Abstract
Purpose:
Epstein-Barr virus (EBV) is a member of the family Gamma Herpes viridae and is usually implicated in malignancies like non-Hodgkin's lymphoma, Hodgkin's lymphoma and Burkitt's lymphoma. The present study was designed with the aim to estimate the seroprevalence of EBV in people with hematological malignancies and further follow up was planned by viral load quantitation by Real time PCR in positive cases.
Methods:
The current study was planned for a period of three years and a total of 272 serum samples were tested from patients with hematological malignancies namely; HL, NHL, ALL, CLL. Serological testing was performed for the presence of IgM and IgG antibodies against EBV viral capsid antigen. Sera of the patients found positive for IgM was further subjected to viral DNA extraction and Real Time Quantitative PCR was performed by a commercial kit.
Results:
The overall seropositivity rate was 89.2% for EBV IgG antibodies and 56.1% for IgM antibodies. The seroprevalence for anti-EBV VCA IgM was found to be highest in the age group <10 years (34.8%) and 11–22 years (20.4%). Of the 109 EBV positive strains by PCR, 27.3% were HL, 35.2% NHL, 24.3% ALL and 13.7% were CLL. The mean viral load was 68.7 × 107 copies/ml DNA.
Conclusion:
Our study showed a higher seroprevalence and a definite causal relationship of EBV in lymphoma patients. Young adults showed a higher risk of hematological malignancies as compared to elder population. This study can prove to be an essential guide and aid to the primary care physicians in identifying the possible risk factors and seroprevalence in various age groups of EBV malignancy patients for their proper follow up and referral to higher speciality centres.
Keywords: Acute Lymphocytic leukemia, chronic lymphocytic leukemia, Epstein-Barr virus, Hodgkin's Lymphoma, Non-Hodgkin's Lymphoma
Introduction
Epstein-Barr virus (EBV) is a widely prevalent DNA virus which is a member of the Gamma Herpesviridae family. It is the causative agent of a common childhood infection ; “infectious mononucleosis (IM) “found worldwide and has a prevalence rate of almost 90%.[1] This virus is usually present in the human saliva and can spread through kissing, sharing of personal food items (also known as 'kissing disease'). After exposure or transmission through these routes, EBV enters the oropharynx and infects the resident epithelium along with the B-cells, followed by replication and spread across the whole body. The incubation period may last from four to seven weeks.[2] EBV disease usually manifests in two forms :primary/latent infection and the reactivation disease. It remains latent for years until reactivation occurs due to immunocompromised states of the body. It is implicated in various forms of malignant diseases ;both hematological and non-hemtological ones. Non-Hodgkin's lymphoma (NHL), Hodgkin's Lymphoma (HL) and Burkitt's Lymphoma (BL) are the common hematological malignant diseases caused by EBV whereas; gastric adenocarcinoma and nasopharyngeal carcinoma are the common non-hematological malignancies.[3] It's causal role has also been implicated in chronic lymphocytic leukemia in adults and childhood leukemia.[4] EBV is also a causative agent in patients undergoing hemodialysis as well as transplant. Post-transplant lymphoproliferative disorder (PTLD) is an entity due to EBV in transplant recipients.[5,6]
The seroprevalence of EBV antibodies is seen to increase with age and varies across different populations based on geographical distribution, ethnicity and socioeconomic status. Nasopharyngeal carcinoma and Burkitt's Lymphoma are mostly endemic in China and eAfrica, respectively, but uncommon elsewhere in the world. Extranodal nasal-type natural killer (NK)/T-cell lymphomas show a high geographical predilection for Asia, South America, and Central American countries and are rarely seen in the United States and Europe.
EBV has varied clinical presentation and can be easily confused with other acute viral diseases caused by other members of the Human Herpes family such as CMV and other hepatitis viruses.[7] The testing assays commonly utilized for the diagnosis of EBV infection can either be nonspecific like heterophile antibodies detection tests (mono spot test) or specific serological assays such as ELISA, EIA, IFA, and IgG avidity tests along with molecular assays for nuclear acid detection.[8] Molecular assays mostly employed nowadays; are Real time PCR techniques, along with In situ hybridization techniques. Diagnosis, monitoring of EBV infection as well as reactivation can now be easily done by latest techniques of Quantitative RT PCR, and blotting methods like Southern blotting and Dot blotting.[9]
Most of the serological tests employed for EBV diagnosis use the product of structural and non-structural genes against humoral antibodies of EBV. Viral capsid antigens (VCAs), the early antigens (EAs), as well as the genes that code for Epstein–Barr nuclear antigens (EBNAs) are most commonly used genes.[10] At least two serological parameters are needed to detect EBV antibodies in suspected EBV cases: VCA-IgG and VCA-IgM. The humoral response to the VCA complex in the form of IgM and IgG is typically found at the onset of clinical symptoms.[11] Recent primary EBV infection is usually determined by VCA-IgM and is never detected later in life. Whereas; VCA IgG is an indicator of acute, convalescence, as well as past EBV infections.
The present study aimed to determine the Seroprevalence of EBV among different age groups with hematological malignancies over a time period of three years along with the associated epidemiological parameters and risk factors and further followed by viral load quantitation by Real time PCR in positive cases.
Materials & Methods
Place & duration of study
The present observational study was planned at a tertiary Health care institute of North India between January 2017 to December 2019.
Patient selection
In the span of three years study period ;(2017–2019); a total of 272 consecutive serum samples were obtained from patients with hematological malignancies namely; HL, NHL, acute lymphoblastic leukemia (ALL), chronic lymphocytic leukemia (CLL) that was diagnosed at our Oncology centre based on clinical manifestations, laboratory findings (lymph node biopsy and flow cytometry on peripheral blood, bone marrow aspirate, and so on) and other laboratory tests. The inclusion criteria were the positive diagnosis of one of the above mentioned hematological malignancies and written informed consent whereas; the exclusion criteria included patients with other hematological disorders, no definite diagnosis and where written informed consent was not available.
Sample preparation and testing
Serological testing – Venous blood samples were procured from each suspected patient in a 5 ml vial. The sera samples were tested for the presence of EBV IgM and IgG antibodies against the viral capsid antigen (VCA). The presence of anti-EBV capsid antigen (VCA) IgG and IgM was detected by commercially available DRG ELISA kits (DRG International, Inc., USA). The results for both IgG and IgM were interpretated as per the manufacturer's instructions provided in the kit.
Molecular testing – Sera of the patients found positive for IgM was further subjected to viral DNA extraction using Qiagen Viral DNA/RNA kit (QIAamp, USA) according to the manufacturer's instructions. Real Time PCR was also performed as per standard instructions by Geno-Sen's EBV (Rotor Gene) Real Time PCR Kit Quantitative for use with the Rotor GeneTM 2000/3000/6000 (Corbett Research Australia).[12]
Statistical analysis
The results were analysed using the SPSS version 22 software (SPSS Inc., Chicago, IL, USA). The frequencies are shown with 95% confidence intervals (95%CI). The Chi-square and Mann–Whitney U test was used to analyse the statistically significant variables. The statistically significant values were considered as P value <0.05.
Results
Out of the total 272 participants, disease categorization was as: 101 ALL, 69NHL, 64 CLL and 38 HL cases [Figure 1]. Of these cases, 52.5% (143/272) were males and 47.4% (129/272) were females. The median age was 11 years and range was from 1 month to 48 years. The overall seropositivity rate for EBV IgG antibodies was 89.2%, 243/272 (95%CI 86.1–92.3). Comparing the age groups, IgG seropositivity was seen to increase from 63.4% in children <10 years of age to 95.3% in 36–45 year olds, and remained constant thereafter with increasing age (P < 0.001). Speaking of the specific population group, prevalence was seen as lowest in children/adolescents (<32 years) (68.1%) as compared to 89.2-98.9% in adults (P < 0.001). Hence, it was determined that increasing age and older age group was a significant risk factor for the EBV IgG seropositivity [Table 1, Figure 2].
Figure 1.
Distribution of hematological malignancies
Table 1.
Seroprevalence of EBV antibodies (IgG and IgM) according to patient’s characteristics
| Characteristics | Total tested n (%) | EBV VCA IgG n (%) | P | EBV VCA IgM n (%) | P |
|---|---|---|---|---|---|
| Overall cases | 272 (100) | 243 (89.2) | 152 (56.1) | ||
| Gender | 0.150 | 0.006 | |||
| Male | 143 (52.5) | 126 (88.1) | 78 (54.5) | ||
| Female | 129 (47.4) | 119 (92.7) | 74 (57.1) | ||
| Age (years) | |||||
| <10 | 33 (12.2) | 21 (63.4) | <0.001 | 11 (34.8) | <0.001 |
| 11-22 | 58 (21.4) | 39 (68.7) | 12 (20.4) | ||
| 23-32 | 37 (13.5) | 27 (72.3) | 7 (18.6) | ||
| 33-42 | 45 (16.3) | 42 (94.5) | 5 (11.3) | ||
| >43 | 99 (36.6) | 95 (95.6) | 6 (5.8) | ||
| Population group | |||||
| Children/Adolescents | 179 (65.7) | 121 (68.1) | <0.001 | 101 (56.2) | <0.001 |
| Adults/Elderly | 93 (34.3) | 61 (95.7) | 42 (44.8) | ||
| Hematological malignancies | |||||
| Hodgkin’s lymphoma (HL) | 38 (13.9) | 85 (31.4) | <0.001 | 79 (29.2) | <0.001 |
| Non-Hodgkin’s lymphoma (NHL) | 69 (25.3) | 95 (35.1) | 102 (37.7) | ||
| Acute lymphoblastic leukemia (ALL) | 101 (37.1) | 50 (20.8) | 58 (21.4) | ||
| Chronic lymphocytic leukemia (CLL) | 64 (23.5) | 34 (12.7) | 32 (11.7) |
Figure 2.
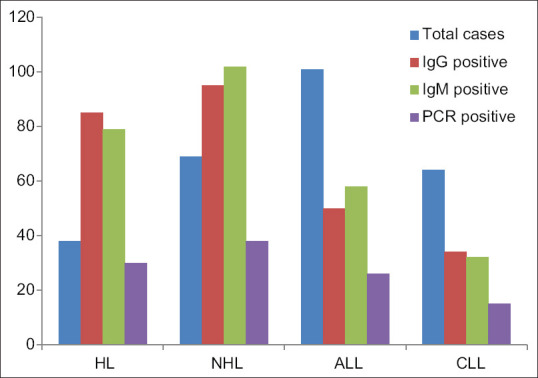
Comparison between different groups of hematological malignancies and their IgG, IgM and PCR positivity
IgM antibodies to EBV were detected in 56.1%, 152/272 (95%CI 52.4 – 59.8) of participants indicating acute infection. No significant difference was found between males and females in the prevalence of IgM antibodies (54.5% vs. 57.1%). The seroprevalence for anti- EBV VCA IgM was found to be highest in the age group <10 years (34.8%) and 11–22 years (20.4%). Prevalence was however, detected in almost all the age groups with 18.6% in 23–32 year olds, 11.3% in 33–42 year olds, and 5.8% in persons >43 years [Table 1, Figure 2].
Regarding the molecular testing by Real time PCR, out of the total 272 samples; 109 were tested as positive giving the positivity rate of 40.03% [Table 1, Figure 2].
Of the 109 EBV positive strains, 27.3% (30/109) were HL, 35.2% (38/109) were NHL, 24.3% (26/109) ALL, 13.7% (15/109) were CLL. Viral load quantitation for positive cases was done by plotting a standard curve using the standard controls of the kit. The mean ± std. deviation of viral loads were 68.75 × 106 ± 30.05 × 107 copies/ml DNA. The highest viral load was seen in the cases of NHL, followed by HL, ALL and CLL. Statistically significant differences was noted between the EBV infectivity and the four groups of hematological malignancies (p < 0.05). NHL and HL group of patients revealed a much higher viral load as compared to ALL and CLL cases; establishing a clear and stronger relationship of EBV pathogenesis in these two malignancies. Statistics also showed a significant difference between age (p = 0.001) and EBV infectivity. Young adults (<34 years) showed a higher risk of hematological malignancies as compared to the elder population. However, no statistical significance was noted between gender variable and EBV infectivity (p = 0.3)
Discussion
Epstein-Barr Virus (EBV) has a seroprevalence of almost 95% and infects nearly all humans upto adulthood. In people with malignancies, the malignant cells harbour the EBV DNA within them and hence, EBV DNA circulates at elevated levels in the plasma. The current study was designed with the aim to determine the Seroprevalence of EBV among different age groups with hematological malignancies and also viral load quantitation by Real time PCR in positive cases.
Our study reported the overall seropositivity rate for EBV IgG antibodies as 89.2%. A study from Iran, showed significantly higher EBV seropositivity in younger age group of children with ALL.[13] A study by Venkitaraman et al.[14] also reported seroprevalence of IgG antibodies to VCA upto age of 5 years as 80%. Our finding was also supported by the results of survey conducted by National Health and Nutrition Examination Surveys (NHANES), United States which showed similar seropositivity rates: 50% in 6–8 years, 59% in 12–14 years, and 89% in 18–19 years.[15] Other similar studies showed that majority of the children are infected with EBV in the early phase of their life. A study from Thailand estimated a seroprevalence rate of 50.4% in children <2 years, and 97.6% in children of 12–14 years.[16] It is usually seen that EBV infection in developed countries like USA often occurs in delayed adolescence, whereas, in developing Asian countries like us, the EBV infection is usually acquired in the first 10 years of life along with seroconversion.[17] Moreover, in developing countries several epidemiological factors, such as crowded living conditions, lower socio-economic status, have also been implicated in the increase of the possibility of being EBV seropositive in the early years of life.[18,19,20]
In our study, the overall prevalence of IgM antibodies was 56.1% in hematological malignancies, indicating an acute EBV infection. VCA-IgM is used as an indicator of recent primary EBV infection and is secreted transitionally. Indeed, VCA-IgM is no more detected after convalescence, and generally it does not occur another time in life.[7] Our study showed that there was no difference in EBV seropositivity between males and females (54.5% vs. 57.1%), which is similar to the other similar studies. A study by Norzuriza et al. also revealed that there was no significant difference in EBV seropositivity between males and females.[21] A recent study in the year 2020 done in United Kingdom also revealed overall increased EBV seroprevalence in the adolescent age group.[22] While a similar study by Winter et al; opined that EBV seroprevalence generally increases until around 24 years of age and remains constant thereafter.[23]
EBV viral load is an important marker for pathogenesis and disease prognosis in patients with hematological malignancies. Epstein–Barr virus DNA detection in patient's serum is even more sensitive than serology and IgG avidity testing.[21] Serological tests are usually adequate for EBV diagnosis in immunocompetent individuals unless the results are indeterminate or invalid.[24] PCR testing is usually required when the EBV related malignancy is strongly suspected with negative serology results.[25] In EBVassociated malignancies, the sample of choice is important based on the type of disease. Serum is highly useful in detecting EBV DNA in hematological malignancy patients as the pathogenesis of the disease involves circulation of EBV DNA in bloodstream through malignant cells. We used serum samples in our current study for EBV viral load quantification via RT-PCR and disease prognosis.[9] We were able to establish a definite causal relationship between EBV and NHL, HL patients. Highest viral load was seen in the class of NHL and HL patients. The mean viral loads in our tested samples were 68.75 × 106 ± 30.05 × 107 copies/ml DNA. Several studies in the hematological malignancy patients also revealed strong correlation in these two malignancies. A Scandinavian study from 2003, a British study from 2009 found EBV positive young adults to be at increased risk of HL.[26,27] An Italian multicenter case-control study from 2000 also demonstrated an increased risk of non-Hodgkin lymphoma (NHL) in EBV positive cases.[28] A statistically significant difference was also noted between age (p = 0.001) and EBV infectivity. Young adults (<34 years) showed a higher risk of hematological malignancies as compared to the elder population. A study by Hjalgrim et al., in Scandinavia revealed an increased risk of EBV-positive HL in young adults.[27] Various risk factors associated with increased risk of EBV infectivity include immuno- suppressive drugs, anti-neoplastic drugs, steroids, and subsequently; may cause a high risk of development of lymphoma and cancers, including leukemia and multiple myeloma.[29,30]
Conclusion
There is still a lack of enough and high quality data on the seroprevalence of EBV among different age groups in various regions of developing Asian countries. The current study highlights the importance of primary care physicians in counselling and educating the patients as well as identifying their possible risk factors which may cause a high risk of development of lymphoma and hematological cancers. Our study comprised a large group of participants expanding over a period of three years and was successful in giving seroprevalence rates among different ages –groups and also established a definite causal relationship of EBV with various hematological mlignacies. However, many more studies and research work is still required to understand the exact pathogenesis of EBV and also to estimate the seroprevalence in other EBV disease groups.
Declarations
Author's contribution
Conception and design of study – Sweta singh, Sangram singh patel, Chinmoy sahu
Acquisition of data- Sweta singh, Sangram singh patel, Chinmoy sahu
Analysis/interpretation of data- Sweta singh, Sangram singh patel, Chinmoy sahu
Drafting of manuscript- Sweta singh, Sangram singh patel, Chinmoy sahu
Critical revision and final draft of manuscript-Ujjala ghoshal, Sangram singh patel, Chinmoy sahu
Laboratory work and investigations- Hemant Verma, Sweta singh.
Ethical statement
The study was approved by the Institute's Ethics Committee. Date of approval : 21/2/2018
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
Not Applicable.
References
- 1.Sarwari NM, Khoury JD, Hernandez CM. Chronic Epstein Barr virus infection leading to classical Hodgkin lymphoma. BMC Hematol. 2016;16:19. doi: 10.1186/s12878-016-0059-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunmire SK, Hogquist KA, Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol. 2015;390:211–40. doi: 10.1007/978-3-319-22822-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paraskevas E, Dimitroulopoulos D. Epstein-Barr virus infection and gastrointestinal diseases. Ann Gastroenterol. 2005;18:386–90. [Google Scholar]
- 4.Laytragoon-Lewin N, Chen F, Avila-Carino J, Zou JZ, Mellstedt H, Ernberg L, et al. Epstein Barr virus (EBV) carrying cells of chronic lymphatic leukemia (CLL) subpopulation express EBNA 1 and LMPs but not ENBA2 in vivo. Int J Cancer. 1995;63:486–90. doi: 10.1002/ijc.2910630404. [DOI] [PubMed] [Google Scholar]
- 5.Schlehofer B, Blettner M, Geletneky K, Haaf HG, Kaatsch P, Michaelis J, et al. Sero-epidemiological analysis of the risk of virus infections for childhood leukemia. Int J Cancer. 1996;65:584–90. doi: 10.1002/(SICI)1097-0215(19960301)65:5<584::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 6.Weikert BC, Blumberg EA. Viral infection after renal transplantation: surveillance and management. Clin J Am Soc Nephrol. 2008;3:S76–86. doi: 10.2215/CJN.02900707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampaio MS, Cho YW, Quazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients: an analysis of US National transplant. Database Transplantation. 2012;94:990–8. doi: 10.1097/TP.0b013e318270bc7b. [DOI] [PubMed] [Google Scholar]
- 8.Klutts JS, Ford BA, Perez NR, Gronowski AM. Evidence-based approach for interpretation of Epstein-Barr virus serological patterns. J Clin Microbiol. 2009;47:3204–10. doi: 10.1128/JCM.00164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess RD. Routine Epstein-Barr virus diagnostics from the laboratory perspective: Still challenging after 35 years. J Clin Microbiol. 2004;42:3381–7. doi: 10.1128/JCM.42.8.3381-3387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein-Barr virus (EBV) load: The significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–19. doi: 10.1002/rmv.582. [DOI] [PubMed] [Google Scholar]
- 11.van Grunsven WM, van Heerde EC, de Haard HJ, Spaan WJ, Middeldorp JM. Gene mapping and expression of two immunodominant Epstein-Barr virus capsid proteins. J Virol. 1993;67:3908–16. doi: 10.1128/jvi.67.7.3908-3916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunmire SK, Hogquist KA, Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol. 2015;390:211–40. doi: 10.1007/978-3-319-22822-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geno-Sen's EBV Real Time PCR kit: Quantitative for Rotor gene 2000/3000/6000 (Corbett research Australia) Genome diagnostics Pvt.Ltd; [Google Scholar]
- 14.Mahjour SB, Ghaffarpasand F, Fattahi MJ, Ghaderi A, Fotouhi Ghiam A, Karimi M. Seroprevalence of human herpes simplex, hepatitis B and Epstein-Barr viruses in children with acute lymphoblastic leukemia in southern Iran. Pathol Oncol Res. 2010;16:579–82. doi: 10.1007/s12253-010-9258-6. [DOI] [PubMed] [Google Scholar]
- 15.Venkitaraman AR, Lenoir GM, John TJ. The seroepidemiology of infection due to Epstein Barr virus in southern India. J Med Virol. 1985;15:11–6. doi: 10.1002/jmv.1890150103. [DOI] [PubMed] [Google Scholar]
- 16.Balfour HH, Jr, Sifakis F, Sliman JA, Knight JA, Schmeling DO, Thomas W. Age specific prevalence of Epstein-Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208:1286–93. doi: 10.1093/infdis/jit321. [DOI] [PubMed] [Google Scholar]
- 17.Pancharoen C, Bhatrarakosol P, Thisyakorn U. Seroprevalence of Epstein-Barr virus infection in Thai children. J Med Assoc Thai. 2001;84:850–4. [PubMed] [Google Scholar]
- 18.Haahr S, Plesner A, Vestergaard B, Höllsberg P. A role of late Epstein–Barr virus infection in multiple sclerosis. Acta Neurol Scand. 2004;109:270–5. doi: 10.1046/j.1600-0404.2003.00221.x. [DOI] [PubMed] [Google Scholar]
- 19.Mekmullica J, Kritsaneepaiboon S, Pancharoen C. Risk factors for Epstein–Barr virus infection in Thai infants. Southeast Asian J Trop Med Publ Health. 2003;34:395–7. [PubMed] [Google Scholar]
- 20.Ozkan A, Kilic S, Kalkan A, Ozden M, Demirdag K, Ozdarendeli A. Seropositivity of Epstein–Barr virus in Eastern Anatolian region of Turkey. Asian Pac J Allerg Immunol. 2003;21:49. [PubMed] [Google Scholar]
- 21.Norzuriza MR, Kon Ken W, Mohammad M, Isahak I, Rahman MM. Epidemiology of Epstein-Barr virus in Malaysia. Bangladesh Vet. 2008;25:82–7. [Google Scholar]
- 22.Kuri A, Benjamin J, Pakpoor J. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health. 2020;20:912. doi: 10.1186/s12889-020-09049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter J, Jackson C, Lewis J, Taylor G, Thomas O. Predictors of Epstein-Barr virus serostatus and implications for vaccine policy: A systematic review of the literature. J Glob Health. 2020;10:010404. doi: 10.7189/jogh.10.010404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer CC, Aberle SW, Popow-Kraupp T, Kapitan M, Hofmann H, Puchhammer-Stockl E. Serum Epstein-Barr virus DNA load in primary Epstein-Barr virus infection. J Med Virol. 2005;75:54–8. doi: 10.1002/jmv.20237. [DOI] [PubMed] [Google Scholar]
- 25.Pitetti RD, Laus S, Wadowsky RM. Clinical evaluation of a quantitative real time polymerase chain reaction assay for diagnosis of primary Epstein-Barr virus infection in children. Pediatr Infect Dis J. 2003;22:736–9. doi: 10.1097/01.inf.0000078157.90639.96. [DOI] [PubMed] [Google Scholar]
- 26.Ikuta K, Saiga K, Deguchi M, Sairenji T. Epstein-Barr virus DNA is detected in peripheral blood mononuclear cells of EBV-seronegative infants with infectious mononucleosis-like symptoms. Virus Genes. 2003;26:165–73. doi: 10.1023/a:1023487413912. [DOI] [PubMed] [Google Scholar]
- 27.Hjalgrim H, Askling J, Rostgaard K, Hamilton-Dutoit S, Frisch M, Zhang JS, et al. Characteristics of Hodgkin's lymphoma after infectious mononucleosis. N Engl J Med. 2003;349:1324–32. doi: 10.1056/NEJMoa023141. [DOI] [PubMed] [Google Scholar]
- 28.Goldacre MJ, Wotton CJ, Yeates DG. Associations between infectious mononucleosis and cancer: Record-linkage studies. Epidemiol Infect. 2009;137:672–80. doi: 10.1017/S0950268808001246. [DOI] [PubMed] [Google Scholar]
- 29.Vineis P, Crosignani P, Sacerdote C, Fontana A, Masala G, Miligi L, et al. Haematopoietic cancer and medical history: A multicentre case control study. J Epidemiol Community Health. 2000;54:431–6. doi: 10.1136/jech.54.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young LS, Yap LF, Murray PG. Epstein–Barr virus: More than 50 years old and still providing surprises. Nat Rev Cancer. 2016;16:789–802. doi: 10.1038/nrc.2016.92. [DOI] [PubMed] [Google Scholar]


