Abstract
Background
Non-human primates are commonly used in neuroimaging research for which general anaesthesia or sedation is typically required for data acquisition. In this analysis, the cumulative effects of exposure to ketamine, Telazol® (tiletamine and zolazepam), and the inhaled anaesthetic isoflurane on early brain development were evaluated in two independent cohorts of typically developing rhesus macaques.
Methods
Diffusion MRI scans were analysed from 43 rhesus macaques (20 females and 23 males) at either 12 or 18 months of age from two separate primate colonies.
Results
Significant, widespread reductions in fractional anisotropy with corresponding increased axial, mean, and radial diffusivity were observed across the brain as a result of repeated anaesthesia exposures. These effects were dose dependent and remained after accounting for age and sex at time of exposure in a generalised linear model. Decreases of up to 40% in fractional anisotropy were detected in some brain regions.
Conclusions
Multiple exposures to commonly used anaesthetics were associated with marked changes in white matter microstructure. This study is amongst the first to examine clinically relevant anaesthesia exposures on the developing primate brain. It will be important to examine if, or to what degree, the maturing brain can recover from these white matter changes.
Keywords: diffusion MRI, general anaesthesia isoflurane, ketamine, neurotoxicity, rhesus monkeys, white matter
Editor's key points.
-
•
The cumulative effects of repeated exposures to ketamine, Telazol® (tiletamine and zolazepam), and isoflurane on early brain development were evaluated in an opportunistic analysis of imaging data in rhesus macaques.
-
•
Diffusion MRI scans from 43 rhesus macaques at either 12 or 18 months of age from two separate primate colonies were analysed for brain structural changes.
-
•
Widespread dose-dependent reductions in white matter structure were observed across the brain as a result of repeated anaesthesia exposures.
-
•
These findings have important translational and experimental implications that require determination of their persistence and possible functional implications.
The adverse impact of prolonged general anaesthesia (GA) on behavioural and brain development was initially not evident in clinical practice until it first became apparent in animal studies. In non-human primates (NHPs), these developmental effects include slower response times and poorer performance on learning tasks, even 3.5 yr after exposure, and increased frequency of anxiety-related behaviours.1,2 Rodent models showed that exposure to a wide range of anaesthetics led to extensive neuronal apoptosis and persistent neurocognitive deficits.3, 4, 5, 6, 7 These seemingly robust results with animal models have been met with some scepticism with regard to the translational implications for clinical practice because only small decreases in cognitive outcome have been detected in children exposed to GA.8 However, an increased risk for more severe cognitive and behavioural conditions attributable to GA has also been reported.8
Limited neuroimaging data on human participants suggest that early GA does not produce long-term loss in grey matter in regions previously identified as vulnerable in laboratory animals.9 However, GA has been associated with altered functional MRI (fMRI) activation patterns during a response inhibition task along with smaller white matter (WM) volumes and disrupted WM microstructure.10,11 These observations on WM volumes are in keeping with histological studies in animals, which found widespread apoptosis of both neurones and oligodendrocytes after long exposures to ketamine, isoflurane, or propofol,1,3, 4, 5, 6, 7,12, 13, 14, 15, 16, 17 with the window of oligodendrocyte vulnerability continuing beyond that of neuronal susceptibility. Because oligodendrocytes play a key role in producing the myelin sheath around axons, anaesthesia-induced loss of oligodendrocytes could mediate some of the adverse neurodevelopmental outcomes observed after GA. In addition, concerns have been raised about the potential for developmental neurotoxicity even with ketamine, a sedative drug that is more widely used in both paediatric practice and with premature infants in the neonatal ICU.18
Human studies typically have the limitation of being retrospective and are difficult to control for the initial clinical condition and subsequent effects of postoperative procedures during recovery. Animal models allow for more-rigorous experimental control. In particular, NHP models are invaluable for investigating the potential impact of anaesthesia on neurodevelopment because of their phylogenetic closeness to humans.19 Prior NHP studies predominantly evaluated exposure times far beyond the clinically relevant duration of 4–24 h., however, the median duration of GA in children under 1 yr is 79 min.20
More systematic research on the unintended consequences of repeated ketamine exposures is indicated, as it is being used more in clinical practice, including with premature infants in neonatal intensive care. The following analysis focuses on the cumulative effect of multiple exposures to anaesthetics commonly used in human and veterinary medicine (ketamine, Telazol® [tiletamine/zolazepam; Zoetis, Parsippany, NJ, USA], and isoflurane21, 22, 23, 24) administered at doses relevant to neuroimaging research in macaques on brain structure. Given previous reports of anaesthesia-induced loss of oligodendrocytes, we were specifically interested in assessing the effects on WM microstructure, which was evaluated via diffusion tensor imaging (DTI), as a possible explanation for emotional and cognitive deficits observed in prior studies.
Methods
Subjects
The analysis focused on two independent cohorts of rhesus macaques originating from similar neurodevelopmental studies, but neither had examined the effects of GA as its primary aim. Only neuroimaging data from healthy animals were analysed. GA exposure resulted from the need for sedation and immobilisation to acquire imaging data, for sample collection, or during routine veterinary care. The significant range in the extent of GA exposure across subjects enabled us to conduct a secondary analysis of the effect of GA on the developing brain.
Neuroimaging data on 28 subjects were obtained from the Wisconsin Neurodevelopment Rhesus Database, Harlow Primate Laboratory (HPL).25 The research protocol was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin. Care and treatment of the animals at HPL met and exceeded the guidelines specified in the Guide for the Care and Use of Laboratory Animals of the National Research Council.
The second data set (N=15) was acquired from a study conducted at the Yerkes National Primate Research Center (YNPRC).26 All study procedures were performed in accordance with the Animal Welfare Act and Guide and were approved by the Emory Institutional Animal Care and Use Committee.
A summary of the subject characteristics is presented in Table 1.
Table 1.
Descriptive statistics for the two cohorts of monkeys used in the analysis. Note that for the YNPRC cohort, subjects were assessed only with either no or four prior MRI scan exposures. Additional exposures to anaesthesia for other reasons were included in the analyses. HPL, Harlow Primate Laboratory; YNPRC, Yerkes National Primate Research Center.
| Number of prior MRI exposures |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPL |
YNPRC |
|||||||||
| 0 (n=2) | 1 (n=4) | 2 (n=10) | 3 (n=6) | 4 (n=6) | 0 (n=6) | 1 (n=0) | 2 (n=0) | 3 (n=0) | 4 (n=9) | |
| Age at scan (postnatal days) | 360–363 | 315–380 | 313–407 | 320–393 | 346–402 | 531–559 | 530–555 | |||
| Weight at scan (kg) | 2.06 (0.16) | 2.20 (0.09) | 2.22 (0.27) | 2.11 (0.21) | 2.45 (0.22) | 2.95 (0.26) | 2.99 (1.36) | |||
| Mean age at first scan (postnatal days) | 361.5 (1.5) | 226.3 (30.4) | 115.3 (19.5) | 50.7 (17.6) | 20.8 (7.1) | 541.7 (9.3) | 14 (4) | |||
| Sex ratio (male:female) | 1:1 | 2:2 | 5:5 | 3:3 | 5:1 | 3:3 | 4:5 | |||
Anaesthesia exposure
Harlow Primate Laboratory
The subjects were administered a pre-scan dose of ketamine hydrochloride (10 mg kg−1 i.m.) for transport to the MRI facility. For infants younger than 6 months of age, GA for scanning was achieved with isoflurane (1.5 vol%). Monkeys older than 7 months of age were anaesthetised throughout the scan with dexmedetomidine 0.015 mg kg−1 i.m. The effects were reversed at the end of the scanning session by administering atipamezole 0.15 mg kg−1 i.v. Plane of anaesthesia was monitored with a pulse oximeter to track HR and oxygen saturation. Overall exposure time was ∼2 h. A few subjects received additional exposures to ketamine for routine veterinary procedures.
Yerkes National Primate Research Center
The subjects were transported with their mother from the YNPRC Field Station to the YNPRC Imaging Center on the day before being scanned. Initial induction of anaesthesia was achieved using Telazol(R) (tiletamine HCl and zolazepam HCl) injection (4.79–5.13 mg kg−1, i.m.) to allow preparation for the scan (tracheal intubation and placement of an i.v. catheter). Scans were acquired under isoflurane anaesthesia kept at the lowest possible concentration (0.8–1 vol%, to effect) throughout the neuroimaging session. This range of isoflurane is below that used in previous papers on macaques, which reported patterns of coherent fMRI blood-oxygen-level-dependent signal fluctuations similar to those observed in awake and behaving monkeys.27, 28, 29, 30 Average isoflurane concentrations were recorded every 15 min during the scan session, and HR and oxygen saturation were monitored continuously. After the scan session and full recovery from anaesthesia, each infant was returned to its mother, and the pair was returned to their social group on the following day. Outside of these scanning sessions, animals were periodically exposed to anaesthetics (Telazol [∼5 mg kg−1 body weight] or ketamine [∼10 mg kg−1 i.m.]) for routine animal care (i.e. semi-annual physical examinations and tuberculosis screening), care for unexpected injuries common in socially housed primates (i.e. digit injury), and for collection of experimental samples at 12 and 18 months of age, as a part of the planned developmental research study.
Normalised exposure
The analysis focused on exposure to the two major anaesthetics, ketamine and isoflurane, which were aggregated into one total normalised exposure (TNE) index. Cumulative exposures to other anaesthetics, such as dexmedetomidine (HPL cohort) and Telazol (YNPRC cohort), were not considered because they were used in only one of the research facilities and could not be normalised across cohorts. High correlation with TNE (0.94 for Telazol and 0.73 for dexmedetomidine) within cohorts precludes adding these two anaesthetics as covariates into separate models for each cohort to estimate their effects in the corresponding sample.
Anaesthesia was normalised per session across the scan sessions, type of anaesthetic, and the two cohorts to generate a consistent metric. The average anaesthesia exposure per MRI session in the HPL cohort was used as a reference, where mean isoflurane per session was 2.0 vol% and mean ketamine per session was 10 mg kg−1. Thus, TNE was counted as TNE=total ketamine×10−1+total isoflurane×2−1, where all anaesthesia exposures were normalised per prior MRI-related exposure, and then aggregated per subject into a single TNE. This TNE captured the cumulative amount of anaesthesia to which a monkey had been exposed. Whilst being an estimate of total exposure, it provided a good approximation of cumulative anaesthetic exposure across the two cohorts (Supplementary Table S1).
Subjects that had received at least one prior MRI-related anaesthetic were referred to being in the ‘repeated’-exposure group, whereas subjects without a prior MRI-related exposure were assigned to the ‘single’-exposure group.
Statistical analysis
To analyse the effect of multiple anaesthesia exposures on WM, a linear model was used with the omnibus of DTI properties: axial (AD), mean (MD), and radial diffusivity (RD), and fractional anisotropy (FA) as independent endpoints, and age, sex, and TNE as covariates. For the YNPRC cohort, age was excluded from the analysis because all monkeys were scanned at the same age. More details about the monkeys, their housing, the MRI protocol, diffusion MRI processing, and statistical procedures are provided in the Supplementary material. Raw data from the Wisconsin cohort are available publicly online at https://www.nitrc.org/projects/uncuw_macdevmri/.
Results
MRI data were analysed from the two monkey cohorts (HPL and YNPRC) to determine the summative impact of anaesthesia exposure. There was a significant correlation between TNE and the extent of impact on WM microstructure. Higher TNE was associated with lower FA and higher AD, MD, and RD, indicating an overall reduction in the integrity of WM tracts. There was not a significant interaction with age or sex.
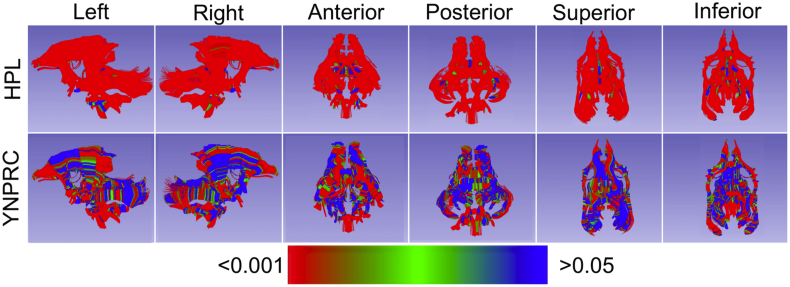
Figure 1 shows false-discovery-rate-corrected P-values for the effect of TNE on the omnibus of DTI properties. Significant effects were found throughout the brain with local P-values <0.01. Additional details on the local P-values for TNE are presented in the Supplementary material. Supplementary Figures S3 and S4 show that when DTI properties were tested separately, effects of anaesthesia exposure remain highly significant across the four diffusion indices and were evident in all of the analysed brain tracts.
Fig 1.
Omnibus of DTI properties FDR corrected p-values from the GLM. In both cohorts there is widespread significant effect of TNE across all regions of the brain and all major tracts. HPL, Harlow Primate Laboratory; YNPRC, Yerkes National Primate Research Center.
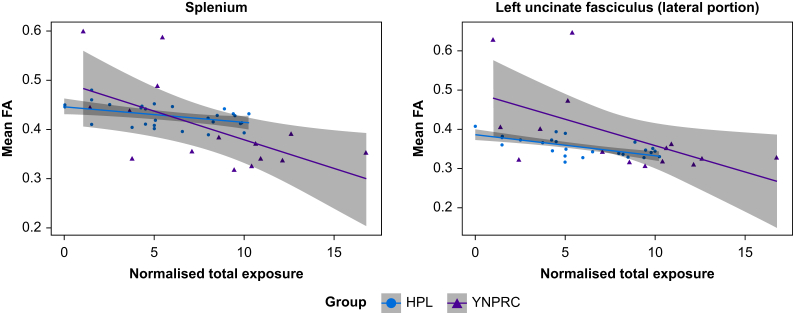
Figure 2 illustrates the effects of anaesthesia exposures used to acquire prior MRI scans. The TNE is plotted with respect to mean FA across two tracts (splenium and lateral portion of the left uncinate fasciculus). In both monkey cohorts, there was a negative relationship between TNE and mean FA for each tract, with more pronounced effects in the YNPRC cohort that experienced repeated anaesthesia sessions of longer duration (Supplementary Table S1). There were two outliers in the YNPRC cohort; excluding those two subjects did not affect the findings.
Fig 2.
Plots showing the change in FA as a function of the TNE to general anaesthesia for two selected tracts. In tracts across the brain a steady decrease in FA was seen as the exposure increases. Note that the effect is more extensive in the YNPRC cohort, in part due to the longer exposure to anaesthesia. FA, fractional anisotropy; HPL, Harlow Primate Laboratory; YNPRC, Yerkes National Primate Research Center.
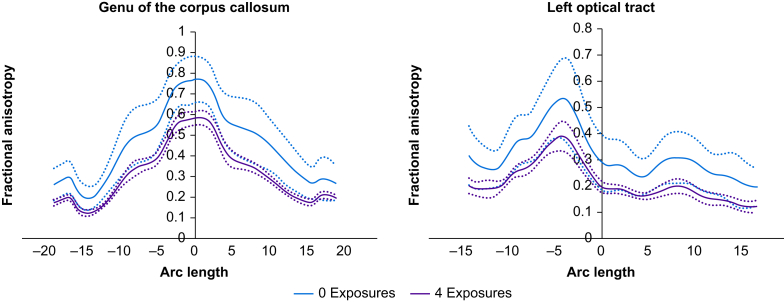
Local FA tract profiles were compared between single- and repeated-exposure groups from the YNPRC cohort (Fig. 3). Whilst the shape of the tract profiles is similar, the profile for the longer-exposure group is shifted lower, in keeping with predictions from the previously discussed effects on tract integrity. Supplementary Figure S5 shows the same anaesthesia effects for AD, MD, and RD, indicating an overt decrease in overall diffusion across these tracts.
Fig 3.
Visualization of the change in FA across the length of the tract averaged over all subjects for two particular tracts (genu and left optic tract) in the YNPRC cohort. Mean FA values are plotted as solid lines and dotted lines indicate standard deviation. There is a clear delination between the groups of a single (green, no prior MRI session) and repeated (red, 4 prior MRI sessions) exposures of anaesthesia. Similar figures for AD, MD, and RD are shown in thr Supplemental Material Figure S5.
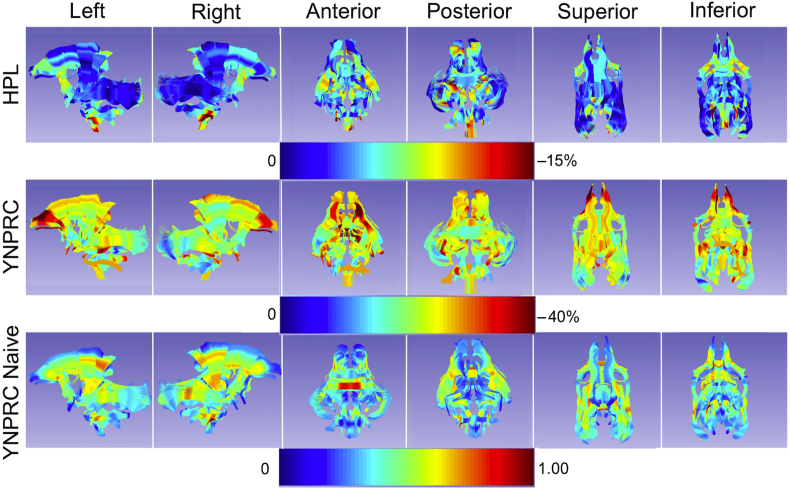
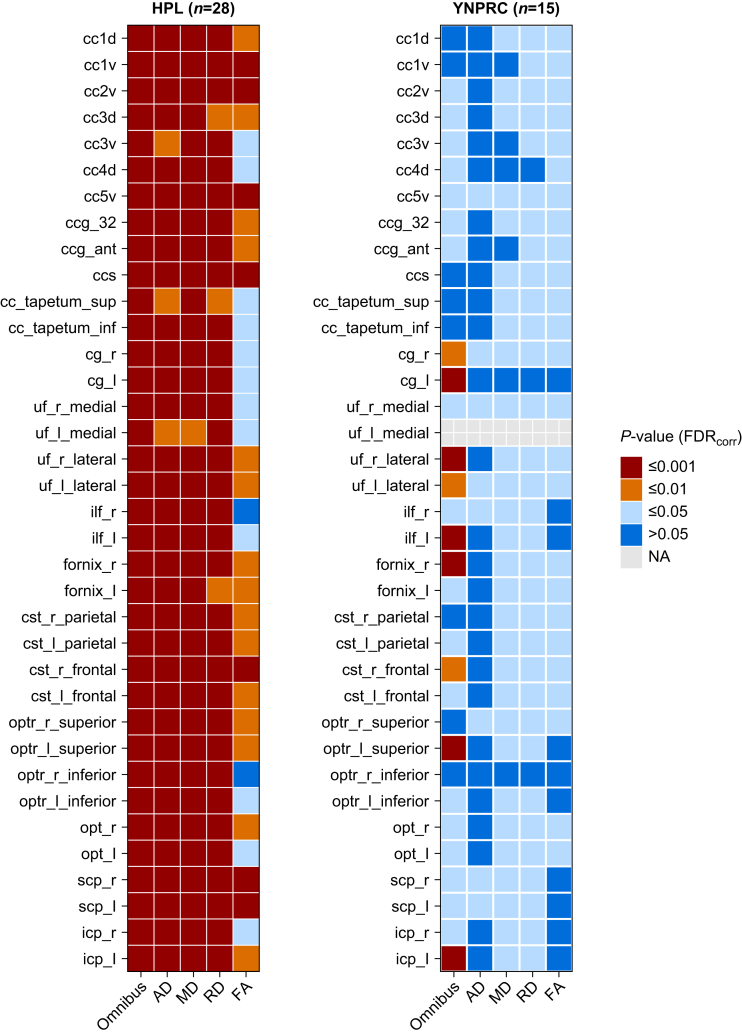
To illustrate the effect of repeated GA exposure on WM, the calculated effect (beta-TNE) was visualised by scaling it relative to prior anaesthesia for a single MRI session (Fig. 4). For the HPL cohort, the anticipated decrease in FA was 2–7% per scan session. For the YNPRC cohort, which had longer exposures, the reduction was 10–20% per scan-related exposure. These effects were not localised, but rather were widespread across the brain. Figure 5 illustrates the statistical significance of the effects of TNE as determined from post hoc testing for each tract and each property.
Fig 4.
Visualization of the expected, average effect of the anaesthesia exposure in the HPL (top) and YNPRC (middle) cohort as a percent change in local FA per MRI session related exposure. For comparison, the mean FA values at 18 months of age in the single exposure group are shown in the bottom row. Note the different color scale used for each cohort. HPL, Harlow Primate Laboratory; YNPRC, Yerkes National Primate Research Center.
Fig 5.
Post hoc p values for normalized total exposure effect. Tracts without values indicate exclusion of that tract during the quality control phase. Red indicates p-values ≤0.001, orange ≤0.01, green ≤0.05, and blue >0.05. Tracts are listed in anterior to posterior anatomical order. AD, axial diffusivity; FA, fractional anisotropy; FDR, false discovery rate; HPL, Harlow Primate Laboratory; MD, mean diffusivity; NA, not applicable due to exclusion; RD, radial diffusivity; YNPRC, Yerkes National Primate Research Center.
Discussion
Multiple exposures to general anaesthetics commonly used in human and veterinary medicine, with exposure durations of ∼2 h typical for MRI sessions, had substantial effects on WM integrity of the developing brain. Total normalised exposure time was associated with magnitude of decrease in AD, MD, and RD, and FA, indicative of widespread impacts on WM integrity.
Alterations in DTI measures were evident in monkeys up to 18 months of age, which is roughly equivalent to early school age in humans. The possible persistence of these effects needs to be examined in future research at older ages. Both the duration and magnitude of the effects are concerning, given that WM integrity has been related to cognitive ability, language, and visual–spatial working memory in children at older ages.31, 32, 33, 34 The effects observed in monkeys may help explain later neurocognitive deficits after early anaesthesia exposures. However, because we did not directly assess cognitive performance or social interactions, the implications for neurobehavioral competence still need to be investigated.
The effect sizes of TNE on the DTI parameters were noticeably different between the two cohorts. Procedural differences between the two facilities may explain the different effect sizes. The most parsimonious explanation is more frequent and longer scan exposures for the YNPRC monkeys, whereas ketamine and other sedatives were not used as regularly during routine veterinary procedures at the HPL, except for neuroimaging scans during the first year of life. The YNPRC monkeys were also exposed to Telazol (containing tiletamine, a ketamine-like N-methyl-D-aspartate receptor antagonist35 that might enhance TNE, whereas the dexmedetomidine administered to HPL monkeys may buffer against ketamine-induced injury to neuronal stem cells.36
Although the final-scan age for the YNPRC group was 18 months and for the HPL group was 12 months, this age difference is unlikely to explain the variation in the effects of anaesthesia exposure. The expected maturational change in diffusion properties from 12 to 18 months of age in monkeys37 is considerably less than the observed effects of anaesthesia exposure. Other differences, including type of scanner, DTI acquisition protocols, age at first exposure, use of multiple anaesthetic drugs, and husbandry, are detailed further in the Supplementary material. Because of these procedural differences, statistical evaluations were conducted separately for each cohort. The fact that the results were congruent in multiple analyses despite procedural differences provides additional confidence that the conclusions generalise.
The duration of GA episodes was normalised to the average duration per imaging session as used for the HPL cohort. Although this approach precluded quantifying the impact of any specific anaesthetic drug, it enabled us to examine typical GA episodes used in monkey imaging research. The study designs and the sample sizes did not permit interrogation of the contribution of each anaesthetic separately in the statistical analysis. Instead, we focused on an integrated metric (TNE) over multiple anaesthetic episodes from birth to last scan.
A limitation of our study is the lack of longitudinal follow-up to address whether the effects on WM described here at just one early juvenile age persist at later ages, or whether there is sufficient neuroplasticity to overcome alterations in the developmental trajectory of myelination. Prior research on oligodendrocyte precursors identified reactive proliferation in isoflurane-exposed infant monkeys that might serve as a compensatory mechanism.12 However, the total brain volume of monkeys at the ages they were scanned is already close to adult size, and the rate of myelination slows down. Additional brain maturational changes, particularly involving development of WM, occur during the pubertal transition between 3 and 4 yr of age and through late adolescence (up to 6 yr). These include cortical thinning and growth of WM,38 but full recovery would require that the observed effects of anaesthesia were attributable only to slowing of the pace of maturation, rather than an irreversible effect.
We also assessed the impact of a single vs repeated exposure to isoflurane, which was the primary anaesthetic used at the YNPRC, which indicated that inhibition of WM might already be initiated after the first session. The literature is inconsistent about whether one exposure is sufficient to induce a large effect.8 Two recent papers on anaesthesia exposure in macaques focused on longer exposures for 4–5 h during the first month of life.39,40 Only multiple bouts of GA induced a long-lasting effect on the development of motor reflexes and anxious emotional behaviour.39 Further research is needed to resolve the significance of anaesthesia duration and repeat exposures.
There exist important limitations of this study, as it was opportunistic and based on a post-hoc analysis of previously acquired DTI scans. Thus, it was not possible to titrate exposure to anaesthesia in a dose-escalation manner in advance, requiring the surrogate index of TNE. In addition, data were pooled from two different cohorts of monkeys in different facilities. However, the use of MRI data acquired with different experimental designs did provide the opportunity to determine if the conclusions generalise. Finally, all exposures to anaesthesia were weighted equally, even though earlier exposures at younger ages might potentially have a larger impact than later ones. Ideally, a future prospective study either would include a sham condition or separately administer anaesthesia to monkeys without the additional procedures required for MRI scanning.
Our findings from two different MRI studies of developing monkeys provide confirmatory evidence that repeated exposure to general anaesthetics used to acquire neuroimaging data can cause a large reduction in overall WM integrity. They also raise concerns about the potential for overuse of sedatives in veterinary practice when it is possible to conduct certain procedures on conscious animals, such as routine collection of blood samples or annual physical examinations. In vivo brain scanning under GA is an integral aspect of many NHP models of psychiatric and neurological conditions, and thus, the effects of repetitive anaesthetic exposure may be additive and interact with the effects of main variables. Therefore, it needs to be included in analyses as a confounder. If the effects on WM translate to young children and the types of anaesthesia used in clinical practice, there are also important implications for the use of anaesthesia during formative periods when maturational trajectories and regulatory set points for brain development are being established.
Authors' contributions
Study conception: JTY, RMV, YS, MS
Data acquisition: CLC, GRL, ALA, MS, XH, EM, BRH
Data analysis: JTY, RMV, YS, MS, JN, MN
Data interpretation: RCK, MGP, GC, KIK
Result interpretation: JTY, RMV, YS, MS, CLC, GRL, ALA, XH, EM, BRH
Drafting/revising of paper: all authors
Final approval: all authors
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
US National Institute of Mental Health (MH901645, MH091645-S1, MH100031, MH086633, P50 MH064065, MH070890, Roadmap Grant U54 EB005149-01, P50 MH078105, and MH078105-S1); National Institute of Child Health and Human Development (HD003352, HD003110, HD079124, HD053000, and HD055255); National Institutes of Health (UNC Intellectual and Developmental Disabilities Research Center P30 HD03110, MH091645, and Office of Research Infrastructure Programs/Office of the Director grant OD11132 [Yerkes National Primate Research Center Base Grant]).
Handling editor: Hugh C Hemmings Jr
Footnotes
This article is accompanied by an editorial: Developmental exposure to general anaesthesia: missed connections? by Baxter & Fehr, Br J Anaesth 2021:126:756–758, doi: 10.1016/j.bja.2021.01.013
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.12.029.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Paule M.G., Li M., Allen R.R. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–230. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raper J., Alvarado M.C., Murphy K.L., Baxter M.G. Multiple anesthetic exposure in infant monkeys alters emotional reactivity to an acute stressor. Anesthesiology. 2015;123:1084–1092. doi: 10.1097/ALN.0000000000000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saito S., Fujita T., Igarashi M. Effects of inhalational anesthetics on biochemical events in growing neuronal tips. Anesthesiology. 1993;79:1338–1347. [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V., Hartman R.E., Izumi Y. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young C., Jevtovic-Todorovic V., Qin Y.Q. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu L.X., Yon J.H., Carter L.B., Jevtovic-Todorovic V. General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis. 2006;11:1603–1615. doi: 10.1007/s10495-006-8762-3. [DOI] [PubMed] [Google Scholar]
- 7.Cattano D., Young C., Straiko M.M.W., Olney J.W. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–1714. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 8.Davidson A.J., Sun L.S. Clinical evidence for any effect of anesthesia on the developing brain. Anesthesiology. 2018;128:840–853. doi: 10.1097/ALN.0000000000001972. [DOI] [PubMed] [Google Scholar]
- 9.Backeljauw B., Holland S.K., Altaye M., Loepke A.W. Cognition and brain structure following early childhood surgery with anesthesia. Pediatrics. 2015;136:e1–12. doi: 10.1542/peds.2014-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taghon T.A., Masunga A.N., Small R.H., Kashou N.H. A comparison of functional magnetic resonance imaging findings in children with and without a history of early exposure to general anesthesia. Paediatr Anaesth. 2015;25:239–246. doi: 10.1111/pan.12606. [DOI] [PubMed] [Google Scholar]
- 11.Block R.I., Thomas J.J., Bayman E.O., Choi J.Y., Kimble K.K., Todd M.M. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 12.Brambrink A.M., Evers A.S., Avidan M.S. Ketamine-induced neuroapoptosis in the fetal and neonatal rhesus macaque brain. Anesthesiology. 2012;116:372–384. doi: 10.1097/ALN.0b013e318242b2cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creeley C.E., Dikranian K.T., Dissen G.A., Back S.A., Olney J.W., Brambrink A.M. Isoflurane-induced apoptosis of neurons and oligodendrocytes in the fetal rhesus macaque brain. Anesthesiology. 2014;120:626–638. doi: 10.1097/ALN.0000000000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma D., Williamson P., Januszewski A. Xenon mitigates isoflurane-induced neuronal apoptosis in the developing rodent brain. Anesthesiology. 2007;106:746–753. doi: 10.1097/01.anes.0000264762.48920.80. [DOI] [PubMed] [Google Scholar]
- 15.Slikker W.J., Zou X., Hotchkiss C.E. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–158. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 16.Zou X., Patterson T.A., Divine R.L. Prolonged exposure to ketamine increases neurodegeneration in the developing monkey brain. Int J Dev Neurosci. 2009;27:727–731. doi: 10.1016/j.ijdevneu.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Brambrink A.M., Evers A.S., Avidan M.S. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112 doi: 10.1097/ALN.0b013e3181d049cd. 834–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dong C., Anand K.J.S. Developmental neurotoxicity of ketamine in pediatric clinical use. Toxicol Lett. 2013;220:53–60. doi: 10.1016/j.toxlet.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 19.Lacreuse A., Herndon J.G. Nonhuman primate models of cognitive aging. In: Bizon J.L., Woods A., editors. Animal models of human cognitive aging. Humana Press; New York, USA: 2009. pp. 29–58. [Google Scholar]
- 20.Bartels D.D., McCann M.M., Davidson A.J., Polaner D.M., Whitlock E.L., Bateman B.T. Estimating pediatric general anesthesia exposure: quantifying duration and risk. Paediatr Anaesth. 2018;28:520–527. doi: 10.1111/pan.13391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durrmeyer X., Vutskits L., Anand K.J.S., Rimensberger P.C. Use of analgesic and sedative drugs in the NICU: integrating clinical trials and laboratory data. Pediatr Res. 2010;67:117–127. doi: 10.1203/PDR.0b013e3181c8eef3. [DOI] [PubMed] [Google Scholar]
- 22.Hall R.W., Shbarou R.M. Drugs of choice for sedation and analgesia in the neonatal ICU. Clin Perinatol. 2009;36:215–226. doi: 10.1016/j.clp.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Kohrs R., Durieux M.E. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 24.Miyabe-Nishiwaki T., Masui K., Kaneko A., Nishiwaki K., Shimbo E., Kanazawa H. Hypnotic effects and pharmacokinetics of a single bolus dose of propofol in Japanese macaques (Macaca fsucata fsucata) Vet Anaesth Analg. 2010;37:501–510. doi: 10.1111/j.1467-2995.2010.00564.x. [DOI] [PubMed] [Google Scholar]
- 25.Young J.T., Shi Yu, Niethammer M. The UNC-Wisconsin rhesus macaque neurodevelopment database: a structural MRI and DTI database of early postnatal development. Front Neurosci. 2017;11:1–11. doi: 10.3389/fnins.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Howell B.R., McMurray M.S., Guzman D.B. Maternal buffering beyond glucocorticoids: impact of early life stress on corticolimbic circuits that control infant responses to novelty. Soc Neurosci. 2017;12:50–64. doi: 10.1080/17470919.2016.1200481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vincent J.L., Patel G.H., Fox M.D. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 28.Hutchison R.M., Womelsdorf T., Gati J.S., Leung L.S., Menon R.S., Everling S. Resting-state connectivity identifies distinct functional networks in macaque cingulate cortex. Cereb Cortex. 2012;22:1294–1308. doi: 10.1093/cercor/bhr181. [DOI] [PubMed] [Google Scholar]
- 29.Li C.X., Patel S., Auerbach E.J., Zhang X. Dose-dependent effect of isoflurane on regional cerebral blood flow in anesthetized macaque monkeys. Neurosci Lett. 2013;541:58–62. doi: 10.1016/j.neulet.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang C.Y., Ramani R. Functional connectivity and anesthesia. Int Anesthesiol Clin. 2016;54:143–155. doi: 10.1097/AIA.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 31.Short S.J., Elison J.T., Goldman B.D. Associations between white matter microstructure and infants’ working memory. Neuroimage. 2013;64:156–166. doi: 10.1016/j.neuroimage.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S.J., Steiner R.J., Yu Y. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc Natl Acad Sci U S A. 2017;114:148–153. doi: 10.1073/pnas.1604658114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deoni S.C.L., O’Muircheartaigh J., Elison J.T. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct. 2016;221:1189–1203. doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Muircheartaigh J., Dean D.C., Dirks H. Interactions between white matter asymmetry and language during neurodevelopment. J Neurosci. 2013;33:16170–16177. doi: 10.1523/JNEUROSCI.1463-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olney J.W., Labruyere J., Price M.T. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 36.Lu P., Lei S., Li W. Dexmedetomidine protects neural stem cells from ketamine-induced injury. Cell Physiol Biochem. 2018;47:1377–1388. doi: 10.1159/000490823. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y., Short S.J., Knickmeyer R.C. Diffusion tensor imaging-based characterization of brain neurodevelopment in primates. Cereb Cortex. 2013;23:36–48. doi: 10.1093/cercor/bhr372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knickmeyer R.C., Styner M., Short S.J. Maturational trajectories of cortical brain development through the pubertal transition: unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman K., Robertson N.D., Dissen G.A. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarado M.C., Murphy K.L., Baxter M.G. Visual recognition memory is impaired in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2017;119:517–523. doi: 10.1093/bja/aew473. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.