collected, de-identified and aggregated outcome data.


