Abstract
Background:
Penicillin allergy is commonly reported and has clinical and financial consequences for patients and hospitals. A penicillin evaluation program can safely delabel patients and optimize antibiotic therapy. Pharmacists who perform this task have focused on a detailed interview or penicillin skin testing (PST). Antibiotic graded challenge after PST requires more resources and is more costly than going directly to a two-step challenge.
Objective:
To determine whether a pharmacist-driven penicillin allergy evaluation and a testing protocol that primarily uses direct oral challenges can safely delabel patients.
Methods:
Adult patients (ages >18 years) with a penicillin allergy in their electronic medical record (EMR) who were admitted between September 2019 and June 2020 were eligible. Although all patients with penicillin allergy were eligible, priority was given to patients who required antibiotics. Patients were interviewed, and, if indicated, based on an institutional protocol, were tested by using PST and/or two-step oral challenge. If the patient passed the challenge, then the penicillin allergy label was removed in the EMR and the patient counseled. Demographic information, allergy questionnaire results, testing results, and changes in antimicrobial therapy were collected.
Results:
Fifty patients were evaluated from September 2019 to June 2020. Ninety-six percent of the patients were delabeled, and antibiotic therapy changed for 54%. Twenty patients were delabeled with an interview alone, and 30 patients underwent oral two-step challenge. Only one patient required PST.
Conclusion:
A pharmacist-driven penicillin allergy evaluation program focused on direct oral graded challenges and bypassing PST can effectively delabel admitted patients. However, more safety data are needed before implementation of similar programs to optimize antibiotic treatment.
Keywords: Penicillin Allergy, PST, Oral 2-step Challenge, pharmacist, pharmacist-driven, beta-lactam allergy
Penicillin allergy is the most commonly reported medication allergy, with a rate of ∼10–15%; however, when formally evaluated, <10% of patients who report a penicillin allergy are truly allergic.1 Reasons for this include mislabeling an adverse event as an allergy, waning sensitivity over time, and misreported history from family members.2,3 A penicillin allergy label is associated with longer hospital stays and receipt of second-line antibiotics that may be less effective, more costly, and associated with more adverse effects,4,5 such as Clostridioides difficile infections, colonization with methicillin-resistant Staphylococcus aureus, or vancomycin-resistant enterococci.6,7 Hospital readmission within 28 days is more common, with many of these patients (81%) readmitted with major infections.8 Penicillin allergy is also associated with an increased risk of mortality.9
Although penicillin skin testing (PST) and allergy evaluation typically fall under the purview of allergy/immunology, a scarcity of allergists in the inpatient setting, particularly in nonacademic centers, limits access to testing. As a result, other health care workers, including infectious diseases specialists, nurses, and pharmacists, manage penicillin allergy evaluations. Some programs involve a detailed interview only,10,11 whereas others involve interviewing, along with PST.12,13 PST is often cost-prohibitive, time-consuming, and requires specialized training; it also requires additional resources, such as benzylpenicilloyl polylysine and intravenous room access. The cost of penicillin evaluation and testing dropped from $220 to $84 without PST.14
Pharmacy allergy interventions focus less on direct oral challenges, although at least one non-U.S. site used pharmacists in this manner,15 and a recent U.S. study revealed that direct oral challenge is safe and efficacious, but the protocol continued to use an initial PST ∼50% of the time.15,16 A protocol that allows a greater proportion of patients to bypass PST would be easier and more cost-effective to implement, and evidence of safety is increasing. Several studies have bypassed skin testing in favor of direct oral challenges. Reaction rates of 2.6% (3/4 mild delayed rashes and one immediate itching) and 2.1% (mild immediate reactions) have been reported, with up to 3.8% developing nonimmediate T-cell–mediated reactions.17,18 Other studies similarly reported low rates of reaction to direct challenges, which supports their safety.19 Although those studies excluded more severe reactions, e.g. anaphylaxis, another direct challenge study included patients with a reported history of respiratory or multisystem reactions and found a reaction rate of 1.5%, all mild reactions.20
METHODS
Setting and Participants
A pharmacist-driven penicillin allergy evaluation and testing program designed with input from a board-certified allergist (SJ) began in September 2019 and collected data through June 2020 in our 500-bed academic medical center. The study was approved by the Oregon Health and Science University Institutional Review Board. Patients were selected by referral or via a report of inpatients with penicillin allergy constructed within the electronic medical record (EMR) and evaluated by the pharmacist (YH). Inpatients ages ≥18 years with a penicillin allergy were eligible, with a hierarchy assigned based on need. Patients with active infections whose antibiotics would change based on testing received the highest priority, whereas patients not actively undergoing treatment received a lower priority. Pregnant patients were excluded, whereas patients who were critically ill or receiving medications that would interfere with testing were evaluated only when referred. Evaluation and testing took place between the hours of 9 A.M. and 5 P.M. on weekdays. Verbal consent was obtained before the interview and testing. The Pharmacist (YH) underwent training with an Allergy physician (SJ) to ensure proficiency with the protocols for penicillin allergy evaluation, oral two-step challenge, and administration of PST. The allergy physician (SJ) was also available for discussion of complicated cases.
Procedure.
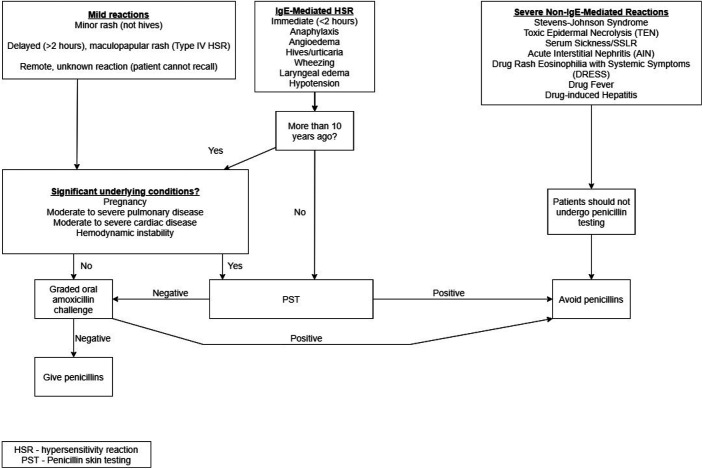
The pharmacist (YH) reviewed the patient's EMR for previous penicillin administration. The patients were then interviewed and asked what penicillins they reacted to in the past, symptoms of the reaction and any treatments received, time elapsed since the reaction, and receipt of any penicillin since the reaction. Our institutional algorithm (Fig. 2) allowed a patient to proceed directly to a challenge if the reaction was mild and did not seem to be immunoglobulin E (IgE) mediated, if the patient did not remember the reaction, or if an IgE-mediated reaction occurred >10 years ago. PST occurred only if the patient had a history suggestive of an IgE-mediated reaction <10 years ago. The primary team, nurse, floor pharmacist, and infectious diseases consult team received notification that the patient met eligibility criteria for testing or that the patient could be delabeled based on the interview. If the patient reported receiving a penicillin with no reaction and administration could be confirmed, then the allergy was removed. If administration could not be confirmed, then the patient underwent a challenge. If the patient received a medication that potentially blunts a histamine response, then a histamine response was confirmed via prick test before oral two-step challenge.
Figure 2.
An institutional algorithm for evaluation, penicillin skin testing (PST), and oral challenge; most patients qualified for the oral challenge route after initial evaluation, bypassing PST.
An oral two-step challenge order set or a PST order panel was used. The PST was a two-stage process, with a skin-prick stage with PRE-PEN (ALK-Abelló, Inc. Port Washington, NY) (major determinant), penicillin G (minor determinant), saline solution (negative control) and histamine (positive control), and a second intradermal stage with PRE-PEN, penicillin G, and saline solution control. For ease of workflow and to prevent multiple order sets, the PST in our study was followed by a standard two-step challenge. The first dose consisted of 25 mg of amoxicillin, and, if the patient did not react within 15 minutes, then a second dose of 250 mg was administered and the patient was monitored for an hour. Rescue medications were available as a part of the order set.
Once PST and/or challenge was complete, the primary team and floor pharmacist were notified, and the patient counseled. The patient received a brochure that contains information about the implications of testing, who else he or she should notify, and the type of test performed. Also included was contact information for an allergy physician should the patient experience a delayed reaction. The results of the evaluation and testing were documented as a progress note, the allergy section was updated to reflect the results of the test, and then the allergy was deleted from the profile. The allergy was deleted so that it would not trigger further alerts; however, testing information was added before deletion to make it visible in the allergy history for future encounters. Data collection after delabeling allowed evaluation of antibiotic regimen modification due to testing.
RESULTS
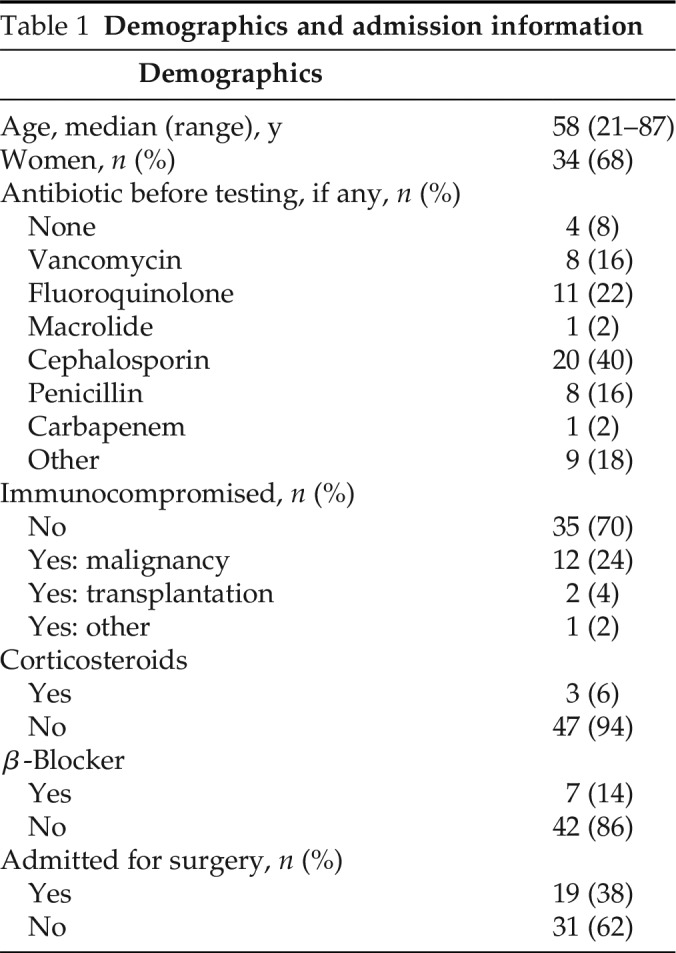
Approximately 24,000 hospital admissions occurred during the study period. Of these, we estimated that 3000 unique individuals possessed a penicillin allergy in their medical record. Fifty patients (1.7%) underwent the penicillin allergy delabeling process. Baseline characteristics are provided in Table 1. The median age of the participants was 58 years (range, 21–87 years). The majority (68%) were women. Forty-six patients (92%) were receiving an antibiotic before evaluation and testing, most commonly a cephalosporin (40%), followed by a fluoroquinolone (22%), vancomycin (16%), or penicillin (16%). Nineteen patients (38%) had been admitted for a surgical procedure. Overall, 50% of the patients had an admitting diagnosis of an infectious process, whereas 50% had a noninfectious diagnosis. Among the 30 patients who received direct challenge only, 14 patients had been admitted with an infection, whereas 16 were admitted with noninfectious diagnoses.
Table 1.
Demographics and admission information
Seventeen patients (34%) could not recall the penicillin to which they reacted. Among the patients who were able to recall, amoxicillin and penicillin ranked the highest, at 32% each (Table 2). The majority of reactions (92%) occurred >10 years ago, with three patients reporting a reaction in the past 1–10 years and one patient reporting a reaction within the past year. One patient who reported a reaction in the past 1–10 years received a PST before a two-step challenge. Two of the patients who reported a reaction in the past 1–10 years actually received a cephalosporin, not a penicillin, which was discovered during a detailed interview and a review of the medical record, and did not require a PST. One patient reported a reaction within the past year, which was subsequently identified as a yeast infection and did not require a PST.
Table 2.
Initial interview responses and evaluation
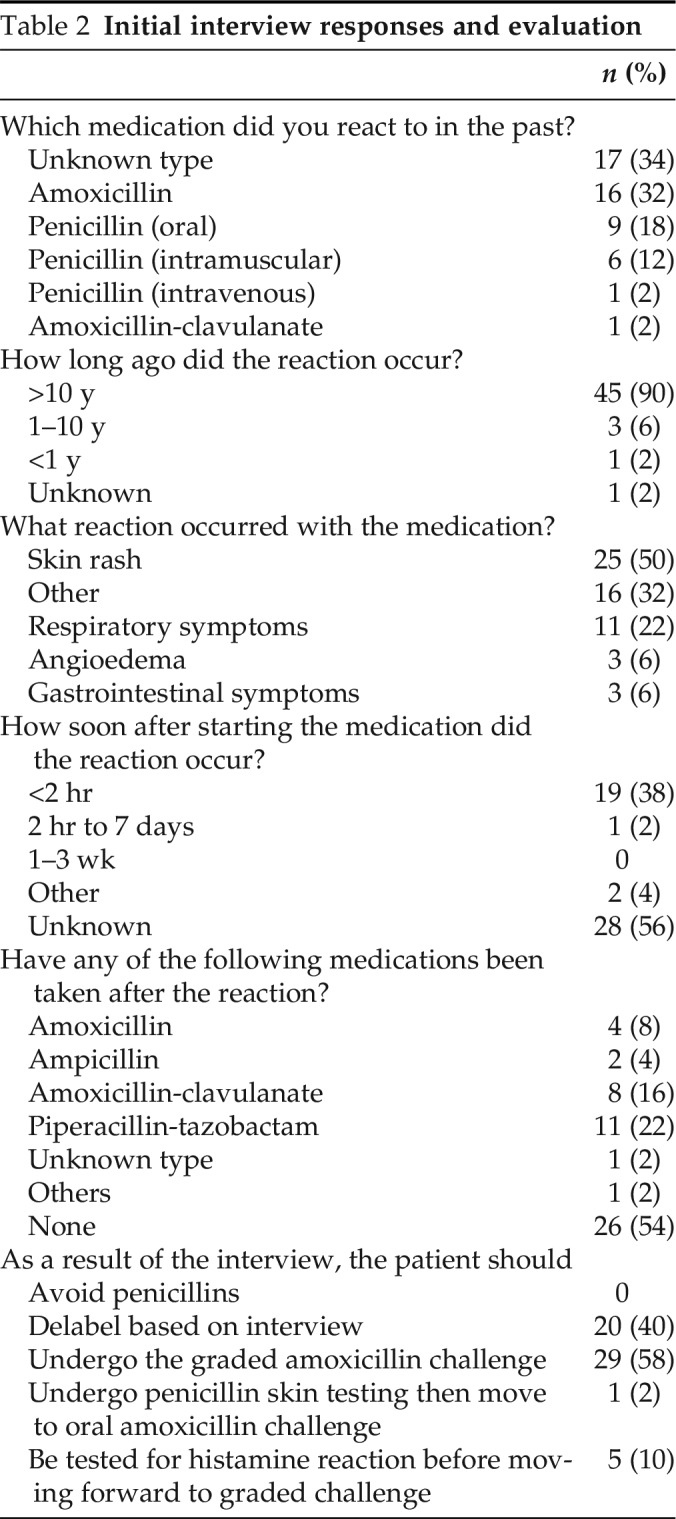
Overall, skin rash was the most common reaction reported (50%), with 14 patients (28%) reporting hives. Eleven patients (22%) reported respiratory symptoms, of which shortness of breath was the most common, and three patients each reported angioedema or gastrointestinal (GI) symptoms. Sixteen patients (32%) could not recall their reaction. Of the 30 patients challenged, 6 patients reported more than one reaction. Three patients reported respiratory symptoms and skin rash, two of those who reported the combination of shortness of breath and hives. Two reported skin rashes and GI symptoms, whereas one patient reported skin rash and serum sickness–like symptoms, which, in the interview, were determined not to be true serum sickness reaction.
More than half of the patients (56%) could not recall how soon after starting the medication the reaction occurred. Nineteen patients (38%) reported that their reaction occurred within 2 hours of exposure. One patient reported a reaction between 2 hours and 7 days after receipt of the medication. The majority of the patients (56%) could not recall receiving any treatment for their reaction to penicillin, and 11 patients (22%) reported that they had received no treatment. Eight patients (16%) recalled receiving antihistamines, whereas three patients each (6%) recalled being treated with epinephrine or steroids. More than half of the patients (52%) reported that they had not received any penicillin since their reaction; however, 24 patients reported receiving a penicillin since their reaction, with piperacillin-tazobactam being the most common (22%).
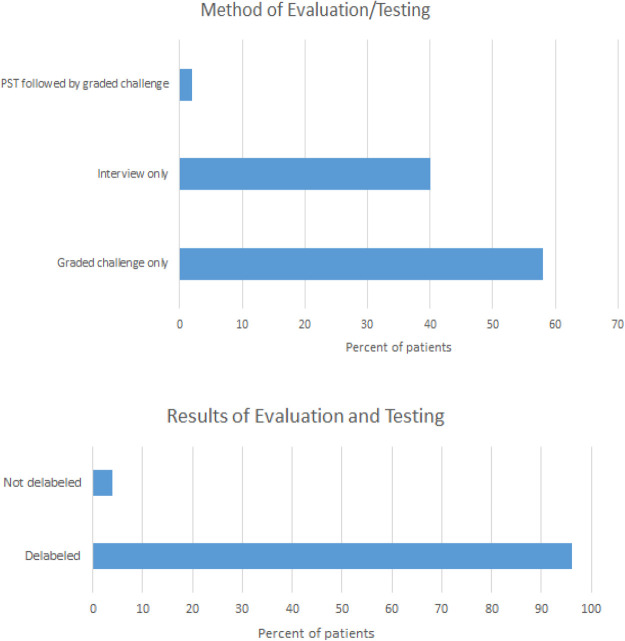
Forty-eight patients (96%) were delabeled. Delabeling occurred in 20 patients (40%) based on interview only. Thirty patients (60%) underwent an oral two-step challenge, and only one patient required a PST before challenge (Fig. 1). Among the 30 patients challenged, 9 reported a history of respiratory reactions, such as lump or swelling in the throat, shortness of breath, or anaphylaxis, whereas 3 patients reported angioedema. Twelve patients who were challenged reported hives. All patients with likely IgE-mediated reactions who were directly challenged reported that their last reaction occurred >10 years ago.
Figure 1.
The initial method of evaluation and the results of evaluation and testing. The patients were evaluated alone; evaluated and direct oral challenged; or evaluated, skin tested, and then oral challenged. The second chart shows the outcomes of all methods of evaluation.
Five patients received a histamine control before two-step challenge because they recently received an antihistamine. Two patients (4%) retained their penicillin allergy label due to mild reactions during the two-step challenge. The first patient, who reported a history of angioedema, developed mild lip tingling on administration of the 25-mg dose without any additional signs or symptoms. No rescue medications were needed and the challenge was discontinued. The second patient, with a reported history of GI upset, developed GI upset with the 25-mg dose. Diphenhydramine was administered and the patient was observed. No further signs of a reaction occurred. The two reactions most likely represented subjective challenge reactions and did not meet criteria for clinically significant IgE-mediated penicillin allergy. Fifty-four percent of the patients changed antibiotic therapy as a direct result of delabeling. Fifty percent of the patients switched to a penicillin therapy and 4% switched to cephalosporin therapy (Fig. 3). No change in therapy occurred in 16% of the patients due to receipt of a penicillin during admission and penicillin allergy labels removed on that basis. In addition, 8% of the patients underwent testing without immediate clinical need secondary to proactive evaluation based on a penicillin allergy label in the EMR.
Figure 3.
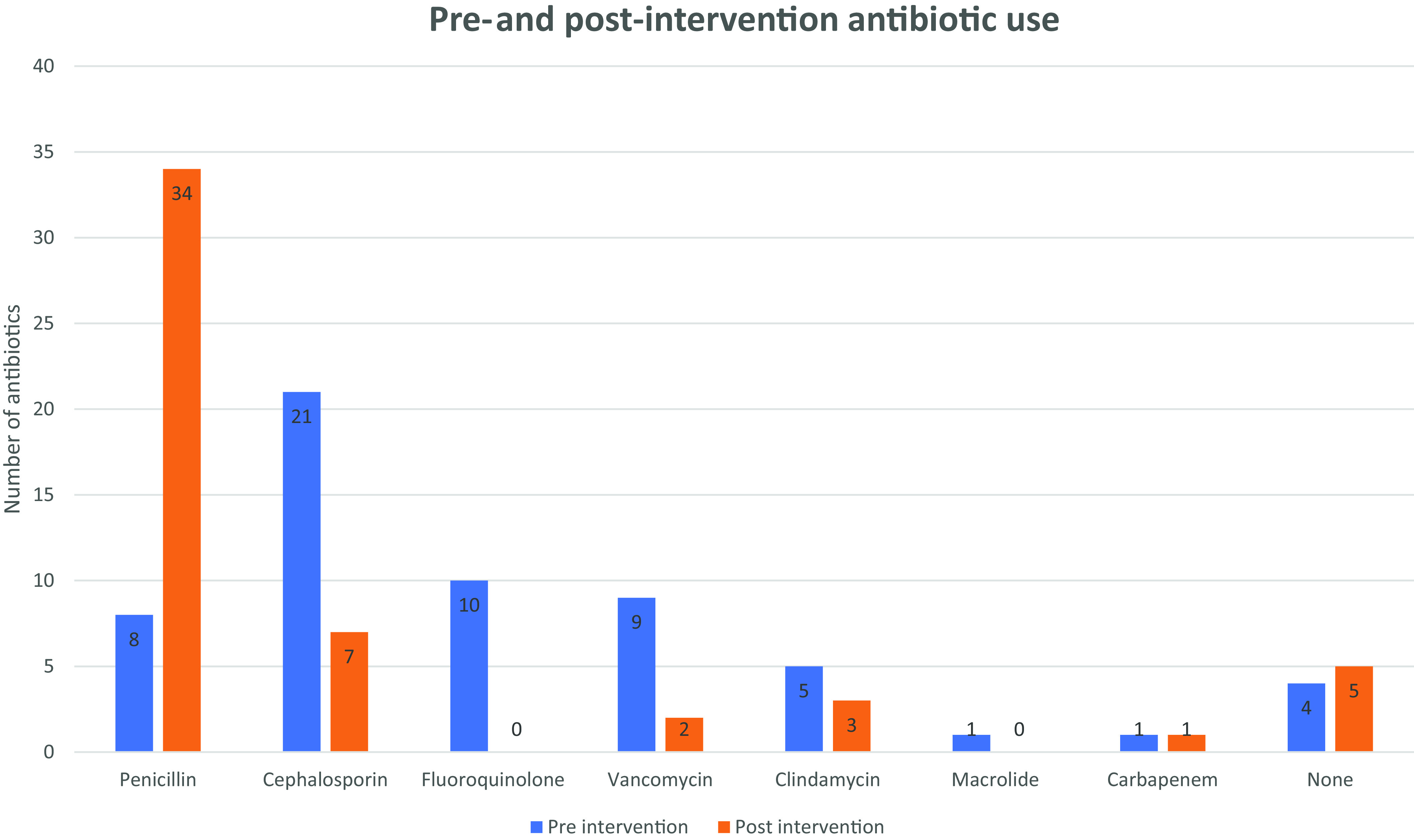
Antibiotic therapy before and after evaluation and testing; the greatest percentage increase was seen in the administration of penicillin, and the greatest percentage decrease was seen in administration of fluoroquinolones. Note: the number of antibiotics may not add up to 50 due to patients on combination therapies.
DISCUSSION
In our series of 50 patients evaluated via an interview with or without allergy testing, 96% could be delabeled. In patients who required allergy testing, proceeding directly to an oral two-step challenge was effective and efficient. In the two patients who reacted to the oral challenge, the adverse effects were mild, likely not IgE mediated, and did not require epinephrine. Most pharmacist-led penicillin allergy programs rely heavily on PST,21–23 but skin testing is not without limitations. It is costly, time-consuming, and requires special training to administer, often an insurmountable hurdle for most hospitals, particularly smaller, more rural institutions. Even for larger hospitals, resources may be scarce without dedicated staff to perform evaluations and testing.
Proceeding directly to an oral two-step challenge allows hospitals to implement this critical program for antimicrobial stewardship with fewer resources. In New Zealand, du Plessis et al.,15 reported results similar to ours and found pharmacist-led efforts that bypass skin testing to be safe and effective. Ramsey et al.16 also reported a protocol that bypassed skin testing but limited their direct oral challenge to patients with mild reactions. Our protocol allowed for oral challenge of patients who had a moderate-to-severe IgE-mediated reaction provided that the reaction occurred >10 years ago (Fig. 2). This protocol allowed bypassing of PST in all but one patient eligible for testing, with no serious reactions. A protocol limited to directly challenging cutaneous reactions, for example, would have required skin testing in at least 12 of our patients who were directly challenged (9 with a distant history of respiratory reactions and 3 with a distant history of angioedema). An additional 12 patients would have required skin testing if a history of hives required PST.
Although our protocol included these patients, only two patients developed mild subjective reactions, whereas the remainder of the patients who were challenged reported no reaction. One patient with a remote history of angioedema reported slight lip tingling and did not require any rescue medications. Although other protocols have been similar in allowing for a history of more severe reactions to be directly challenged, more safety data are required in this area before universal acceptance.20 Expert guidance from an allergist (SJ) was critical in launching our protocol, and continued support will be important as safety data gathering continues. Until safety can be more robustly established, institutions should remain cautious about direct challenges in patients at higher risk, such as those with a history of anaphylaxis. Fifty-four percent of patients experienced antibiotic regimen modification secondary to challenge (Fig. 3), often changing to antibiotics associated with fewer adverse effects, better efficacy, and less expense.4
Given the well-documented clinical and financial downsides associated with a documented penicillin allergy, institutions should consider implementation of antibiotic allergy testing programs to improve patient health and safety. Pharmacists are well suited to the task because of their knowledge of antibiotics and associated adverse reactions, and can be helpful in gathering additional safety data under the purview of an allergist. Analysis of our data demonstrated the viability of a pharmacist-driven program that bypassed PST in a significant majority of patients. Although our pool of patients who were directly challenged was small, a large portion reported a history of noncutaneous reactions. More data gathered support of allergy specialists will, it is hoped, demonstrate the safety of such a program and add to the promising results of our study, which lends support to other hospital programs.
Limitations of our study included a small number of patients and a short follow-up period in some patients, which minimized our ability to assess for allergy relabeling or delayed reactions. An additional limitation in assessing the safety of direct challenges was the inclusion of patients who were simply delabeled with a detailed interview. Although the purpose of our program was to delabel by interview or testing, this limited our pool of patients who received a challenge, which made assessing safety difficult.
CONCLUSION
A pharmacist-driven penicillin allergy evaluation and testing program effectively delabeled the vast majority of patients with documented penicillin allergy. A protocol that bypasses PST in the majority of patients lowers the barrier for hospitals that seek to implement an antibiotic allergy testing program; however, more data are required to further evaluate the safety of such a protocol.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
No external funding sources reported
REFERENCES
- 1. Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010; 105:259–273. [DOI] [PubMed] [Google Scholar]
- 2. Lee CE, Zembower TR, Fotis MA, et al. The incidence of antimicrobial allergies in hospitalized patients: implications regarding prescribing patterns and emerging bacterial resistance. Arch Intern Med. 2000; 160:2819–2822. [DOI] [PubMed] [Google Scholar]
- 3. Shenoy ES, Macy E, Rowe T, et al. Evaluation and management of penicillin allergy: a review. JAMA. 2019; 321:188–199. [DOI] [PubMed] [Google Scholar]
- 4. Jeffres MN, Narayanan PP, Shuster JE, et al. Consequences of avoiding β-lactams in patients with β-lactam allergies. J Allergy Clin Immunol. 2016; 137:1148–1153. [DOI] [PubMed] [Google Scholar]
- 5. Reilly CA, Backer G, Basta D, et al. The effect of preoperative penicillin allergy testing on perioperative non-beta-lactam antibiotic use: a systematic review and meta-analysis. Allergy Asthma Proc. 2018; 39:420–429. [DOI] [PubMed] [Google Scholar]
- 6. Charneski L, Deshpande G, Smith SW. Impact of an antimicrobial allergy label in the medical record on clinical outcomes in hospitalized patients. Pharmacotherapy. 2011; 31:742–747. [DOI] [PubMed] [Google Scholar]
- 7. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014; 133:790–796. [DOI] [PubMed] [Google Scholar]
- 8. Knezevic B, Sprigg D, Seet J, et al. The revolving door: antibiotic allergy labelling in a tertiary care centre. Intern Med J. 2016; 46:1276–1283. [DOI] [PubMed] [Google Scholar]
- 9. Blumenthal KG, Lu N, Zhang Y, et al. Recorded penicillin allergy and risk of mortality: a population-based matched cohort study. J Gen Intern Med. 2019; 34:1685–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Campbell S, Hauler G, Immler EL, et al. Pharmacist-led penicillin allergy assessment in the emergency department reduced empiric fluoroquinolone use. Clin Infect Dis. 2020; 7:e506–e508. [DOI] [PubMed] [Google Scholar]
- 11. Mann KL, Wu JY, Shah SS. Implementation of a pharmacist-driven detailed penicillin allergy interview. Ann Pharmacother. 2020; 54:364–370. [DOI] [PubMed] [Google Scholar]
- 12. Englert E, Weeks A. Pharmacist-driven penicillin skin testing service for adults prescribed nonpreferred antibiotics in a community hospital. Am J Health Syst Pharm. 2019; 76:2060–2069. [DOI] [PubMed] [Google Scholar]
- 13. Harmon S, Richardson T, Simons H, et al. The clinical and financial impact of a pharmacist-driven penicillin skin testing program on antimicrobial stewardship practices. Hosp Pharm. 2020; 55:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blumenthal KG, Li Y, Banerji A, et al. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018; 6:1019–1027.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ramsey A, Mustafa SS, Holly AM. Direct challenges to penicillin-based antibiotics in the inpatient setting. J Allergy Clin Immunol Pract. 2020; 8:2294–2301. [DOI] [PubMed] [Google Scholar]
- 16. du Plessis T, Walls G, Jordan A, et al. Implementation of a pharmacist-led penicillin allergy de-labelling service in a public hospital. J Antimicrob Chemother. 2019; 74:1438–1446. [DOI] [PubMed] [Google Scholar]
- 17. Iammatteo M, Alvarez Arango S, Ferastraoaru D, et al. Safety and outcomes of oral graded challenges to amoxicillin without prior skin testing. J Allergy Clin Immunol Pract. 2019; 7:236–243. [DOI] [PubMed] [Google Scholar]
- 18. Mill C, Primeau M-N, Medoff E, et al. Assessing the diagnostic properties of a graded oral provocation challenge for the diagnosis of immediate and nonimmediate reactions to amoxicillin in children. JAMA Pediatr. 2016; 170:e160033. [DOI] [PubMed] [Google Scholar]
- 19. Kuruvilla M, Shih J, Patel K, et al. Direct oral amoxicillin challenge without preliminary skin testing in adult patients with allergy and at low risk with reported penicillin allergy. Allergy Asthma Proc. 2019; 40:57–61. [DOI] [PubMed] [Google Scholar]
- 20. Tucker MH, Lomas CM, Ramchandar N, et al. Amoxicillin challenge without penicillin skin testing in evaluation of penicillin allergy in a cohort of Marine recruits. J Allergy Clin Immunol Pract. 2017; 5:813–815. [DOI] [PubMed] [Google Scholar]
- 21. Wall GC, Peters L, Leaders CB, et al. Pharmacist-managed service providing penicillin allergy skin tests. Am J Health Syst Pharm. 2004; 61:1271–1275. [DOI] [PubMed] [Google Scholar]
- 22. Chen JR, Tarver SA, Alvarez KS, et al. A proactive approach to penicillin allergy testing in hospitalized patients. J Allergy Clin Immunol Pract. 2017; 5:686–693. [DOI] [PubMed] [Google Scholar]
- 23. Bland CM, Bookstaver PB, Griffith NC, et al. A practical guide for pharmacists to successfully implement penicillin allergy skin testing. Am J Health Syst Pharm. 2019; 76:136–147. [DOI] [PubMed] [Google Scholar]