Abstract
Wernicke's encephalopathy (WE) is an acute neurological condition characterized by the triad of ophthalmoparesis with nystagmus, ataxia, and global confusion. WE is a life-threatening illness caused by thiamine deficiency, primarily affecting the peripheral and central nervous systems. Thiamine deficiency is predominantly associated with chronic alcoholism, but various other causes have also been reported, including severe malnutrition, prolonged parenteral nutrition, malignancies, immunodeficiency syndromes, liver disease, hyperthyroidism and severe anorexia nervosa, and hyperemesis gravidarum. We, hereby, report a unique case of WE induced by hyperemesis gravidarum that presented in mid-trimester of pregnancy in a rather extremely unusual way with focal seizures and secondary generalization but fortunately ended up with a good feto-maternal outcome.
Keywords: Epilepsy, hyperemesis gravidarum, pregnancy, seizures, thiamine, Wernicke's encephalopathy
Introduction
Wernicke's encephalopathy (WE) is a potentially reversible acute neurological disease resulting from thiamine deficiency, characterized by the classical triad of ataxia, confusion, and ophthalmoplegia. Although typically observed among chronic alcoholics, it can be also associated with hyperemesis gravidarum (HG), malnutrition, malignancy, gastrointestinal disorders and its surgery, chronic kidney disease, thyrotoxicosis, anorexia nervosa, organ transplantation, or total parenteral nutrition.[1] WE is still a frequently under-diagnosed condition in pregnancy and is often masquerade as devastating neurological diseases like autoimmune encephalitis, pituitary apoplexy, cerebral venous thrombosis, ischemic stroke, neuroinfection, etc., In WE secondary to HG, thiamine deficiency results from the inability of existing body stores to meet heightened metabolic demands during pregnancy as well as thiamine stores being depleted owing to poor consumption or excessive vomiting.[2] We report an unusual case of WE complicating HG, presenting to us with seizures in the mid-trimester of her pregnancy.
Case History
A 20-year-old woman (gravida 2, parity 0) presented to the emergency department in the 16th week of her second pregnancy with a chief complaint of recurrent convulsions and altered sensorium for 1 day. Her mother reported that she had odd eye movements and drooping of eyelids for the last 2 weeks. For the last 1 month, she also had developed an insidious onset, gradually progressive gait disturbance needing the support of the walls while going for her daily needs. This was also associated with the lady having lost all her interests in her daily activities, utter indifference, and refusal to talk to her near and dear ones for 1 month. Her family also reported that she had been telling incongruent stories that never seemed to have happened and experiencing difficulties in recollecting recent events. She had a history of persistent uncontrollable nausea, vomiting, severe anorexia, and weight loss since the 4th week of gestation. Vomiting was chiefly post-prandial, occurred nine to ten times daily, was non-projectile, and could not be satisfactorily relieved by any medication. There was no history of any addiction or previous psychiatric or neurologic illness. Her previous obstetric history revealed excessive vomiting in her previous pregnancy and a subsequent mid-trimester fetal loss. Her medication comprised iron and calcium tablets along with some anti-emetics she took from her antenatal clinic.
On examination, the patient presented with focal seizures affecting the entire left side of the body with secondary generalization. She was afebrile, dehydrated, tachycardiac, tachypneic, normotensive, and euglycemic. On neurological examination, she was drowsy, disoriented to time, place, and person (Glasgow Coma scale score [GCS]: E2M5V2) without any signs of meningeal irritation. Both pupils were equally and sluggishly reacting to light. All her deep tendon reflexes were depressed. Plantar responses were bilaterally extensor. Fundoscopy did not show any papilledema. A review of other systems was essentially normal. She was immediately given intravenous lorazepam (4 mg slowly, followed by repeat dose after 10 min) which terminated the ictus. Besides, she was put on levetiracetam 500 mg twice daily intravenously. She was also prescribed intravenous fluids (0.9% sodium chloride and Hartmann's solution) and intravenous ondansetron 4 mg thrice-a-day.
Routine blood reports (complete hemogram, serum electrolytes, urea, creatinine, lipid profile, urinary examination, liver and thyroid function tests, viral serology, blood culture, beta-human chorionic gonadotropin) were normal except mild hyponatremia (serum sodium- 130 mEq/L). Cerebrospinal fluid study, antinuclear antibody, antiphospholipid antibody profile, autoimmune encephalitis profile, syphilis serology, serum ceruloplasmin, and 24-h urinary copper excretion levels were within the normal range. Repeated ultrasonographies for fetoplacental profile and anomaly scans were done and they all showed a single live fetus without any anomaly.
Electroencephalography revealed the normal study. Contrast-enhanced magnetic resonance imaging (MRI) revealed hyperintensity at bilateral dorsomedial thalami, bilateral basal ganglia [Figure 1], peri-aqueductal region [Figure 2], and mammillary bodies [Figure 3] along with white matter based hyperintense cortical lesions at both frontoparietal regions [Figure 4] in fluid-attenuated inversion recovery (FLAIR) sequence. Neuroradiological findings were consistent with the diagnosis of WE. MR venography of cerebral veins was normal.
Figure 1.
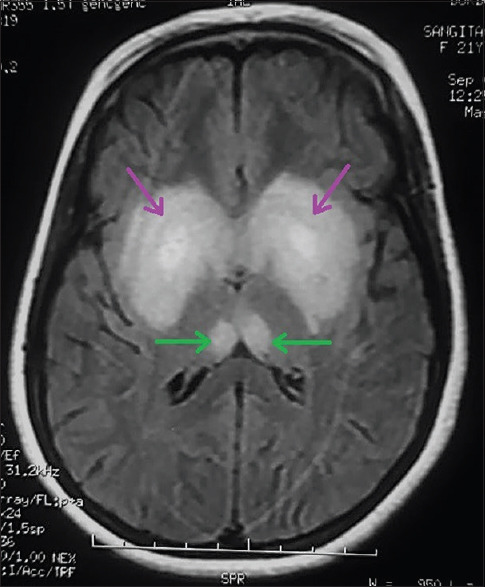
Cerebral MRI showing FLAIR hyperintensity of both dorsomedial thalami (green arrows) and both basal ganglia (purple arrows)
Figure 2.
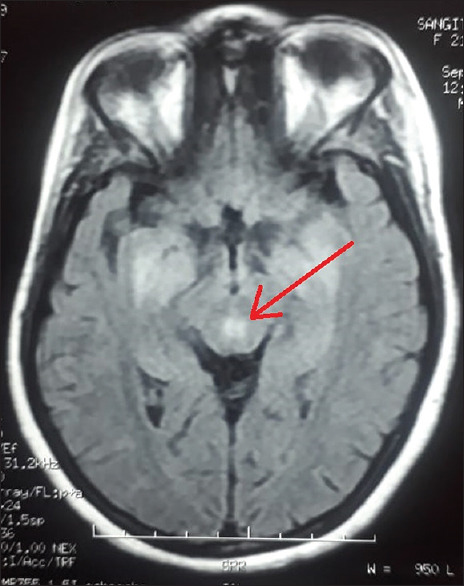
Cerebral MRI showing FLAIR hyperintensity of the peri-aqueductal region (red arrow) which is highly specific for WE
Figure 3.
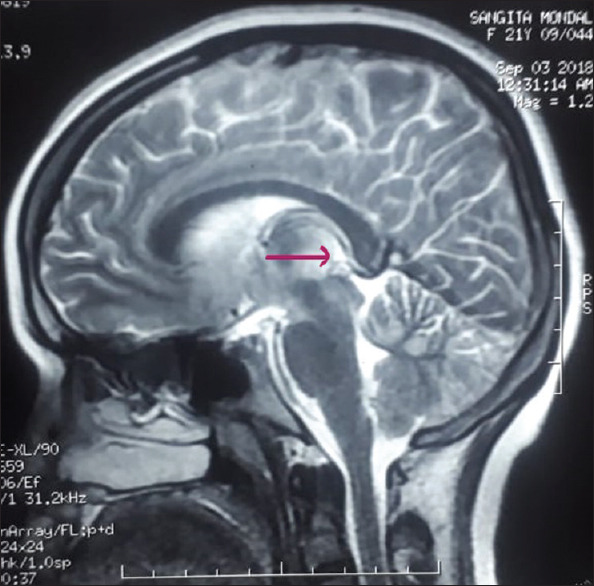
Cerebral MRI brain showing FLAIR hyperintensity of the mammillary body (pink arrow) which is also quite specific for WE
Figure 4.
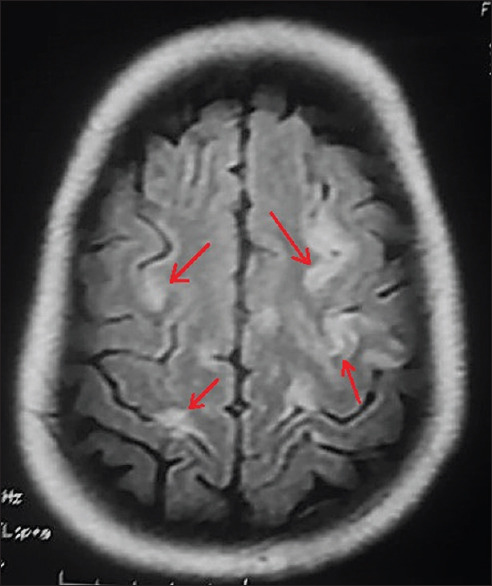
Cerebral MRI showing hyperintense cortical lesions at both frontoparietal regions (red arrows) in FLAIR, a quite uncommon finding in WE
Serum thiamine estimation unveiled an eminently low level of the vitamin (2.84 mol/L; reference level: 70–180 mmol/L) sealing the diagnosis. The patient was put on high dose parenteral thiamine (500 mg 8 hourly).
For the next few days, her neurological status remained the same, no further convulsions were reported. It was only on hospital day (hd) 6 some responses to verbal commands were noted (GCS- E2M5V4). On hd8, ophthalmologic examination revealed bilateral ptosis, upward and lateral gaze restrictions, conjugate gaze palsy, and bidirectional nystagmus. On hd10, there was no ptosis or medial rectus palsy. However, horizontal bidirectional nystagmus was still present. By the end of the second week, she had only conjugate gaze restriction. On hd14, the patient started walking with support improved (truncal ataxia), she was oriented to time, place, and person with deficits in registration, attention, and recall (minimental status examination score [MMSE]- 24/30). Follow-up MRI about a month later showed near resolution of the previous abnormalities, although some hyperintensities at the head of the caudate nucleus and globus pallidi and paraventricular areas persisted [Figure 5]. On discharge, she had MMSE = 30/30, no ataxia, and minimal gaze palsy. She was discharged with oral thiamin (100 mg twice a day), doxylamine thrice daily, and levetiracetam (500 mg twice a day). Levetiracetam was eventually withdrawn after gradual dose tapering after 6 months. With the timely institution of therapy, we were able to salvage her pregnancy this time.
Figure 5.
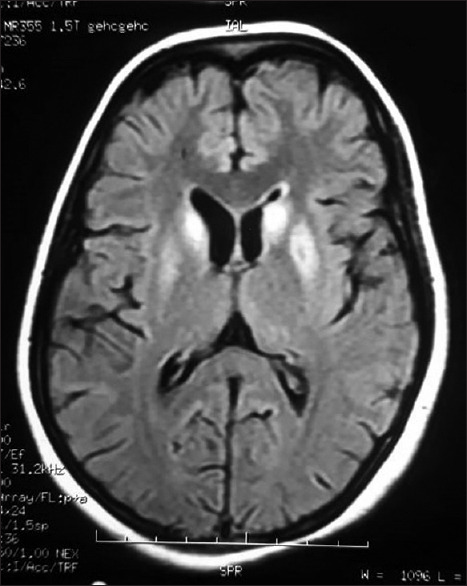
Cerebral MRI showing resolution of previous lesions after therapy and minimal persistence of FLAIR hyperintensities of the caudate head (magenta arrows) and globus pallidi (green arrows) and normal-appearing periaqueductal gray mater and dorsomedial thalami
Discussion
Thiamine (vitamin B1) is an essential water-soluble vitamin. Its body store is about 25–30 mg that may last for nearly 18 days. The recommended daily allowance (RDA) is 0.4 mg/1000 kcal which is generally met by an average adult diet. However, its daily requirement depends on the metabolic rates, with the greatest need during periods of high metabolic demand and high glucose intake. In pregnancy, the requirement of thiamine may increase up to 1.5 mg/day.[3] Thiamine serves as a cofactor for several key enzymes such as transketolase, alpha-ketoglutarate dehydrogenase, and pyruvate dehydrogenase. Thiamine-dependent enzymes function as a connection between glycolytic and citric acid cycles. Deficiency of thiamine leads to decreased levels of these enzymes and hence accumulation of lactate and pyruvate resulting in metabolic imbalances leading to neurotoxicity.[4]
In a comprehensive analysis of 49 cases of WE from HG, the classic triad of WE i.e., encephalopathy, oculomotor dysfunction, and gait ataxia were present among 46.9% cases.[5] Our patient presented with all three and with a rather unusual presentation – seizures. About etiopathogenesis, epilepsy was attributed to cortical involvement in WE (which occurs rarely), bilateral thalamus involvement, and associated electrolyte and metabolic disturbances due to hyperemesis. Till date only 13 cases have been reported due to non-alcoholic WE presenting with epileptic seizures in literature.[6] Only two of them presented with seizures as an initial manifestation (our case is the third reported case in this regard) and only six of those cases had cortical lesions (ours is the seventh being reported). In our case, both cortical and bilateral thalamus involvement explained the source of seizure activity even in the absence of any demonstrable electrolyte imbalance.
A high index of suspicion is a must for diagnosing WE following HG as delay in diagnosis worsens the prognosis.[7] Diagnosis of WE is usually based on its distinctive neurological manifestations, with supportive evidence of thiamine deficiency, characteristic MRI findings, and reversal on treatment with thiamine. In our case, thiamine deficiency was objectively proven by high-performance liquid chromatography which supplanted the need for transketolase activity testing. MRI played an important role in diagnosing this condition. Reversible cytotoxic edema is the most characteristic lesion in WE, readily seen on T2, diffusion-weighted (DW), and FLAIR images. Periventricular regions of diencephalon, mesencephalon, brainstem, and superior vermis of the cerebellum are particularly sensitive to thiamine deficiency and are commonly seen as hyperintensities on T2 images. Typical findings include areas of increased T2 and FLAIR signals, decreased T1 signal, and diffusion abnormality around the aqueduct and third ventricle and in the medial thalamus, dorsal medulla, tectal plate, and mammillary bodies.[8] Atypical lesions can be observed in the cerebellum, red, dentate, caudate nuclei, cerebellum, and cerebral cortex, which have mostly been described in non-alcoholics.[8,9] MRI of our patient showed typical involvement of symmetrical subcortical areas along with frontoparietal cortices, the latter indicated advanced disease and was associated with poor pregnancy outcomes in previous literature.[10] Repeat MRI scans showed significant resolution over time thus further supporting our diagnosis and effectiveness of treatment.
Although WE, in general, has a favorable prognosis with proper thiamine supplementation, the maternal mortality rate may reach 20%.[11] Pregnancy outcomes among mothers with WE have been worse (nearly 50% of mothers had fetal demise).[5,12] In this case, although the patient in an advanced stage presented with seizures, prompt diagnosis, and management, we were able to prevent any fetal complications, and the patient had an uncomplicated vaginal delivery.
Although the mainstay of treatment lies in the timely administration of high dose thiamine, consensus guidelines specific to WE in HG are still lacking regarding its optimum dosage, duration, and routes of administration.[13] The preferred dose of thiamine treatment for WE may be as high as 500 mg given one to three times daily, parenterally. Magnesium may need to be supplemented if levels are decreased as it is also a cofactor for the transketolase.[14] Oral thiamine 200 mg/day after the parenteral dose must be given for at least 3 months.
Conclusion
In conclusion, WE secondary to HG is being diagnosed and reported with increasing frequency although relatively uncommon.[15,16] The presented case is peculiar for presentation in a very advanced stage with seizures and radiologically proven cortical involvement. Despite poor prognostic indicators, we could save the life of mother and baby due to prompt diagnosis, appropriate intervention, and vigilance. Pregnant females with excessive vomiting, history of HG, or on parenteral nutrition should receive thiamine supplements as prophylactic measures at primary healthcare settings considering the vitamin is innocuous, cheap, and capable of preventing a neurological catastrophe. Pregnant females with HG should undergo thiamine determination.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke encephalopathy-clinical pearls. Mayo Clin Proc. 2019;94:1065–72. doi: 10.1016/j.mayocp.2019.02.018. [DOI] [PubMed] [Google Scholar]
- 2.Stephens A, Patel K, Rao A, Browne P, Raley S, Street L. Recurrent Wernicke's encephalopathy in pregnancy: A case report. Nutr Neurosci. 2019;22:528–30. doi: 10.1080/1028415X.2017.1416941. [DOI] [PubMed] [Google Scholar]
- 3.Oudman E, Wijnia JW, Oey M, van Dam M, Painter RC, Postma A. Wernicke's encephalopathy in hyperemesis gravidarum: A systematic review. Eur J Obstet Gynecol Reprod Biol. 2019;236:84–93. doi: 10.1016/j.ejogrb.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Vemuganti R, Kalluri H, Yi JH, Bowen KK, Hazell AS. Gene expression changes in thalamus and inferior colliculus associated with inflammation, cellular stress, metabolism and structural damage in thiamine deficiency. Eur J Neurosci. 2006;23:1172–88. doi: 10.1111/j.1460-9568.2006.04651.x. [DOI] [PubMed] [Google Scholar]
- 5.Chiossi G, Neri I, Cavazzuti M, Basso G, Facchinetti F. Hyperemesis gravidarum complicated by Wernicke encephalopathy: Background, case report, and review of the literature. Obstet Gynecol Surv. 2006;61:255–68. doi: 10.1097/01.ogx.0000206336.08794.65. [DOI] [PubMed] [Google Scholar]
- 6.Shang W, Chen X, Li X, Chen H, Tang S, Hong H. Epileptic seizures in nonalcoholic Wernicke's encephalopathy: A case report and literature review. Metab Brain Dis. 2017;32:2085–93. doi: 10.1007/s11011-017-0106-1. [DOI] [PubMed] [Google Scholar]
- 7.Ashraf VV, Prijesh J, Praveenkumar R, Saifudheen K. Wernicke's encephalopathy due to hyperemesis gravidarum: Clinical and magnetic resonance imaging characteristics. J Postgrad Med. 2016;62:260–3. doi: 10.4103/0022-3859.191005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke's encephalopathy: Review of the literature. AJR Am J Roentgenol. 2009;192:501–8. doi: 10.2214/AJR.07.3959. [DOI] [PubMed] [Google Scholar]
- 9.Zuccoli G, Santa Cruz D, Bertolini M, Rovira A, Gallucci M, Carollo C, et al. MR imaging findings in 56 patients with Wernicke encephalopathy: Nonalcoholics may differ from alcoholics. AJNR Am J Neuroradiol. 2009;30:171–6. doi: 10.3174/ajnr.A1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhong C, Jin L, Fei G. MR imaging of nonalcoholic Wernicke's encephalopathy: A follow-up study. AJNR Am J Neuroradiol. 2005;26:2301–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Wedisinghe L, Jayakody K, Arambage K. Wernicke's encephalopathy: A preventable cause of maternal death. Br J Hosp Med (Lond) 2011;72:31–4. doi: 10.12968/hmed.2011.72.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Di Gangi S, Gizzo S, Patrelli TS, Saccardi C, D'Antona D, Nardelli GB. Wernicke's encephalopathy complicating hyperemesis gravidarum: From the background to the present. J Matern Fetal Neonatal Med. 2012;25:1499–504. doi: 10.3109/14767058.2011.629253. [DOI] [PubMed] [Google Scholar]
- 13.Aneja T, Chaturvedula L, Nair PP, Barathi D, Keepanasseril A. Complete recovery in Wernicke's encephalopathy complicating hyperemesis gravidarum. BMJ Case Rep. 2019;12:e227530. doi: 10.1136/bcr-2018-227530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLean J, Manchip S. Wernicke's encephalopathy induced by magnesium depletion. Lancet. 1999;353:1768. doi: 10.1016/S0140-6736(99)00182-8. [DOI] [PubMed] [Google Scholar]
- 15.Žigrai M, Smetanová V, Gmitterová K, Klepancová P, Vyskočil M. Wernicke encephalopathy-a rare complication of hyperemesis gravidarum. Eur J Clin Nutr. 2020;74:663–5. doi: 10.1038/s41430-020-0592-9. [DOI] [PubMed] [Google Scholar]
- 16.Meggs WJ, Lee SK, Parker-Cote JN. Wernicke encephalopathy associated with hyperemesis gravidarum. Am J Emerg Med. 2020;38:690. doi: 10.1016/j.ajem.2019.09.012. e3-e5. [DOI] [PubMed] [Google Scholar]


