Abstract
Background
Several devices record and interpret patient brain activity via electroencephalogram (EEG) to aid physician assessment of anaesthetic effect. Few studies have compared EEG monitors on data from the same patient. Here, we describe a set-up to simultaneously compare the performance of three processed EEG monitors using pre-recorded EEG signals from older surgical patients.
Methods
A playback system was designed to replay EEG signals into three different commercially available EEG monitors. We could then simultaneously calculate indices from the SedLine® Root (Masimo Inc., Irvine, CA, USA; patient state index [PSI]), bilateral BIS VISTA™ (Medtronic Inc., Minneapolis, MN, USA; bispectral index [BIS]), and Datex Ohmeda S/5 monitor with the Entropy™ Module (GE Healthcare, Chicago, IL, USA; E-entropy index [Entropy]). We tested the ability of each system to distinguish activity before anaesthesia administration (pre-med) and before/after loss of responsiveness (LOR), and to detect suppression incidences in EEG recorded from older surgical patients receiving beta-adrenergic blockers. We show examples of processed EEG monitor output tested on 29 EEG recordings from older surgical patients.
Results
All monitors showed significantly different indices and high effect sizes between comparisons pre-med to after LOR and before/after LOR. Both PSI and BIS showed the highest percentage of deeply anaesthetised indices during periods with suppression ratios (SRs) > 25%. We observed significant negative correlations between percentage of suppression and indices for all monitors (at SR >5%).
Conclusions
All monitors distinguished EEG changes occurring before anaesthesia administration and during LOR. The PSI and BIS best detected suppressed periods. Our results suggest that the PSI and BIS monitors might be preferable for older patients with risk factors for intraoperative awareness or increased sensitivity to anaesthesia.
Keywords: bispectral index (BIS), brain monitoring, EEG, entropy, patient state index (PSI), processed EEG
Editor's key points.
-
•
Devices to measure EEG during general anaesthesia include the SedLine® Root patient state index (PSI), bilateral BIS VISTA™ bispectral index (BIS), and Datex Ohmeda S/5 monitor with Entropy™ Module E-entropy index.
-
•
The authors developed an anaesthesia EEG playback system allowing for a collected EEG data set to be inputted simultaneously to these three EEG devices (PSI, BIS, and Datex Ohmeda).
-
•
When using the playback system on 29 EEG recordings from older surgical patients, all three devices distinguished EEG changes occurring during loss of responsiveness. The playback system also suggests the PSI and BIS could be better than the Datex Ohmeda at detecting EEG suppression.
-
•
This method comparing EEG devices head to head with the same EEG recording can provide an opportunity to understand the advantages and disadvantages of each EEG monitoring device relative to one another.
To aid physician assessment of anaesthetic effect whilst patients are anaesthetised, several commercially available devices record and interpret brain activity via EEG. Such processed EEG monitors output unique indices based on proprietary EEG analyses. The SedLine® Root (Masimo Inc., Irvine, CA, USA), bilateral BIS VISTA™ (Medtronic Inc., Minneapolis, MN, USA), and the Datex Ohmeda S/5 monitor with the Entropy™ Module (GE Healthcare, Chicago, IL, USA) are commonly used monitors that output the patient state index (PSI), bispectral index (BIS), and E-entropy index (Entropy), respectively.1,2
Initially, these devices were developed to lower the incidence of intraoperative awareness,3, 4, 5, 6 and reassuringly their output indices all strongly correlate with clinical assessments of alertness.7,8 Additionally, monitor use reduces the amount of anaesthetic delivered9,10 and decreases time to emergence.8 However, other evidence argues that there is no outcome difference in intraoperative awareness, haemodynamic instability, or adverse events between monitored and unmonitored groups.9
More recently, processed EEG monitors have been used to titrate anaesthetic administration to avoid profoundly deep anaesthesia (characterised by prolonged periods of EEG suppression). Specifically, the length of time a patient has EEG suppression11,12 or has low BIS values13, 14, 15 is correlated with negative postoperative cognitive outcomes. This is also true for patients sensitive to anaesthesia (indicated by EEG suppression at lower volatile concentrations).16 Although titrating anaesthesia to avoid EEG suppression might not decrease delirium incidence,17 processed EEG monitors can help physicians predict the risk of negative cognitive outcomes.
To compare monitoring devices, investigators have connected electrode strips on a patient to two monitors (BIS and Entropy connectors are adaptable),7,18,19 compared EEG-monitored vs vital-sign-monitored patient outcomes,4,9 randomised patients to different monitoring devices,20 or built devices to examine monitors offline.21, 22, 23, 24 However, a uniform evaluation platform to simultaneously compare the PSI, BIS, and Entropy indices on previously recorded EEG signals has not been implemented. Using the same EEG data sets would eliminate data heterogeneity and publication bias, and reduce clinical trial costs by decreasing the number of studies needed to compare all devices. As such, a system like this could be widely applied in diverse clinical cases to compare processed EEG indices and guide physician decisions on which processed EEG monitor to use.
In this paper, we describe the first set-up to simultaneously compare the three monitor indices: PSI, BIS, and Entropy. We test the clinical relevance of our anaesthesia EEG playback system (henceforth ‘playback system’) to distinguish subtle changes in EEG before induction and before/after loss of responsiveness (LOR), and to detect EEG suppression periods in older patients on beta-adrenergic block, a traditionally hard-to-monitor patient cohort.
Methods
Playback system specifications
Our playback system enables previously recorded EEG data sets to be replayed to multiple processed EEG monitoring devices simultaneously. Here, we describe the set-up to compare the SedLine Root (Root), bilateral BIS VISTA (BIS), and the Datex Ohmeda S/5 monitor with the Entropy Module with the unilateral sensors (Entropy; Fig. 1a). For the SedLine Root, we use ‘Root’ to describe the physical monitor in the methods and ‘PSI’ to describe the index the monitor generates. The BIS with bilateral sensors outputs two independent BIS values: BIS-L for the left electrodes and BIS-R for the right electrodes. The Entropy outputs two values as well: the state entropy (Entropy-S) and the response entropy (Entropy-R). Indices from these monitors range from 0 to 100 (with the exception of Entropy-S, which ranges from 0 to 91),7,19 where 0 indicates maximally deep anaesthesia and 100 (91 for Entropy-S) indicates maximally awake states (Table 1).
Fig 1.
The anaesthesia playback system equipment set-up. (a) Pre-recorded EEG files are converted and replayed on the National Instruments (NI) PXIe-8821 to the NI PXI-6733 digital-to-analogue converter, which then directs EEG signals to the NI CA-1000. The NI CA-1000 is directly connected to and outputs EEG to individual monitors simultaneously. Outputs from the three monitors are collected on a laptop with RugloopII and ADC software programs installed (these programs collect and store the monitor indices). Arrows indicate the direction of data flow through the playback system. (b) Processed EEG monitors approximate electrode locations for the SedLine Root (red), bilateral BIS VISTA (blue), and the Datex Ohmeda S/5 monitor with the Entropy Module (orange). The Root and Entropy electrode locations closely follow those of the standard 10–20 electrode locations, as indicated. However, the BIS electrode positions (labeled LT and RT) are located on the zygomatic arch as it extends laterally approximately an inch anterior and a quarter of an inch superior from the external acoustic meatus.
Table 1.
Recommended ranges of processed EEG monitors. BIS, bispectral index; PSI, patient state index.
| Processed EEG monitor (index) | Above general anaesthesia | Anaesthetised (recommended ranges) | Deeply anaesthetised |
|---|---|---|---|
| BIS VISTA (BIS) | 60–100 | 40–60 | <40 (deep) <20 (EEG suppression) |
| Datex Ohmeda S/5 anaesthesia monitor with Entropy Module (state and response entropy) | 60–91 (state) 60–100 (response) |
40–60 (both state and response) | <40 (deep) 0 (persistently suppressed EEG) (both state and response) |
| SedLine Root monitor (PSI) | 50–100 | 25–50 | <25 |
Previously recorded EEG from any electrophysiology device can be replayed so long as the electrode positions are at or near the recording positions used for the monitoring devices (Fig. 1b), and EEG data need to be collected at equal or greater than a 100 Hz sampling frequency. We reasoned that 100 Hz is sufficient, as monitors have reported using low-frequency ratios as part of their index calculations.19,25,26 The monitors have a patient electrode interface designed for an analogue input from the patient. Thus, the raw digital EEG file must first be converted into an analogue file to be recognised by the monitors. The module that stores and streams the EEG files is the National Instruments (Austin, TX, USA) NI PXIe-8821 (Fig. 1a). Custom MATLAB code sends previously recorded EEG activity to an NI PXI-6733 module, which converts the incoming digital signal into an analogue signal before sending it to the NI CA-1000 (Fig. 1a). The NI CA-1000 houses a customised voltage divider circuit to convert (voltage adjust) the analogue EEG signal to ensure that output to the patient monitors accurately reflects the number and position of electrodes each device expects. We determined these specifications based on the manufacturer instructions and electrode configurations of the accessory electrodes.
The EEG signals from the NI CA-1000 were transmitted by customised monitor patient cables (normally attached to their adhesive scalp EEG strips) to individual monitors. The proprietary indices from the BIS and Entropy devices were routed to a receiving laptop and captured by RugloopII© (http://www.demed.be/rugloop.htm; revision 14.02). Data from the Root were exported to the same laptop and captured by a proprietary Masimo ADC program (Pulse Ox Automated Data Collection, version 3.2.1.4; Masimo Inc.). Before using the playback system, the impedance checking was turned off for all monitors (as it will corrupt data if left on).
Preprocessing and replay of EEG from older adults
To test the playback system, we replayed EEG data collected using a Masimo SedLine Legacy monitor (Masimo Inc.) from older patients (≥65 yr old). This data set included 29 patients (19 males) with a median age of 76 (65–88) yr, who were exposed to general anaesthesia for elective abdominal procedures and were premedicated with beta-adrenergic blockers (beta blockers).27,28 Patients were induced with fentanyl (1–3 μg kg−1), propofol (1–2 mg kg−1), and neuromuscular blocking agent (if required) using either rocuronium (0–1 mg kg−1) or vecuronium (0.1 mg kg−1). After intubation, maintenance anaesthesia consisted of sevoflurane in oxygen with 50–60% nitrous oxide. Such a patient population poses unique challenges to anaesthetic effect monitoring,27,29 as the cardiovascular disturbances from inadequate anaesthesia are often masked by beta-blocker therapy.27,29 All EEG recordings were acquired under an approved protocol from the Stanford School of Medicine Administrative Panel on Human Subjects in Medical Research (ClinicalTrials.gov, NCT00938782).
The EEG data were originally collected using the USB port from the SedLine Legacy monitor (Masimo Inc.). Files were converted into MATLAB format and saved in a 32-bit unsigned integer array on a laptop independent from the playback equipment.
Such preprocessing is critical to ensure that our data best match the expected incoming data of each processed EEG monitoring device. This is because differences in the signal input can greatly influence the calculations of the proprietary indices.23,30 As electrode locations are the same for the SedLine Legacy and the Root monitor, we could replay these data to the new device without adjustments. For the Entropy monitor, we used EEG data from the AFz, Fp1, and F7 (with 20% gain adjustment; see explanation in Supplementary material) locations, as the expected input is unilateral from these approximate locations (Fig. 1b). For the BIS VISTA monitor, we used electrode locations AFz as the reference (C), ground at FpZ (G), left electrode (LE) was Fp1, left temporal (LT) position was F7 (gain increased by 20%), right electrode (RE) was Fp2, and right temporal (RT) was F8 (gain increased by 20%; Fig. 1b). Electrode abbreviations correspond to those on the BIS adhesive electrode strip and BIS manual (Supplementary Fig. S1).
The BIS monitor was set at a 15 s smoothing rate with 2014 hardware and software revision 1.15. We used the Root monitor 2018 hardware with DSP firmware version 2000. The Entropy Module included 2009-12 hardware.
We used MATLAB programs on the NI PXIe-8821 to apply a 0.5 Hz high-pass filter. The MATLAB programs also included an initiation pulse that was sent through the playback system and stored by the ADC software program.
Monitor comparisons
We show the spectral EEG activity from the F7 electrode and F8 electrode for an entire anaesthetic case (Fig. 2). Spectrograms were created with the Chronux toolbox for MATLAB (chronux.org)31 using a time–bandwidth product of 5 with nine tapers and limiting frequency ranges calculated up to 50 Hz. Each monitor has a recommended range for its respective index for anaesthesia administration (Table 1). The upper bound is intended to avoid inadequate anaesthesia and intraoperative awareness. To compare this across monitors, we tested the ability of the monitors to discriminate between the subtle EEG changes that occur before anaesthesia was given, and before and after LOR. We compared the mean monitor indices between 20 s clips before anaesthesia and before/after the patient lost a response to verbal command (LOR). To identify LOR, patients were given verbal commands approximately every 5 s during induction. Using this time point, 20 s artifact-free clips were identified within a 4 min window surrounding LOR (see Supplementary materials for clip selection). Raw EEG clips and spectrograms were visually inspected by three of the authors (DRD, MBM, and SLE) to ensure they were artifact free (n=27 patients). We tested whether indices after LOR were significantly different from before anaesthesia administration and before LOR by calculating the effect size of the difference using Cohen's d (a difference in the means divided by the pooled standard deviation).
Fig 2.
Example of brain activity and monitor trajectories for a full anaesthesia case. Spectrograms of the EEG activity throughout an entire anaesthesia case (top F7; middle F8). Displayed in the bottom-line plot is the patient state index (PSI), bispectral index from the left (BIS-L) and right (BIS-R) electrodes, response entropy (Entropy-R), and state entropy (Entropy-S). Note that the entropy measures are only calculated unilaterally. The moment when the patient no longer responded to verbal commands (loss of response [LOR]) and recovery of response (ROR) to verbal commands are indicated with dashed vertical lines at the beginning and end of the case, respectively. The patient lightening at ∼20 min (indicated by increase in high-frequency power and indices) is when surgical stimulation began and nitrous oxide was added for anaesthetic maintenance.
The lower bound of the recommended range for anaesthesia administration is meant to avoid excessive anaesthesia exposure and profoundly deep anaesthesia. We reasoned that the presence of EEG suppression is a clinical sign that physicians use to determine whether their patients are too deeply anaesthetised. Thus, we tested how often monitors displayed deeply anaesthetised indices during periods of EEG suppression. An expert anaesthesiologist who uses EEG in clinical practice (DRD) visually scored the EEG files to identify periods of suppression using RemLogic™ software (Embla Systems, Thornton, CO, USA). We then used an objective, descriptive measure of EEG suppression described by Rampil and colleagues.32 After this definition, 4 s epochs were used to calculate a suppression ratio (SR) from physician scoring. Epochs were combined into 28 s blocks to account for monitor delays. Blocks were identified, in which EEG suppression exceeded 25%; the mean index values for each individual monitor were then calculated for those corresponding blocks. We reasoned that for EEG proficiency, this percentage would catch the attention of physicians and alert them that their patients were too deeply anaesthetised. The percentage of time the values for each monitor indicated deep anaesthesia (Table 1) was calculated and compared. We excluded cases without EEG suppression. In general, we had a low incidence of EEG suppression. There were only 116 incidences of SR >5% in five patients and 70 incidences of SR >25% in five patients. From the five patients who had lengthy EEG suppression, we examined the percentage of time the monitors displayed corresponding deeply anaesthetised index values at SR >25%. This provided us with an estimate of the accuracy of the monitor indices to detect EEG suppression. We also calculated the correlation between the SR and monitor indices using Spearman correlation when SR >5%. We reasoned that increasing anaesthetic depth marked by increasing percentage of suppression ought to be synchronised with decreasing monitor indices. Additionally, we evaluated monitor EEG suppression performance by calculating the area under the curve for receiver operating characteristic curves, which is discussed in the Supplementary material.
Statistical analysis
Preliminary testing for the normality of monitor indices using Lilliefors test revealed the 20 s clip data were not normally distributed. Thus, the after LOR indices were compared with the before anaesthesia and before LOR separately using the Wilcoxon signed-rank test. All significance values reported are uncorrected (note that Bonferroni correction for multiple comparisons would place the signficance threshold at 0.01; all our results reported were well below this threshold).
Results
Monitor dynamics across an anaesthetic case
During EEG monitoring, the electrodes are attached to the patients when they enter the operating theatre, and an EEG record should contain awake-like activity at the beginning and end of a case with variable levels of anaesthetised activity in the middle. For our data collected, all three monitors reliably reproduced this expected EEG pattern (Fig. 2).
Monitor comparison: distinguishing loss of response
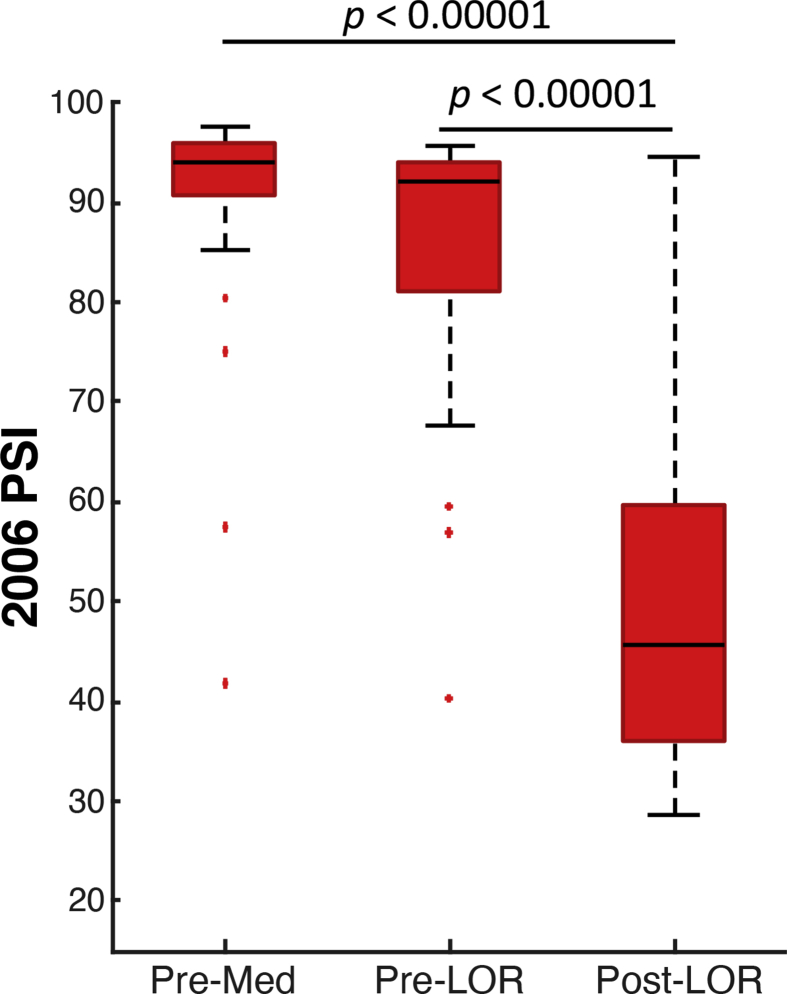
To test the monitor discrimination of the EEG differences occurring before anaesthesia (pre-med) and when patients are responsive (pre-LOR) and not responsive (post-LOR), we identified three 20 s artifact-free clips from these periods and compared averaged monitor indices. The spectral dynamics of this activity for F7 (Fig. 3a) and F8 (Fig. 3b) show a strong correlation to monitor index trajectories (Fig. 3c). All monitors showed significantly different indices after LOR compared with before anaesthesia and before LOR (Fig. 3d). To gauge the change in magnitude, we calculated the effect sizes using Cohen's d for the same comparisons. An effect size of 0.8 or greater is considered a large effect size, 0.5 a medium effect size, and 0.2 or less a small effect size. Effect sizes are listed pre-med to post-LOR and pre-LOR to post-LOR. The PSI had the highest effect size (1.82, 1.82); however, the BIS index (BIS-L 0.67, 0.58; BIS-R 1.37, 1.07), and the Entropy index (Entropy-R 1.42, 0.81; Entropy-S 1.18, 0.73) also had medium to high effect sizes. Notably, all index ranges during these two 20 s periods consistently showed greater variability around LOR illustrating the variability in subjective LOR assessment and patient response to anaesthesia (Fig. 3d).
Fig 3.
Representative patient around loss of response (LOR) to verbal stimuli. Density spectral arrays showing EEG spectral activity around LOR for (a) F7 and (b) F8. (c) The trajectories of the indices outputted by the monitors in the same time period are shown. All monitors show appropriate decreases in indices around LOR as anaesthetic depth increases, although some have more abrupt transitions than others. (d) The index ranges outputted by the processed EEG monitors from 27 patients during 20 s segments before anaesthesia are given (pre-med) and before (Pre-LOR) and after (Post-LOR) LOR. All indices show decreases across patients. The central mark on the box plot indicates the median; the bottom and top edges are the 25th and 75th percentiles, respectively; and the whiskers extend to the most extreme data points not considered outliers. The ‘+’ indicates individual outlier data points. Wilcoxon signed-rank significance values (P) reported are uncorrected.
Monitor comparison: detecting EEG suppression
To demonstrate our manual suppression detection, we show an example of EEG activity during times our physician scorer (DRD) detected EEG suppression (Fig. 4a). In this figure, the patient was highly sensitive to the anaesthetic, so the patient transitioned from an awake state directly to EEG suppression. Additionally, the EEG shows significant reductions in spectral power during suppressed periods (Fig. 4b). We demonstrate a correspondence between suppressed activity identification (Fig. 4a), reductions in spectral content (Fig. 4b), and increased percentage suppression (Fig. 4c). The monitor index trajectories during this time all showed significant decreases from awake values to deeply anaesthetised values, excluding the Entropy monitor (Fig. 4d).
Fig 4.
Example of brain activity and monitor trajectories from awake to EEG suppression during patient induction. (a) A 6 min clip of raw EEG activity (blue trace) from F7 is shown, where period of suppressed EEG has been identified in red. Suppressed periods were identified manually. (b) Clear decreases in the EEG spectral activity can be seen for periods of suppression in the corresponding density spectral array. (c) The percentage of suppression (per 4 s epoch) closely tracks EEG suppression onset and offset. (d) The trajectories of the outputted indices synchronised with this EEG activity.
Another example of a patient who exhibited four significant bouts of intraoperative EEG suppression is shown in Fig 5. The corresponding spectral changes for F7 (Fig. 5a) and F8 (Fig. 5b) are shown along with the percentage of EEG suppression (Fig. 5c) with inset examples of different percentages of suppressed periods and corresponding index trajectories (Fig. 5d).
Fig 5.
Example of brain activity and monitor trajectories in a patient exhibiting several incidences of intraoperative EEG suppression. The synchronised density spectral array from (a) F7 and (b) F8 is shown with (c) corresponding percentages of EEG suppression in 4 s epochs (F7, blue traces; F8, grey traces). Two insets above the percentage of EEG suppression represent 4 s clips of EEG activity from corresponding time points indicated by the arrows and red vertical bars. These represent F7 clips with 31% suppression (left trace) and 77% suppression (right trace) with identified suppressed periods in red (c, insets). Left and right red bars correspond to the left and right traces, respectively. (d) The trajectories of the synchronised monitor indices are shown with labels indicating relevant clinical events. BIS-L, bispectral index from the left; BIS-R, bispectral index from the right; Entropy-R, response entropy; Entropy-S, state entropy; PSI, patient state index.
To test the lower-bound detection of the too deeply anaesthetised state, we evaluated the co-occurrence of high SRs and low monitor indices indicating deep anaesthesia (Table 1). We initially calculated the percentage of time that our manual EEG suppression scoring showed SR >25% and indices were in the deeply anaesthetised range (n=5 patients; 67 incidents total). The percentage of corresponding PSI values showing deeply anaesthetised indices was 97%, BIS-L 97%, BIS-R 96%, and Entropy-R and Entropy-S were both near chance at 48%. We examined the SR correlation and indices when SR >5% (113 incidences). All correlations were negatively correlated with SR (PSI r=–0.54, P<0.001; BIS-L r=–0.51, P<0.001; BIS-R r=–0.47, P<0.001; Entropy-R r=–0.27, P<0.005; Entropy-S r=–0.30, P<0.002).
Discussion
We described a playback system allowing for simultaneous offline comparison of processed EEG indices using the PSI, BIS, and Entropy devices. As a first use case, we tested monitor performance using EEG data from traditionally hard-to-monitor older patients receiving beta blockers. Similar to previous reports, we found a strong correlation between monitor indices and the general trend in clinical response to anaesthesia7,9,22 (Fig. 2). Our data show that all monitors significantly discriminate between the EEG activity that occurs after LOR and that before anaesthesia administration and before LOR.
The patients in our study were anaesthetised with boluses of propofol (a common clinical induction technique), and thus, EEG activity during our LOR transitions is likely more abrupt compared with slow infusions.7,19 Thus, our analysis and conclusions may not extend to slower infusions of propofol. One observation is that all index ranges consistently showed greater variability around LOR (Fig. 3d). The pre-LOR variability is likely attributable to the heterogeneity in patient sensitivity to anaesthesia and subjectivity of physician judgement for LOR.19 The decreased variability after LOR may indicate that all brains converge to a similar steady state with more homogeneous EEG patterns.
Our patient cohort also had five patients with lengthy episodes of EEG suppression. Within those EEG suppression periods (SR >25%), the Root and BIS monitors displayed appropriate indices most frequently. For the current playback system, the Entropy algorithm was the oldest (2009) compared with the BIS (2014) and PSI (2018), which could play a role in the poorer performance. Additionally, all monitor indices were negatively correlated with SRs (>5%), indicating that all monitor indices appropriately decreased when SRs increased.
The goal for this study was to gauge monitor response to real-world clinical situations. However, we recognise that some assumptions made about how each processed EEG monitor handles an EEG signal may limit the accuracy and utility of the playback system. One assumption includes the electrode location adjustment for the BIS and Entropy systems, given that the EEG data were originally collected on a Masimo SedLine Legacy monitor. Additionally, the Legacy monitor collects data at 250 Hz sampling frequency, and we applied a 0.5 Hz high-pass filter to the data. Thus, the EEG data bandwidth extended from 0.5 to 125 Hz. As some of the monitor specifications are unknown, we cannot conclude that this range does not limit performance. Additionally, we recognise the importance of accurately conveying the incoming EEG signals from the correct locations,30 and plan to record new EEG for future studies. Also, we have not included an analysis of how monitors handle artifacts, nor a sensitivity and specificity analysis, which are both important to assess monitor functionality in real-world clinical settings.
Nonetheless, our first use case analyses showed strong agreement between EEG activity and monitor trajectories. The playback system has high utility to determine the strengths and limitations of these monitors to reflect anaesthetic effect in traditionally hard-to-monitor patients. Future work using systems, such as this, will enhance clinical care by allowing physicians to make more informed decisions about how to best monitor their patients, knowing the strengths and limitations of their equipment.
Authors' contributions
Study conception: DRD, SLE
Collection of EEG data: DRD
Running of data through playback set-up: SLE, CMD
Organising of data for analysis: SLE, DRD, CMD
Data analysis: SLE
Supervision of data analysis: DRD, MBM
Writing of paper: all authors
All authors contributed to the intellectual content with rounds of review, and approved the final version of the paper for publication.
Acknowledgements
The authors thank Nicholas Ouellette (Stanford School of Engineering) for helpful discussions on analyses.
Handling editor: Michael Avidan
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bja.2020.12.042.
Declarations of interest
DRD is a consultant for Masimo. Equipment to create the anaesthesia EEG playback system was purchased with an unrestricted grant from Masimo (DRD, MBM, and SLE). A Masimo employee helped the research group to overcome challenges with the software driver used to translate EEG files into the input format needed by the National Instruments analogue module (DRD, MBM, and SLE). Data for the original study performed to collect the EEG data were funded by Hospira (DRD). The rest of the authors declare no additional conflicts of interest.
Funding
Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine to DRD, MBM, and SLE; the Anaesthesia T32 Training Grant in Biomedical Research, US National Institutes of Health (GM089626-09) to SLE; National Institutes of Health, US National Institute of General Medical Sciences Pathway to Independence Award (1K99GM140215-01) to SLE.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Shander A., Lobel G.P., Mathews D.M. Brain monitoring and the depth of anesthesia. Anesth Analg. 2018;126:705–709. doi: 10.1213/ANE.0000000000002383. [DOI] [PubMed] [Google Scholar]
- 2.Fahy B.G., Chau D.F. The technology of processed electroencephalogram monitoring devices for assessment of depth of anesthesia. Anesth Analg. 2018;126:111–117. doi: 10.1213/ANE.0000000000002331. [DOI] [PubMed] [Google Scholar]
- 3.Sebel P.S., Bowdle T.A., Ghoneim M.M. The incidence of awareness during anesthesia: a multicenter United States study. Anesth Analg. 2004;99:833–839. doi: 10.1213/01.ANE.0000130261.90896.6C. table of contents. [DOI] [PubMed] [Google Scholar]
- 4.Myles P.S., Leslie K., McNeil J., Forbes A., Chan M.T.V. Bispectral index monitoring to prevent awareness during anaesthesia: the B-Aware randomised controlled trial. Lancet. 2004;363:1757–1763. doi: 10.1016/S0140-6736(04)16300-9. [DOI] [PubMed] [Google Scholar]
- 5.Schneider G., Gelb A.W., Schmeller B., Tschakert R., Kochs E. Detection of awareness in surgical patients with EEG-based indices—bispectral index and patient state index. Br J Anaesth. 2003;91:329–335. doi: 10.1093/bja/aeg188. [DOI] [PubMed] [Google Scholar]
- 6.Mathews D.M., Clark L., Johansen J., Matute E., Seshagiri C.V. Increases in electroencephalogram and electromyogram variability are associated with an increased incidence of intraoperative somatic response. Anesth Analg. 2012;114:759–770. doi: 10.1213/ANE.0b013e3182455ac2. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt G.N., Bischoff P., Standl T., Hellstern A., Teuber O., Schulte Esch J. Comparative evaluation of the Datex-Ohmeda S/5 entropy module and the bispectral index monitor during propofol-remifentanil anesthesia. Anesthesiology. 2004;101:1283–1290. doi: 10.1097/00000542-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Drover D., Ortega H.R. Patient state index. Best Pract Res Clin Anaesthesiol. 2006;20:121–128. doi: 10.1016/j.bpa.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Drover D.R., Lemmens H.J., Pierce E.T. Patient state index: titration of delivery and recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 2002;97:82–89. doi: 10.1097/00000542-200207000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Mickle A.M., Maybrier H.R., Winter A.C. Achieving milestones as a prerequisite for proceeding with a clinical trial. Anesth Analg. 2018;126:1851–1858. doi: 10.1213/ANE.0000000000002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avidan M.S., Fritz B.A., Maybrier H.R. The Prevention of Delirium and Complications Associated with Surgical Treatments (PODCAST) study: protocol for an international multicentre randomised controlled trial. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soehle M., Dittmann A., Ellerkmann R.K., Baumgarten G., Putensen C., Guenther U. Intraoperative burst suppression is associated with postoperative delirium following cardiac surgery: a prospective, observational study. BMC Anesthesiol. 2015;15:61. doi: 10.1186/s12871-015-0051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown C.H., Azman A.S., Gottschalk A., Mears S.C., Sieber F.E. Sedation depth during spinal anesthesia and survival in elderly patients undergoing hip fracture repair. Anesth Analg. 2014;118:977–980. doi: 10.1213/ANE.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieber F.E., Zakriya K.J., Gottschalk A. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85:18–26. doi: 10.4065/mcp.2009.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kertai M.D., Palanca B.J.A., Pal N. Bispectral index monitoring, duration of bispectral index below 45, patient risk factors, and intermediate-term mortality after noncardiac surgery in the B-Unaware Trial. Anesthesiology. 2011;114:545–556. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 16.Fritz B.A., Maybrier H.R., Avidan M.S. Intraoperative electroencephalogram suppression at lower volatile anaesthetic concentrations predicts postoperative delirium occurring in the intensive care unit. Br J Anaesth. 2018;121:241–248. doi: 10.1016/j.bja.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wildes T.S., Mickle A.M., Ben Abdallah A. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the ENGAGES randomized clinical trial. JAMA. 2019;321:473–483. doi: 10.1001/jama.2018.22005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musialowicz T., Lahtinen P., Pitkänen O., Kurola J., Parviainen I. Comparison of Spectral Entropy and BIS VISTA™ monitor during general anesthesia for cardiac surgery. J Clin Monit Comput. 2011;25:95–103. doi: 10.1007/s10877-011-9280-6. [DOI] [PubMed] [Google Scholar]
- 19.Kaskinoro K., Maksimow A., Långsjö J. Wide inter-individual variability of bispectral index and spectral entropy at loss of consciousness during increasing concentrations of dexmedetomidine, propofol, and sevoflurane. Br J Anaesth. 2011;107:573–580. doi: 10.1093/bja/aer196. [DOI] [PubMed] [Google Scholar]
- 20.Soto R.G., Smith R.A., Zaccaria A.L., Miguel R.V. The effect of addition of nitrous oxide to a sevoflurane anesthetic on BIS, PSI, and entropy. J Clin Monit Comput. 2006;20:145–150. doi: 10.1007/s10877-006-9009-0. [DOI] [PubMed] [Google Scholar]
- 21.Kreuzer M., Kochs E.F., Pilge S., Stockmanns G., Schneider G. Construction of the electroencephalogram player: a device to present electroencephalogram data to electroencephalogram-based anesthesia monitors. Anesth Analg. 2007;104:135–139. doi: 10.1213/01.ane.0000249045.52690.e8. [DOI] [PubMed] [Google Scholar]
- 22.Bibian S., Dumont G.A., Zikov T. Dynamic behavior of BIS, M-Entropy and neuroSENSE brain function monitors. J Clin Monit Comput. 2011;25:81–87. doi: 10.1007/s10877-010-9266-9. [DOI] [PubMed] [Google Scholar]
- 23.Pilge S., Kreuzer M., Karatchiviev V., Kochs E.F., Malcharek M., Schneider G. Differences between state entropy and bispectral index during analysis of identical electroencephalogram signals. Eur J Anaesthesiol. 2015;32:354–365. doi: 10.1097/EJA.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 24.Petersen C.L., Görges M., Massey R., Dumont G.A., Ansermino J.M. A procedural electroencephalogram simulator for evaluation of anesthesia monitors. Anesth Analg. 2016;123:1136–1140. doi: 10.1213/ANE.0000000000001506. [DOI] [PubMed] [Google Scholar]
- 25.Billard V., Gambus P.L., Chamoun N., Stanski D.R., Shafer S.L. A comparison of spectral edge, delta power, and bispectral index as EEG measures of alfentanil, propofol, and midazolam drug effect. Clin Pharmacol Ther. 1997;61:45–58. doi: 10.1016/S0009-9236(97)90181-8. [DOI] [PubMed] [Google Scholar]
- 26.Viertio-Oja H., Maja V., Sarkela M. Description of the Entropy™ algorithm as applied in the Datex-Ohmeda S/5™ entropy module. Acta Anaesthesiol Scand. 2004;48:154–161. doi: 10.1111/j.0001-5172.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- 27.Drover D.R., Schmiesing C., Buchin A.F. Titration of sevoflurane in elderly patients: blinded, randomized clinical trial, in non-cardiac surgery after beta-adrenergic blockade. J Clin Monit Comput. 2011;25:175–181. doi: 10.1007/s10877-011-9293-1. [DOI] [PubMed] [Google Scholar]
- 28.Eagleman S.L., Vaughn D.A., Drover D.R. Do complexity measures of frontal EEG distinguish loss of consciousness in geriatric patients under anesthesia? Front Neurosci. 2018;12:645. doi: 10.3389/fnins.2018.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghosh I., Bithal P.K., Dash H.H., Chaturvedi A., Prabhakar H. Both clonidine and metoprolol modify anesthetic depth indicators and reduce intraoperative propofol requirement. J Anesth. 2008;22:131–134. doi: 10.1007/s00540-007-0606-y. [DOI] [PubMed] [Google Scholar]
- 30.Kreuzer M., Schneider G., García P.S. The input is reflected in the output. Anesth Analg. 2017;124:1734–1735. doi: 10.1213/ANE.0000000000001958. [DOI] [PubMed] [Google Scholar]
- 31.Mitra and H Bokil P. Oxford University Press, New York, NY USA; 2008. Observed brain dynamics. [Google Scholar]
- 32.Rampil I.J., Weiskopf R.B., Brown J.G. I653 and isoflurane produce similar dose-related changes in the electroencephalogram of pigs. Anesthesiology. 1988;69:298–302. doi: 10.1097/00000542-198809000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.