Abstract
Background:
Epinephrine is the first-line therapy for patients with anaphylaxis, and intramuscular (IM) delivery is shown to be superior to subcutaneous (SC) delivery. There currently is no consensus on the ideal body position for epinephrine autoinjector (EAI) administration.
Objective:
We designed this study to investigate whether SC tissue depth (SCTD) is affected by body position (e.g., standing, sitting, supine), which can potentially impact delivery of EAI into the IM space.
Methods:
Volunteer adults (ages ≥ 18 years) from a military medical treatment facility in the United States were recruited to participate in this study. SCTD of the vastus lateralis was measured via ultrasound at standing, sitting, and supine body positions. Subjects' age, sex, and body mass index (BMI) were collected. Statistical analysis was performed to compare average SCTD between body positions, sex, and BMI.
Results:
An analysis of variance of 51 participants (33 men and 18 women) did not reveal statistically significant difference in SCTD among standing, sitting, and supine body positions. It did show a significantly greater SCTD in women than in men (2.72 ± 1.36 cm versus 1.10 ± 0.38 cm; p < 0.001). There was no significant association observed between BMI and SCTD in this study.
Conclusion:
Body position did not seem to significantly change the distance between skin and thigh muscle in adults. This would suggest that there might not be an ideal body position for EAI administration. Therefore, in case of anaphylaxis, prompt administration of epinephrine is recommended at any position.
Keywords: Anaphylaxis, epinephrine, autoinjector, subcutaneous, intramuscular, tissue depth, sex, gender, body mass index, body position
Anaphylaxis is a serious allergic reaction that can be fatal if not promptly treated.1 The current recommendation on the use of epinephrine for the treatment of anaphylaxis includes both subcutaneous (SC) and intramuscular (IM) administration, but most guidelines are in agreement that IM administration is favored due to superior pharmacokinetics.2–5 It is believed that early onset of high plasma epinephrine concentration is critical for reversal of hypotension and survival in the early treatment of anaphylaxis. Previous studies compared pharmacokinetics of epinephrine administered via the SC and IM routes, and concluded that IM administration results in more-rapid achievement of a peak plasma concentration and a higher peak plasma concentration.6,7
Epinephrine autoinjectors (EAI) are commonly prescribed by clinicians for outpatient self-treatment of anaphylaxis.8,9 Although current consensus favors IM administration of EAI in the lateral thigh (vastus lateralis muscle),2–5 there has been no guidance with regard to the optimal body position to assume during EAI administration. Current guidelines on EAI use do not specify a particular body position (i.e., standing, sitting, or supine), and there is no mention of body position on package inserts for EpiPen (Mylan Specialty LP, Basking Ridge, NJ) or AUVI-Q (Kaleo, Inc., Richmond, VA), two commonly prescribed EAIs, although their package illustrations demonstrate the EAIs being self-administered while standing.10,11 Surveys from survivors of anaphylaxis revealed mixed responses with regard to the body position assumed while administering or receiving the EAI: 51% reported the sitting position, 28% the standing position, 16% the supine position, and 5% unable to recall.12
There have been no studies that examined if body position has any effect on epinephrine delivery. Previous studies on EAIs also do not have uniformity on the study subject's body position. For example, some studies were carried out with the subjects in the standing position,13,14 whereas other studies were carried out with subjects in the supine position.15,16 In addition, the adequacy of available EAI needle lengths has become controversial. Given the high prevalence of obesity in the United States,17,18 some have raised concern that the needle length of current EAIs is inadequate for IM delivery.19–21 A study that compared adult male and female thighs demonstrated that an EpiPen injection would most commonly result in SC administration among the female population due to greater SC tissue depth (SCTD).13 In the pediatric population, a study demonstrated that approximately one-fifth of the children may not receive EpiPen injections IM but rather SC.15
If SCTD is dependent on body position, this has important implications on the ability of the EAI to penetrate the IM space, particularly when the adequacy of the EAI needle length is questioned. To our knowledge, there have been no published studies to date that examined body position and SCTD or delivery of EAI. Therefore, we designed this study to investigate whether SCTD is a function of body position to determine if there is an ideal body position for EAI administration in an adult population.
METHODS
This study was conducted at Madigan Army Medical Center, Tacoma, Washington. The study protocol was approved by the institutional review board at Madigan Army Medical Center (protocol 219050). Between March 2019 and February 2020, the participants were recruited via posters at different clinics and direct recruiting of patients by ultrasound technicians. Oral consent was obtained before participation in the study per institutional review board protocol. The inclusion criterion was adults ages ≥ 18 years. A pediatric population was not examined in this study. The exclusion criteria were anyone with amputated or unformed limbs, unable to stand for measurements, or unable to follow instructions to change body positions (i.e., severe dementia, language barrier). The primary outcome studied was whether SCTD varied among body positions. The secondary outcome studied was whether SCTD varied between the sexes and body mass index (BMI).
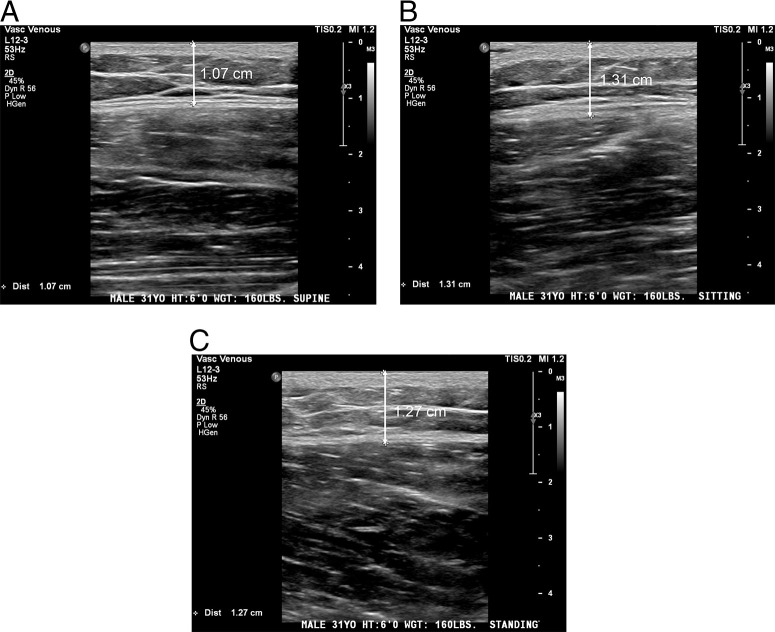
Demographic data, including sex, age, height, and weight, were collected. BMI was later calculated from the height and weight data. Ultrasound measurements were performed by trained ultrasound technicians by using either the Philips Epic 7 (Philips, Amsterdam, Netherlands), Canon Aplio i900 (Canon Medical Systems, Tustin, CA), or GE Logiq E9 (GE Healthcare, Chicago, IL). All the images were measured by using a high-frequency linear transducer. SCTD of the right anterolateral thigh were obtained by ultrasound technicians applying the minimum pressure needed for measurement from the surface of the skin to the fascia of the muscle (vastus lateralis) and were recorded with the subjects in (1) supine, (2) sitting, and (3) standing positions (Fig. 1).
Figure 1.
Sample ultrasound data at (A) supine, (B) sitting, and (C) standing positions. The arrow marks the subcutaneous tissue depth measurement from the surface of the skin to the fascia of the vastus lateralis.
A standardized approach was established to assure that the measurements were obtained in the same order from the identical anatomic positions to minimize interexaminer variability. The supine position required the subjects to lie horizontally on the examination table with the legs completed extended; the sitting position required the subjects to place both feet on the ground with knees bent at ∼90°; and the standing position required the subjects to stand in the anatomic position. All the images were stored, with de-identified subject information, on FUJI Synapse PACS (picture archiving and communication system) (Fujifilm Medical Systems, Lexington, MA), by ultrasound technicians and later transcribed into a Microsoft Excel (Microsoft, Redmond, WA) sheet by research staff.
Data analysis was performed by using SPSS for Windows version 24 (IBM Corp, Armonk, NY). The relationship between SCTD and body position was examined by using univariate analysis of variance by randomized block design. A subset analysis of the sexes was performed by using a two-tailed, independent samples t-test. Pearson correlation coefficients were calculated for independent associations between BMI and SCTD. Data are presented as the mean ± standard deviation (SD). A level of significance was set at 5% (p ≤ 0.05), with appropriate 95% confidence intervals (CI).
RESULTS
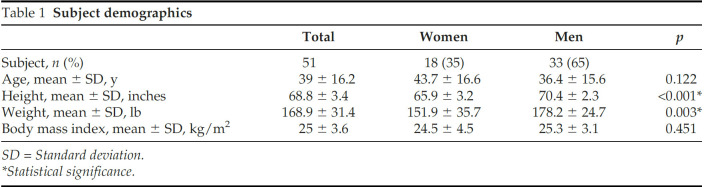
This study included a total of 51 participants, 33 were men (65%), and 18 were women (35%). The mean ± SD age was 39 ± 16.2 years (range, 23–83 years), and the mean ± SD BMI was 25 ± 3.6 kg/m2 (range, 17.72–37.8 kg/m2). The majority of the subjects were either normal weight (n = 28 [BMI, 18–25 kg/m2]) or overweight (n = 17 [BMI, 25–30 kg/m2]); one subject was underweight (BMI < 18 kg/m2), and five were obese (BMI ≥ 30 kg/m2). Age and BMI did not differ significantly between the sexes (Table 1). The mean ± SD standing SCTD was 1.62 ± 1.02 cm (95% CI, 1.33–1.91 cm), mean ± SD sitting SCTD was 1.76 ± 1.31 cm (95% CI, 1.38–2.06 cm), and mean ± SD supine SCTD was 1.72 ± 1.21 cm (95% CI, 1.38–2.06 cm). No statistically significant difference in SCTD was observed between the body positions (p = 0.106) (Fig. 2).
Table 1.
Subject demographics
SD = Standard deviation.
*Statistical significance.
Figure 2.
The body position and the subcutaneous tissue depth (SCTD). The mean SCTD at standing, sitting, and supine body positions. The error bar represents the 95% confidence interval (CI). The dashed line represents the average EpiPen needle length (1.43 cm).
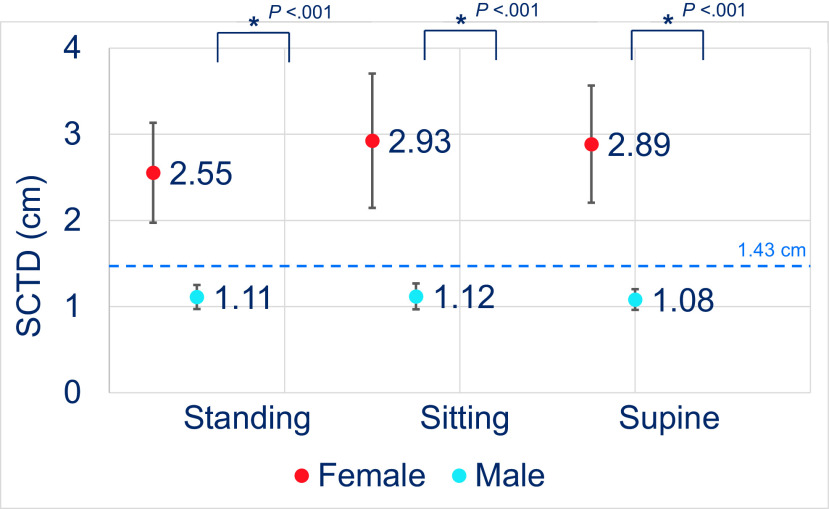
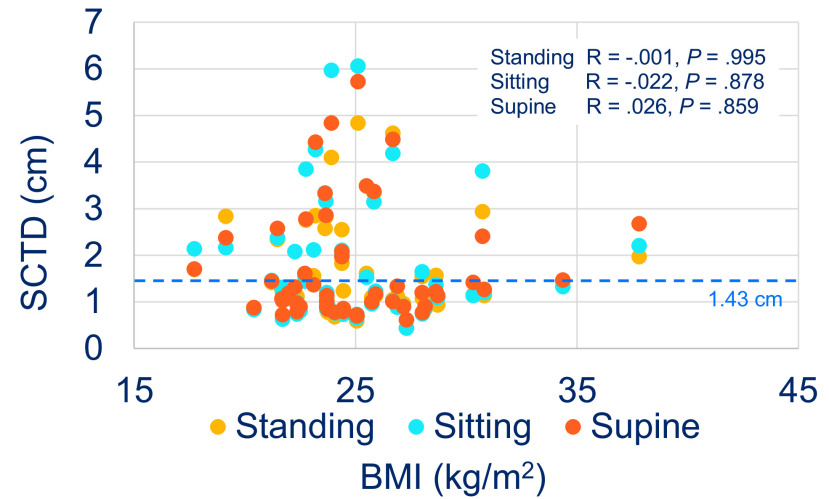
In the women, the mean ± SD standing SCTD was 2.55 ± 1.17 cm (95% CI, 1.97–3.13 cm), mean ± SD sitting SCTD was 2.93 ± 1.58 cm (95% CI, 2.15–3.71 cm), and mean ± SD supine SCTD was 2.89 ± 1.36 cm (95% CI, 2.21–3.57 cm); whereas, in the men, the mean ± SD standing SCTD was 1.11 ± 0.38 cm (95% CI, 0.97–2.08 cm), mean ± SD sitting SCTD was 1.12 ± 0.43 cm (95% CI, 0.97–1.27 cm), and mean ± SD supine SCTD was 1.08 ± 0.33 cm (95% CI, 0.96–1.2 cm). An analysis showed no statistically significant difference in SCTD among the body positions within the sexes. However, statistically significant difference in SCTD was observed between the sexes for each body position (p < 0.001) (Fig. 3). The mean SCTDs in the subjects who were underweight to normal weight (BMI < 25 kg/m2) and in the subjects who were overweight to obese (BMI ≥ 25 kg/m2) were not significantly different within each body position. Correlation analysis also did not reveal any significant association between SCTD and BMI at sitting, standing, and supine positions (Fig. 4).
Figure 3.
The subcutaneous tissue depth (SCTD) between the men and the women. The mean SCTD between the men and the women at standing, sitting, and supine body positions. The error bar represents the 95% confidence interval (CI). *Statistical significance between the sexes. The dashed line represents the average EpiPen needle length (1.43 cm).
Figure 4.
The body mass index (BMI) and the subcutaneous tissue depth (SCTD). The distribution of the SCTD versus the BMI at standing, sitting, and supine body positions. The dashed line represents the average EpiPen needle length (1.43 cm). R = Pearson correlation coefficient.
DISCUSSION
All guidelines recommend immediate administration of epinephrine in the treatment of anaphylaxis, and most anaphylaxis guidelines agree that epinephrine should be administered IM into the lateral thigh.2–5 However, there is no specific guidance on the best body position for EAI administration. Ring et al.22 recommend that the patient should be positioned according to symptom severity during anaphylaxis, such as placing the patient in the supine position to avoid further physical exertion or the Trendelenburg position to improve hemodynamics, but this is not a consensus. The package inserts for the EpiPen and AUVI-Q also do not specify a particular body position, although package illustrations demonstrate EAIs being self-administered at a standing position.10,11 In addition, AUVI-Q has an illustration of a caretaker administering EAI in a patient in the supine position.11
Our study compared thigh SCTD at standing, sitting, and supine positions. There was no statistical significance observed between SCTD and body position despite a trend toward lower SCTD with the standing position. Because the body position did not seem to significantly change the distance between skin and thigh muscle, it would suggest that there might not be an ideal body position for EAI administration. Therefore, in case of anaphylaxis, prompt administration of epinephrine is recommended at any position.
Although there was no statistical significance in SCTD between the body positions, statistical significance was observed between the male and female subjects at standing, sitting, and supine body positions. The female subjects had a mean thigh SCTD more than double that of their male counterparts across all the body positions, despite similar BMI between the sexes. This result further supported findings from previous studies that demonstrate significant differences in SCTD between the sexes13,14,16 and speaks to the underlying physiologic differences between human females and males.23
Previous studies questioned the ability of the EAI needle length to adequately reach the IM space.13–16 In our study, the mean SCTD at sitting, standing, and supine positions were all longer than the standard EAI needle length of 1.43 cm from the EpiPen,20 and the mean SCTD at sitting, standing, and supine positions for the women were even greater than the Emerade (Medeca Pharma, Uppsala, Sweden), which, at a needle length of 2.5 cm,20 is the longest EAI needle currently available in some countries. The results of our study would suggest that the standard available EAIs would likely fail to penetrate the IM space if adequacy is judged purely based on needle length. However, a previous study, which used pig models, demonstrated that IM delivery is affected by thigh compression and propulsion pressure of the autoinjector.24 Epinephrine is not delivered at the tip of the needle but beyond due to multiple factors. In this study, SCTD was not measured by taking into context the compression or propulsion depth of the anterolateral thigh, and future studies that address this issue would be helpful.
Trends from the most recent National Health and Nutrition Examination Survey report17 conducted by the U.S. Centers for Disease Control and Prevention show that the percentage of adults who were overweight and those who were obese continues to rise in the United States.17,18 As a consequence of obesity, studies have expressed concerns that the needle length of current EAIs may not reach the IM space.13,14 However, this study did not reveal any significant association between SCTD and BMI. Conflicting evidence exists on SCTD and BMI. Song et al.16 demonstrated no difference in BMI and SCTD as measured by computed tomography, whereas Bhalla et al.13 demonstrated a significant association between BMI and SCTD as measured by ultrasound. One explanation for the discrepancy between Bhalla et al.13 and the results of our study may be that the majority of our study subjects were of normal weight or were overweight; few subjects were obese. It is possible that a significant association was not achieved due to the limited number of subjects at the extremes of BMI being studied.
There were several limitations to this study. One was its small sample size, the majority of the subjects were men, with BMI < 30 kg/m2, which may have led to an underpowered assessment of its primary outcome. The study was also limited to a single military medical treatment facility where the majority of study subjects were active duty soldiers and may not represent the general population or the allergic population most at risk for anaphylaxis. In addition, our study was only able to theorize the effects of body position on EAI delivery based solely on SCTD measurements and did not take into account other factors that can effect epinephrine delivery, such as tissue compression and propulsive pressure of the autoinjector.24 The most accurate way to test for EAI delivery adequacy would be to inject patients at various body positions and subsequently measure their plasma concentration levels, but such a study design would have been highly impractical.
CONCLUSION
This was the first study to our knowledge to examine whether thigh SCTD is significantly affected by body position, and results of this study can potentially guide patient counseling for epinephrine injection. Thigh SCTD does not seem to be affected by body position based on the results of this study. Therefore, the decision as to how or when EAI is to be administered in a patient during anaphylaxis should, in no way, be influenced by the position of the patient, and prompt administration of epinephrine regardless of position is strongly recommended. Future studies in female and/or obese individuals would be helpful to assess adequacy of EAI use in these special populations.
ACKNOWLEDGEMENT
We thank Alexander Pierron, Nicole Dela Cruz, and the rest of the Madigan Army Medical Center ultrasound staff for their support with this project.
Footnotes
T.T. Song reports acting as a consultant for Boehringer-Ingelheim and Genentech. The remaining authors have no conflicts of interest to declare pertaining to this study
No external funding sources reported
The views expressed are those of the authors and do not reflect the official policy of the Department of the Army, the Department of Defense, or the U.S. government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45 CFR 46
REFERENCES
- 1. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017; 140:335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lieberman P, Nicklas RA, Randolph C, et al. Anaphylaxis–a practice parameter update 2015. Ann Allergy Asthma Immunol. 2015; 115:341–384. [DOI] [PubMed] [Google Scholar]
- 3. Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy. 2014; 69:1026–1045. [DOI] [PubMed] [Google Scholar]
- 4. Shaker MS, Wallace DV, Golden DB, et al. Anaphylaxis - a 2020 practice parameter update, systematic review and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol. 2020; 145:1082–1123. [DOI] [PubMed] [Google Scholar]
- 5. Simons FE, Ardusso LR, Bilo MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J. 2014; 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simons FE, Roberts JR, Gu X, et al. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol. 1998; 101(Pt 1):33–37. [DOI] [PubMed] [Google Scholar]
- 7. Simons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001; 108:871–873. [DOI] [PubMed] [Google Scholar]
- 8. Davis JE. Self-injectable epinephrine for allergic emergencies. J Emerg Med. 2009; 37:57–62. [DOI] [PubMed] [Google Scholar]
- 9. Sicherer SH, Simons FER, Section on Allergy and Immunology. Epinephrine for first-aid management of anaphylaxis. Pediatrics. 2017; 139:e20164006. [DOI] [PubMed] [Google Scholar]
- 10. EpiPen Prescribing Information. EpiPen (updated 2018). Available online at https://www.epipen.com/-/media/epipencom/assets/pdf/epipen-prescribing-information-aug_2018.pdf; accessed February 24, 2020.
- 11. AUVI-Q Prescribing Information: Auvi-Q (updated September 2019). Available online at https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=6180fb40-7fca-4602-b3da-ce62b8cd2470&type=display; accessed June 1, 2020.
- 12. Simons FE, Clark S, Camargo CA., Jr Anaphylaxis in the community: learning from the survivors. J Allergy Clin Immunol. 2009; 124:301–306. [DOI] [PubMed] [Google Scholar]
- 13. Bhalla MC, Gable BD, Frey JA, et al. Predictors of epinephrine autoinjector needle length inadequacy. Am J Emerg Med. 2013; 31:1671–1676. [DOI] [PubMed] [Google Scholar]
- 14. Tsai G, Kim L, Nevis IF, et al. Auto-injector needle length may be inadequate to deliver epinephrine intramuscularly in women with confirmed food allergy. Allergy Asthma Clin Immunol. 2014; 10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stecher D, Bulloch B, Sales J, et al. Epinephrine auto-injectors: is needle length adequate for delivery of epinephrine intramuscularly? Pediatrics. 2009; 124:65–70. [DOI] [PubMed] [Google Scholar]
- 16. Song TT, Nelson MR, Chang JH, et al. Adequacy of the epinephrine autoinjector needle length in delivering epinephrine to the intramuscular tissues. Ann Allergy Asthma Immunol. 2005; 94:539–542. [DOI] [PubMed] [Google Scholar]
- 17. Hales CM, Fryar CD, Carroll MD, et al. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007-2008 to 2015-2016. JAMA. 2018; 319:1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flegal KM, Kruszon-Moran D, Carroll MD, et al. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016; 315:2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greenberger PA, Wallace DV, Lieberman PL, et al. Contemporary issues in anaphylaxis and the evolution of epinephrine autoinjectors: what will the future bring? Ann Allergy Asthma Immunol. 2017; 119:333–338. [DOI] [PubMed] [Google Scholar]
- 20. Song TT, Lieberman P. Epinephrine auto-injector needle length: what is the ideal length? Curr Opin Allergy Clin Immunol. 2016; 16:361–365. [DOI] [PubMed] [Google Scholar]
- 21. Song TT, Worm M, Lieberman P. Anaphylaxis treatment: current barriers to adrenaline auto-injector use. Allergy. 2014; 69:983–991. [DOI] [PubMed] [Google Scholar]
- 22. Ring J, Beyer K, Biedermann T, et al. Guideline for acute therapy and management of anaphylaxis: S2 Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Association of German Allergologists (AeDA), the Society of Pediatric Allergy and Environmental Medicine (GPA), the German Academy of Allergology and Environmental Medicine (DAAU), the German Professional Association of Pediatricians (BVKJ), the Austrian Society for Allergology and Immunology (ÖGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Anaesthesiology and Intensive Care Medicine (DGAI), the German Society of Pharmacology (DGP), the German Society for Psychosomatic Medicine (DGPM), the German Working Group of Anaphylaxis Training and Education (AGATE) and the patient organization German Allergy and Asthma Association (DAAB). Allergo J Int 2014; 23:96–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bredella MA. Sex differences in body composition. Adv Exp Med Biol. 2017; 1043:9–27. [DOI] [PubMed] [Google Scholar]
- 24. Song TT, Merrill NL, Cole JW. Delivery depth of epinephrine by auto-injector into the subcutaneous tissue of pig. Ann Allergy Asthma Immunol. 2013; 111:143–145. [DOI] [PubMed] [Google Scholar]