Abstract
Background
Studies of pulmonary denitrogenation (pre-oxygenation) in obstetric populations have shown high flow nasal oxygen therapy (HFNO) is inferior to facemask techniques. HFNO achieves median end-tidal oxygen fraction (FE′O2) of 0.87 after 3 min. As HFNO prolongs safe apnoea times through apnoeic oxygenation, we postulated that HFNO would still extend safe apnoeic times despite the lower FE′O2 after pre-oxygenation.
Methods
The Interdisciplinary Collaboration in Systems Medicine simulation suite, a highly integrated, high-fidelity model of the human respiratory and cardiovascular systems, was used to study the effect of varying FE′O2 (60%, 70%, 80%, and 90%) on the duration of safe apnoea times using HFNO and facemask techniques (with the airway open and obstructed). The study population consisted of validated models of pregnant women in active labour and not in labour with BMI of 24, 35, 40, 45, and 50 kg m−2.
Results
HFNO provided longer safe apnoeic times in all models, with all FE′O2 values. Labour and increased BMI reduced this effect, in particular a BMI of 50 kg m−2 reduced the improvement in apnoea time to 1.8–8.5 min (depending on the FE′O2), compared with an improvement of more than 60 min in the subject with BMI 24 kg m−2.
Conclusions
Despite generating lower FE′O2, HFNO provides longer safe apnoea times in pregnant subjects in labour. Care should be taken when used in patients with BMI ≥50 kg m−2 as the extension of the safe apnoea time is limited.
Keywords: apnoea, computer simulation, high-flow nasal oxygenation, obesity in pregnancy, obstetrics
Editor's key points.
-
•
In pregnant women in active labour, high flow nasal oxygen therapy (HFNO) is less effective than with facemask oxygenation in increasing median end-tidal oxygen fraction (FE′O2) before induction of anaesthesia.
-
•
This simulation study indicates that HFNO after induction of anaesthesia effectively delays the onset of hypoxia, although its efficacy is not ideal in women with BMI ≥50 kg m−2.
Achieving a safe apnoea time through adequate pre-oxygenation remains a key component of safe general anaesthesia in obstetrics. Pre-oxygenation using a standard facemask technique to an end-tidal oxygen fraction (FE′O2) of at least 90% is recommended before inducing general anaesthesia.1 However, it is well recognised that several factors make this difficult to achieve in obstetric practice. Time pressure, human factors, and maternal distress can all impede the optimal use of a tight-fitting facemask and lead to sub-optimal pre-oxygenation.2 Even with adequate pre-oxygenation, the physiological changes of pregnancy result in more rapid deoxygenation and a shorter safe apnoea time (defined here as the time to desaturate to <90%) for labouring women.3
Prolonging safe apnoea times through apnoeic oxygenation has grown in popularity in non-obstetric anaesthesia in recent years.4,5 During the induction of anaesthesia, apnoeic oxygenation can be achieved by continuing oxygen delivery via the application of a tight-fitting facemask until airway instrumentation. This can be supplemented by the addition of nasal cannula that can also deliver oxygen during airway instrumentation. Utilising high-flow, humidified, nasal oxygen (HFNO) at ≥70 L min−1 to provide such apnoeic oxygen supplementation is appealing in obstetrics, because the hands-free nature and lack of a requirement for a tight facemask seal have clear benefits over traditional facemask pre-oxygenation. However, there have not yet been any RCTs of HFNO in obstetric airway management; indeed, this would present a particular challenge given the preference for regional anaesthesia and the unpredictable nature of most airway management scenarios in obstetric practice. Instead, recent work has studied the efficacy of HFNO in pre-oxygenation; three studies have demonstrated that pre-oxygenation with HFNO is inferior to a tight-fitting facemask in healthy pregnant volunteers.6, 7, 8 HFNO achieved a mean FE′O2 of 87% at 3 min (95% confidence interval, 86.5–89%) with tidal breathing.7 Meanwhile, in a separate study using HFNO with up to 20 vital capacity breaths,8 the median maximum FE′O2 was 82% (inter-quartile range [IQR], 75–86%) and 73% (IQR, 63–84%) with mouth closed and mouth open, respectively. These studies also identified outliers with final FE′O2 <75%. Increasing pre-oxygenation time to 8 min does not improve the proportion of healthy pregnant volunteers reaching FE′O2 90 with HFNO.9 As current HFNO devices prevent measurement of FE′O2 in clinical practice, the results of the studies are potentially concerning.
Given that HFNO facilitates apnoeic oxygenation to extend safe apnoea times, we postulate that even with a lower FE′O2, apnoeic oxygenation will extend the safe apnoea period beyond that seen with facemask techniques in obstetric airway management.
Owing to the ethical and practical difficulties in addressing this question in patients, we used a high-fidelity computational simulation to investigate the effects of various FE′O2 values on the safe apnoea time for both HFNO and facemask pre-oxygenation for obstetric patients.
Methods
The Interdisciplinary Collaboration in Systems Medicine (ICSM) simulation suite is a high-fidelity, highly integrated model of the respiratory and cardiovascular systems, based on the Nottingham Physiology Simulator,10 which has been previously described in detail.10, 11, 12 It is validated for investigation of pre-oxygenation and apnoea in adults,13,14 and specifically in pregnancy.15, 16, 17 The model has been recently extended to include cardiogenic gaseous oscillations, gas mixing within the pulmonary deadspace and pharyngeal pressure oscillations during the use of HFNO at 70 L min−1.18 Details of the model are described in the Supplementary material.
Ten virtual subjects were created, based on published physiological data; these were similar to those used in our previous works.17,19,20 These subjects had BMI of 24 kg m−2 (BMI24), 35 kg m−2 (BMI35), 40 kg m−2 (BMI40), 45 kg m−2 (BMI45), and 50 kg m−2 (BMI50), and included subjects in active labour and not in labour. Where physiological data were not available, values were inferred from study data from non-pregnant individuals and established physiological theory (Table 1).17
Table 1.
Baseline physiological values used to configure the virtual obstetric subjects.15, 16, 17,21, 22, 23 BMI 24/35/40/45/50, body mass index 24/35/40/45/50 kg m−2, respectively.
| BMI24 | BMI24 and labour | BMI35 | BMI35 and labour | BMI40 | BMI40 and labour | BMI45 | BMI45 and labour | BMI50 | BMI50 and labour | |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight (kg) | 75 | 75 | 94 | 94 | 108 | 108 | 122 | 122 | 135 | 135 |
| Tidal volume (ml) | 630 | 850 | 690 | 900 | 700 | 840 | 710 | 900 | 730 | 950 |
| Ventilatory frequency (bpm) | 16 | 23 | 16 | 23 | 16 | 23 | 16 | 23 | 19 | 23 |
| Functional residual capacity (ml) | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 | 1600 |
| Deadspace (%) | 140 | 140 | 140 | 140 | 140 | 140 | 140 | 140 | 140 | 140 |
| Shunt fraction (%) | 9 | 9 | 13 | 13 | 15 | 15 | 17 | 17 | 18 | 18 |
| Cardiac output (L min−1) | 6.9 | 8.3 | 8.0 | 9.6 | 8.6 | 9.8 | 8.8 | 10.0 | 9.0 | 10.5 |
| Heart rate (beats min−1) | 90 | 100 | 90 | 100 | 90 | 100 | 90 | 100 | 90 | 100 |
| Total blood volume (L) | 6.75 | 6.75 | 6.82 | 6.82 | 7.88 | 7.88 | 8.91 | 8.91 | 9.85 | 9.85 |
| Haemoglobin concentration (g dl−1) | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 | 11.3 |
| Oxygen consumption (ml min−1) | 270 | 322 | 338 | 404 | 388 | 464 | 439 | 524 | 450 | 619 |
| Respiratory quotient | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 |
Each virtual subject underwent pulmonary pre-oxygenation via tidal breathing with FiO2 1.0 to reach FE′O2 of 60%, 70%, 80%, and 90% before apnoea commenced. During apnoea, 100% or 21% oxygen was provided at the open glottis to simulate the continuation of HFNO or the removal of facemask during airway manipulation. Simulation runs also included a closed glottis, and in this scenario, ambient oxygen fraction was irrelevant as it did not pass the glottis. At the onset of apnoea, functional residual capacity (FRC) was reduced by 20% in the BMI24 subject21 and by 30% in the remaining subjects24 simultaneously, oxygen consumption (VO2) was reduced by 65 ml min−1 to simulate the effect on metabolic oxygen consumption of general anaesthesia and use of rocuronium as a choice of neuromuscular blocking agent.25 Apnoea continued until arterial haemoglobin oxygen saturation (SaO2) reached 40%.
The SaO2 was recorded every 5 ms from the start of pre-oxygenation until termination of the protocol. Model simulations ran on a 64 bit Intel Core i7 3.7 GHz Windows 10 personal computer, running Matlab version R2018a.v9 (MathWorks Inc., Natick, MA, USA).
Results
Table 2, Table 3 show the safe apnoea times for the various FE′O2 values studied in subjects not in labour and in active labour, respectively.
Table 2.
Times (min) to reach SaO2 90% and 40% during apnoeic oxygenation for different end-tidal oxygen fractions in the subjects not in labour. BMI 24/35/40/45/50, body mass index 24/35/40/45/50 kg m−2, respectively; FiO2, inspired fraction of O2; HFNO, high flow nasal oxygen therapy; n.a., not achieved (i.e. is SaO2 does not decrease to 40%, but in 120 min of apnoea, SaO2 reaches 82%, 82%, 82%, and 83% with end-tidal oxygen fractions 60%, 70%, 80%, and 90%, respectively); SaO2, arterial oxygen saturation.
| End-tidal oxygen fraction (%) | BMI24 |
BMI35 |
BMI40 |
BMI45 |
BMI50 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | |
| Time from onset of apnoea to SaO290% (min) | |||||||||||||||
| 60 | 3.1 | 3.7 | 66.9 | 2.9 | 3.1 | 19.8 | 2.7 | 2.8 | 12.8 | 2.4 | 2.5 | 7.9 | 2.1 | 2.2 | 3.9 |
| 70 | 3.8 | 4.1 | 63.9 | 3.4 | 3.6 | 21.4 | 3.1 | 3.2 | 13.9 | 2.7 | 2.8 | 8.9 | 2.4 | 2.4 | 4.3 |
| 80 | 4.5 | 4.8 | 63.8 | 3.7 | 3.9 | 22.3 | 3.3 | 3.4 | 14.9 | 3.0 | 3.0 | 9.8 | 2.7 | 2.7 | 4.9 |
| 90 | 6.6 | 7.8 | 65.2 | 5.6 | 6.5 | 34.2 | 4.8 | 5.5 | 25.9 | 4.1 | 4.6 | 19.5 | 3.4 | 3.6 | 12.1 |
| Time from onset of apnoea to SaO240% (min) | |||||||||||||||
| 60 | 6.0 | 7.0 | n.a. | 6.1 | 6.7 | 44.8 | 5.7 | 6.0 | 32.9 | 5.1 | 5.5 | 25.9 | 4.7 | 5.0 | 20.4 |
| 70 | 6.7 | 7.5 | n.a. | 6.6 | 7.3 | 45.8 | 6.1 | 6.5 | 33.9 | 5.5 | 5.9 | 26.7 | 5.0 | 5.3 | 21.4 |
| 80 | 7.5 | 8.4 | n.a. | 7.0 | 7.6 | 46.4 | 6.3 | 6.7 | 34.6 | 5.7 | 6.1 | 27.0 | 5.3 | 5.6 | 22.2 |
| 90 | 9.8 | 11.6 | n.a. | 9.0 | 10.6 | 54.4 | 8.0 | 9.2 | 42.5 | 7.0 | 8.1 | 34.9 | 6.2 | 6.9 | 29.0 |
Table 3.
Time (min) to reach SaO2 90% and 40% during apnoeic oxygenation for different end-tidal oxygen fractions in the subjects in labour. BMI 24/35/40/45/50, body mass index 24/35/40/45/50 kg m−2, respectively; FiO2, inspired fraction of O2; HFNO, high flow nasal oxygen therapy; SaO2, arterial oxygen saturation.
| End-tidal oxygen fraction (%) | BMI24 and labour |
BMI35 and labour |
BMI40 and labour |
BMI45 and labour |
BMI50 and labour |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | Closed glottis | FiO2 21% | HFNO | |
| Time from onset of apnoea to SaO290% (min) | |||||||||||||||
| 60 | 3.1 | 3.3 | 22.7 | 2.4 | 2.6 | 11.4 | 2.5 | 2.6 | 8.6 | 2.4 | 2.4 | 4.9 | 2.0 | 2.2 | 3.7 |
| 70 | 3.6 | 3.8 | 23.7 | 2.8 | 3.0 | 12.6 | 2.7 | 2.8 | 9.4 | 2.6 | 2.6 | 5.5 | 2.2 | 2.4 | 4.0 |
| 80 | 4.1 | 4.4 | 25.4 | 3.2 | 3.4 | 14.3 | 2.9 | 3.0 | 9.9 | 2.7 | 2.7 | 6.3 | 2.4 | 2.6 | 4.3 |
| 90 | 5.8 | 6.9 | 33.9 | 4.5 | 5.4 | 23.6 | 3.8 | 4.5 | 18.7 | 3.4 | 3.7 | 13.4 | 3.0 | 3.5 | 12.0 |
| Time from onset of apnoea to SaO240% (min) | |||||||||||||||
| 60 | 6.0 | 6.6 | 45.7 | 4.6 | 5.0 | 23.8 | 4.6 | 4.9 | 19.7 | 4.4 | 4.7 | 17.9 | 4.5 | 4.7 | 16.2 |
| 70 | 6.5 | 7.1 | 46.1 | 5.0 | 5.4 | 24.8 | 4.8 | 5.2 | 20.4 | 4.6 | 4.9 | 18.5 | 4.7 | 5.0 | 16.7 |
| 80 | 7.0 | 7.8 | 47.4 | 5.4 | 5.9 | 25.8 | 4.9 | 5.3 | 21.0 | 4.8 | 5.1 | 19.4 | 4.8 | 5.1 | 16.9 |
| 90 | 8.9 | 10.5 | 52.7 | 6.8 | 8.1 | 34.3 | 6.1 | 7.1 | 28.4 | 5.6 | 6.5 | 25.5 | 5.6 | 6.6 | 24.0 |
Increasing the FE′O2 from 60% to 90% at the end of pre-oxygenation markedly extended the time to reach SaO2 90% and SaO2 40% during apnoeic oxygenation in all subjects. There is a significant increase in time to SaO2 90% time between FE′O2 80% and 90% in all groups except BMI24 not in labour.
The use of HFNO significantly delayed haemoglobin desaturation in all subjects in comparison with the standard facemask technique. In the BMI24 subject, who was not in active labour, the lowest SaO2 was 82% after 120 min of apnoea with an open airway.
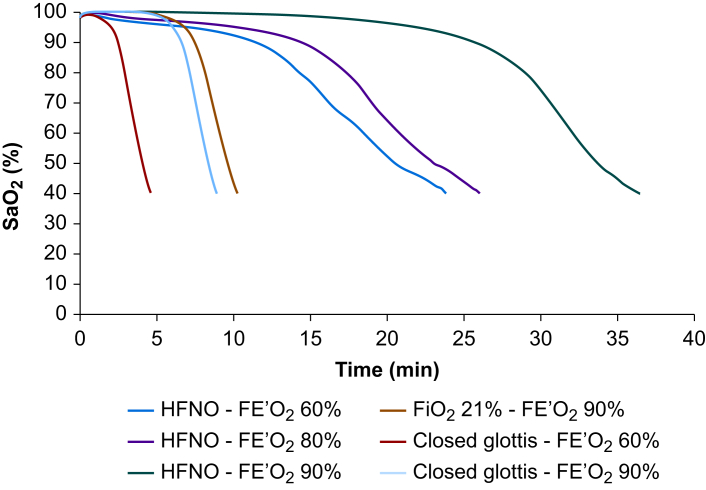
HFNO not only delayed desaturation overall, but also changed the shape of the desaturation curve with respect to time. Desaturation occurred more slowly overall and the gradient of the steep part of the curve was reduced. The decrease in SaO2 from 90% to 40% took 12.5 min in the subject with the highest BMI (i.e. BMI50, in labour, FE′O2 60%) with HFNO, compared with 3.7 min in the subject with the lowest BMI (i.e. BMI24, FE′O2 90%) with facemask. For HFNO, when the FE′O2 was 90%, we also observed an initial plateau phase, where SaO2 was maintained at 100%, before a gradual decrease to 90%. When FE′O2 was less than 90%, this plateau phase of the curve was short, such that there was a slow decrease in SaO2 after the first 1–2 min of apnoea. These changes are illustrated in Figure 1 for the BMI35 labouring subject, and they occurred in all models for HFNO at all FE′O2 values, with shorter timescales as BMI increased or end-tidal oxygen concentration decreased.
Fig 1.
Oxygen saturation during apnoea (SaO2) in the virtual subject BMI35 kg m−2 in labour, during HFNO, closed glottis, and open airway with FiO2 21%. Closed glottis simulates failure of HFNO whereas FiO2 21% with end-tidal oxygen fraction (FE′O2) of 90% simulates facemask technique. HFNO, high flow nasal oxygen therapy.
The presence of labour, increased BMI, or both significantly shortened the safe apnoea time in all subjects. Despite the safe apnoeic time being greatest with HFNO at all FE′O2 values in comparison with facemask techniques, there was a profound reduction in the observed increase in safe apnoeic times for HFNO in the BMI50 subject (Table 2). In that subject, compared with the facemask models, HFNO increased safe apnoeic time by only 1.8–8.5 min, dependent upon the FE′O2. This contrasts with increases of about 60 min in the BMI24 subject at all values of FE′O2.
In active labour, using HFNO with FE′O2 60%, safe apnoea time was reduced by approximately 66% (from 66.9 to 22.7 min) in the BMI24 subject, by 42% (from 19.8 to 11.4 min) in the BMI35 subject, by 33% (from 12.8 to 8.6 min) in the BMI40 subject, by 38% in the BMI45 subject (from 7.9 to 4.9 min), and by 7% (from 3.9 to 3.7 min) in the BMI50 subject.
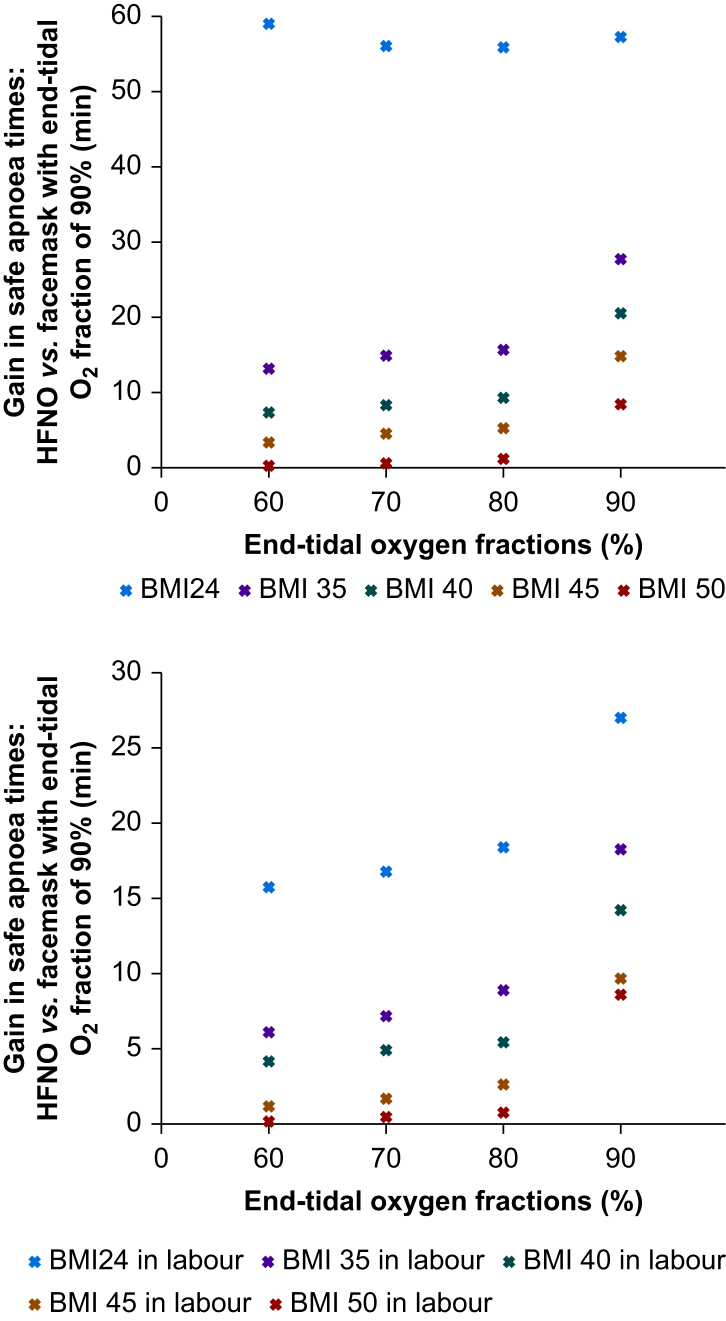
Figure 2 shows the difference in min to reach SaO2 90% between HFNO with the different FE′O2 (i.e. 60%, 70%, 80%, 90%) and facemask with FE′O2 of 90% in the subjects not in labour (upper panel) and in subjects in labour (lower panel). In every configuration and every subject, HFNO offered a longer safe apnoea time, although with the increase of BMI and with the labour the gain is less marked.
Fig 2.
Gain in safe apnoea times, calculated as difference in min to reach SaO2 90% between HFNO with end-tidal oxygen fraction (FE′O2) of 60%, 70%, 80%, 90%, and facemask with FE′O2 of 90%. HFNO, high flow nasal oxygen therapy.
Discussion
Our computational modelling of apnoeic oxygenation predicted that pre-oxygenation to a lower FE′O2 value does not prevent HFNO from providing a clinically significant increase in the safe apnoea time in obstetric populations. Even when starting with FE′O2 60%, HFNO increased safe apnoea time to more than 60 min in a non-obese parturient (similar to findings in non-obstetric populations).4,13,14 HFNO provided superior safe apnoeic times in all models compared with face mask pre-oxygenation with the same starting FE′O2. Furthermore, HFNO provided a superior safe apnoea time from FE′O2 60% than standard facemask pre-oxygenation to FE′O2 90% in all modelled subjects. Greater increases in safe apnoea times were seen with higher FE′O2 values for HFNO, particularly when FE′O2 was increased from 80% to 90%. This reinforces the importance of appropriate use of HFNO to maximise patients' FE′O2 – applying HFNO as soon as the patient enters the operating theatre, providing the maximum time for pre-oxygenation at the maximum tolerated flow rate.
The best initial flow rate remains an area of uncertainty, with concerns of patient tolerance of flows as high as 60–70 L min–19; the minimum flow rate required for pre-oxygenation remains unknown. Pre-oxygenation in HFNO studies has used a mixture of starting flow rates. Two started at 30 L min−1 and either gradual ramped the flow to 60 L min–17 or increased to 60 L min−1 after a set period of time,6 whereas two others started at 50 L min−1.8,9 As our current model is validated for HFNO using the maximum flow rate of 70 L min−1, we cannot comment further on what would be the best flow rate to balance comfort and the highest generated FE′O2, although this an area for further research.
As our previous work demonstrated,14,19 HFNO also alters the shape of the desaturation curve, reducing the gradient of the initial decrease in SaO2 to 90% and the subsequent steeper decrease (from 90% to 40%); this extends the available time for critical thinking and enacting airway rescue plans. Our data show that this effect is maintained regardless of the FE′O2 achieved during HFNO pre-oxygenation. If the FE′O2 achieved during pre-oxygenation is less than 90% (i.e. the likely real-life scenario6, 7, 8, 9), our modelling also predicted a slow decrease in SaO2 soon after apnoea (Fig. 1). Along with the relatively larger decreases in safe apnoea time with a decrease from FE′O2 90% to 80%, this finding implies that the physiological mechanisms underpinning apnoeic oxygenation are more effective with a higher FE′O2. Clinically, this also highlights that a slight reduction in SaO2 should be expected with HFNO after the onset of apnoea, but this may also help a clinician detect the failure of apnoeic oxygenation. Should this occur, the SaO2 will follow the closed glottis curve (Fig. 1), and a more rapid, accelerating decrease in SaO2 to 90% will be seen, rather than the slower decline to 90% with successful apnoeic oxygenation.
Increasing BMI was observed to reduce the extent of the improvement in safe apnoea time using HFNO. Increased closing capacity, lower airway collapse, atelectasis, the subsequent increase in the shunt fraction seen under the effects of GA, and muscle relaxation in this cohort could explain these effects, which cannot be completely overcome.24 Being in active labour had a similar effect to increasing BMI. The greatest benefit when using HFNO for subjects in labour and high BMI were seen when FE′O2 reached 90%, which may be difficult to achieve.6, 7, 8, 9 Our modelling in the highest BMI subjects (BMI45 and BMI50) predicts much smaller increases in safe apnoeic time with HFNO and an FE′O2 of less than 90% compared with facemask pre-oxygenation to an FE′O2 90%. An increase of 3.4–5.2 min for BMI45 and 0.3–1.3 min for BMI50 dependent on FE′O2 was seen (Fig. 2). Given that this cohort is at greater risk of airway obstruction, these patients are also at a greater risk of failure of apnoeic oxygenation and rapid deoxygenation. Our modelling predicts that when apnoeic oxygenation fails, a short safe apnoeic period ensues, especially when FE′O2 90% has not been reached; this remains a concern. These patients are more likely to have difficult airway management and to desaturate rapidly after facemask pre-oxygenation; therefore, they should benefit most from HFNO. However, findings of this study suggest that they receive the least benefit. Although any additional increase in safe apnoea time may be lifesaving, the modest increase in safe apnoea time, particularly in the BMI50 group, combined with the risk of failure of apnoeic oxygenation may not outweigh the benefits of instant access to bag and mask ventilation; thus, we recommend that HFNO be used cautiously in this group.
We have utilised computational modelling to address an issue that would be extremely difficult to address using clinical methods. Of course, the quality of the data and the assumptions built into the model represent a potential weakness of this approach; however, the ICSM simulation suite has been extensively validated in reproducing the physiological changes during apnoea in obstetric and non-obstetric populations, and it provides a suitable methodological approach given the ethical concerns with intentionally inadequate pre-oxygenation, and in studying the limits of safe apnoeic times in an obstetric population.
In contrast to our previous modelling studies,15, 16, 17 we chose to model the use of rocuronium instead of suxamethonium for muscle paralysis. This replicates the current practice in many obstetric units, and an increasing trend across obstetric practice.26 This change removes the transient increase in VO2 seen with suxamethonium. This could be argued to extend the safe apnoeic times compared with our previous modelling studies, but this would affect each of our study subjects similarly.
HFNO offers additional benefits beyond apnoeic oxygenation for obstetrics. Theoretically, it provides a hands-free method of pre-oxygenation that does not require a tight facemask seal and can be applied as soon as the patient enters the theatre, limiting the impact of the many detrimental factors which influence pre-oxygenation in obstetric general anaesthesia.3 HFNO therefore could be argued to improve the overall safety of obstetric airway management, despite concerns of a lower final FE′O2 after pre-oxygenation with HFNO. Apnoeic oxygenation will still extend the safe apnoeic time significantly, providing an extra safety net for airway management, even with an FE′O2 of only 60%. The additional benefits of HFNO for apnoeic oxygenation can therefore be cautiously extended to obstetric airway management. The exception may be when at the extremes of BMI, as the gains in safe apnoeic times are significantly reduced, particularly if FE′O2 90% is not achieved. Unfortunately, it is in these most obese patients where difficult airway management and rapid deoxygenation are often encountered. The advantages of the standard facemask technique to facilitate measurement of FE′O2, and to facilitate bag mask ventilation, if required should not be ignored in this situation. It may be that by combining nasal cannula oxygen with facemask pre-oxygenation (so called low-flow apnoeic oxygenation) will be of value in this cohort. This technique could enable the benefits of facemask pre-oxygenation, apnoeic oxygenation, and easier rescue bag-mask ventilation. Further modelling work comparing lower flow nasal cannula apnoeic oxygenation techniques with HFNO should be undertaken to help clarify the benefits of different models of apnoeic oxygen in obstetrics.
In summary, our modelling shows that HFNO provides superior safe apnoea times in pregnant subjects despite generating a lower FE′O2 after pre-oxygenation and supports cautious application to obstetric anaesthetic practice. Care should be taken when used in BMI ≥50 kg m−2 as the gain in safe apnoea time is limited.
Author's contributions
Design of study: DS, ML, AP, JGH
Design of computational models: JGH
Simulation runs: ML
Configuration of subjects and interpretation of data: DS, ML, AP, JGH
Writing and final approval of manuscript: DS, ML, AP, JGH
Handling editor: Takashi Asai
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.12.031.
Declarations of interest
JGH is the associate editor-in-chief of the British Journal of Anaesthesia. JGH accepts fees for the provision of advice to the police, Crown Prosecution Service, coroners, and solicitors. The other authors have no conflicts to declare.
Funding
This work was supported by EPSRC grant ‘Personalised Simulation Technologies for Optimising Treatment in the Intensive Care Unit: Realising Industrial and Medical Applications’ (Grant No. EP/P023444/1). Fisher and Paykel Healthcare (New Zealand): sponsorship of research and a PhD studentship in apnoea and mechanisms of gas exchange during the use of Optiflow™.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mushambi M.C., Kinsella S.M., Popat M. Obstetric Anaesthetists' Association and Difficult Airway Society guidelines for the management of difficult and failed tracheal intubation in obstetrics. Anaesthesia. 2015;70:1286–1306. doi: 10.1111/anae.13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter R., Wrench I.J., Freeman R. Preoxygenation for general anaesthesia in pregnancy: is it adequate? Int J Obstet Anesth. 2011;20:363–365. doi: 10.1016/j.ijoa.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Douglas M.J., Preston R.L. The obstetric airway: things are seldom as they seem. Can J Anesth. 2011;58:494. doi: 10.1007/s12630-011-9492-8. [DOI] [PubMed] [Google Scholar]
- 4.Patel A., Nouraei S.A. Transnasal Humidified Rapid-Insufflation Ventilatory Exchange (THRIVE): a physiological method of increasing apnoea time in patients with difficult airways. Anaesthesia. 2015;70:323–329. doi: 10.1111/anae.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle A.J., Stolady D., Mariyaselvam M. Preoxygenation and apneic oxygenation using transnasal humidified rapid-insufflation ventilatory exchange for emergency intubation. J Crit Care. 2016;36:8–12. doi: 10.1016/j.jcrc.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Tan P.C.F., Millay O.J., Leeton L., Dennis A.T. High-flow humidified nasal preoxygenation in pregnant women: a prospective observational study. Br J Anaesth. 2019;122:86–91. doi: 10.1016/j.bja.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 7.Shippam W., Preston R., Douglas J., Taylor J., Albert A., Chau A. High-flow nasal oxygen vs. standard flow-rate facemask pre-oxygenation in pregnant patients: a randomised physiological study. Anaesthesia. 2019;74:450–456. doi: 10.1111/anae.14567. [DOI] [PubMed] [Google Scholar]
- 8.Al-Sulttan S., Bampoe S., Howle R. A prospective, up-down sequential allocation study investigating the effectiveness of vital capacity breaths using high-flow nasal oxygenation versus a tight-fitting face mask to pre-oxygenate term pregnant women. Int J Obstet Anesth. 2020 doi: 10.1016/j.ijoa.2020.08.004. Advance access published in August. [DOI] [PubMed] [Google Scholar]
- 9.Au K., Shippam W., Taylor J., Albert A., Chau A. Determining the effective pre-oxygenation interval in obstetric patients using high-flow nasal oxygen and standard flow rate facemask: a biased-coin up-down sequential allocation trial. Anaesthesia. 2020;75:609–616. doi: 10.1111/anae.14995. [DOI] [PubMed] [Google Scholar]
- 10.Hardman J.G., Bedforth N.M., Ahmed A.B., Mahajan R.P., Aitkenhead A.R. A physiology simulator: validation of its respiratory components and its ability to predict the patient's response to changes in mechanical ventilation. Br J Anaesth. 1998;81:327–332. doi: 10.1093/bja/81.3.327. [DOI] [PubMed] [Google Scholar]
- 11.Bedforth N.M., Hardman J.G. Predicting patients' responses to changes in mechanical ventilation: a comparison between physicians and a physiological simulator. Intensive Care Med. 1999;25:839–842. doi: 10.1007/s001340050961. [DOI] [PubMed] [Google Scholar]
- 12.Hardman J.G., Bedforth N.M. Estimating venous admixture using a physiological simulator. Br J Anaesth. 1999;82:346–349. doi: 10.1093/bja/82.3.346. [DOI] [PubMed] [Google Scholar]
- 13.Hardman J.G., Wills J.S., Aitkenhead A.R. Investigating hypoxemia during apnea: validation of a set of physiological models. Anesth Analg. 2000;90:614–618. doi: 10.1097/00000539-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 14.McNamara M.J., Hardman J.G. Hypoxaemia during open-airway apnoea: a computational modelling analysis. Anaesthesia. 2005;60:741–746. doi: 10.1111/j.1365-2044.2005.04228.x. [DOI] [PubMed] [Google Scholar]
- 15.McClelland S.H., Bogod D.G., Hardman J.G. Pre-oxygenation in pregnancy: an investigation using physiological modelling. Anaesthesia. 2008;63:259–263. doi: 10.1111/j.1365-2044.2007.05346.x. [DOI] [PubMed] [Google Scholar]
- 16.McClelland S.H., Bogod D.G., Hardman J.G. Apnoea in pregnancy: an investigation using physiological modelling. Anaesthesia. 2008;63:264–269. doi: 10.1111/j.1365-2044.2007.05347.x. [DOI] [PubMed] [Google Scholar]
- 17.McClelland S.H., Bogod D.G., Hardman J.G. Pre-oxygenation and apnoea in pregnancy: changes during labour and with obstetric morbidity in a computational simulation. Anaesthesia. 2009;64:371–377. doi: 10.1111/j.1365-2044.2008.05785.x. [DOI] [PubMed] [Google Scholar]
- 18.Laviola M., Das A., Chikhani M., Bates D.G., Hardman J.G. Computer simulation clarifies mechanisms of carbon dioxide clearance during apnoea. Br J Anaesth. 2019;122:395e401. doi: 10.1016/j.bja.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Laviola M., Niklas C., Das A., Bates D.G., Hardman J.G. Effect of oxygen fraction on airway rescue: a computational modelling study. Br J Anaesth. 2020;125:69–74. doi: 10.1016/j.bja.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillai A., Chikhani M., Hardman J.G. Apnoeic oxygenation in pregnancy: a modelling investigation. Anaesthesia. 2016;71:1077–1080. doi: 10.1111/anae.13563. [DOI] [PubMed] [Google Scholar]
- 21.Wahba R.W.M. Perioperative functional residual capacity. Can J Anaesth. 1991;38:384–400. doi: 10.1007/BF03007630. [DOI] [PubMed] [Google Scholar]
- 22.Knuttgen H.G., Emerson K. Physiological response to pregnancy at rest and during exercise. J Appl Physiol. 1974;36:549–553. doi: 10.1152/jappl.1974.36.5.549. [DOI] [PubMed] [Google Scholar]
- 23.Eliasson A.H., Phillips Y.Y., Stajduhar K.C., Carome M.A., Cowsar J.D., Jr. Oxygen consumption and ventilation during normal labour. Chest. 1992;102:467–471. doi: 10.1378/chest.102.2.467. [DOI] [PubMed] [Google Scholar]
- 24.Damia G., Mascheroni D., Croci M., Tarenzi L. Perioperative changes in functional residual capacity in morbidly obese patients. Br J Anaesth. 1988;60:574–578. doi: 10.1093/bja/60.5.574. [DOI] [PubMed] [Google Scholar]
- 25.Jakobsson J., Vadman S., Hagel E., Kalman S., Bartha The effects of general anaesthesia on oxygen consumption: a meta-analysis guiding future studies on perioperative oxygen transport. Acta Anaesthesiol Scand. 2019;63:144–153. doi: 10.1111/aas.13265. [DOI] [PubMed] [Google Scholar]
- 26.Desai N., Wicker J., Sajayan A., Mendonca C. A survey of practice of rapid sequence induction for caesarean section in England. Int J Obstet Anesth. 2018;36:3–10. doi: 10.1016/j.ijoa.2018.05.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.