Abstract
Background:
Osteoporosis (OP) is a silent systemic disease characterized by decrements in bone mineral density (BMD) and bone microstructure. This study aimed to determine the status of bone quality as well as to investigate the relationship between the glycaemic; lipid; bone profiles; and the BMD.
Methods:
A retrospective study was conducted at KFHU, Al Khobar, Saudi Arabia analysis of patients who underwent BMD testing between the periods of 2016 to 2018. Based on the T scores, patients were classified as follows: normal (>−1.0), osteopenic (−1.0 to −2.5), or osteoporotic (≤−2.5). Details about medical and demographic information as well as metabolic and bone profiles (fasting blood glucose [FBG], glycated haemoglobin [HbA1c], cholesterol [Chol], high-density lipoprotein [HDL], low-density lipoprotein [LDL], triglycerides [TG], calcium [Ca], phosphorus [Ph], alkaline phosphatase [ALP], vitamin D 25OHD [Vit D]) were extracted from the medical records system.
Results:
Out of 4838 extracted profiles, there were 4196 females (85.1%). The mean glycaemic variables of patients showed an abnormal profile (FBG 118 ± 49 and HbA1c 6.6 ± 2). The mean T score showed that the majority of patients had either osteopenic (40%) or osteoporotic (24%) changed. Significant increase in mean HbA1c (7.6 ± 1.7) was obvious among the osteopenic patients when judged against the normal (7.5 ± 1.6; P < 0.033) and osteoporotic (7.4 ± 1.8; P < 0.037). Meanwhile, the mean serum ALP was significantly lower (81 ± 26) in the normal group than in the osteopenic (86 ± 33; P < 0.006) and osteoporotic groups (90 ± 40; P < 0.001). Finally, a linear, logistic regression analysis was found that Ca and ALP levels were significant predictors.
Conclusion:
This study finds that the main cause that affects bone quality in Saudi Arabia is diabetes mellitus and/or its related metabolic alteration. These results suggest that bone health is clinically significant and should be carefully assessed in diabetes patients.
Keywords: Alkaline phosphatase, bone mineral density, calcium, glycaemic profile, osteoporosis
Introduction
Osteoporosis (OP) is a systematic chronic metabolic skeletal disorder characterized by decrement in bone mineral density (BMD) and bone microstructure that conferring a fracture burden.[1] Unfortunately, OP is not diagnosed until a fracture occurs and prior to that it exists as a silent disease “because routine spinal radiographs are not a useful method of detecting osteoporosis until 30% of the bone is lost“. According to the recommendations of the National Osteoporosis Foundation, the diagnosis of OP is based on quantification of BMD by Dual-Energy X-ray Absorptiometry (DEXA) of the forearm, spine and hip“with T-score of ≤−2.5“.[2]
OP and/or its associated fractures are considered a public health problem globally. According to published data, over 200 million people worldwide have OP, consequently, responsible for near nine million fractures per year.[3] In Saudi Arabia, a previous report (2018) showed that 37.8% of women and 28.2% of Saudi men aged 50–79 years are osteoporotic.[4]
There are very few reports available on the risk factors of OP in Saudi Arabia.[5] Therefore, this studied to investigate the relationship between the glycaemic profile (glycated haemoglobin [HbA1c], fasting blood glucose [FBG]); lipid profile (cholesterol [Chol], low-density lipoprotein [LDL], high-density lipoprotein [HDL], triglycerides [TG]);bone profile (calcium [Ca], phosphorus [Ph], vitamin D 25OHD[Vit D], alkaline phosphatase [ALP]); and the BMD Tscoresin the spine and hip regions of patients in King Fahd University Hospital (KFHU).
Materials and Methods
The present study was a retrospective (hospital-based) analysis of adult old patients who have undergone BMD testing using DEXA scan during the period of 2016–2018 at KFHU, Al Khobar.[6]
Ethical approval for this study was obtained from the institutional review board (IRB-2018-01-257) of Imam Abdulrahman Bin Faisal University, Saudi Arabia.
Data were collected using the medical recording software system (PACS, General Electric GE, Massachusetts, Boston, United States).
We used DEXA (Discovery QDR Series; Hologic, Marlborough, MA, USA) with Discovery QDR Series (Asian) software (Hologic) to assess BMD. Assessment provided data on T scores and data were interpreted according to the World Health Organization (WHO). T scores represented the number of standard deviations above or below the average BMD, and they were expressed in g/cm2. Based on the T scores, patients were classified as follows: [normal (>−1.0), osteopenic (−1.0 to −2.5), orosteoporotic (≤−2.5)]. According to the WHO, an increase in T-score indicates a worsening bone condition, i.e., the more negative the score is, the worse the condition.[7]
Details about demographic and medical data, including contact information, age, sex, inpatient hospitalization, visits to outpatient clinic, medications, and diagnosis as well as laboratory results (FBS, HbA1c, Lipid profile, Ca, Ph, Vit D and ALP) were extracted from the medical records system (QuadrMed). All laboratory information was acquired according to standard operating laboratory procedures.
Patients were included if they had undergone a DEXA scan for the lumbar spine and hip and were between the ages of 18 and 90 years.
Statistical analysis
Analysis was performed using IBM SPSS 26® software (IBM Corp., Armonk, NY, USA). A descriptive analysis was used to determine mean ± standard deviation (SD) and a Chi-squared (χ2) test was used for categorical variables. Finally, a linear, logistic regression analysis was performed to predict the influence of the independent variables on the T score in order to explore the relationships among all factors. Data are presented as 95% confidence intervals (CIs), and P < 0.05 was considered to be statistically significant.
Results
Out of 4838 extracted profiles, there were 4196 females (85.1%), as shown in Figure 1. The characteristics of patients are shown in Table 1. The mean age was 58.1 ± 13 years. The glycaemic variables showed an abnormal profile (FBG 118 ± 49 and HbA1c 6.6 ± 2).
Figure 1.

Sex distribution (n)
Table 1.
Characteristics of the sample
| Mean | SD | |
|---|---|---|
| Age (years) | 58.1 | 13 |
| FBG (mg/dL) | 118.4 | 49 |
| HbA1c | 6.6 | 2 |
| Chol (mg/dL) | 185.6780 | 42 |
| HDL (mg/dL) | 55.4294 | 15 |
| LDL (mg/dL) | 110.5124 | 35 |
| TG (mg/dL) | 111.2785 | 57 |
| Ca (mg/dL) | 9.1077 | 0.5 |
| Ph (mg/dL) | 3.8642 | 0.7 |
| ALP (IU/L) | 82.0086 | 29 |
| VitD (ng/mL) | 28.0140 | 13 |
SD=standard deviation, FBG=fasting blood glucose, Chol=cholesterol, HDL=high-density lipoproteins, LDL=low-density lipoproteins, TG=triglycerides, Ca=Calcium, ALP=alkaline phosphatase, Vit D=vitamin D 25OHD, IU=international unit, ng=nanogram
On the basis of the mean T score, the majority of patients had either osteopenic (40%) or osteoporotic (24%) changes [Figure 2], and as expected, we found that females had significantly lower T scores compared to males [Figure 3]. Moreover, the mean T score percentage in females was significantly higher in osteopenic (93.1/46.7) and osteoporotic groups (24.1/25.4) than the normal group (36.7/27.8), as shown in Figure 3.
Figure 2.
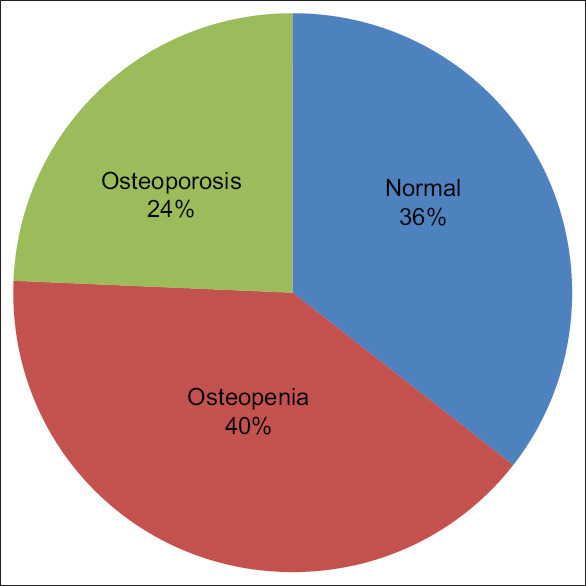
Bone status in all patients (%)
Figure 3.
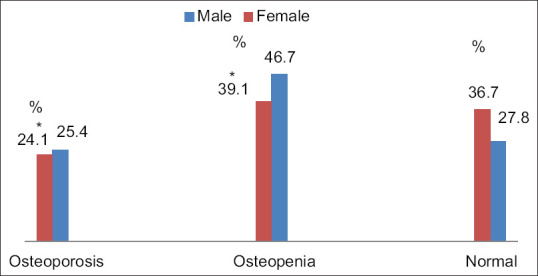
T score for males and females (%)
The data obtained from the study were tabulated, compiled, and subjected to further statistical analysis. Patients were divided into three groups based on their mean T score. The osteopenic group had a significantly higher mean HbA1c (7.6 ± 1.7) than the normal (7.5 ± 1.6; P < 0.033) and osteoporotic (7.4 ± 1.8; P < 0.037) groups [Table 2]. Meanwhile, the mean serum ALP was significantly lower (81 ± 26) in the normal group than in the osteopenic (86 ± 33; P < 0.006) and osteoporotic groups (90 ± 40; P < 0.001), as shown in Table 2.
Table 2.
Age, glycaemic profile, and bone profile of the patients based on the mean T score groups
| Normal Mean±SD (P) | Osteopenia Mean±SD (P) | Osteoporosis Mean±SD (P) | |
|---|---|---|---|
| Age | 56±12 (<0.001 b) (<0.001 c) | 59±13 | 60±14 |
| FBG | 140±56 | 143±64 | 152±75 |
| HbA1C | 7.5±1.6 (NS) | 7.6±1.7 (0.033 a) (0.037 c) | 7.4±1.8 |
| Chol | 176±49 | 175±46 | 179±49 |
| HDL | 53±13 | 53±16 | 52±16 |
| LDL | 102±35 | 100±30 | 106±40 |
| TG | 125±57 | 124±60 | 123±59 |
| Ca | 9.0±0.4 | 9.1±0.5 | 9.1±0.6 |
| Ph | 3.8±0.7 | 3.9±0.8 | 3.8±0.7 |
| ALP | 81±26 0.006 b 0.001 c | 86±33 | 90±40 |
| Vit D | 27.5±13 | 29.6±15 | 28.6±13 |
a. Significantly higher than normal group. b. Significantly higher than osteopenic group. c. significantly higher than osteoporotic group. SD=standard deviation, FBG=fasting blood glucose, Chol=cholesterol, HDL=high-density lipoproteins, LDL=low-density lipoproteins, TG=triglycerides, Ca=Calcium, ALP=alkaline phosphatase, Vit D=vitamin D 25OHD, IU=international unit, ng=nanogram
There was a significant abrupt loss of bone in the osteopenic and osteoporosis groups observed after the age of 59 ± 13 (p = 0.006) and 60 ± 14 (p = 0.001) years, respectively, whereas a normal mean T-score was found in the 56 ± 12 years age group. Meanwhile, the mean lipid profile (chol, HDL, LDL, and TG), Ca, Ph and Vit D did not differ significantly in the three categories [Table 2].
When the T score was predicted, it was found that Ca (beta = –0.113, P < 0.017) and ALP (beta = 0.30, P < 0.002) levels were significant predictors. The overall model fit was (F[15.7] = 2.193, P < 0.006), with an R2 of 0.042 [Table 3].
Table 3.
Predictors of the measured T score identified by multiple linear regression
| β* (95% CI) | P | |
|---|---|---|
| Sex | -0.118 (-0.264; 0.028) | 0.112 |
| Address | 0.012 (-0.022; 0.046) | 0.482 |
| DX | 0.036 (-0.071; -0.142) | 0.511 |
| TDM | 0.002 (-0.015; 0.018) | 0.848 |
| THAT | 0.003 (-0.031; 0.037) | 0.866 |
| FBG | 0.001 (0.000; 0.002) | 0.247 |
| HbA1C | -0.003 (-0.042; 0.035) | 0.861 |
| Chol | 0.001 (-0.002; 0.004) | 0.674 |
| HDL | -0.003 (-0.008; 0.002) | 0.258 |
| LDL | 0.002 (-0.002; 0.005) | 0.332 |
| TG | -0.001 (-0.002; 0.000) | 0.057 |
| Ca | -0.113 (-0.207; -0.020) | 0.017 |
| Ph | -0.013 (-0.083; 0.057) | 0.715 |
| ALP | 0.300 (0.100; 0.400) | 0.002 |
| Vit D | 0.003 (-0.001; 0.007) | 0.102 |
CI=confidence interval’ SD=standard deviation, FBG=fasting blood glucose, Chol=cholesterol, HDL=high-density lipoproteins, LDL=low-density lipoproteins, TG=triglycerides, Ca=Calcium, ALP=alkaline phosphatase, Vit D=vitamin D 25OHD, IU=international unit, ng=nanogram
Discussion
The findings of this study showed an abnormal glycaemic profile of all the studied categories and higher prevalence of osteopenia, as well as loss of bone minerals in osteopenic and osteoporotic groups. Moreover, by using multiple logistic regression analysis tests, serum ALP and Ca were found to be the independent predictors of osteopenia and osteoporosis.
The female patients represented a larger proportion of the group. This may be due to the practice of performing a DEXA scan for females in the >65 years age group, as they are perceived to be more prone to having lower BMDs after menopause.[8]
Vit D is an essential hormone in the maintenance of optimal bone health. The results of the present work showed no significant effect of Vit D on T scores in males or females. This finding is consistent with prior Saudi study,[9] which can be attributed to the achievement of an effective local Vit D screening protocol.[6]
Due to the significant correlation between high glycaemic profile and normal serum Vit D, we emphasize that diabetes mellitus could be considered as a potential cause of bone loss. Diabetes mellitus was present as a co-morbid condition in most of the affected patients as well as the normal T score population, a pattern that has been noted in many epidemiological studies.[10]
Progression of type 2 diabetes and low levels of insulin may cause reductions in BMD through several mechanisms. Hyperglycaemia can generate advanced glycation end products that may stimulate apoptosis of osteoblasts and interact with bone, resulting in bone loss in patients with diabetes.[10,11] Furthermore, microvascular disturbance in diabetes mellitus leads to impaired blood flow to bone and the metabolic disturbance of diabetes can cause hypercalciuria, leading to decreased levels of Ca in the extracellular fluid, and this may contribute to poor bone quality and bone fragility, thus interfering with bone mineral metabolism and potentially altering the BMD.[12]
Some medications used to treat diabetic-related sequences, such as calcium channel blockers[13] and dipeptidyl peptidase-4 inhibitors,[14] have been found to possibly affect BMD. Other external factors can affect the BMD. Sedentary lifestyle, body mass index, smoking, female sex, drugs, alcohol, caffeine consumption, low calcium intake, lack of sun exposure, non-O blood group, and low sex hormones were associated with an increased severity of OP.[15,16]
Among the Middle East and North Africa Region, Saudi Arabia has the fourth highest number of people with diabetes (17.7%).[17] A previous study performed in the Eastern province of Saudi Arabia addressing this topic did not find any relationship between diabetes and the incidence of osteoporosis or osteopenia. However, this study was limited to one sex and had a smaller sample size.[18]
Studies have shown that diabetes mellitus is one of the fastest growing health problems in Saudi Arabia, due to rapid economic growth, which has led to the adoption of a Westernized lifestyle described as “increased consumption of junk foods and sugar rich beverages“. Simultaneously, technological advances, such as elevators, escalators, cars, and remotes, have led to a decrease in the level of physical activity.[19,20] The International Diabetes Federation predicts that “approximately one in four adults in Saudi Arabia will have diabetes by 2045“.[17]
Bone-specific ALP isoenzyme is a protein found in hypertrophic chondrocytes in the epiphyseal growth plate, in mature osteoblasts and in calcifying matrix vesicles. It is of an important diagnostic value in many disorders including metabolic bone disease.[21] ALP is an essential factor needed for the synthesis and mineralization bone and elevated as a result of increased osteoblastic activity due to increased bone turnover.[21]
Detection of the early bone changes using ALP as a simple biochemical predictor holds the management of OP and can be a good indicator that reflects the efficiency of management's key for OP.[22] Our results showed that ALP a major predictor and reflected the bone quality of studied patients, along with Ca.
According to recent reports found that primary care physicians perceive OP as a silent disorder and that is obscure by several health problems as well as it receives less attention manage.[23,24] The present study will help ensure that primary care physicians take the needed responsibility and for following up bone quality of osteoporotic patients.
A simple screening blood tests for ALP and Ca to old patients can improve the early detection of the osteopenia and osteoporosis and prevent the unlikely outcomes of OP.
Conclusion
Every diabetic patient should be screened for bone health and OP at a young age, according to the guidelines established by The International Society for Clinical Densitometry and The National Osteoporosis Foundation. Early diagnosis and appropriate management of low BMD are highly recommended in Saudi Arabia, especially among those with high-risk factors, including diabetic patients, women, patients with high ALP and patients with low Vit D levels and low calcium intake.
Limitations
There were some limitations to the present study. Firstly, it was a hospital-based study conducted on a specific population, and therefore, it cannot be generalized with the same confidence. No other bone biomarkers were studied. In addition, no multivariate analysis was carried out to evaluate the effects of other confounding factors, such as lifestyle and environmental factors, on BMD. In addition, only a single measurement of all variables was performed.
Financial support and sponsorship
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Marcucci G, Brandi ML. Rare causes of osteoporosis. Clin Cases Miner Bone Metab. 2015;12:151–6. doi: 10.11138/ccmbm/2015.12.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of the Surgeon General (US) Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville (MD): Office of the Surgeon General (US); 2004. PMID: 20945569. [PubMed] [Google Scholar]
- 3.Sozen T, Ozisik L, Calik Basaran N. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4:46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abdulaziz Alomary S, El Mourad Senior Advisor M, Al Najjar Y, et al. National plan for osteoporosis prevention and management in the Kingdom of Saudi Arabia 2018 Saudi Osteoporosis Society-SoS. 2018 [Google Scholar]
- 5.Al-Hariri M, Aldhafery B, Affatato S. Association of Hypertension and Lipid Profile with Osteoporosis. Scientifica (Cairo) 2020;2020:7075815. doi: 10.1155/2020/7075815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadat-Ali M, Almomen AW, AlOmar HK, AlAlwan SA, Gullenpet AH, FM The current issues on osteoporosis among male Saudi Arabians. J Mens health. 2017:13. doi: 10.22374/1875-6859.13.2.7. [Google Scholar]
- 7.Kanis JA, Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report? Osteoporos Int. 1994;4:368–81. doi: 10.1007/BF01622200. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 8.Gourlay ML, Overman RA, Ensrud KE. Bone density screening and re-screening in postmenopausal women and older men. Curr Osteoporos Rep. 2015;13:390–8. doi: 10.1007/s11914-015-0289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhenizan A, Mahmoud A, Hussain A, Gabr A, Alsoghayer S, Eldali A. The relationship between 25 (oh) d levels (vitamin d) and bone mineral density (bmd) in a saudi population in a community-Based Setting. PLoS One. 2017;12:e0169122. doi: 10.1371/journal.pone.0169122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Hariri M. Sweet bones: The pathogenesis of bone alteration in diabetes? J Diabetes Res. 2016;2016:6969040. doi: 10.1155/2016/6969040. doi: 10.1155/2016/6969040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40:345–53. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogt MT, Cauley JA, Kuller LH, Nevitt MC. Bone mineral density and blood flow to the lower extremities: The study of osteoporotic fractures. J Bone Miner Res. 1997;12:283–9. doi: 10.1359/jbmr.1997.12.2.283. [DOI] [PubMed] [Google Scholar]
- 13.Ağaçayak KS, Güven S, Koparal M, Güneş N, Atalay Y, Atılgan S. Long-term effects of antihypertensive medications on bone mineral density in men older than 55 years. Clin Interv Aging. 2014;27;9:509–13. doi: 10.2147/CIA.S60669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: A meta-analysis of randomized clinical trials. Diabetes Care. 2011;34:2474–6. doi: 10.2337/dc11-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu BB, Li KH. Association between ABO blood groups and osteoporosis severity in Chinese adults aged 50 years and over. J Int Med Res. 2011;39:929–33. doi: 10.1177/147323001103900327. [DOI] [PubMed] [Google Scholar]
- 16.Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. 2018;14:2029–49. doi: 10.2147/TCRM.S138000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Federation ID. IDF Diabetes Atlas Eighth edition 2017 [Google Scholar]
- 18.Al-Elq AMH, Sadat-Ali M. Diabetes mellitus and male osteoporosis? Is there a relationship. Saudi Med J. 2006;27:1729–33. [PubMed] [Google Scholar]
- 19.Fareed M, Salam N, Khoja A, Abdulrahman M. Life style related risk factors of type 2 diabetes mellitus and its increased prevalence in Saudi Arabia: A brief review. Heal Sci. 2017;6:125–32. [Google Scholar]
- 20.Alotaibi A, Perry L, Gholizadeh L, Al-Ganmi A. Incidence and prevalence rates of diabetes mellitus in Saudi Arabia: An overview. J Epidemiol Glob Health. 2017;7:211–8. doi: 10.1016/j.jegh.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arjumand S, Tabassum H, Ali MN, Al-Jameil N. Seasonal variation in status of vitamin-D, serum bone profile and thyroid function in adult population of Saudi Arabia. Biomed Res. 2016;27:1385–9. [Google Scholar]
- 22.Kuo TR, Chen CH. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark Res. 2017;5:18. doi: 10.1186/s40364-017-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salminen H, Piispanen P, Toth-Pal E. Primary care physicians' views on osteoporosis management: A qualitative study. Arch Osteoporos. 2019;14:48. doi: 10.1007/s11657-019-0599-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCloskey E, Rathi J, Heijmans S, Blagden M, Cortet B, Czerwinski E, et al. The osteoporosis treatment gap in patients at risk of fracture in European primary care: a multi-country cross-sectional observational study. Osteoporos Int. 2020 doi: 10.1007/s00198-020-05557-z. doi: 101007/s00198-020-05557-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


