Weekly topical treatment with imiquimod is effective in promoting regression of cervical high-grade squamous intraepithelial lesions.
Abstract
OBJECTIVE:
To evaluate the histologic response rate of high-grade squamous intraepithelial lesions (HSIL) of the cervix after topical application of 5% imiquimod cream.
METHODS:
In this phase II trial, women with cervical HSIL (cervical intraepithelial neoplasia [CIN] 2–3) were randomly assigned to 250 mg of 5% imiquimod cream applied to the cervix weekly for 12 weeks, followed by loop electrosurgical excision procedure (LEEP) without preceding treatment. The sample size was calculated based on the HSIL regression rates previously reported by Grimm et al. The primary outcome was rate of histologic regression (to CIN 1 or less) in LEEP specimens. Prespecified secondary endpoints included surgical margin status and adverse events. Outcomes were stratified by human papillomavirus type and lesion grade (CIN 2 or CIN 3). Results were reported according to per protocol (PP) and intention-to-treat (ITT) analyses.
RESULTS:
Ninety women were enrolled: 49 in the experimental group and 41 in the control group. In the PP population, histologic regression was observed in 23 of 38 participants (61%) in the experimental group compared with 9 of 40 (23%) in the control group (P=.001). Surgical margins were negative for HSIL in 36 of 38 participants (95%) in the experimental group and 28 of 40 (70%) in the control group (P=.004). In the ITT population, rates of histologic regression also were significantly higher in the experimental group. Rates of adverse events in the experimental group were 74% (28/38) in the PP population and 78% (35/45) in the ITT population. Adverse events were mild, with abdominal pain being the most common. Three patients in the experimental group had grade 2 adverse events, including vaginal ulcer, vaginal pruritus with local edema, and moderate pelvic pain.
CONCLUSION:
Weekly topical treatment with imiquimod is effective in promoting regression of cervical HSIL.
CLINICAL TRIAL REGISTRATION:
ClinicalTrials.gov, NCT03233412.
Histologically confirmed high-grade squamous intraepithelial lesions (HSIL) of the cervix is induced by human papillomavirus (HPV)1 and is a precursor of cervical cancer. Surgical excision by either cold knife conization, laser conization, or loop electrosurgical excision procedure (LEEP) is the gold standard treatment.2 However, surgical excision is associated with obstetric complications such as preterm delivery, premature rupture of amniotic membranes, chorioamnionitis, low birth weight, admission of the newborn to the intensive care unit, and an increase in perinatal morbidity.3–6
Imiquimod is an imidazoquinoline amine that binds to Toll-like receptors 7 and 8 of macrophages, producing cytokines and interferons (IFNs), specifically IFN-alpha and IFN-beta, that limit viral replication and stimulate natural killer cells.7 Additionally, these cytokines and IFNs stimulate dendritic cells, thereby generating proliferation of CD4 T lymphocytes, IFN-gamma, cytokines and activation of CD8 T lymphocytes, all of which are toxic to HPV.8,9
The aim of this study was to evaluate the histologic response of cervical HSIL after topical application of 5% imiquimod cream, with histologic response defined as histologic regression to cervical intraepithelial neoplasia (CIN) 1 or less in the LEEP specimen. Secondary objectives included the effect of imiquimod treatment on LEEP margin status, adverse events, and tolerance of treatment.
METHODS
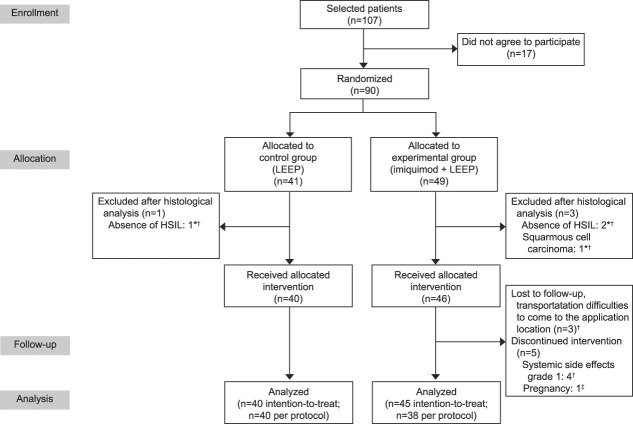
Patients aged 25–50 years with a confirmed diagnosis of CIN 2–3 were prospectively enrolled at Barretos Cancer Hospital in Barretos, Brazil, from August 2017 through April 2019. All patients had had participated in our screening program for cervical cancer prevention and had abnormal cervical cytology. Colposcopy was performed by a gynecologist using acetic acid 5%, followed by Lugol's solution 1% at magnification increments from 6× to 40× with directed cervical biopsy. Findings were classified according to the 2011 Colposcopic Terminology of the International Federation for Cervical Pathology and Colposcopy.10 In cases of histologically confirmed CIN 2 or CIN 3, women were invited to participate in this study. Exclusion criteria included: 1) suspected or confirmed invasive squamous carcinoma or in situ or invasive adenocarcinoma by colposcopy, biopsy, or cytology; 2) current pregnancy or lactation; 3) immunosuppression due to HIV or organ transplantation; and 4) previous treatment for HSIL. Eligible patients who agreed to participate in the study provided informed consent. The CONSORT (Consolidated Standards of Reporting Trials) flow diagram11 is shown in Figure 1.
Fig. 1. CONSORT (Consolidated Standards of Reporting Trials) flow chart of randomization and clinical trial progress. *Patients redrawn from intention-to-treat analysis. †Patients redrawn from per protocol analysis. ‡Excluded from per protocol and intention-to-treat analysis because they became pregnant during treatment and had not yet undergone loop electrosurgical excision procedure (LEEP). HSIL, high-grade squamous intraepithelial lesion.
Fonseca. Topical Imiquimod for HSIL of the Cervix. Obstet Gynecol 2021.
Video 1. Imiquimod application in the cervix. Application of 5% imiquimod cream on the cervix with the aid of a disposable brush. Video created by Bruno de Oliveira Fonseca, MD. Used with permission.
The study was a randomized phase II trial, without blinding and with parallel groups (ClinicalTrials.gov Identifier: NCT03233412). The study was approved by the Barretos Cancer Hospital Research Ethics Committee (No. 2,133,654). Patients were randomly assigned to two parallel groups: 1) imiquimod followed by LEEP and 2) LEEP without preceding treatment. After randomization, all pathology samples were reanalyzed by two pathologists with specialized training in gynecologic cancers to confirm HSIL.
The randomization sequence was in blocks of eight using the R software 3.4.3 by function sample. This list was loaded on the REDCap12 platform where a simple random allocation.
Patients in both groups underwent molecular testing for high-risk HPV (COBAS 4800 test). The Brazilian cervical cancer screening program is based only on cervical cytology. These patients underwent the COBAS HPV test only on the date of entry into the study, that is, 3 months after the collection of cytology in the screening program and 1 month after the initial colposcopy and confirmatory HSIL biopsy.
Patients in the experimental group underwent application of imiquimod directly to the cervix (Video 1) once a week for 12 weeks followed by LEEP. The women underwent a weekly gynecologic examination during which a speculum was used to visualize the cervix, and 250 mg of 5% imiquimod cream was applied using a disposable brush (Viba-Brush) (Fig. 2). The entire transformation zone of the cervix was covered during the application (Fig. 3). Sexual abstinence was advised for at least 72 hours after application. Before the seventh week of treatment, the women underwent a repeat colposcopic examination to assess clinical response by a doctor different from the one responsible for the application of imiquimod.
Fig. 2. Disposable brush.

Fonseca. Topical Imiquimod for HSIL of the Cervix. Obstet Gynecol 2021.
Fig. 3. Application of the imiquimod immunomodulator to the cervix. Beginning of the application (A); imiquimod reaching the entire transformation zone (B); imiquimod covering all lesion and transformation zone (C).
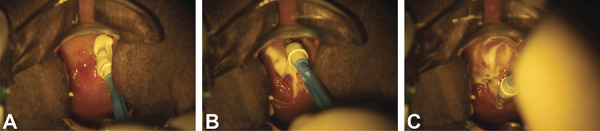
Fonseca. Topical Imiquimod for HSIL of the Cervix. Obstet Gynecol 2021.
In both the experimental and control groups, patients underwent LEEP with local anesthesia (with mepivacaine) performed by the same surgeon (B.O.F.), per local guidelines. Our patient follow-up schedule was identical for the control group and the experimental group: cytology, high-risk HPV testing, and colposcopy every 6 months for at least 2 years.
All pathology slides from biopsy and LEEP were evaluated by two pathologists with specialized training in gynecologic cancers. Consensus was achieved for discordant cases. Histologic diagnosis categories included cervicitis–benign, CIN 1, CIN 2, CIN 3, and invasive cancer. If HSIL could not be precisely graded as CIN 2 or 3, it was defined as high-grade CIN. In cases of uncertain diagnosis, complementary immunohistochemical examination was performed. In cases positive for p16, the final diagnosis was high-grade CIN. When p16 staining was inconclusive, Ki-67 staining was performed. The size of the LEEP specimens and the status of the surgical margins (endocervical, ectocervical, or both) were recorded. Response was categorized as: regression, defined as CIN 1 or cervicitis–benign; persistence, defined as presence of HSIL; or progression, defined as presence of invasive cervical carcinoma.
Adverse events were documented weekly according to patient reports and findings on the gynecologic examination. They were graded according to the Common Terminology Criteria for Adverse Events guidelines v.4.0313 from grade 0 (no symptoms) to grade 5 (death). In patients with grade 1 adverse events, treatment was continued as long as the patient was willing; treatment for symptoms was prescribed if necessary. In patients with grade 2 adverse events, treatment was suspended for 7 days and then reassessment was performed to determine if treatment could be restarted. In patients with grade 3 or 4 adverse events, treatment would be suspended and a LEEP scheduled as soon as possible.
Based on the HSIL regression rates previously reported by Grimm et al,14 of a 34% response difference (39% and 73% in the placebo and imiquimod groups, respectively) with a significance level of 5% and a power of 85%, a sample size of 41 was estimated for each group (G-Power software 3.1.9.6). Assuming a rate of loss to follow-up of 20% for the experimental group and in an effort to ensure equal numbers of patients in the two groups at the end of the study, eight additional patients were included in the experimental group, for a total sample size of 90 patients.
Analyses were undertaken in two populations: the per protocol (PP) population, defined as patients who completed the entire study protocol, excluding patients with protocol violations, and the intention-to-treat (ITT) population, defined as patients who fully or partially completed the study protocol. A mandatory interim analysis for imiquimod efficacy was done in the middle of the study recruitment and showed that 60% (ITT population) to 80% (PP population) of patients treated with imiquimod had histologic regression of HSIL by analysis of the LEEP specimens.
Patient characteristics were summarized with mean and SD for quantitative variables and relative and absolute frequencies for qualitative variables. Normality of the data was verified using the Shapiro-Wilk and Kolmogorov-Smirnov tests. The average number of patients who would need to receive the intervention for the outcome to occur, the number needed to treat (NNT), was calculated as the inverse of absolute risk reduction. Absolute risk reduction was defined as the percentage of patients with histologic regression in the control group subtracted from the percentage of patients with histologic regression in the experimental group. The difference in response rates between the experimental and control groups was calculated with a 95% CI. For group comparisons, we used Pearson's χ2 test, the Fisher exact test, or the Mann-Whitney U test. Characteristics with P<.20 in the aforementioned analyses were selected to fit the multiple logistic regression model, through which the odds ratio (OR) and its 95% CI were estimated. The final model was adjusted with variables with P<.05. The level of significance assumed for the other tests was 5%.
RESULTS
The study included 41 participants in the control group and 49 in the experimental group. As a result of findings on reevaluation of the pathology samples after randomization, one patient was excluded from the control group because HSIL was not confirmed, and three patients were excluded from the experimental group, two because HSIL was not confirmed and one because invasive squamous cell carcinoma was diagnosed. Therefore, we included for treatment allocation 40 patients in the control group and 46 patients in the experimental group.
In the experimental group, after treatment allocation, one patient was excluded from per protocol and ITT analysis because she became pregnant during treatment and she had not yet undergone LEEP. This patient had her treatment interrupted in the fifth week of pregnancy when she had already undergone eight applications of imiquimod. She underwent regular prenatal care, and no teratogenic effects were observed during pregnancy. At the end of the study, the infant was 5 months old and the patient was being followed up at the colposcopy clinic according to Barretos Cancer Hospital's standard care protocol. Seven more women in the experimental group were excluded from per protocol analysis because of systemic side effects (four patients) and transportation problems making it difficult to come to the hospital (three patients). Therefore, in the control group, we included 40 patients in the per protocol and ITT analyses, and in the experimental group, we included 45 patients in the ITT analysis and 38 patients in the per protocol analysis (Fig. 1). All patients removed from the study analysis were treated at the Barretos Cancer Hospital in accordance with the standard institutional protocol.
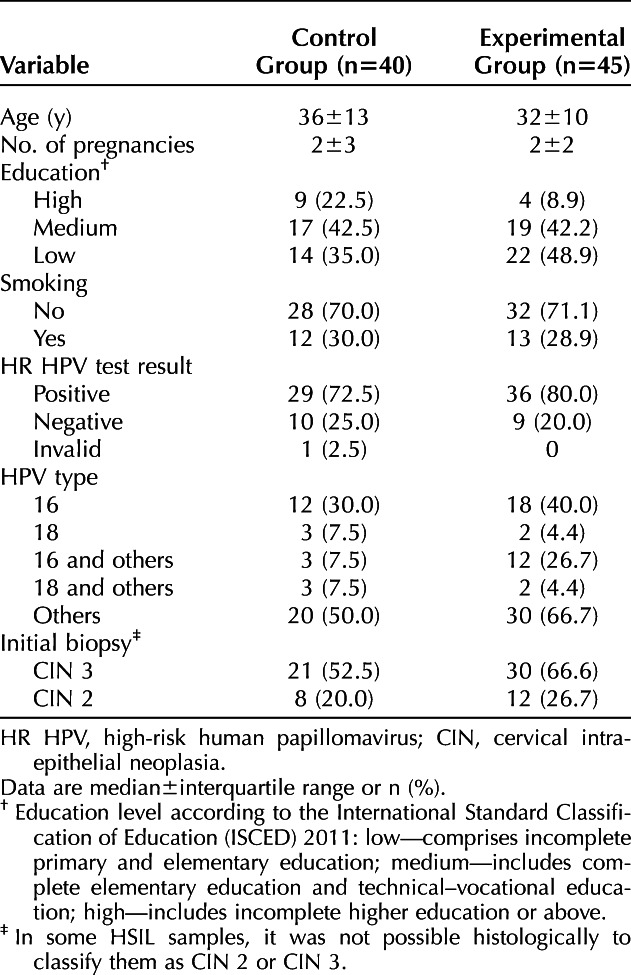
Sociodemographic and clinical characteristics were balanced between the two groups in the ITT population (Table 1). Characteristics were also balanced between groups in the PP population. In 14 patients, HSIL could not be graded as CIN 2 or CIN 3, and these lesions were classified as high-grade CIN.
Table 1.
Allocation of Cases Analyzed by Intention To Treat According to Sociodemographic and Clinical Characteristics
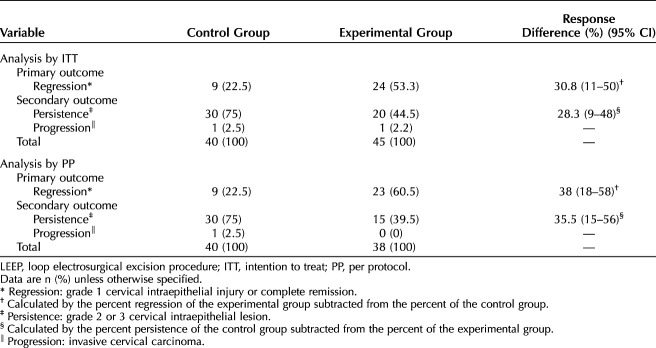
The rate of histologic regression was higher in the experimental group than in the control group in both populations analyzed (Table 2). In the PP population, histologic regression occurred in 22.5% of the LEEP specimens in the control group and in 60.5% of the specimens in the experimental group (P=.001), resulting in a NNT of 2.63 (95% CI 1.7–5.6). High-grade squamous intraepithelial lesions persisted in 75% of the specimens in the control group and in 39.5% of those in the experimental group (P=.002). In the ITT population, histologic regression occurred in 22.5% of the LEEP specimen in the control group and 53.3% of the specimens in the experimental group (P=.004), resulting in a NNT of 3.25 (95% CI 2.0–9.1). High-grade squamous intraepithelial lesions persisted in 75% of the specimens in the control group and in 44.5% of those in the experimental group (P=.008). The analysis of histologic regression only in CIN 3 is showed in Appendix 1, available online at http://links.lww.com/AOG/C298, and the comparison of the response in CIN 2 and CIN 3 is showed in Appendix 2, also available online at http://links.lww.com/AOG/C298.
Table 2.
Histologic Evolution After Evaluation of the LEEP Surgical Specimen
One patient in the control group had progression of the lesion in the LEEP specimen. The diagnosis was superficially invasive squamous cell carcinoma, stage IA1. She underwent laparoscopic hysterectomy and bilateral salpingectomy. The pathology report showed HSIL (CIN 3) without residual invasive neoplasia, with negative vaginal margins. In the ITT population, one patient in the experimental group had progression of the lesion. This patient underwent nine applications of imiquimod, after which she discontinued treatment. She subsequently underwent LEEP. The pathology report showed stage IA1 invasive squamous cell carcinoma. Treatment was complemented with laparoscopic hysterectomy and bilateral salpingectomy. The surgical pathology report did not show any residual invasive lesion and showed margins negative for precursor lesion.
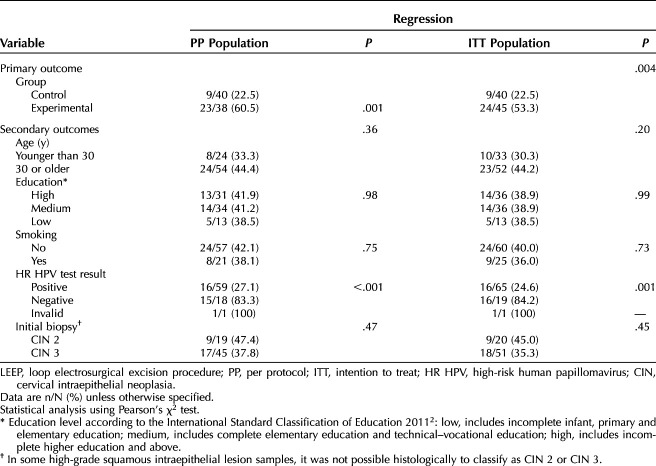
Rates of histologic regression stratified by sociodemographic and clinical characteristics are shown in Table 3. As in both the PP and ITT populations, the histologic regression rate and the positivity of the high-risk HPV test varied significantly by treatment group (experimental or control: P<.20 for both) a multiple logistic regression model was designed (Table 4).
Table 3.
Histologic Regression After Evaluation of the LEEP Surgical Specimen Stratified by Sociodemographic and Clinical Characteristics
Table 4.
Multiple Logistic Regression Model for Group Variables and High-Risk Human Papillomavirus Test in Relation to High-Grade Squamous Intraepithelial Lesions Histologic Regression (Secondary Outcomes)
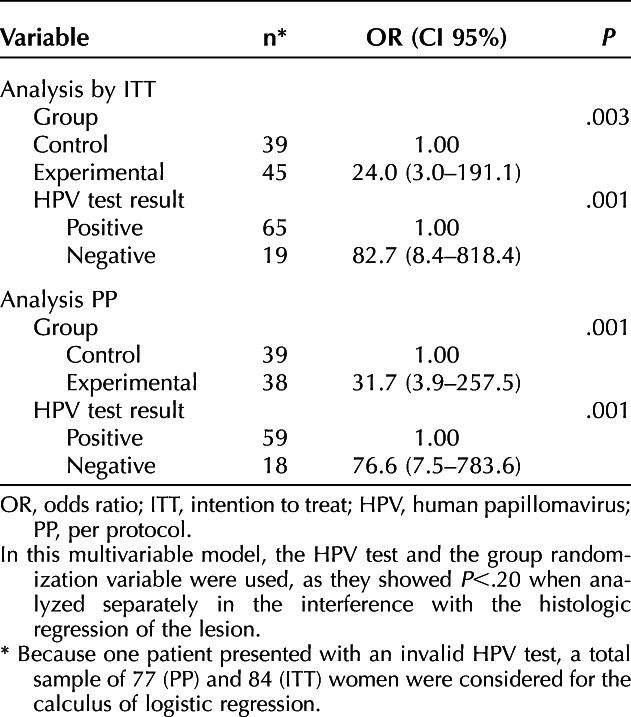
As one patient had an invalid HPV test, 77 women were included in the PP population, and 84 were included in the ITT population. In the PP population, the OR was 31.7 (95% CI 3.9–257.5) (P<.001) for HSIL regression in patients in the experimental group, compared with patients in the control group. In the ITT population, the OR was 24.0 (95% CI 3.0–191.1) (P=.003) for HSIL regression in patients in the experimental group compared with those in the control group.
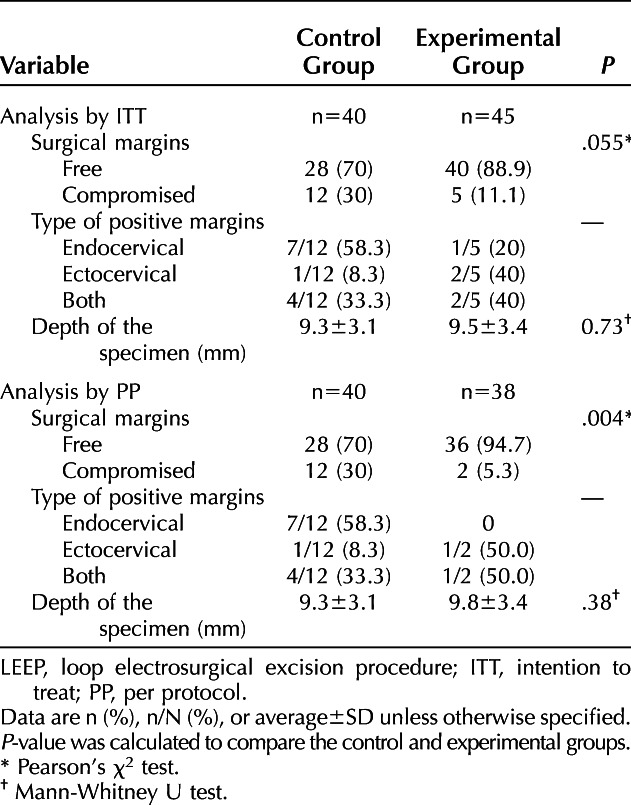
The status of the surgical margins in the LEEP specimen is summarized in Table 5. In the control group, the surgical margins were negative for intraepithelial lesion in 28 of 40 patients (70.0%). In the experimental group, the surgical margins were negative in 36 of 38 patients (94.7%) in the PP population (P=.004) and 40 of 45 patients (88.9%) in the ITT population (P=.055). The depth of the surgical specimens was equivalent in the two groups.
Table 5.
Comparison of Surgical Margins and Depth of LEEP Parts Between Groups (Secondary Outcomes)
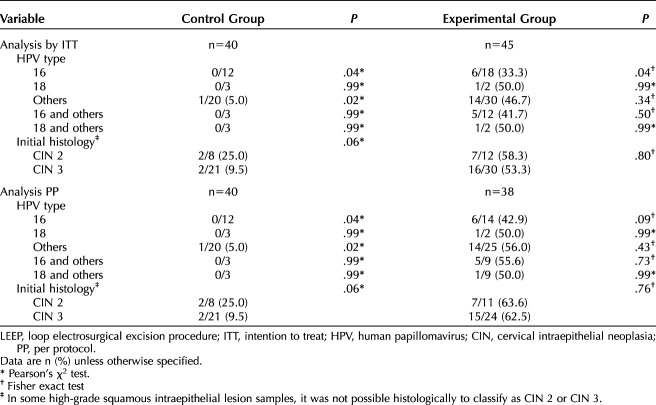
The mean interval between the diagnosis of HSIL and the LEEP procedure was 16.0±6.1 weeks in the control group and 21.0±2.6 weeks in the experimental group (P<.001). We analyzed whether this delay could interfere in lesion regression, persistence or progression. The mean interval between HSIL diagnosis and LEEP was 17.6±5.8 weeks in patients with histologic regression and 19.7±4.4 weeks in patients with persistent disease or progression (P=.09). The rate of histologic regression was higher in the experimental group than in the control group regardless of high-risk HPV type or histologic grade (Table 6).
Table 6.
Histologic Regression After Evaluation of the LEEP Surgical Specimen Divided by Allocation Group and Stratified by the Type of Human Papillomavirus and Histologic Grade at the Time of Inclusion in the Study (Secondary Outcomes)
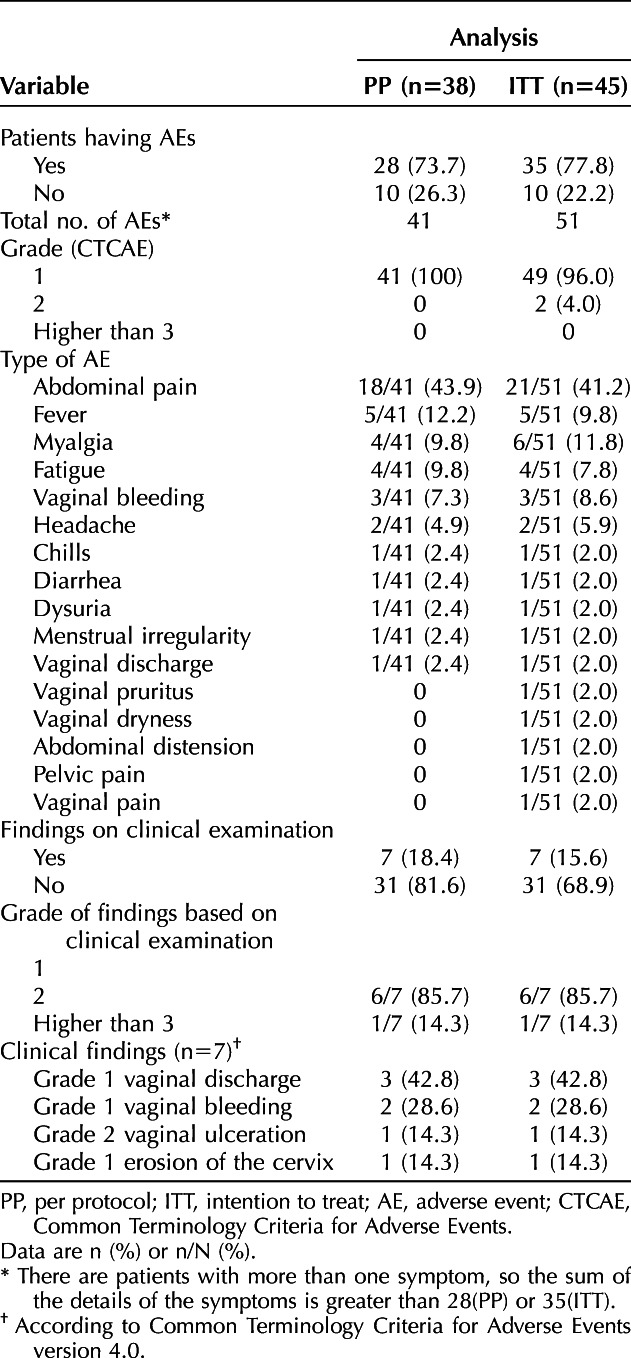
The side effects are summarized in Table 7. Twenty-eight of 38 women (73.7%) in the PP population and 35 of 45 (77.8%) in the ITT population reported adverse events. Two patients (4%) in the ITT population had grade 2 symptoms. One of them reported intermittent vaginal pruritus with local edema on the day of previous application of imiquimod with spontaneous resolution within 24 hours. The other patient reported, when she came to the hospital for the fourth application of imiquimod, that she had experienced moderate pelvic pain that limited her daily activities for less than 24 hours after the two previous applications. These two patients chose not to complete treatment with imiquimod. On clinical examination, seven abnormalities were documented, six (85.7%) classified as grade 1 and one (14.3%) was classified as grade 2. Among the six patients with grade 1 findings, three had increased vaginal discharge, two had mild vaginal bleeding on the speculum examination, and one had focal and superficial erosion of the cervix. The patient with a grade 2 abnormality had a vaginal ulcer in the vaginal introitus, already undergoing epithelialization, that was diagnosed before the fourth application of imiquimod. After improvement of her condition, she completed the 12 weeks of treatment without recurrence of the ulcerated lesion.
Table 7.
Proportion of Adverse Events Observed in Patients in the Experimental Group
DISCUSSION
Weekly topical treatment with imiquimod for 12 weeks is effective in promoting regression of cervical HSIL. One clinical application of these findings is the potential to use in larger lesions to achieve a higher rate of free surgical margins. We observed histologic regression (to CIN 1 or less) in more than half of patients, which suggests this might be an alternative treatment strategy to a cervical excision procedure.
Imiquimod is approved by the U.S. Food and Drug Administration for use in the treatment of external genital and perianal warts, small superficial basal cell carcinomas, and clinically typical actinic keratoses.15 Its off-label use in vulvar intraepithelial neoplasia and vaginal intraepithelial neoplasia is common and is supported by a solid base of evidence in the literature16–26; however, few studies have focused on the effect of topical imiquimod treatment in patients with CIN.14,27–29
Most prior studies that evaluated the efficacy of imiquimod in cervical intraepithelial lesions included patients with low-grade lesions (CIN 1) (Jung PS, Kim JH, Kim D. Application of topical imiquimod for treatment of cervical intraepithelial neoplasia in young women: a preliminary result of a pilot study [abstract]. Gynecol Oncol 2016;141:103–4).28,29 It is difficult to compare results of those studies with our results because our study only included patients with HSIL. Topical imiquimod for exclusive treatment of HSIL was examined in two randomized clinical trials.14,30 Koeneman et al30 interrupted their study because of poor accrual after 12 patients had been recruited. Grimm et al14 demonstrated histologic regression in 73% of patients in the group treated with imiquimod, resulting in a NNT of 2.9. However, histologic regression was evaluated only with colposcopy-directed biopsy, not removal of the entire transformation zone.14 In our clinical trial, all patients underwent LEEP, which allowed a thorough assessment of histologic regression. In our study, the rate of positive surgical margins in the LEEP specimens was 5.3% in the experimental group, lower than rates reported in the literature after LEEP without imiquimod, which range from 27% to 46.5%.31–34 There is evidence that the higher degree of lesion, depth of the conization specimen, and higher parity are risk factors for margin involvement.31–34 The low frequency of positive margins in our study after imiquimod and LEEP might mean that even if there is no histologic regression, the topical treatment has reduced the lesion length.
Adverse events were frequent among the patients in our current study, with abdominal pain being the most common. In a recent case series, three patients had immunomodulatory treatment discontinued because of severe adverse events such as hyponatremia, severe headache, and corneal erosion, which required hospitalization of two of the patients.35 Temporary hair loss also was reported in two patients treated with imiquimod as a vaginal suppository.36 Grimm et al14 observed adverse events in 97% of patients treated with imiquimod. In our population, no adverse event was higher than grade 2. This lower intensity of adverse effects might be related to the direct application of imiquimod to the cervix, minimizing absorption outside the target organ. In addition, we believe that the once-a-week frequency of application of imiquimod might have reduced local adverse effects.
The limitations of our study include the distance between the patients' cities of residence and Barretos Cancer Hospital, which resulted in missed follow-up visits and imiquimod applications. The delay between CIN 2 and CIN 3 diagnosis and the LEEP procedure in the control group could be a limitation, but we analyzed whether this delay could interfere in lesion regression, persistence or progression. As we showed, this period did not affect the lesion evolution.
Authors' Data Sharing Statement
Will individual participant data be available (including data dictionaries)? Participants' individual data (including data dictionaries) will be made available in compliance with data protection laws. The identifying variables will be hidden for any data extraction.
What data in particular will be shared? The results of cytological exams, histological exams, COBAS HRHPV test and colposcopy exams will be shared.
What other documents will be available? The study protocol, statistical analysis plan, consultation templates and the consent form will still be available.
When will data be available (start and end dates)? The data will be available after approval for publication and will always be available.
By what access criteria will data be shared (including with whom, for what types of analyses, and by what mechanism)? All data will be uploaded to the Figshare database with accessible link via a URL.
Footnotes
The 5% imiquimod cream was provided free of charge to the investigators by Laboratório Farmoquímica S.A. for use in the study. Laboratório Farmoquímica S.A. was not involved in the study design, data collection, interpretation, or analysis.
Financial Disclosure Júlio C. Possati-Resende disclosed they received money from Becton, Dickinson, and Company. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C299.
Figure.
No available caption
REFERENCES
- 1.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. doi: [DOI] [PubMed] [Google Scholar]
- 2.Martin-Hirsch PP, Paraskevaidis E, Bryant A, Dickinson HO, Keep SL. Surgery for cervical intraepithelial neoplasia. The Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No.: CD001318. doi: 10.1002/14651858.CD001318.pub2 [DOI] [PMC free article] [PubMed]
- 3.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489–98. doi: 10.1016/S0140-6736(06)68181-6 [DOI] [PubMed] [Google Scholar]
- 4.Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ 2008;337:a1284. doi: 10.1136/bmj.a1284.PMID:18801868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conner SN, Cahill AG, Tuuli MG, Stamilio DM, Odibo AO, Roehl KA, et al. Interval from loop electrosurgical excision procedure to pregnancy and pregnancy outcomes. Obstet Gynecol 2013;122:1154–9. doi: 10.1097/01.AOG.0000435454.31850.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin G, LanLan Z, Li C, Dan Z. Pregnancy outcome following loop electrosurgical excision procedure (LEEP) a systematic review and meta-analysis. Arch Gynecol Obstet 2014;289:85–99. doi: 10.1007/s00404-013-2955-0 [DOI] [PubMed] [Google Scholar]
- 7.Sauder DN. Immunomodulatory and pharmacologic properties of imiquimod. J Am Acad Dermatol 2000;43:S6–11. doi: 10.1067/mjd.2000.107808 [DOI] [PubMed] [Google Scholar]
- 8.Slade HB, Owens ML, Tomai MA, Miller RL. Imiquimod 5% cream (Aldara). Expert Opin Investig Drugs 1998;7:437–49. doi: 10.1517/13543784.7.3.437 [DOI] [PubMed] [Google Scholar]
- 9.Miller RL, Gerster JF, Owens ML, Slade HB, Tomai MA. Imiquimod applied topically: a novel immune response modifier and new class of drug. Int J Immunopharmacol 1999;21:1–14. doi: 10.1016/s0192-0561(98)00068-x [DOI] [PubMed] [Google Scholar]
- 10.Bornstein J, Bentley J, Bosze P, Girardi F, Haefner H, Menton M, et al. 2011 colposcopic terminology of the International Federation for cervical pathology and colposcopy. Obstet Gynecol 2012;120:166–72. doi: 10.1097/AOG.0b013e318254f90c [DOI] [PubMed] [Google Scholar]
- 11.Schulz KF, Altman DG, Moher D, Group C. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063–70. doi: 10.1097/AOG.0b013e3181d9d421 [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v4.03. National Cancer Institute; 2010. [Google Scholar]
- 14.Grimm C, Polterauer S, Natter C, Rahhal J, Hefler L, Tempfer CB, et al. Treatment of cervical intraepithelial neoplasia with topical imiquimod: a randomized controlled trial. Obstet Gynecol 2012;120:152–9. doi: 10.1097/AOG.0b013e31825bc6e8 [DOI] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. Drugs@FDA: FDA-approved drugs. Accessed December 19, 2019. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020723 [Google Scholar]
- 16.Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomised, double-blinded study. Gynecol Oncol 2007;107:219–22. doi: 10.1016/j.ygyno.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 17.van Seters M, van Beurden M, ten Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. N Engl J Med 2008;358:1465–73. doi: 10.1056/NEJMoa072685 [DOI] [PubMed] [Google Scholar]
- 18.Buck HW, Guth KJ. Treatment of vaginal intraepithelial neoplasia (primarily low grade) with imiquimod 5% cream. J Low Genit Tract Dis 2003;7:290–3. doi: 10.1097/00128360-200310000-00011 [DOI] [PubMed] [Google Scholar]
- 19.Tainio K, Jakobsson M, Louvanto K, Kalliala I, Paavonen J, Nieminen P, et al. Randomised trial on treatment of vaginal intraepithelial neoplasia-Imiquimod, laser vaporisation and expectant management. Int J Cancer 2016;139:2353–8. doi: 10.1002/ijc.30275 [DOI] [PubMed] [Google Scholar]
- 20.Lawrie TA, Nordin A, Chakrabarti M, Bryant A, Kaushik S, Pepas L. Medical and surgical interventions for the treatment of usual-type vulval intraepithelial neoplasia. The Cochrane Database of Systematic Reviews 2016, Issue xx. Art. No.: CD011837. doi: 10.1002/14651858.CD011837.pub2 [DOI] [PMC free article] [PubMed]
- 21.de Witte CJ, van de Sande AJ, van Beekhuizen HJ, Koeneman MM, Kruse AJ, Gerestein CG. Imiquimod in cervical, vaginal and vulvar intraepithelial neoplasia: a review. Gynecol Oncol 2015;139:377–84. doi: 10.1016/j.ygyno.2015.08.018 [DOI] [PubMed] [Google Scholar]
- 22.Tristram A, Hurt CN, Madden T, Powell N, Man S, Hibbitts S, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT(3)VIN): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 2014;15:1361–8. doi: 10.1016/S1470-2045(14)70456-5 [DOI] [PubMed] [Google Scholar]
- 23.Policiano AC, Lopes JP, Barata SA, Colaco AM, Calhaz-Jorge C. Topical therapy with imiquimod for vaginal intraepithelial neoplasia: a case series. J Low Genit Tract Dis 2016;20:e34–6. doi: 10.1097/LGT.0000000000000214 [DOI] [PubMed] [Google Scholar]
- 24.Preti M, Igidbashian S, Costa S, Cristoforoni P, Mariani L, Origoni M, et al. VIN usual type-from the past to the future. Ecancermedicalscience 2015;9:531. doi: 10.3332/ecancer.2015.531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tranoulis A, Laios A, Mitsopoulos V, Lutchman-Singh K, Thomakos N. Efficacy of 5% imiquimod for the treatment of Vaginal intraepithelial neoplasia-A systematic review of the literature and a meta-analysis. Eur J Obstet Gynecol Reprod Biol 2017;218:129–36. doi: 10.1016/j.ejogrb.2017.09.020 [DOI] [PubMed] [Google Scholar]
- 26.Haidopoulos D, Diakomanolis E, Rodolakis A, Voulgaris Z, Vlachos G, Intsaklis A. Can local application of imiquimod cream be an alternative mode of therapy for patients with high-grade intraepithelial lesions of the vagina? Int J Gynecol Cancer 2005;15:898–902. doi: 10.1111/j.1525-1438.2005.00152.x [DOI] [PubMed] [Google Scholar]
- 27.Koeneman MM, Kruse AJ, Kooreman LFS, Zur Hausen A, Hopman AHN, Sep SJS, et al. TOPical Imiquimod treatment of high-grade Cervical intraepithelial neoplasia (TOPIC trial): study protocol for a randomized controlled trial. BMC Cancer 2016;16:132. doi: 10.1186/s12885-016-2187-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pachman DR, Barton DL, Clayton AC, McGovern RM, Jefferies JA, Novotny PJ, et al. Randomized clinical trial of imiquimod: an adjunct to treating cervical dysplasia. Am J Obstet Gynecol 2012;206:42 e1–7. doi: 10.1016/j.ajog.2011.06.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin CT, Qiu JT, Wang CJ, Chang SD, Tang YH, Wu PJ, et al. Topical imiquimod treatment for human papillomavirus infection in patients with and without cervical/vaginal intraepithelial neoplasia. Taiwan J Obstet Gynecol 2012;51:533–8. doi: 10.1016/j.tjog.2012.09.006 [DOI] [PubMed] [Google Scholar]
- 30.Koeneman MM, Kruse AJ, Kooreman LF, Zur Hausen A, Hopman AH, Sep SJ, et al. Preliminary stop of the TOPical Imiquimod treatment of high-grade Cervical intraepithelial neoplasia (TOPIC) trial. BMC Cancer 2017;17:110. doi: 10.1186/s12885-017-3108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun XG, Ma SQ, Zhang JX, Wu M. Predictors and clinical significance of the positive cone margin in cervical intraepithelial neoplasia III patients. Chin Med J (Engl) 2009;122:367–72. [PubMed] [Google Scholar]
- 32.Panna S, Luanratanakorn S. Positive margin prevalence and risk factors with cervical specimens obtained from loop electrosurgical excision procedures and cold knife conization. Asian Pac J Cancer Prev 2009;10:637–40. [PubMed] [Google Scholar]
- 33.Costa S, De Nuzzo M, Terzano P, Santini D, De Simone P, Bovicelli A, et al. Factors associated with cone margin involvement in CIN patients undergoing conization-equivalent electrosurgical procedure. Acta Obstet Gynecol Scand 2000;79:586–92. [PubMed] [Google Scholar]
- 34.O'Shea AS S CK. The impact of LEEP margin status on subsequent abnormal cervical cytology. Proc Obstet Gynecol 2014;4:1–8. doi: 10.17077/2154-4751.1249 [DOI] [Google Scholar]
- 35.Wouters T, Hendriks N, Koeneman M, Kruse AJ, van de Sande A, van Beekhuizen HJ, et al. Systemic adverse events in imiquimod use for cervical intraepithelial neoplasia—a case series. Case Rep Womens Health 2019;21:e00105. doi: 10.1016/j.crwh.2019.e00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borst C, Grimm C, Tanew A, Radakovic S. Imiquimod-induced effluvium after intravaginal application for treatment of cervical intraepithelial neoplasia. JAAD Case Rep 2019;5:602–4. doi: 10.1016/j.jdcr.2019.04.01510.1016/j.jdcr.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]