Abstract
Metastatic triple-negative breast cancer (mTNBC) is the most aggressive breast cancer subtype. Programmed death ligand 1 (PD-L1) on immune cells (IC) using the VENTANA SP142 assay is linked to improved clinical outcome in atezolizumab plus nab—paclitaxel-treated patients with mTNBC in the IMpassion130 study. The goal of the current study was to evaluate prevalence of VENTANA SP142 PD-L1 assay by anatomic location in 670 histologically confirmed TNBC cases from subjects with metastatic disease screened for the phase 1 study PCD4989g (NCT01375842). PD-L1 immunohistochemistry was centrally tested on tumor cells (TC) and on tumor infiltrating IC, following manufacturer’s instructions. At a 1% cutoff, tumor PD-L1 was more prevalent in IC than TC: 46% were PD-L1 IC+/TC−, 3% were PD-L1 IC−/TC+, and 10% were PD-L1 IC+/TC+. PD-L1 IC and TC immunostaining correlated with CD274 RNA expression, as assessed by fluidigm. Analyses of anatomic locations suggest that prevalence of PD-L1 IC+ was highest in lymph nodes (65.0%), lowest in liver metastases (26.9%), while breast tissue was intermediate (57.1%). Matched paired samples from the same subject collected synchronously or asynchronously showed a PD-L1 IC status agreement of 80% (8/10) and 75% (15/20), respectively. Our results suggest that the anatomic location of metastases and time of collection may influence the detection of PD-L1.
Key Words: PD-L1, triple-negative breast cancer (TNBC), atezolizumab, SP142, immune cells
Triple-negative breast cancer (TNBC) is an aggressive, heterogenous disease characterized by lack of estrogen receptor (ER), progesterone receptor (PR), and overexpression of human epidermal growth factor receptor 2 (HER2) protein or amplification of HER2/neu gene on tumor cells (TC).1 It represents 10% to 15% of diagnosed breast cancer. Compare with other breast cancer subtypes, patients with TNBC have a poor prognosis and limited treatment potions.2 Patients with metastatic TNBC (mTNBC) have median overall survival of only 12 to 17 months with standard-of-care chemotherapy.3 New treatment options, such as targeted therapy using PARP inhibitors in patients carrying BRCA1/2 mutations and immune checkpoint inhibitors for patients whose tumors are programmed death ligand 1 (PD-L1) positive have increased clinical benefits in this hard to treat patient population.2
PD-L1 is a cell membrane protein expressed on TC and immune cells (IC).4,5 Binding of PD-L1 to PD-1 on T cells reduces T-cell activation and inhibits antitumor immune response.6 PD-L1 immunohistochemistry is used as a companion diagnostic to treat some cancer patients with PD-1/PD-L1 inhibitors. In the atezolizumab monotherapy phase 1 clinical trial PCD4989g (NCT01375842), patients with mTNBC whose disease expressed PD-L1 in at least 1% of tumor infiltrating IC as percentage of tumor area (IC ≥1%) were more likely to respond to atezolizumab and had longer overall survival.7 Furthermore, the IMpassion130 clinical trial demonstrated that patients with mTNBC whose tumors expressed PD-L1 IC≥1% were more likely to derive progression free and overall survival clinical benefit from atezolizumab in combination with nab-paclitaxel compared with nab-paclitaxel plus placebo,8 leading to the accelerated approval of this combination by the US FDA, and by EMA and EAMS in the UK [Tecentriq (atezolizumab) (package insert), Genentech Inc., South San Francisco, CA].
PD-L1 SP142 immunohistochemistry assay was developed as a diagnostic test for atezolizumab in patients with TNBC, non–small cell lung cancer and urothelial carcinoma.8,9 The assay evaluates PD-L1 staining on both TC and tumor infiltrating IC (Package insert, Ventana PD-L1 (SP142) Assay, Ventana Medical Systems, Tucson, AZ). To characterize better the prevalence and stability of this biomarker in TNBC, we evaluated in the current study the frequency of PD-L1 in tumor infiltrating IC and TC categorized by anatomic locations, and agreement of PD-L1 IC status in matched samples from the same patient collected at the same (synchronous) or different (asynchronous) times.
MATERIALS AND METHODS
Specimens
Formalin-fixed, paraffin-embedded (FFPE) tumor tissue from histologically confirmed TNBC samples was collected from patients with mTNBC (locally diagnosed) screened for enrollment in the clinical trial PCD4989g (NCT01375842).7,10 Specimens tested were obtained either from primary or metastatic tumors. Samples were PD-L1-evaluable if they had at least 50 viable TC with associated stroma and no technical artifacts occurred during staining. Samples with insufficient or no tumor, no invasive tumor or where tissue was washed off the slide were excluded, and samples with technical artifacts: control tissues not showing appropriate staining or staining artifacts. Exclusion for technical reasons was <1% of all samples. Acceptable specimens were core needle biopsies and surgical resections, while fine-needle aspiration, brushing, cell pellets from pleural effusion, and lavage samples and tissue microarrays specimens were not accepted. Anatomic locations of the samples were derived from the information in the pathology reports submitted by the local clinical sites. All samples were tested following standard recommendations provided in the package insert for PD-L1 (SP142) assay (formalin fixed and paraffin embedded with 6 to 72 hours of fixation time and were within the cut slide stability range), to limit the effect of preanalytical variables. The patients provided informed consent for the analysis of these samples.
PD-L1 SP142 Immunohistochemistry
PD-L1 expression on IC and TC was evaluated centrally using the VENTANA SP142 immunohistochemistry (IHC) assay (Ventana Medical Systems), as described previously.9 PD-L1 expression in TC was assessed as the percentage of TC with membrane PD-L1 immunostaining of any intensity, and both partial and complete membrane staining was scored as positive. Expression in IC (lymphocytes, macrophages, dendritic cells, and granulocytes) was assessed as the proportion of tumor area (viable TC with associated intratumoral stroma and contiguous peritumoral stroma) occupied by PD-L1-positive IC of any intensity. IC exhibit punctate staining in lymphocytes, which is perinuclear in location. Although it seems to be cytoplasmic, given the fact that lymphocytes do not have much cytoplasm it is difficult to distinguish between and membrane and cytoplasmic localization of this type of staining. Dendritic cells and macrophages show membrane and sometimes associated cytoplasmic staining. The scoring algorithm was as follows: IC0 (<1%); IC1 (≥1% and <5%); IC2 (≥5% and <10%); IC3 (≥10%); TC0 (<1%); TC1 (≥1% and <5%); TC2 (≥5% and <50%); and TC3 (≥50%). When accessing lymph node (LN) samples, same scoring method as primary samples was used. In tumors with clearly identified desmoplastic stroma in a LN metastasis, contiguous desmoplastic stroma was used for assessment of peritumoral stroma. In cases where tumors did not have much desmoplastic stroma, the peritumoral stroma included only the line of lymphocytes that are touching the edge of the tumor. Native lymphoid tissue was not considered toward tumor area; neither was any IC staining in native tissue scored to provide positive/negative status. More than 1 pathologist at a single laboratory scored these samples, but each sample was scored by a single pathologist. All pathologists who participated in scoring for this study were trained and considered proficient in scoring PD-L1 (SP142) assay. The concordance of pathologist scores to consensus scores provides evidence of interobserver agreement. Reproducibility of the assay performed by trained pathologists was reported in a recent global study.11
CD274 RNA Fluidigm
FFPE tumor sections were macrodissected to enrich for tumor content when the tumor content in the sample was below 60% area, as assessed by a pathologist. RNA was isolated using the High Pure FFPE RNA Micro Kit (Roche Applied Sciences, Indianapolis, IN) according to the manufacturer’s protocol. CD274 (PD-L1) RNA gene-expression analysis was performed using the BioMark HD real-time PCR Platform as previously described.10 The assay was performed according to manufacturer’s guidelines. All samples were assayed in triplicate. A geometric mean of the cycle threshold (Ct) values for 4 of the reference genes (SP2, GUSB, VPS338, SDHA) was calculated for each sample, and expression levels were determined using the delta Ct (dCt) method as follows: Ct(Target Gene)−GeoMean Ct(Reference Genes).
Statistics
Correlation was analyzed with Spearman index for nonparametric analysis. Jonckheere trend test was applied to test the pattern of CD274 mRNA expression across PD-L1 IC/TC categories. The prevalence distribution of PDL1 IC/TC by tumor samples was evaluated by Fisher exact t test.
RESULTS
Tumor Sample Collection from TNBC Patients
A total of 759 tumor samples from 675 subjects were collected (Fig. 1). Eighty-eight percent (670/759) of the samples had evaluable tumor content. A total of 303 samples were from breast, 60 samples were from LN, and 82 were from various anatomic locations including liver, lung, skin, chest wall, brain, bone and others (225 samples had no information on anatomic location).
FIGURE 1.
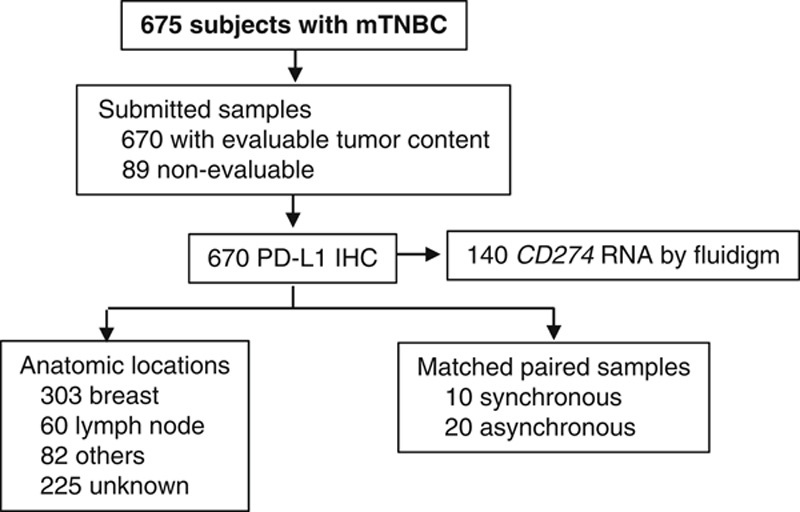
Study flowchart. The flowchart shows number of subjects with metastatic triple-negative breast cancer (mTNBC) who submitted samples for the PCD4989g clinical study. Of the 670 samples evaluated by PD-L1 IHC, 140 had RNA extracted and CD274 was evaluated by fluidigm. Primary/metastases and anatomic locations were annotated as inscribed by the participating local clinical site. Synchronous: samples collected on the same date; asynchronous: samples collected on different dates. IHC indicates immunohistochemistry; PD-L1, programmed death ligand 1.
Prevalence of PD-L1 in IC) and TC
SP142 PD-L1 in TC and tumor infiltrating IC had distinctive immunostaining patterns (Fig. 2A). Prevalence of PD-L1 IC+ (IC≥1%, IC1/2/3) was 56% (374/670), while PD-L1 TC+ (TC≥1%, TC1/2/3) was found in only 14% (92/670) of the samples (Fig. 2B). PD-L1 TC+ mostly co-occurred in PD-L1 IC+ specimens: prevalence of PD-L1 IC+/TC+ was 10%, while prevalence of PD-L1 IC+/TC-, PD-L1 IC-/TC+ was 46% and 3%, respectively (Fig. 2B). A more granular evaluation of the IC/TC distribution showed that 34% was IC1, 14% was IC2, and 8% for IC3, while 9% was TC1, 4% was TC2 and 1% was TC3 (Table 1).
FIGURE 2.
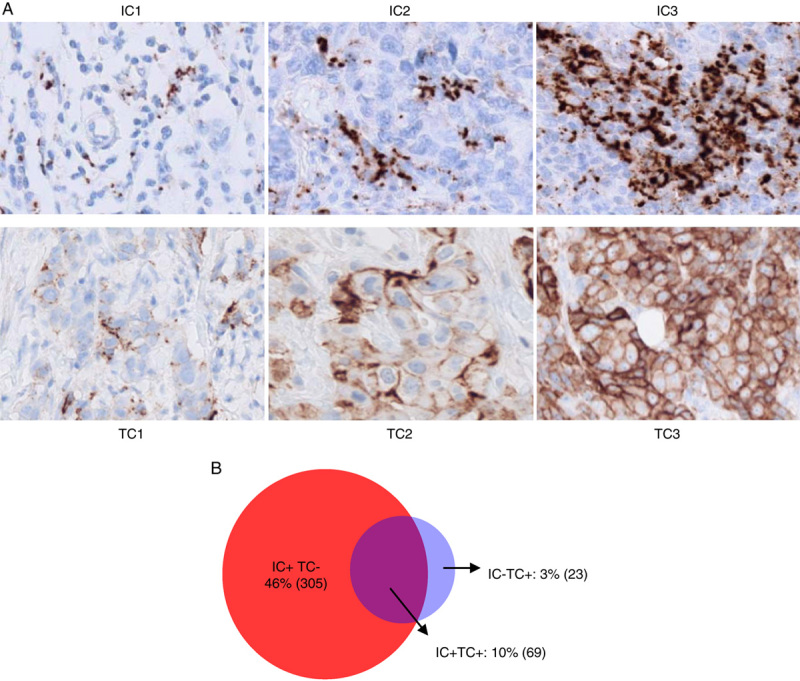
PD-L1 expression in TNBC in tumor infiltrating immune cells and tumor cells. A, Range of PD-L1 expression in immune cells as assessed by SP142 IHC by PD-L1 subgroup. B, Prevalence of PD-L1 IC+ (IC≥1%) and PD-L1 TC+ (TC≥1%). IC indicates immune cells; PD-L1, programmed death ligand 1; TC, tumor cells; TNBC, triple-negative breast cancer.
TABLE 1.
Prevalence of PD-L1 by IC and TC Subgroup
| N (%) | |||||
|---|---|---|---|---|---|
| PD-L1 | TC0 | TC1 | TC2 | TC3 | Total |
| IC0 | 273 (40.7) | 20 (3.0) | 2 (0.3) | 1 (0.1) | 296 (44.2) |
| IC1 | 202 (30.1) | 15 (2.2) | 9 (1.3) | 3 (0.5) | 229 (34.2) |
| IC2 | 66 (9.9) | 15 (2.2) | 12 (1.8) | 0 (0.0) | 93 (13.9) |
| IC3 | 37 (5.5) | 11 (1.6) | 3 (0.4) | 1 (0.1) | 52 (7.8) |
| Total | 578 (86.3) | 61 (9.1) | 26 (3.9) | 5 (0.7) | 670 (100.0) |
PD-L1 in IC: IC0 (IC <1%), IC1 (IC≥1% and <5%), IC2 (IC≥5% and <10%) and IC3 (IC≥10%). PD-L1 in TC: TC0 (TC <1%), TC1 (TC≥1% and <5%), TC2 (TC≥5% and <50%) and TC3 (TC≥50%).
IC indicates immune cells; PD-L1, programmed death ligand 1; TC, tumor cells.
CD274 mRNA Association to PD-L1 Immunostained Tumor and Tumor-infiltrating IC
To validate the SP142 PD-L1 results with an independent methodology, we evaluated the association of PD-L1 expression on TC and IC at the scoring cutoffs with increasing CD274 (PD-L1) mRNA levels as an independent measure of PD-L1 expression. Indeed, CD274 mRNA levels correlated significantly with PDL1 IC and TC categories (Fig. 3; P<0.0001), further confirming the specificity of the test.
FIGURE 3.
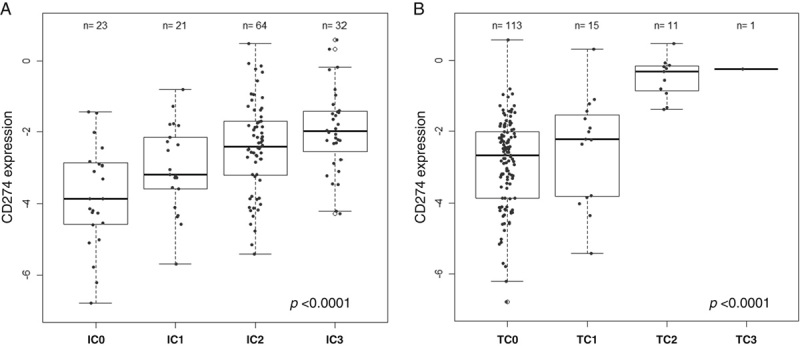
Association of CD274 mRNA and PD-L1 immunostaining. CD274 RNA, as assessed by fluidigm, was associated to PD-L1 IC (A) and PD-L1 TC (B). PD-L1 in IC categories: IC0 (IC <1%), IC1 (IC≥1% and <5%), IC2 (IC≥5% and <10%) and IC3 (IC≥10%). PD-L1 in TC categories: TC0 (TC <1%), TC1 (TC≥1% and <5%), TC2 (TC≥5% and <50%) and TC3 (TC≥50%). P-value derived from Joncheree trend test. IC indicates immune cells; PD-L1, programmed death ligand 1; TC, tumor cells.
PD-L1 IC and TC Prevalence by Anatomic Locations
Prevalence by anatomic location was heterogenous for PD-L1 IC (Fig. 4A and Table 2). Evaluation of PD-L1 IC as a continuum indicated that median percentage of PD-L1 IC was lowest in specimens from bone (median <1%, n=4), soft tissue (median <1%, n=7), and liver (median: <1%, n=26), while median prevalence was highest in lung (median: 2%, n=15) and LNs (median: 3%, n=60), and breast was intermediate (median: 1%, n=303). Median of PD-L1 TC was 0%, irrespective of the anatomic location (Table 2). Categorical evaluation of PD-L1 IC positive cases (≥1% of IC) demonstrated a significantly higher prevalence in LNs (prev.: 65.0%, n=60) compared with breast (prev.: 57.1%, n=303) and other metastatic sites (prev.: 42.1%, n=76) (P=0.0186), while PD-L1 TC+ was not significantly different between LNs, breast and other anatomic locations (prev.: 18.3% vs. 13.5%, vs. 15.8%, respectively; P=0.5443) (Table 2).
FIGURE 4.
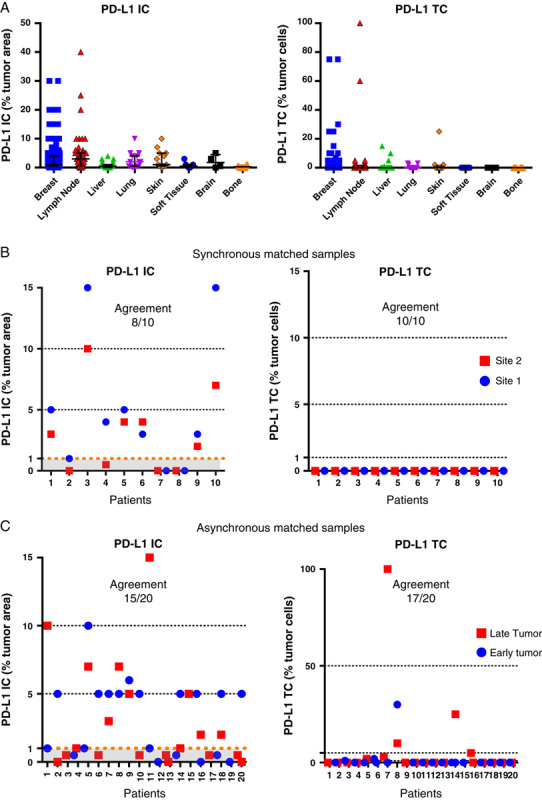
Prevalence of PD-L1 IC and TC by anatomic location and in paired matched samples collected synchronously or asynchronously. Expression of PD-L1 by anatomic location as a continuum variable (A). PD-L1 on IC and TC was evaluated in samples collected at the same time (B) or at different times (C). The dotted lines discriminate the PD-L1 subgroups by their respective cutoffs. “Late” versus “Early” were classified relative to dates of sample collection. IC indicates immune cells; PD-L1, programmed death ligand 1; TC, tumor cells.
TABLE 2.
PD-L1 IC+ and TC+ Prevalence by Anatomic Location
| Breast | Lymph Node | Liver | Lung | Skin | Soft Tissue | Brain | Bone | |
|---|---|---|---|---|---|---|---|---|
| Samples, N | 303 | 60 | 26 | 15 | 14 | 7 | 4 | 4 |
| PD-L1 IC | ||||||||
| Median (IQTL 25%, 75% range) | 1.00 (0.50, 4.00) | 3.00 (0.50, 5.00) | 0.50 (0.0, 1.00) | 2.00 (0.50, 4.00) | 1.00 (1.00, 5.00) | 0.50 (0.00, 1.00) | 1.75 (0.13, 4.50) | 0.25 (0.0, 0.88) |
| IC≥1% (%) | 57.1 | 65.0 | 26.9 | 53.3 | 57.1 | 42.9 | 50.0 | 25.0 |
| PD-L1 TC | ||||||||
| Median (IQTL 25%, 75% range) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.50) | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.00) | 0.0 (0.0, 0.0) |
| TC≥1% (%) | 13.5 | 18.3 | 15.4 | 20.0 | 21.4 | 0.0 | 0.0 | 0.0 |
PD-L1 in IC: IC0 (IC <1%), IC1 (IC≥1% and <5%), IC2 (IC≥5% and <10%) and IC3 (IC≥10%). PD-L1 in TC: TC0 (TC <1%), TC1 (TC≥1% and <5%), TC2 (TC≥5% and <50%) and TC3 (TC≥50%).
IC indicates immune cells; IQTL, interquartile; PD-L1, programmed death ligand 1; TC, tumor cells.
PD-L1 IC Expression in Matched Synchronous and Asynchronous Tumor Specimens
Some patients provided multiple tumor samples collected at the same (synchronous) or on different dates (asynchronous), enabling the evaluation of PD-L1 IC and TC agreement synchronously and asynchronously. At a 1% cutoff, PD-L1 IC agreement in synchronous samples was 80% (8/10 pairs). Six pairs had PD-L1 IC≥1% in both lesions, 2 pairs were <1% in both locations, and 2 pairs had 1 lesion ≥1% and the other <1% (Fig. 4B). The anatomic origin of these pairs was: 7 were both from breast; 1 pair had both specimens from LN; 1 pair had 1 sample from breast and the other from LN, 1 pair had 1 sample from lung and the other from unknown tissue sources. PD-L1 TC was negative in all evaluated specimens.
PD-L1 IC agreement in asynchronous matched samples was 75% (15/20 pairs). At the same time, PD-L1 TC agreement in asynchronous matched samples was higher at 85% (17/20 pairs) using 1% cutoff. The median time difference between samples collection was 282 days (range, 17 d to >4 y). Six pairs from breast; 2 pairs from skin; 5 pairs were from different tissues (lung vs. LN, neck vs. LN, 2 pairs of breast vs. skin breast vs. liver); and 7 pairs had at least 1 specimen with unknown anatomic location. Ten matched pairs were PD-L1 IC+ in both early and late samples, and 5 pairs were PD-L1 IC- in both early and late tumors. Of the discordant cases, 4 pairs whose early lesions were PD-L1 IC+ switched to PD-L1 IC- in the late specimen, while 1 pair of PD-L1 IC- in the early tumor converted in PD-L1 IC+ in the late specimen (Fig. 4C). Although the small numbers of pairs did not allow very meaningful statistical analysis, these results suggest that PD-L1 status may change in a fraction of patients with TNBC.
DISCUSSION
Tumor expression of PD-L1 in TC and IC is a predictive biomarker to select patients more likely to respond to PD-L1/PD-1 immune checkpoint inhibitors in several cancer indications.8,12–14 The investigational use only (IUO) SP142 PD-L1 IHC is approved as a complementary diagnostic for atezolizumab monotherapy in patients with 2L non–small cell lung cancer (PD-L1 IC≥1% or TC≥1%), and as companion diagnostic in 1L cisplatin-ineligible urothelial cancer (PD-L1 IC ≥5%).12,15,16 More recently, the FDA approved the SP142 PD-L1 IHC assay as companion diagnosis for atezolizumab in combination with nab-paclitaxel in 1L locally advanced or metastatic TNBC treated with atezolizumab plus nab-paclitaxel (PD-L1 IC ≥1%) based on the results from the IMpassion130 clinical study,8 [Tecentriq (atezolizumab) (package insert), Genentech Inc.]. The current prevalence study was performed in samples from heavily pretreated mTNBC screened subjects from the large phase 1 atezolizumab monotherapy study PCD4989g.7
PD-L1 expression in the tumors of TNBC is mainly IC rather than TC. Non–small cell lung cancer has been reported to have a higher rate of PD-L1 TC+ (≥1% TC expressing PD-L1) (32%) using the SP142 assay. In that study, SP142 also detected a higher prevalence of PDL1 in IC (59%) than in TC.17 Emens and colleagues showed that prevalence of PD-L1 IC+ in TNBC samples from the IMpassion130 study was 41%, while prevalence for TC+ was 9%. Although the results in IMpassion130 and the current report are not the same, there is a similar pattern of higher prevalence of IC+ than TC+ and that most of the PDL1 TC+ cases were also IC+ (in the IMpassion130 study 2% of cases were TC+/IC− vs. 7% TC+/IC+).18
Comparison of PD-L1 prevalence studies in TNBC has been challenging, as each study had different reagents, cutoffs, and samples of different stages of disease.19–22 A PD-L1 IHC comparative study in TNBC using tumor microarrays showed that SP142 stained fewer TC and IC compared with the E1L3N and 28-8, antibodies.23 Similar results were also reported in analysis of NSCLC tumors.24 The reasons for discrepancy between the assays remain to be addressed. Collectively, PD-L1 diagnosis can be influenced by many factors. Depending on the treatment and disease indication/stage, 1 assay with its defined cut off can be more efficient in identifying the patient population that can benefit a specific treatment than other assays.
In our study, PD-L1 prevalence varied according to anatomic location. Metastatic sites such as liver, skin, soft tissue, and bone had lower prevalence of PD-L1 IC+ compared with breast, while LNs, lung and brain may have higher prevalence, though caution should be taken considering the small number of samples tested for some of the locations. The liver, an organ that in homeostasis ensures tolerance to circulating antigens and endotoxins from the gut, possesses intrinsic immune tolerogenic characteristics that contribute to spontaneous allogeneic organ acceptance.25 Therefore, the liver’s intrinsic immunosuppressive microenvironment may explain for the very low prevalence of PD-L1 IC in the liver metastases. A similar pattern of low PD-L1 IC prevalence has been observed in other tumor types beyond breast cancer (bladder, lung, melanoma, kidney; unpublished data). Importantly, in the IMpassion130 study patients with mTNBC who have liver metastases and are PD-L1 IC+ (identified either in the liver or in other anatomic location) derive clinical benefit from atezolizumab plus nab-paclitaxel compared with placebo plus nab-paclitaxel.8
Szekely et al26 described that the metastatic tissue of TNBC have lower levels of immune genes compared with the tissue of the primary tumor. Although that study compared primary versus metastases in the same patient, a major difference to our study is that the subjects in our study had received in many cases multiple lines of therapy before sample submission to our study. Some therapeutic interventions may modulate the tumor immune microenvironment, hence PD-L1 IC may change between primary and metastases. Supporting this hypothesis, we have reported the case of a patient with mTNBC who had multiple tissue samples collected in the course of her clinical history and had changed the PD-L1 status from negative in the primary to positive in the metastases after exposure to different chemotherapy and experimental treatments.27
PD-L1 expression may change as a function of anatomic location or treatment exposure. In the current study we were able to evaluate the agreement of PD-L1 IC status in a small set of matched samples, either collected at the same time or at different times in the course of the patients’ clinical history. Although the number of paired biopsies were small, we observed discrepancy of 20% to 25% in synchronous and asynchronous. Reasons for such discrepancies could be heterogeneity in the tumor and change of PD-L1 status during disease progression or treatment. Such heterogeneity of PD-L1 raises the possibility that a fraction of patients may be diagnosed as PD-L1 negative even if PD-L1 can be expressed in another uncollected tumor site. In addition, it remains to be investigated whether the on-treatment change of PD-L1 status of patient being treated with PD-L1/PD-1 blockade can provide valuable information for clinical decision.
Footnotes
Y.L., C.-W.C., D.T., R.N., T.S., P.H., and L.M. are employees of Genentech Inc. B.V. is employee of Ventana Medical Systems.
REFERENCES
- 1.Bianchini G, Balko JM, Mayer IA, et al. Tripple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13:674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehanna J, Haddad FG, Eid R, et al. Triple-negative breast cancer: current perspective on the evolving therapeutic landscape. Int J Womens Health. 2019;11:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yardley DA, Coleman R, Conte P, et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: results from the tnAcity trial. Ann Oncol. 2018;29:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 5.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Emens LA, Cruz C, Eder JP, et al. Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a phase 1 study. JAMA Oncol. 2019;5:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid P, Adams S, Rugo HS, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative rreast cancer. N Engl J Med. 2018;379:2108–2121. [DOI] [PubMed] [Google Scholar]
- 9.Vennapusa B, Baker B, Kowanetz M, et al. Development of a PD-L1 complementary diagnostic immunohistochemistry assay (SP142) for atezolizumab. Appl Immunohistochem Mol Morphol. 2019;27:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dennis E Kockx M Harlow G, et al. Effective and globally reproducible digital pathologist training program on PD-L1 immunohistochemistry scoring on immune cells as a predictive biomarker for cancer immunotherapy in triple negative breast cancer [abstract]. Proceedings of the 2019 San Antonio Breast Cancer Symposium; 2019 December 10–14; San Antonio, TX. Philadelphia, PA: AACR; Cancer Res. 2020;80(suppl): Abstract no. PD5-02.
- 12.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393:2404–2415. [DOI] [PubMed] [Google Scholar]
- 14.Emens LA, Esteva FJ, Beresford M, et al. Overall survival (OS) in KATE2, a phase II study of programmed death ligand 1 (PD-L1) inhibitor atezolizumab (atezo)+trastuzumab emtansine (T-DM1) vs placebo (pbo)+T-DM1 in previously treated HER2+ advanced breast cancer (BC). Ann Oncol. 2019;30(suppl 5):v104–v142. [Google Scholar]
- 15.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 16.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kowanetz M, Zou W, Gettinger SN, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A. 2018;115:E10119–E10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emens LA Loi S Rugo HS, et al. IMpassion130: Efficacy in immune biomarker subgroups from the global, randomized, double-blind, placebo-controlled, phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic triple-negative breast cancer [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 December 4–8; San Antonio, TX. Philadelphia, PA: AACR; Cancer Res 2019;79(suppl): Abstract no. GS1-04.
- 19.Botti G, Collina F, Scognamiglio G, et al. Programmed death ligand 1 (PD-L1) tumor expression is associated with a better prognosis and diabetic disease in triple negative breast cancer patients. Int J Mol Sci. 2017;18:459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nanda R, Chow LQ, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative rreast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34:2460–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brockhoff G, Seitz S, Weber F, et al. The presence of PD-1 positive tumor infiltrating lymphocytes in triple negative breast cancers is associated with a favorable outcome of disease. Oncotarget. 2018;9:6201–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang M, Sun H, Zhao S, et al. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8:31347–31354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun WY, Lee YK, Koo JS. Expression of PD-L1 in triple-negative breast cancer based on different immunohistochemical antibodies. J Transl Med. 2016;14:173–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol. 2017;3:1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heymann F, Tacke F. Immunology in the liver--from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016;13:88–110. [DOI] [PubMed] [Google Scholar]
- 26.Szekely B, Bossuyt V, Li X, et al. Immunological differences between primary and metastatic breast cancer. Ann Oncol. 2018;29:2232–2239. [DOI] [PubMed] [Google Scholar]
- 27.Molinero L, Li Y, Chang CW, et al. Tumor immune microenvironment and genomic evolution in a patient with metastatic triple-negative breast cancer and a complete response to atezolizumab. J Immunother Cancer. 2019;7:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]


