Abstract
Background
Novel preventive therapies are needed for postoperative delirium, which especially affects older patients. A mouse model is presented that captures inflammation-associated cortical slow wave activity (SWA) observed in patients, allowing exploration of the mechanistic role of prostaglandin-adenosine signalling.
Methods
EEG and cortical cytokine measurements (interleukin 6, monocyte chemoattractant protein-1) were obtained from adult and aged mice. Behaviour, SWA, and functional connectivity were assayed before and after systemic administration of lipopolysaccharide (LPS)+piroxicam (cyclooxygenase inhibitor) or LPS+caffeine (adenosine receptor antagonist). To avoid the confounder of inflammation-driven changes in movement which alter SWA and connectivity, electrophysiological recordings were classified as occurring during quiescence or movement, and propensity score matching was used to match distributions of movement magnitude between baseline and post-LPS administration.
Results
LPS produces increases in cortical cytokines and behavioural quiescence. In movement-matched data, LPS produces increases in SWA (likelihood-ratio test: χ2(4)=21.51, P<0.001), but not connectivity (χ2(4)=6.39, P=0.17). Increases in SWA associate with interleukin 6 (P<0.001) and monocyte chemoattractant protein-1 (P=0.001) and are suppressed by piroxicam (P<0.001) and caffeine (P=0.046). Aged animals compared with adult animals show similar LPS-induced SWA during movement, but exaggerated cytokine response and increased SWA during quiescence.
Conclusions
Cytokine-SWA correlations during wakefulness are consistent with observations in patients with delirium. Absence of connectivity effects after accounting for movement changes suggests decreased connectivity in patients is a biomarker of hypoactivity. Exaggerated effects in quiescent aged animals are consistent with increased hypoactive delirium in older patients. Prostaglandin-adenosine signalling may link inflammation to neural changes and hence delirium.
Keywords: cytokines, delirium, electroencephalography, functional connectivity, slow wave activity
Editor's key points.
-
•
Inflammation is thought to be implicated in the pathophysiology of delirium, but distinguishing causation from association is challenging.
-
•
There are no good animal models for delirium, which makes it difficult for scientists to characterise the neurobiology of delirium and to develop interventions targeted accordingly.
-
•
This interesting rodent study showed that lipopolysaccharide-induced inflammation produces electroencephalographic (slow waves) and behavioural (decreased movement) features in wakefulness—an important methodological strength of this work—and these are consistent with certain characteristics of delirium in humans.
-
•
Lipopolysaccharide-treated rodents displayed features of hypoactive delirium in response to an inflammatory challenge, which is consistent with the common subtype of delirium, but raises the question why hyperactivity was not seen at all if this is a reliable animal model of delirium.
Inflammation is a hypothesised mechanism of many neurological disorders, be they chronic, such as dementia, or acute, such as delirium.1, 2, 3, 4, 5, 6 Even in less severe cases, acute inflammation probably affects brain function with illnesses including the common cold or influenza, causing neurological effects such as somnolence.7 Elucidating how inflammation might affect brain function could highlight therapeutic targets to reduce the burden of these conditions.
Delirium is an acute disturbance of consciousness characterised by reduced attention, disorganised thinking, and fluctuating arousal levels that often affect sick older patients, especially those undergoing high-risk surgery.8, 9, 10, 11, 12 The electrophysiological hallmark of delirium is EEG slow wave activity (SWA),13,14 similar to that observed during non-rapid eye movement sleep. SWA during delirium seems to particularly involve posterior brain regions.14 Although evidence suggests that SWA during natural overnight sleep is restorative and enhances cognitive function, SWA during wakefulness, as occurs in delirium, is associated with cognitive deficits.15 We have suggested that SWA may precipitate cognitive disintegration in delirium, such that patients are awake and confused.1 Inflammation might be the predominant acute cause of delirium1,14,16; it drives somnolence and enhances SWA in sleep,17, 18, 19 and inflammation may similarly drive SWA in delirium.20 In older patients, EEG SWA correlates with delirium severity, plasma cytokines, and EEG connectivity.14 Our overarching hypothesis is that inflammation drives the observed acute changes in SWA13,14 and disrupted cortical connectivity14,21 during wakefulness, triggering sudden and profound impairment in cognition. Herein, we test the link between inflammation and changes in brain activity and connectivity in a mouse model.
Progress on developing therapeutic interventions for delirium has been limited, perhaps partly because of the lack of an established animal model to provide insights into its pathogenesis. A critical limitation has been in identifying translational biomarkers of this complex human cognitive disorder. Recognising the difficulty in establishing an animal model for these cognitive deficits, we focus on a translational biomarker of delirium, SWA in the EEG, building on previous work.22,23 In an advance from this earlier work, we focus on (i) SWA specific to active wakefulness, (ii) inflammation as the primary trigger, and (iii) how age may modulate these two factors, consistent with age being a key predisposing factor to delirium. Furthermore, we test interventions that attenuate the behavioural consequences of lipopolysaccharide (LPS) administration. Based on prior studies showing that cyclooxygenase inhibitors attenuate acute behavioural changes induced by LPS by inhibiting the prostaglandin response,3,24 and that prostaglandins act as somnogens via adenosine signalling,25,26 we investigated the role of prostaglandin-adenosine signalling in linking inflammation to changes in neural activity and connectivity.
Methods
Further methodological details can be found in Supplementary Methods online.
Data collection
All procedures with animals were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and in full accordance with Research Animal Resources and Compliance. Adult (2–8 months old; n=68) and aged (16–24 months old, n=14) c57Bl/6J mice were used in this study (Supplementary Table S1). Of these 82 mice, 72 were instrumented for skull screw EEG recordings (bilateral parietal and frontal electrodes). After 5–7 days of recovery, animal activity, resting-state EEG, and anterior-posterior functional connectivity were assayed during the animals' dark (active) phase during a 1 h baseline period and for several hours after treatments, after which animals were euthanised and their brains frozen for later cytokine enzyme-linked immunosorbent assay (ELISA) (Supplementary Figs S1 and S7). For LPS-alone experiments, animals were recorded 1 h before and for 4 h after intraperitoneal (i.p.) LPS administration at t=0 h (vehicle=NaCl 0.9%, low LPS=12.5 or 25 μg kg−1, high LPS=125 μg kg−1). For piroxicam experiments, recordings commenced at t=−2 h, piroxicam (10 mg kg−1 i.p.) was administered at t=−1 h, followed by LPS (25 μg kg−1) at t=0 h, and euthanasia at t=4 h. Because caffeine has a short half-life in mice (<1 h),27 three i.p. injections of caffeine citrate (30 mg kg−1) were administered: the first at t=0 h (along with LPS 25 μg kg−1), then at t=1 h and t=2 h. To monitor movement and activity levels, video was recorded for the duration of electrophysiological recording and analysed offline.
Data analysis
Band power analysis of EEG data proceeded according to standard techniques,28 with power calculated in 4-s sliding windows in the delta (i.e. SWA, 2–4 Hz), theta (4–12 Hz), alpha (13–20 Hz), beta (20–30 Hz), and gamma (30–80 Hz) bands and normalised by total power. Functional connectivity was assayed using the alpha band debiased weighted phase lag index (wPLI),29 calculated in 20-s sliding windows between anterior and posterior channel pairs for each hemisphere, and averaged across hemispheres. We chose alpha band wPLI a priori because it is a standard metric of functional connectivity28 and is used especially in other papers on delirium.14,21,30 A movement signal, derived from the smoothed and normalised frame-by-frame video difference signal, was aligned in time with the simultaneously recorded EEG signal, and the movement signal averaged in each 4-s or 20-s epoch for band power and wPLI analysis, respectively. Epochs with non-zero estimated movement were used to calculate electrophysiological parameters corresponding to active wakefulness. To ensure that drug-induced changes in activity level for epochs classified as active wakefulness did not influence measured electrophysiological parameters, distributions of movement signal magnitude were matched between baseline and treatment periods using propensity score matching (PSM; Supplementary Fig. S2).31
Cytokine quantification was applied to brains from 46 mice with EEG recordings and 10 uninstrumented mice subjected to identical drug treatments (Supplementary Table S1). The cytokines interleukin 6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) were quantified by multiplex ELISA performed by Eve Technologies (#MDF10, Calgary, AB, Canada). IL-6 was selected a priori as the primary cytokine of interest, as brain levels of IL-6 increase dramatically in the hours after peripheral treatment with LPS,32 with IL-6 having a relatively long half-life compared with other cytokines,33 and IL-6 tends to be higher in hip fracture surgical patients with delirium compared with those without.34 Additionally, MCP-1 was chosen based on our recent findings in humans showing that MCP-1 correlates with delirium severity and EEG SWA.14
To compare LPS-driven changes in EEG and activity measures across time, data were averaged across epochs during a ‘peak effect’ period (t=1–3 h post-injection) and compared with the average across epochs during the ‘baseline’ pre-injection period (t=−1–0 h). LPS effects on band power, movement, and wPLI were assessed by fitting linear mixed effects models.35 Fixed effects were treatment group, experiment epoch (baseline vs peak effect), and their interaction, with random effects for animal to account for repeated measures and for multiple electrodes within animals when appropriate. Models fit to cytokine data used group and region (anterior/posterior) as fixed effects.
LPS dose categories were as follows: ‘vehicle’ (n=7) corresponded to 0 μg kg−1 (i.e. NaCl 0.9% alone), ‘low LPS’ to 12.5 μg kg−1 (n=8) and 25 μg kg−1 (n=15) (grouped together as the outcomes of statistical analyses were unchanged by grouping the low doses), and ‘high LPS’ (n=8) to 125 μg kg−1. Effects of LPS were tested by comparing models with and without the group-by-epoch interaction (or group-by-region for cytokine data) using likelihood ratio tests. Caffeine and the associated saline controls were fit in separate models from the other groups because of their differing experiment schedule, which may have affected behaviour and precluded direct comparisons of these groups to the others. Post hoc comparisons used the Kenward-Roger method and P-values were adjusted for the family of relevant multiple comparisons by estimating a multivariate t distribution using the emmeans package for R (R Foundation for Statistical Computing, Vienna, Austria).36
For relationships between cytokine levels and changes in movement-matched SWA, we fit a linear model to all data in the vehicle, low LPS, and high LPS groups to estimate SWA as a function of cytokine concentration. We then predicted SWA changes based on cytokine levels observed in the piroxicam+low LPS group and tested whether the mean residual differed from zero using a one-sample t-test.
Results
lipopolysaccharide injection increases inflammatory markers in the brain
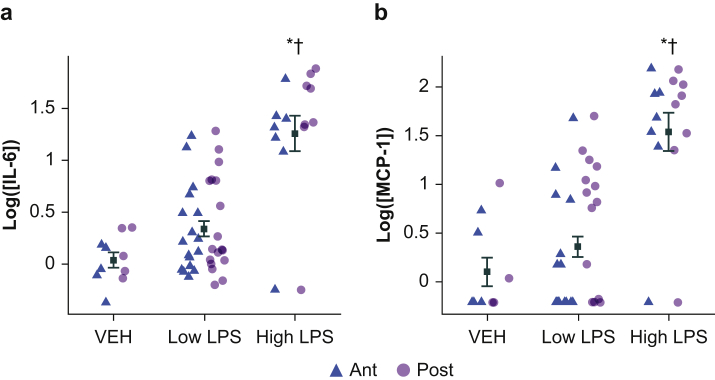
We first used ELISA to measure protein levels of proinflammatory cytokines IL-6 and MCP-1 (Fig. 1a and b) in anterior or posterior mouse neocortex (purple and green, respectively) 4 h after i.p. injection of LPS. There was no interaction between region and LPS dose for IL-6 (likelihood ratio test adding location: χ2(4)=4.30, P=0.37) or MCP-1 (likelihood ratio test adding location: χ2(4)=6.85, P=0.14); thus, we averaged anterior and posterior samples. IL-6 and MCP-1 concentrations were elevated after LPS injection, and there was a significant overall effect of LPS group on cytokine concentration (Table 1A and B). Cytokine concentrations in vehicle animals were not different from low LPS animals, although both groups had significantly lower cytokine concentrations compared with high LPS.
Fig 1.
Proinflammatory cytokine levels in neocortex after lipopolysaccharide treatment. (a) Shown are IL-6 protein concentrations measured via enzyme-linked immunosorbent assay in neocortical homogenate samples from mice (including animals with and without EEG implant) euthanised 4 h after LPS injection. Each point represents log IL-6 concentration (pg ml−1) from samples obtained bilaterally from anterior (ant) or posterior cortex (post). Overlaid symbols (black) represent the within-group mean across all samples. Error bars represent ± standard error of the mean. (b) MCP-1 protein concentration values are shown. MCP-1 samples below the threshold of detection were set to the square root of the lowest observed quantity (1.13). ∗Indicates significant difference from VEH. †Indicates significant difference from low LPS. IL-6, interleukin 6; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; VEH, vehicle.
Table 1.
Statistical modelling results. Shown are results of likelihood ratio tests for both the main models and models comparing caffeine experiments, along with associated sample counts (n1, n2), P-values and the results of pairwise comparisons. Effects of LPS were tested by comparing models with and without the group-by-epoch interaction (or group itself for cytokine data) using likelihood ratio tests. Caffeine experiments were fit in separate models together with equivalent saline controls; since there are only two factor levels no pairwise comparisons are needed. Post hoc comparisons used the Kenward-Roger method and P-values for pairwise comparisons were adjusted for multiple comparisons by estimating a multivariate t-distribution using the emmeans package for R.36 IL-6, interleukin 6; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; Movt, movement; PXM, piroxicam; SWA, slow wave activity; VEH, vehicle; wPLI, weighted phase lag index.
| Measure | Likelihood ratio test/pairwise comparison | n1, n2 | P-value | Measure | Likelihood ratio test/pairwise comparison | n1, n2 | P-value | ||
|---|---|---|---|---|---|---|---|---|---|
| A | IL-6 | χ2(4)=29.0 | <0.0001 | B | MCP-1 | χ2(4)=29.1 | <0.0001 | ||
| VEH vs low LPS | 5, 18 | 0.68 | VEH vs low LPS | 5, 18 | 0.87 | ||||
| VEH vs high LPS | 5, 8 | 0.0004 | VEH vs high LPS | 5, 8 | 0.0003 | ||||
| Low LPS vs high LPS | 18, 8 | 0.0002 | Low LPS vs high LPS | 18, 8 | <0.0001 | ||||
| VEH vs PXM+low LPS | 5, 12 | 0.23 | VEH vs PXM+low LPS | 5, 12 | 0.12 | ||||
| Low LPS vs PXM+low LPS | 18, 12 | 0.69 | Low LPS vs PXM+low LPS | 18, 12 | 0.17 | ||||
| Low LPS vs aged low LPS | 18, 13 | 0peak.0033 | Low LPS vs aged low LPS | 18, 13 | 0.014 | ||||
| C | SWA | χ2(4)=87.8 | <0.0001 | D | gamma | χ2(4)=78.6 | <0.0001 | ||
| VEH vs low LPS | 7, 17 | <0.0001 | VEH vs low LPS | 7, 17 | <0.0001 | ||||
| VEH vs high LPS | 7, 8 | <0.0001 | VEH vs high LPS | 7, 8 | <0.0001 | ||||
| Low LPS vs high LPS | 17, 8 | 0.041 | Low LPS vs high LPS | 17, 8 | 0.043 | ||||
| VEH vs PXM+low LPS | 7, 8 | 0.72 | VEH vs PXM+low LPS | 7, 8 | 0.5 | ||||
| Low LPS vs PXM+low LPS | 17, 8 | <0.0001 | Low LPS vs PXM+low LPS | 17, 8 | <0.0001 | ||||
| Low LPS vs aged low LPS | 17, 14 | 0.025 | Low LPS vs aged low LPS | 17, 14 | 0.36 | ||||
| Caffeine | χ2(1)=36.6 | 8, 10 | <0.001 | Caffeine | χ2(1)=61.5 | 8, 10 | <0.0001 | ||
| E | wPLI | χ2(4)=26.2 | <0.0001 | F | Movt | χ2(4)=18.8 | 0.00085 | ||
| VEH vs low LPS | 7, 17 | 0.011 | VEH vs low LPS | 7, 17 | 0.014 | ||||
| VEH vs high LPS | 7, 8 | 0.002 | VEH vs high LPS | 7, 8 | 0.0005 | ||||
| Low LPS vs high LPS | 17, 8 | 0.61 | Low LPS vs high LPS | 17, 8 | 0.25 | ||||
| VEH vs PXM+low LPS | 7, 8 | 0.063 | VEH vs PXM+low LPS | 7, 8 | 0.23 | ||||
| Low LPS vs PXM+low LPS | 17, 8 | 1 | Low LPS vs PXM+low LPS | 17, 8 | 0.83 | ||||
| Low LPS vs aged low LPS | 17, 12 | 0.031 | Low LPS vs aged low LPS | 17, 14 | 0.99 | ||||
| Caffeine | χ2(1)=18.2 | 8, 10 | 0.0002 | Caffeine | χ2(1)=18.8 | 8, 10 | <0.0001 | ||
| G | SWAMovt-matched | χ2(4)=21.5 | 0.00025 | H | SWAQuiescent | χ2(4)=19.7 | 0.00057 | ||
| VEH vs low LPS | 7, 16 | 0.0086 | VEH vs low LPS | 6, 16 | 0.67 | ||||
| VEH vs high LPS | 7, 5 | 0.0057 | VEH vs high LPS | 6, 5 | 0.32 | ||||
| Low LPS vs high LPS | 16, 5 | 0.77 | Low LPS vs high LPS | 16, 5 | 0.82 | ||||
| VEH vs PXM+low LPS | 7, 7 | 1 | VEH vs PXM+low LPS | 6, 7 | 1 | ||||
| Low LPS vs PXM+low LPS | 16, 7 | 0.013 | Low LPS vs PXM+low LPS | 16, 7 | 0.83 | ||||
| Low LPS vs aged low LPS | 16, 13 | 0.99 | Low LPS vs aged low LPS | 16, 13 | 0.0086 | ||||
| Caffeine | χ2(1)=3.98 | 8, 8 | 0.046 | Caffeine | χ2(1)=2.61 | 8, 8 | 0.11 | ||
| I | wPLIMovt-matched | χ2(4)=6.39 | 0.17 | J | wPLIQuiescent | χ2(4)=4.77 | 0.31 | ||
| Caffeine | χ2(1)=7.0 | 8, 6 | 0.0082 | Caffeine | χ2(1)=1.49 | 8, 8 | 0.22 | ||
lipopolysaccharide injection increases slow wave activity and decreases antero-posterior connectivity
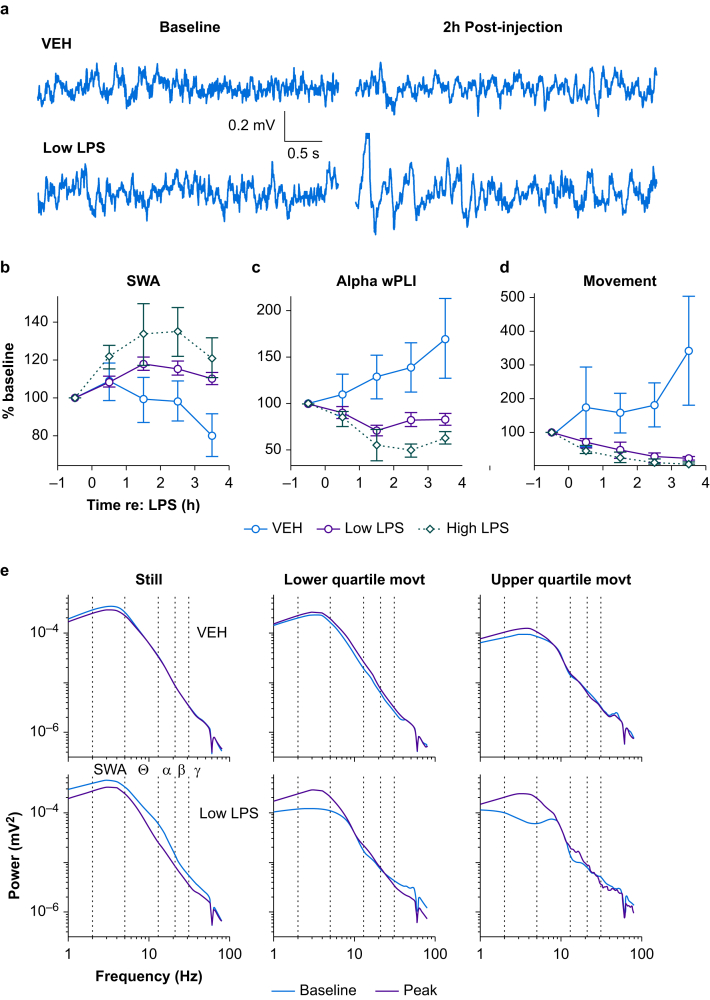
LPS administration was followed by a slowing of resting-state brain activity, manifest as an increase in SWA in the EEG signal (Fig. 2a and b) and a concomitant decrease in amplitude in higher frequency bands, such as gamma (Supplementary Fig. S3). SWA band power showed clear changes after LPS injection, with the effect reaching a peak between 1 and 3 h post-injection (‘peak LPS’; Fig. 2b). Increases in SWA from baseline to peak LPS significantly depended on LPS dose (Table 1C). Similarly, decreases in gamma power after LPS treatment were dose-dependent (Supplementary Fig. S4a; Table 1D). In a regional analysis of SWA we found a significant interaction between anterior/posterior region, group, and time (likelihood ratio test: χ2(3)=8.82, P=0.032). However, this effect was limited to a larger posterior compared with anterior increase in the high LPS animals, which could be explained by ceiling effects specifically in the high LPS condition (at baseline, anterior SWA was greater than posterior power in all groups). As animals in all subsequent experiments received either vehicle or low LPS, and because the LPS effect was comparable in anterior and posterior channels in the low LPS group, we did not alter our a priori statistical plan to combine anterior and posterior channels in analyses of power.
Fig 2.
Generalised slowing of EEG and disrupted functional connectivity after lipopolysaccharide injection. (a) Representative time-domain EEG signals during the pre-injection baseline hour (left) and 2 h post-injection (right) are shown for animals that received either a saline ‘vehicle’ (top) or 25 μg kg−1 ‘low’ LPS injection (bottom). Traces were selected based on having mean SWA values approximately equal to the mean SWA over the entire hour. (b) The time series of SWA (2–4 Hz) normalised to mean spectral power (2–80 Hz) are shown for the different LPS doses. Symbols represent the mean percent change in SWA from baseline across all animals at each LPS dose at each recording hour. Error bars represent plus or minus standard error of the mean. (c) Time series of alpha band (13–20 Hz) anterior-posterior wPLI, a measure of functional connectivity. (d) The time series of movement for each LPS dose is shown. Symbols represent the mean percent change in movement from baseline across all animals at each dose of LPS at each recording hour. Error bars represent plus or minus standard error of the mean. (e) Example EEG power spectra separated according to movement magnitude. Power spectra were calculated in overlapping 4-s windows and aligned with movement epochs, then data were binned into quiescence (i.e. zero movement, left), lower quartile movement (middle), or upper quartile movement (right), and averaged. Pre-injection spectra (‘Baseline’) and average spectra of 1–3 h post-injection (‘Peak’), averaged across four EEG channels, are shown from animals that received either vehicle (top) or a 25 μg kg−1 ‘low’ dose of LPS (bottom). LPS, lipopolysaccharide; movt, movement; SWA, slow wave activity; VEH, vehicle; wPLI, weighted phase lag index.
Previous reports have suggested that cortical functional connectivity (measured by alpha band wPLI) is disrupted in delirious patients,14,21 and systemic LPS can alter connectivity in human volunteers.37 Consistent with these previous observations, we observed decreased alpha band wPLI after injection of LPS (Fig. 2c). Alpha band connectivity decreased more in low LPS and high LPS animals compared with vehicle, although there was no difference between LPS doses (Table 1E).
Lipopolysaccharide decreases movement
Animals injected with LPS exhibited sickness behaviour typical of systemic inflammation, including piloerection, hunched posture, and reduced locomotion and grooming activity.38 The effect of LPS on overall activity level was quantified by the magnitude of the movement signal derived from the video recordings (Supplementary Fig. S5, black; see Methods). Consistent with prior observations,39 LPS caused a dramatic decrease in movement from baseline to peak LPS hours (Fig. 2d). Movement decreased more in low LPS and high LPS animals compared with vehicle, although there was no significant difference between LPS doses (Table 1F).
Slow wave activity in lipopolysaccharide-treated animals increases after correcting for changes in movement
Because EEG delta power is higher and gamma power is lower during sleep and quiescent wakefulness compared with active wakefulness,40,41 the changes in movement after LPS injection could themselves account for the observed slowing in EEG signals. In all animals, movement was negatively correlated with SWA (Supplementary Fig. S5; Supplementary Fig. S6a; r2=0.313, P<0.05). Because delirium in patients is characterised by elevated delta power during wakefulness,13,14 we tested whether LPS caused a slowing of the EEG after accounting for the LPS-induced decrease in movement.
In an exploratory analysis, we calculated power spectral density in overlapping 4-s windows and averaged the power spectra over epochs within lower or upper quartile movement ranges, and quiescence (Fig. 2e, top). As expected, under baseline conditions, the power spectra changed based on the magnitude of movement, with SWA suppressed during movement compared with quiescence, and in the highest quartile of activity compared with the lowest. Importantly, the same analysis applied to data recorded after administration of LPS did not show changes in the power spectra with increases in movement (Fig. 2e, bottom). Instead, after LPS administration, SWA was elevated even during movement, rendering power spectra during movement similar to those recorded during quiescence.
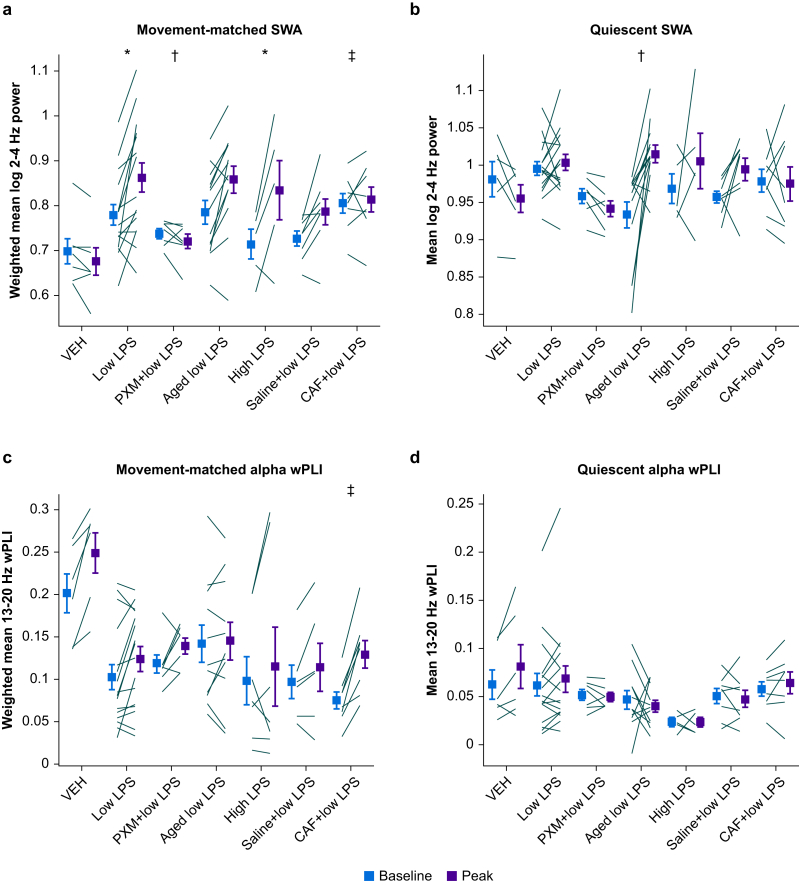
The analysis of Figure 2e suggests an effect of LPS on SWA during active wakefulness. However, to quantify this effect, we would want to compare SWA recorded during periods with identical movement profiles. To achieve this, we applied PSM (Supplementary Fig. S2) to compare SWA across comparable distributions of movement recorded during baseline and peak LPS. LPS caused a steep increase in movement-matched SWA (Fig. 3a), although there was no significant difference between LPS doses (Table 1G). SWA during quiescent periods was increased by LPS treatment (Fig. 3b), although this effect was mainly driven by aged animals (discussed later) and no direct comparisons showed differences between the adult LPS-only groups (Table 1H).
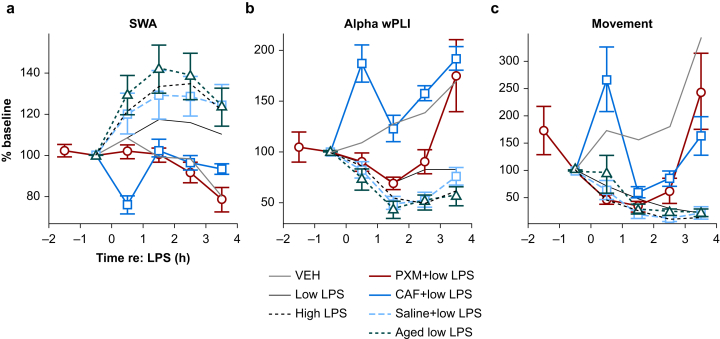
Fig 3.
Changes in slow wave activity and alpha band wPLI during movement or quiescence. (a) Movement-matched SWA values at baseline or peak LPS. Lines indicate mean log SWA values for individual animals and symbols represent group averages. Error bars indicate plus or minus standard error of the mean. (b) SWA during quiescence. (c) Movement-matched alpha band wPLI. (d) Alpha wPLI during quiescence. ∗Indicates significant difference of differences from vehicle. †Indicates significant difference of differences from low LPS. ‡Indicates significant difference of differences from saline+low LPS. CAF, caffeine citrate; LPS, lipopolysaccharide; PXM, piroxicam; SWA, slow wave activity; VEH, vehicle; wPLI, weighted phase lag index.
Cortical connectivity is unchanged in lipopolysaccharide after correcting for changes in activity
Under baseline conditions, we observed that alpha band wPLI was positively correlated with movement (Supplementary Fig. 6b; r2=0.435, P<0.05), suggesting that the decrease in wPLI observed in LPS could be attributable to the reduction in movement after injection of LPS. This is indeed what was observed when we applied PSM to the relationship between wPLI and movement. To obtain accurate measures of wPLI, we expanded the time window for movement analysis to 20 s, slightly reducing the temporal resolution of changes in activity level. The movement-matched alpha band wPLI was unaffected by LPS (Fig. 3c; Table 1I), indicating the absence of changes in wPLI after accounting for LPS-induced decreases in movement. wPLI during quiescence was also unaffected by LPS treatment (Fig. 3d; Table 1J).
Neocortical cytokine levels correlate with changes in movement-matched slow wave activity
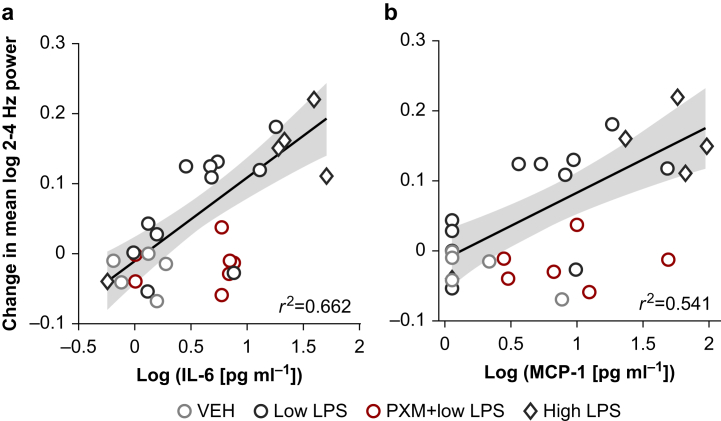
We observed increases in cytokine levels after injection of LPS and systematic increases in SWA after accounting for the effect of LPS on movement. We next sought to determine if the magnitude of the increase in cytokine levels and the magnitude of the changes in brain activity were related for the measures that showed significant LPS effects. IL-6 levels correlated with increases in movement-matched SWA for LPS-only groups (Fig. 4a; r2=0.662, P<0.001), as did MCP-1 levels (Fig. 4b; r2=0.541, P=0.0014).
Fig 4.
Cytokine correlations with changes in movement-matched slow wave activity. (a) Scatterplot of the change in movement-matched SWA from baseline to peak LPS vs log IL-6 concentration. Markers represent values for individual animals. The line indicates the polynomial least-squares fit (using the MATLAB function ‘regress’) for LPS-only groups, and the shading indicates the 95% prediction interval of the regression line. The r2 value for the fit is also indicated. (b) Scatterplot of the change in movement-matched SWA from baseline to peak vs log MCP-1 concentration. IL-6, interleukin-6; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; PXM, piroxicam; SWA, slow wave activity; VEH, vehicle.
Piroxicam attenuates the EEG slowing in lipopolysaccharide without affecting brain IL-6 levels or acute decreases in movement
Piroxicam is a non-selective cyclooxygenase (COX) inhibitor previously shown to attenuate acute behavioural changes induced by LPS.3,24 We first determined that piroxicam 10 mg kg−1 administration before low LPS did not affect neocortical levels of IL-6 or MCP-1 compared with low LPS alone (Supplementary Fig. S7a and b; Table 1A), indicating any effect of piroxicam electrophysiologically or behaviourally would be downstream of the initial pro-inflammatory cytokine response, similar to prior reports.
Piroxicam blunted the LPS-induced increase of overall SWA (i.e. before accounting for movement; Fig. 5a red; Table 1C) and gamma power (Supplementary Fig. S4b; Table 1D) but did not decrease the impact of LPS on wPLI (Fig. 5b; Table 1E). Piroxicam did not alter movement during the peak hours after LPS injection compared with low LPS-only animals (Fig. 5c). However, the difference in movement between baseline and the final recording hour (the fourth hour post-LPS, not included in ‘peak LPS’) was significantly smaller in ‘piroxicam+low LPS’ relative to low LPS alone (likelihood ratio test: χ2(1)=16.625, P<0.001), suggesting that piroxicam-treated animals may recover more quickly. Piroxicam attenuated the LPS-induced increase in movement-matched SWA (Fig. 3a; Table 1G). As with low LPS alone, piroxicam did not change SWA during quiescent periods (Fig. 3b; Table 1H). Further, piroxicam-treated animals showed smaller movement-matched SWA changes than would have been predicted from IL-6 (Fig. 4a red; for piroxicam prediction residuals, one-sample t-test vs zero: t=−3.78, degrees of freedom =6, P=0.0092) or MCP-1 concentrations (Fig. 4b red; for piroxicam prediction residuals, one-sample t-test vs zero: t=−3.54, degrees of freedom =6, P=0.012).
Fig 5.
EEG and movement time course summary across groups. (a) The time series of SWA (2–4 Hz) normalised to mean spectral power (2–80 Hz) are shown for all LPS groups (vehicle, low LPS, and high LPS are the same as in Fig. 2). Symbols represent the mean percent change in SWA from baseline across all animals at each LPS dose at each recording hour. Error bars represent plus or minus standard error of the mean. (b) Gamma power time series. (c) Alpha wPLI time series. (d) Movement time series. Bars indicate plus or minus standard error of the mean. CAF, caffeine citrate; LPS, lipopolysaccharide; PXM, piroxicam; SWA, slow wave activity; VEH, vehicle; wPLI, weighted phase lag index.
Given prior data showing that piroxicam blunts the prostaglandin response to LPS by inhibiting COX activity,3,24 and that prostaglandin D2 acts as a powerful somnogen via downstream effects on adenosine signalling,25,26 we further investigated the role of this pathway in LPS-induced SWA by testing the effect of caffeine citrate.
Repeated injection of caffeine diminishes lipopolysaccharide-induced EEG changes
Caffeine promotes wakefulness through antagonism at the A2A adenosine receptor,42,43 which is a downstream mediator of the somnogenic prostaglandin D2 that is known to affect cortical arousal.26,44,45 When animals were administered LPS in combination with caffeine citrate, they showed lower overall SWA (Fig. 5a blue), higher gamma power (Supplementary Fig. 4b), increased alpha band wPLI, and increased movement compared with animals administered LPS with saline (Fig. 5b and c; Table 1C–F). Caffeine blunted the effects of LPS on the movement-matched SWA compared with animals treated with LPS plus saline (Fig. 3a; Table 1G). Caffeine did not alter SWA during quiescence compared with saline-treated animals (Fig. 3b; Table 1H). The effect of caffeine citrate treatment plus LPS on movement-matched wPLI relative to animals treated with saline plus LPS (Fig. 3c) was strikingly similar to the effect of vehicle vs low LPS reported above (compare Fig. 3c), although here the difference between the two was statistically significant (Table 1I). Caffeine pretreatment did not alter wPLI during quiescence relative to saline (Fig. 3d; Table 1J).
Aged animals exhibit exaggerated EEG slowing during quiescence
As delirium is most relevant in older patients,46 we repeated LPS experiments in aged mice and compared results with adult animals. As expected,47 aged mice compared with adults showed higher neocortical IL-6 and higher MCP-1 protein levels in response to LPS treatment (Supplementary Fig. S6a and b; Table 1A and B). Aged animals demonstrated an increased overall SWA response to LPS compared with adult animals (Fig. 5a green; Table 1C), although this was not observed for gamma power (Supplementary Fig. 4b; Table 1D). Decreases in wPLI were exaggerated in aged animals (Fig. 5b; Table 1E). LPS-driven decreases in movement were not different between aged animals and adults (Fig. 5c; Table 1F). Increases in movement-matched SWA attributable to LPS were not different from adult animals (Fig. 3a; Table 1G). Instead, SWA during quiescence was greatly increased in aged compared with adult animals (Fig. 3b; Table 1H), and this was the only significant contrast in the model. As stated above, changes in both movement-matched and quiescent alpha wPLI after LPS treatment were not significant in models including aged animals (Fig. 3c and d; Table 1I and J).
Discussion
Inflammation-induced changes in cortical activity
Inflammation has been associated with acute changes in brain activity and connectivity in the hippocampus, where changes in synaptic plasticity may underlie cognitive deficits observed during delirium.48,49 Less is known about the effects of inflammation on neocortical activity. Our results show some alignment with previous findings in rodents,23,50 where a much higher dose of LPS (1 mg kg−1) slowed hippocampal theta rhythms independently of changes in locomotion, although comparable effects were not observed in the prefrontal cortex, suggesting the slowing is region-selective. The data presented here suggest a similar regional heterogeneity, as posterior increases in SWA were larger than anterior in high LPS animals, but this observation requires confirmation with further experiments. Posterior cortical SWA appears particularly important in delirium.14 Previous studies have also described EEG slowing and decreases in alpha antero-posterior wPLI,50 but did not account for effects on movement40 and also used a much higher dose of LPS (1 mg kg−1), making their results more likely to reflect somnolence rather than wakeful EEG activity.39
Our findings with aged animals indicate an exaggerated biochemical and electrophysiological response to inflammation, but since the differences between aged and adult animals were only observed in quiescence, a limitation is that those electrophysiological effects could be secondary to increased hypoactivity. Further work is needed to determine whether this increase in SWA in mice during quiescence reflects hypoactive wakefulness or sleep, but the data suggest that inflammation in aged animals induces a hypoactive phenotype that is particularly prevalent in older patients.46
Inflammation-induced changes in cortical connectivity
The finding that alpha band wPLI connectivity changes were attributable to changes in movement is potentially of clinical significance. The association of impaired alpha band wPLI with delirium stems from work in postoperative patients where delirium is predominantly hypoactive.14,21,30 In contrast, a study of delirium on emergence from anaesthesia in young children, which is typically hyperactive, found increased alpha band connectivity during delirium.51 In this context, reduced alpha band wPLI may represent a specific marker of hypoactive delirium and this possibility should be tested in a cohort of patients including hypoactive and hyperactive delirium. The absence of hyperactivity in this study is a potential limitation.
Cytokine cascades contributing to inflammation
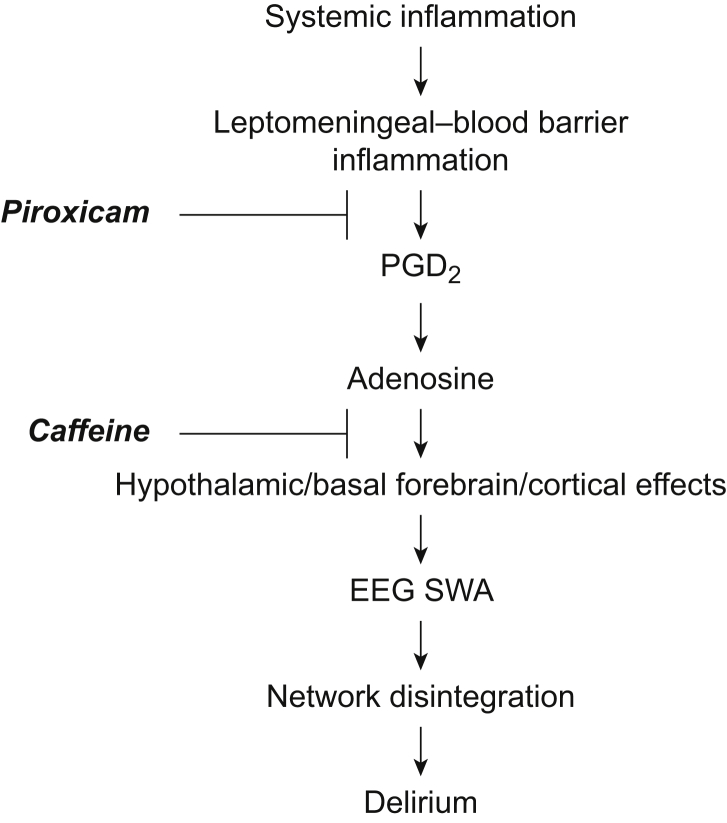
We acknowledge important differences in approaches to the study of inflammation in the mouse and human models, which are potential limitations. Notably we induced systemic inflammation in the mouse model using LPS, but focused on brain inflammation as a surrogate of neuroinflammatory hypotheses of delirium. In our recent work on delirium14 we studied plasma cytokines, a more distant surrogate of neuroinflammation. In the current study, we specified a priori that IL-6 would be the primary cytokine of interest because of its sensitivity to LPS,32 long half-life,33 and prior data from CSF studies of delirium.52 We complemented this with study of MCP-1 based on our recent paper.14 Given redundancy in cytokine cascades, the next step is to understand local neuronal, immune, and circuit dynamics of these inflammatory stimuli. We suggest that inflammation drives EEG changes through induced release of prostaglandin D2 and subsequent effects on adenosine signalling, most likely in sleep and arousal centres in the hypothalamus and basal forebrain (Fig. 6).26 However, prostaglandin D synthase is expressed by the leptomeninges and found in CSF,53 and so we cannot exclude additional direct cortical actions to induce SWA. Understanding whether the observed SWA reflects global or local slow waves seems a critical next step to determine whether delirium involves activation of sleep centres (likely inducing global slow waves that typically occur early in the night) or also local changes that support more restricted SWA (that may reflect local changes in adenosine related to prostaglandin D2 or metabolism). Verification of the locus of adenosine's actions, and investigation of possible direct actions of adenosine on cortical circuits, awaits further experiments. As NSAID should be avoided in vulnerable older patients, future studies should focus on the therapeutic benefit of targeted manipulation of adenosine signalling in this mouse model and in a clinical setting.
Fig 6.
Working model of inflammation-driven slow wave activity. Our working model of the generation of wakeful SWA relevant to delirium begins with systemic inflammation leading to leptomeningeal–blood barrier inflammation, release of prostaglandin D2 and, via prostaglandin D receptor activation, release of adenosine. The enrichment of prostaglandin D receptors on the basal forebrain and hypothalamus suggests that adenosine then acts at the nearby arousal centres to promote sleep, and cortical SWA during wakefulness. PGD2, prostaglandin D2; SWA, slow wave activity.
Translational relevance
Our focus has been on objective translational features of delirium that can be feasibly studied in the rodent. In contrast to studies of sepsis, where LPS is viewed as a suboptimal model,54 LPS is commonly used in rodent studies of the mechanisms of delirium because it produces a profound and consistent proinflammatory response.3,23,49 Among current theories of the aetiology of delirium, neuroinflammatory hypotheses for delirium are heavily supported in the literature55,56 and we based our experimental paradigm on these hypotheses. The choice to use LPS instead of a surgical model for delirium was based on the following: (1) there is extensive evidence that peripheral inflammation and the sickness response are components critical to the mechanism for delirium,57 and LPS triggers both; (2) LPS has been used previously in animal models of delirium58, 59, 60; (3) the simplicity and repeatability of LPS vs surgery, which enhances the scientific rigor (e.g. the rapid inflammatory response to LPS is predictable in time); (4) inflammatory pathways common to both damage-associated molecular patterns and pathogen-associated molecular patterns (e.g. activation of Toll-like receptor 4, release of cytokines and chemokines including IL1-beta and TNF-alpha,61 and MCP-1 and IL-6,62 which correlate with delirium severity14); (5) delirium does not just occur in the setting of surgery but also with inflammation resulting from infections and severe illness.46 However, certain aspects of damage-vs pathogen-associated molecular patterns are distinct, such as the degree to which they stimulate innate immune pathways and coagulation,63 and LPS produces a stronger central immune response compared with other inflammatory stimuli,64 suggesting that surgical and LPS models will also differ. Future experiments are needed to test whether surgical animal models show the same effects as those we observe here.
Our approach was to model how this inflammatory response affects EEG activity during movement to avoid the confounder of sleep. As elevated SWA during wakefulness is a key criterion for delirium and is often associated with inattention, another key criterion, our model has a plausible association with delirium. The cognitive features that define delirium include impaired attention, arousal, executive function, orientation to the environment, and memory and perceptual disturbances. Because inflammatory agents such as LPS affect motivation and motor function in animal models, investigating the cognitive correlates of inflammation behaviourally in mice is complicated.39 Instead, we present an animal model of inflammation-related SWA during wakefulness; establishing this animal model opens opportunities for testing hypotheses about mechanisms and treatments for delirium and other inflammatory brain disorders. We consider this work to be complementary to work done with cognitive testing,3,65 which itself has limitations regarding the characterisation of a complex human disorder in mice. Importantly, we recently showed that inflammation-driven SWA correlates with delirium severity in humans,14 hence the ability to model this effect in mice and study mechanisms represents a major methodological advance. Furthermore, delirium, cognitive decline, and dementia are profound cognitive disorders associated with inflammation2,4,5 and changes in SWA.13,66,67 Hence understanding the mechanisms whereby inflammation drives SWA may illuminate the key pathophysiological mechanisms of cognitive impairment in a variety of disorders.
Future directions
The data presented here motivate future investigations into the mechanisms linking inflammation to changes in neural activity and connectivity. For example, previous studies have shown that the acute behavioural effects of LPS are driven by peripheral IL-1β49; thus, it would be illuminating to measure plasma inflammatory markers in addition to measuring cortical cytokines. Future experiments should also identify specific receptor subtypes and other aspects of the signalling pathways involved in the link between neuroinflammation and changes in brain activity. For example, we tested caffeine, which is a non-specific adenosine receptor antagonist and has potential clinical application. Elucidating the roles of adenosine A1 vs A2 receptors would allow for more targeted drug development. In addition, future studies should include the effects of inflammation in mice modelling disorders associated with delirium, such as dementia. More broadly, the model presented here opens opportunities for testing the roles of specific neuronal, glial, and immune cell types in the signalling cascade linking inflammation to changes in brain function.66,67
Authors' contributions
Study design: ZWS, ERJ, BMK, CAM, RDS, MIB
Data collection: ZWS, ERJ, SMG
Data analysis: ZWS, ERJ, BMK, SMG
Manuscript revision: ZWS, BMK, SMG, CAM, RDS, MIB
Writing first draft of paper: ERJ, RDS, MIB
Acknowledgements
The authors thank Elizabeth A. Townsend, Payge A. Barnard, Jens Mellby, Hazel Bastien, and Matthieu Darracq for technical assistance.
Handling editor: Michael Avidan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2020.12.040.
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
Supported by US National Institutes of Health (R01 GM109086 to MIB; R01 AG063849 to RDS), and the Department of Anesthesiology, School of Medicine and Public Health, University of Wisconsin, Madison, WI, USA.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sanders R.D. Hypothesis for the pathophysiology of delirium: role of baseline brain network connectivity and changes in inhibitory tone. Med Hypotheses. 2011;77:140–143. doi: 10.1016/j.mehy.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham C., Maclullich A.M.J. At the extreme end of the psychoneuroimmunological spectrum: delirium as a maladaptive sickness behaviour response. Brain Behav Immun. 2013;28:1–13. doi: 10.1016/j.bbi.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin É.W., Skelly D.T., Murray C.L., Cunningham C. Cyclooxygenase-1-dependent prostaglandins mediate susceptibility to systemic inflammation-induced acute cognitive dysfunction. J Neurosci. 2013;33:15248–15258. doi: 10.1523/JNEUROSCI.6361-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramanan V.K., Risacher S.L., Nho K. GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain. 2015;138:3076–3088. doi: 10.1093/brain/awv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whittington R.A., Planel E., Terrando N. Impaired resolution of inflammation in Alzheimer’s disease: a review. Front Immunol. 2017;8:1464. doi: 10.3389/fimmu.2017.01464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Merluzzi A.P., Carlsson C.M., Johnson S.C. Neurodegeneration, synaptic dysfunction, and gliosis are phenotypic of Alzheimer dementia. Neurology. 2018;91:e436–e443. doi: 10.1212/WNL.0000000000005901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Bautista E., Chotpitayasunondh T., Gao Z. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 8.Ely E.W., Gautam S., Margolin R. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27:1892–1900. doi: 10.1007/s00134-001-1132-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandharipande P.P., Pun B.T., Herr D.L. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 10.Riker R.R., Shehabi Y., Bokesch P.M. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 11.Pandharipande P.P., Girard T.D., Jackson J.C. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudolph J.L., Jones R.N., Levkoff S.E. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponten S.C., Tewarie P., Slooter A.J.C., Stam C.J., van Dellen E. Neural network modeling of EEG patterns in encephalopathy. J Clin Neurophysiol. 2013;30:545–552. doi: 10.1097/WNP.0b013e3182a73e16. [DOI] [PubMed] [Google Scholar]
- 14.Tanabe S., Mohanty R., Lindroth H. Cohort study into the neural correlates of postoperative delirium: the role of connectivity and slow-wave activity. Br J Anaesth. 2020;125:55–66. doi: 10.1016/j.bja.2020.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nir Y., Andrillon T., Marmelshtein A. Selective neuronal lapses precede human cognitive lapses following sleep deprivation. Nat Med. 2017;23:1474–1480. doi: 10.1038/nm.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casey C.P., Lindroth H., Mohanty R. Postoperative delirium is associated with increased plasma neurofilament light. Brain. 2020;143:47–54. doi: 10.1093/brain/awz354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yasuda T., Yoshida H., Garcia-Garcia F., Kay D., Krueger J.M. Interleukin-1beta has a role in cerebral cortical state-dependent electroencephalographic slow-wave activity. Sleep. 2005;28:177–184. doi: 10.1093/sleep/28.2.177. [DOI] [PubMed] [Google Scholar]
- 18.Taishi P., Churchill L., Wang M. TNFalpha siRNA reduces brain TNF and EEG delta wave activity in rats. Brain Res. 2007;1156:125–132. doi: 10.1016/j.brainres.2007.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger J.M., Clinton J.M., Winters B.D. Involvement of cytokines in slow wave sleep. Prog Brain Res. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plaschke K., Fichtenkamm P., Schramm C. Early postoperative delirium after open-heart cardiac surgery is associated with decreased bispectral EEG and increased cortisol and interleukin-6. Intensive Care Med. 2010;36:2081–2089. doi: 10.1007/s00134-010-2004-4. [DOI] [PubMed] [Google Scholar]
- 21.van Dellen E., van der Kooi A.W., Numan T. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121:328–335. doi: 10.1097/ALN.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 22.Trzepacz P.T., Leavitt M., Ciongoli K. An animal model for delirium. Psychosomatics. 1992;33:404–415. doi: 10.1016/S0033-3182(92)71945-8. [DOI] [PubMed] [Google Scholar]
- 23.Mamad O., Islam M.N., Cunningham C., Tsanov M. Differential response of hippocampal and prefrontal oscillations to systemic LPS application. Brain Res. 2018;1681:64–74. doi: 10.1016/j.brainres.2017.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teeling J.L., Cunningham C., Newman T.A., Perry V.H. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun. 2010;24:409–419. doi: 10.1016/j.bbi.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qu W.-M., Huang Z.-L., Xu X.-H. Lipocalin-type prostaglandin D synthase produces prostaglandin D2 involved in regulation of physiological sleep. Proc Natl Acad Sci U S A. 2006;103:17949–17954. doi: 10.1073/pnas.0608581103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saper C.B., Romanovsky A.A., Scammell T.E. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. doi: 10.1038/nn.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann M., Czok G. Pharmacokinetics of caffeine in mice and its modification by ethanol. Z Ernahrungswiss. 1980;19:215–227. doi: 10.1007/BF02018787. [DOI] [PubMed] [Google Scholar]
- 28.Banks M.I., Krause B.M., Endemann C.M. Cortical functional connectivity indexes arousal state during sleep and anesthesia. NeuroImage. 2020;211:116627. doi: 10.1016/j.neuroimage.2020.116627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinck M., Oostenveld R., van Wingerden M., Battaglia F., Pennartz C.M.A. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. NeuroImage. 2011;55:1548–1565. doi: 10.1016/j.neuroimage.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 30.Numan T., Slooter A.J.C., van der Kooi A.W. Functional connectivity and network analysis during hypoactive delirium and recovery from anesthesia. Clin Neurophysiol. 2017;128:914–924. doi: 10.1016/j.clinph.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Ho D.E., Imai K., King G., Stuart E.A. Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Polit Anal. 2007;15:199–236. [Google Scholar]
- 32.Skelly D.T., Hennessy E., Dansereau M.-A., Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1β, [corrected] TNF-α and IL-6 challenges in C57BL/6 mice. PLoS One. 2013;8 doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oda S., Hirasawa H., Shiga H., Nakanishi K., Matsuda K., Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–175. doi: 10.1016/j.cyto.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Munster B.C.V., Korevaar J.C., Zwinderman A.H., Levi M., Wiersinga W.J., Rooij S.E.D. Time-course of cytokines during delirium in elderly patients with hip fractures. J Am Geriatr Soc. 2008;56:1704–1709. doi: 10.1111/j.1532-5415.2008.01851.x. [DOI] [PubMed] [Google Scholar]
- 35.Bates D., Mächler M., Bolker B., Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 36.Lenth R., Buerkner P., Herve M., Love J., Riebl H., Singmann H. emmeans: estimated marginal means, aka least-squares means. 2020. https://CRAN.R-project.org/package=emmeans Available from:
- 37.Labrenz F., Wrede K., Forsting M. Alterations in functional connectivity of resting state networks during experimental endotoxemia – an exploratory study in healthy men. Brain Behav Immun. 2016;54:17–26. doi: 10.1016/j.bbi.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 38.Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham C., Sanderson D.J. Malaise in the water maze: untangling the effects of LPS and IL-1β on learning and memory. Brain Behav Immun. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen I.H., Agerskov C., Arvastson L., Bastlund J.F., Sørensen H.B.D., Herrik K.F. Pharmaco-electroencephalographic responses in the rat differ between active and inactive locomotor states. Eur J Neurosci. 2019;50:1948–1971. doi: 10.1111/ejn.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maloney K.J., Cape E.G., Gotman J., Jones B.E. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76:541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 42.Huang Z.-L., Qu W.-M., Eguchi N. Adenosine A 2A, but not A 1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- 43.Yacoubi M.E., Ledent C., Ménard J.-F., Parmentier M., Costentin J., Vaugeois J.-M. The stimulant effects of caffeine on locomotor behaviour in mice are mediated through its blockade of adenosine A2A receptors. Br J Pharmacol. 2000;129:1465–1473. doi: 10.1038/sj.bjp.0703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terao A., Matsumura H., Saito M. Interleukin-1 induces slow-wave sleep at the prostaglandin D2-sensitive sleep-promoting zone in the rat brain. J Neurosci. 1998;18:6599–6607. doi: 10.1523/JNEUROSCI.18-16-06599.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scammell T., Gerashchenko D., Urade Y., Onoe H., Saper C., Hayaishi O. Activation of ventrolateral preoptic neurons by the somnogen prostaglandin D2. Proc Natl Acad Sci. 1998;95:7754–7759. doi: 10.1073/pnas.95.13.7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fong T.G., Tulebaev S.R., Inouye S.K. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murtaj V., Belloli S., Di Grigoli G. Age and sex influence the neuro-inflammatory response to a peripheral acute LPS challenge. Front Aging Neurosci. 2019;11:299. doi: 10.3389/fnagi.2019.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D.-S., Zurek A.A., Lecker I. Memory deficits induced by inflammation are regulated by α5-subunit-containing GABAA receptors. Cell Rep. 2012;2:488–496. doi: 10.1016/j.celrep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Skelly D.T., Griffin É.W., Murray C.L. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol Psychiatry. 2019;24:1533–1548. doi: 10.1038/s41380-018-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Albrecht M.A., Vaughn C.N., Erickson M.A., Clark S.M., Tonelli L.H. Time and frequency dependent changes in resting state EEG functional connectivity following lipopolysaccharide challenge in rats. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin J.C., Liley D.T.J., Harvey A.S., Kuhlmann L., Sleigh J.W., Davidson A.J. Alterations in the functional connectivity of frontal lobe networks preceding emergence delirium in children. Anesthesiology. 2014;121:740–752. doi: 10.1097/ALN.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 52.Cape E., Hall R.J., van Munster B.C. Cerebrospinal fluid markers of neuroinflammation in delirium: a role for interleukin-1β in delirium after hip fracture. J Psychosom Res. 2014;77:219–225. doi: 10.1016/j.jpsychores.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urade Y., Kitahama K., Ohishi H., Kaneko T., Mizuno N., Hayaishi O. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc Natl Acad Sci U S A. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lewis A.J., Seymour C.W., Rosengart M.R. Current murine models of sepsis. Surg Infect. 2016;17:385–393. doi: 10.1089/sur.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cerejeira J., Firmino H., Vaz-Serra A., Mukaetova-Ladinska E.B. The neuroinflammatory hypothesis of delirium. Acta Neuropathol (Berl) 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 56.Maldonado J.R. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry. 2018;33:1428–1457. doi: 10.1002/gps.4823. [DOI] [PubMed] [Google Scholar]
- 57.Maclullich A.M.J., Ferguson K.J., Miller T., de Rooij S.E.J.A., Cunningham C. Unravelling the pathophysiology of delirium: a focus on the role of aberrant stress responses. J Psychosom Res. 2008;65:229–238. doi: 10.1016/j.jpsychores.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray C., Sanderson D.J., Barkus C. Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging. 2012;33:603–616. doi: 10.1016/j.neurobiolaging.2010.04.002. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kealy J., Murray C., Griffin E.W. Acute inflammation alters brain energy metabolism in mice and humans: role in suppressed spontaneous activity, impaired cognition, and delirium. J Neurosci. 2020;40:5681–5696. doi: 10.1523/JNEUROSCI.2876-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreuder L., Eggen B.J., Biber K., Schoemaker R.G., Laman J.D., de Rooij S.E. Pathophysiological and behavioral effects of systemic inflammation in aged and diseased rodents with relevance to delirium: a systematic review. Brain Behav Immun. 2017;62:362–381. doi: 10.1016/j.bbi.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 61.Wilson J.E., Mart M.F., Cunningham C. Delirium. Nat Rev Dis Primer. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Klune J.R., Dhupar R., Cardinal J., Billiar T.R., Tsung A. HMGB1: endogenous danger signaling. Mol Med. 2008;14:476–484. doi: 10.2119/2008-00034.Klune. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eppensteiner J., Kwun J., Scheuermann U. Damage- and pathogen-associated molecular patterns play differential roles in late mortality after critical illness. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoogland I.C.M., Houbolt C., van Westerloo D.J., van Gool W.A., van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davis D.H.J., Skelly D.T., Murray C. Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium. Am J Geriatr Psychiatry. 2015;23:403–415. doi: 10.1016/j.jagp.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Babiloni C., De Pandis M.F., Vecchio F. Cortical sources of resting state electroencephalographic rhythms in Parkinson’s disease related dementia and Alzheimer’s disease. Clin Neurophysiol. 2011;122:2355–2364. doi: 10.1016/j.clinph.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura A., Cuesta P., Fernández A. Electromagnetic signatures of the preclinical and prodromal stages of Alzheimer’s disease. Brain. 2018;141:1470–1485. doi: 10.1093/brain/awy044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.