Abstract
Background:
Hereditary angioedema (HAE) is a rare disease that often leads to misdiagnosis. The delay of diagnosis is > 10 years in China. Recurrent and acute abdominal pain is one of the common symptoms of HAE. Because of the high misdiagnosis rate, it usually results in unnecessary surgical procedures. This study focused on the clinical symptoms and management of HAE-related abdominal attacks in Chinese patients to provide some new insight for the emergency department (ED) physicians and gastroenterologists.
Methods:
A Web-based survey was conducted among 107 patients with HAE from 94 unrelated families. Detailed questions with respect to the abdominal attacks were asked, including the frequency, symptoms, and duration before and after confirmed diagnosis. The demographic characteristics, diagnosis process, and treatment outcomes were also included.
Results:
Approximately 70% of the patients with HAE presented with abdominal symptoms during the onset of edema, mostly characterized by pain (94.8%), nausea (83.1%), vomiting (83.1%), diarrhea (59.7%), and constipation (23.4%). The patients were easily misdiagnosed as having gastroenteritis (35.1%) and appendicitis (10.4%), and 24.7% of them received unnecessary appendectomy or laparotomy. Danazol, a widely used drug for long-term prophylaxis of HAE in China, can reduce the attack frequency and alleviate the abdominal symptoms, but the adverse effects are also significant and more severe in women.
Conclusions:
Abdominal symptoms are common and important clinical features of HAE but are easily confused with other gastrointestinal diseases. ED physicians and gastroenterologists should consider HAE when patients experience recurrent and unexplained abdominal pain. Proper medical treatment should be administered in a timely manner if an HAE diagnosis is confirmed and efforts are required to increase access in China to medications both for on-demand treatment and long-term prophylaxis.
Keywords: hereditary angioedema, gastrointestinal angioedema, C1-INH, acute abdomen
Hereditary angioedema (HAE) is a rare and potentially life-threatening disease, with an estimated prevalence of 1/50,000.1 Classic HAE is caused by the mutation of the SERPING1 (Serpin Family G Member 1) gene (OMIM [Online Mendelian Inheritance In Man] https://omim.org/entry/106100?search=serping1&highlight=serping1), which causes C1 inhibitor (C1-INH) deficiency, which can be subdivided into type I (decreased plasma levels of C1-INH) and type II (normal levels but dysfunction). In China, type I HAE accounts for the majority of patients with HAE. The percentage of Chinese patients with type II HAE (<5%)2,3 was lower than expected (<15%).4 C1-INH deficiency causes uncontrolled generation of bradykinin, a vasoactive peptide to increase vascular permeability, and results in edema and inflammation. Up to now, other types of HAE due to the defects in factor XII, plasminogen, or angiopoietin-1 have not been reported in China.
HAE is clinically characterized by recurring and self-limiting episodes of subcutaneous and submucosal edema, which involves the face, extremities, trunk, genitalia, upper airways, and/or gastrointestinal tract.5 The symptoms present large intra- and interindividual variation with respect to the frequency, severity, and locations of edematous attacks. Gastrointestinal reaction is a common symptom, often accompanied with severe abdominal pain, nausea, vomiting, and diarrhea, which may urge patients to seek medical treatment. However, due to its rarity and the variety of clinical manifestations, the abdominal attacks, are easily misdiagnosed, especially when extra-abdominal symptoms are absent, which leads to unnecessary appendectomy or laparotomy. Consequently, gastrointestinal angioedema significantly reduces life quality and aggravates the economic burden of patients with HAE. Known to be an autosomal dominant disease, patients with HAE typically have a positive family history. However, ∼25% of cases developed due to sporadic de novo mutations,6 which makes the diagnosis more difficult. The delay in diagnosis of HAE can be > 10 years for Chinese patients.7
Our study mainly focused on HAE-related abdominal attacks to further describe its symptoms, frequency, and severity. The conditions of diagnosis and treatment in China were also included. we hope these data provide some information to the emergency department (ED) physician and gastroenterologist to consider HAE when patients experience recurrent but inexplicable abdominal pain.
METHODS
Patients
A total of 107 participants from 94 unrelated families with type I or type II HAE, who presented to the Department of Allergy, Peking Union Medical College Hospital, between the years 1983 and 2017, were surveyed. The diagnosis of HAE was based on the medical history (recurrent angioedema without urticaria, involved in skin and/or gastrointestinal tract and/or laryngeal) and laboratory results (repeated confirmation of low plasma levels of C4 and C1-INH in type I HAE; normal or increased level but decreased function of C1-INH in type II HAE). The study protocol was approved by the research and ethics board of the Peking Union Medical College Hospital. Also, the participants were informed of the objectives, the agency that conducts the research, and privacy protection of this survey. Informed consent was obtained from all patients before the study, and the patients signed informed consent with regard to publishing their data.
A questionnaire was developed by using the wjx online survey platform (Changsha Ranxing Information Technology Co., Ltd., Changsha, P.R. China). The patients were asked to recall the characteristics of edema episodes before and after a confirmed diagnosis, including locations, frequency, specific symptoms, and duration. The severity of skin, gastrointestinal tract, and laryngeal edema were assessed by the patients themselves by using a scale weighted by the degree of edema (0, normal; 1–3, mild; 4–7, moderate; 8–10, severe). The conditions of diagnosis and treatment were also investigated. The questionnaire was completed by all the participants, and any incomplete or ambiguous feedback was reconfirmed. The Wilcoxon signed rank test, performed by using IBM SPSS Statistics 25 software (International Business Machines Corporation, New York, United States), was used to evaluate the effects of danazol according to the frequency, severity score, and duration of edema.
RESULTS
Abdominal Attack Presentation
One hundred and seven patients with HAE (49 male and 58 female patients), with a mean age of 38.2 years (range, 11–68 years) were analyzed (Table 1). Type I HAE was present in 103 participants and type II was present in 4. The number of the patients with skin, gastrointestinal, and laryngeal edema was 106 (99.1%), 77 (72.0%), and 65 (60.7%), respectively, and the mean frequency was 9.2 (range, 0.75–25; median [interquartile range {IQR}], 6.5 [4–13.5]), 4.6 (range, 0.12–26; median [IQR], 3 [2–6.25]), and 2.1 (range, 0.1–14; median [IQR], 1[1–2.875]) times per year among the patients who have ever experienced the above symptoms, respectively, before the confirmed diagnosis. Based on the self-assessment of edema severity, the mean score of abdominal symptoms was 7.6 (range, 1–10; median [IQR], 8.25 [6–9]), close to laryngeal edema, of 7.7 (range, 3–10; median [IQR], 8.75 [6.75–9]); and higher than skin, of 6.9 (range, 1–10; median [IQR], 8 [5–9]).
Table 1.
Patient demographics and characteristics of hereditary angioedema attacks (N = 107)
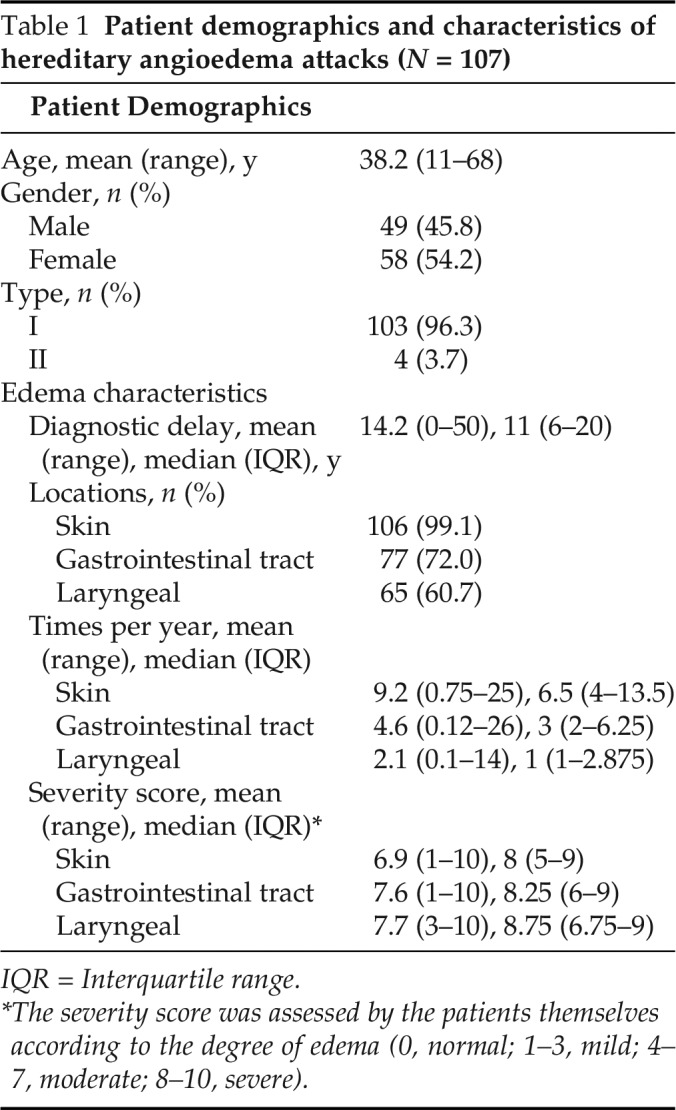
IQR = Interquartile range.
*The severity score was assessed by the patients themselves according to the degree of edema (0, normal; 1–3, mild; 4–7, moderate; 8–10, severe).
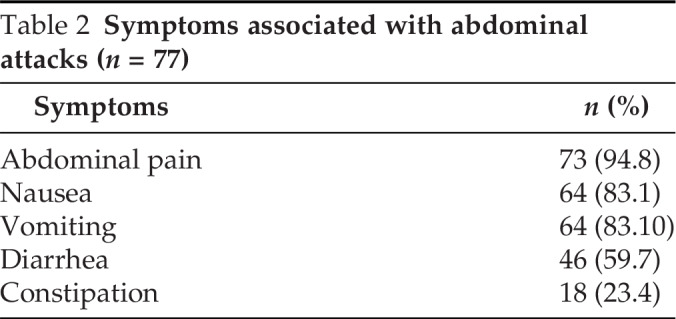
In our survey, all 77 patients who had gastrointestinal edema were diagnosed as having type I HAE. Four patients with type II HAE did not report abdominal symptoms. Abdominal involvement presented at the first episode of HAE in 40 patients, of whom 7 had isolated abdominal symptoms during the first attack. Abdominal pain was the main symptom reported by 73 of 77 patients (94.8%) during abdominal attacks with or without other gastrointestinal symptoms, including nausea (64/77 [83.1%]), vomiting (64/77 [83.1%]), diarrhea (46/77 [59.7%]), and constipation (18/77 [23.4%]) (Table 2). Patients went to the ED due to the abdominal attacks 3.2 on average (range, 0–18, median [IQR], 2 [0–5]) times per year. Sixty-two patients received abdominal ultrasound because of abdominal pain during the onset of edema and ascites was documented in 47 patients. Without intervention, the abdominal pain lasted mean 68.9 hours (range, 8–240 hours; median [IQR], 72 hours [36–72.75 hours]), and most patients (45/47) reported that the ascites disappeared as the abdominal pain improved. Fifty-three patients reported that their family members also had a history of abdominal pain relative to HAE.
Table 2.
Symptoms associated with abdominal attacks (n = 77)
Diagnostic Delays and Misdiagnosis
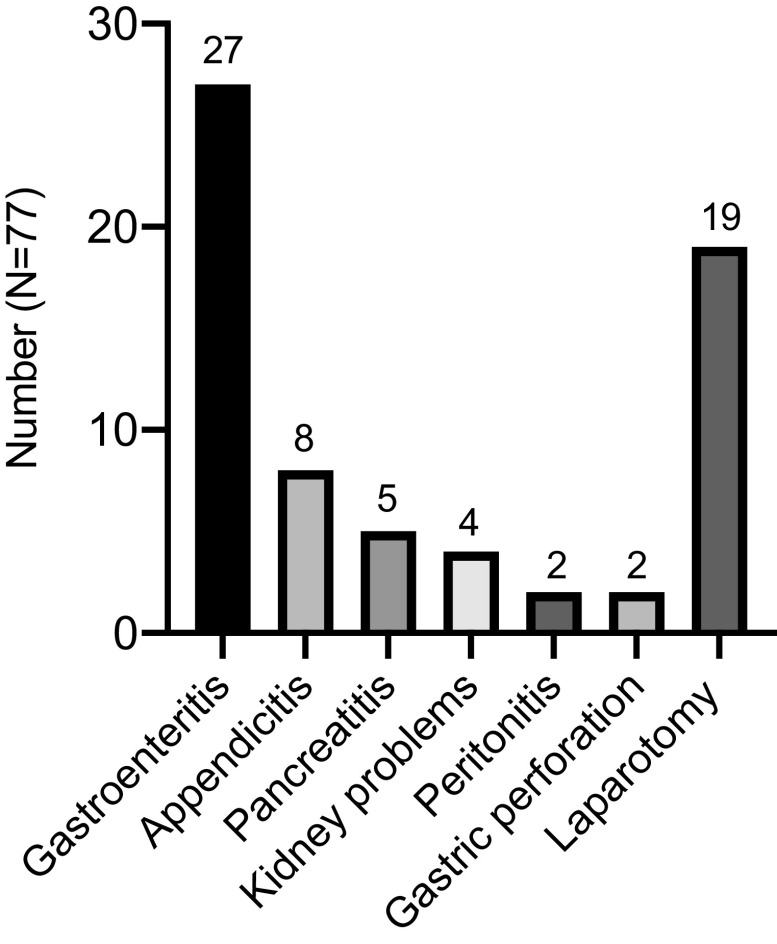
The mean delay from the first episode of HAE to the confirmed diagnosis was 14.2 years (range, 0–50 years; median [IQR], 11 years [6–20 years]) for these 77 patients. It took 16 years before diagnosis in one patient, who only presented with abdominal attacks since the first attack at the age of 23 years. Gastrointestinal angioedema was misdiagnosed as recurrent episodes of gastroenteritis in 27 patients, appendicitis in 8 patients, pancreatitis in 5 patients, and kidney problems in 4 patients (Fig. 1). Two patients were also misdiagnosed with peritonitis (1) and gastric perforation (1). Misdiagnosis of abdominal symptoms led to unnecessary appendectomy or other laparotomy in 19 of 77 patients (24.7%).
Figure 1.
Misdiagnosis and mistreatment of patients with hereditary angioedema (HAE) and with abdominal pain.
Treatment Outcomes
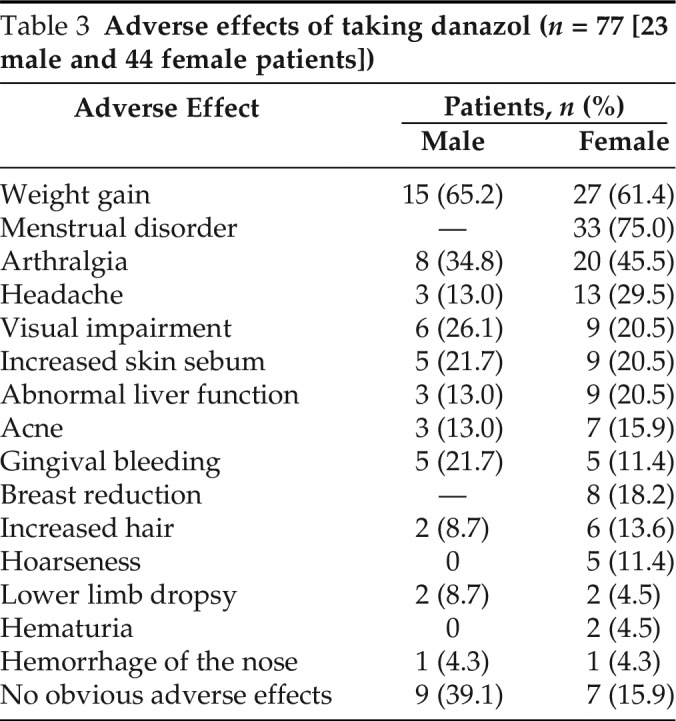
Danazol, one kind of attenuated androgens, was adopted by most of the patients (67/77 [87.0%]) for long-term prophylaxis and the median (IQR) frequency of gastrointestinal edema decreased from 3 (2–6.25) to 1.5 (0–3) (mean, 2.3; range, 0–12) times per year (p < 0.001). The severity score of abdominal symptoms dropped from a median (IQR) of 8.25 (6–9) to 7 (4.25–9) (mean, 6.2; range, 1–10) (p = 0.001), and the duration of HAE attacks was shortened, from a median (IQR) of 72 hours (36–72.75 hours) to 48 hours (24–72 hours) (mean, 53.8 hours; range, 3–264 hours) (p < 0.001). However, 50 patients (74.6%) presented obvious adverse effects (Table 3), with symptoms of menstrual disorder in 75% of the female patients (33/44), weight gain in 61.8% of the patients (42/68), and arthralgia in 41.2% of the patients (28/68). Sixteen patients (23.9%) did not report obvious adverse effects. Men were less likely to have adverse effects than were women. Fresh frozen plasma (FFP) was administered to one patient once to abort a severe abdominal attack and symptoms began to be relieved after 30 minutes and completely disappeared after 2 hours.
Table 3.
Adverse effects of taking danazol (n = 77 [23 male and 44 female patients])
DISCUSSION
Abdominal symptoms are important clinical features of HAE. This study showed that ∼70% of patients presented with an abdominal symptom during the onset of edema, which far exceeded the percentage (34.17%) previously reported in China.7 In addition to the limited sample size, it may be because most of the participants (87.8%) in our survey were the proband of their family, who may present more significant clinical manifestations. Our results showed that abdominal edema occurred twice as frequently as the life-threatening laryngeal edema but with a similar severity score. In another study, >80% of patients rated abdominal attacks as excruciating or severely painful.8 Consequently, a timely diagnosis and proper treatment of gastrointestinal angioedema will greatly reduce the humanistic and economic burden for patients.
The diagnosis of HAE is challenging due to its rarity and various clinical presentations. Previous studies showed a significant delay of 12.6 years in China7 and 8 to 10 years in Europe9–10 and the United States.11 It will be more difficult to recognize HAE-related abdominal attacks, especially for patients with gastrointestinal edema as the main or the only clinical manifestation. Although most of the HAE patients may present multiple sites of edema in their medical history, the episode of gastrointestinal edema is often not accompanied by edema in other locations. According to one survey,12 of 521 attacks with any abdominal pain among 149 HAE patients, 49% presented with isolated abdominal pain only. Also, in another research,8 nearly 30% of the patients reflected that the abdominal attacks seemed long before they ever noted the skin edema. In our survey, there was one patient who had presented only abdominal symptoms in his medical record. Undoubtedly, this kind of patient is more likely to be misdiagnosed.
Abrupt angioedema of the bowel wall will cause partial or complete intestinal obstruction, ascites, and hemoconcentration. According to our investigations, the abdominal symptoms are mostly characterized by pain (94.8%), nausea (83.1%), vomiting (83.1%), and diarrhea (59.7%), and ∼25% of patients had constipation, which is in agreement with previous overseas reports.8,13 Ascites, which would disappear along with the edema remission, was found in 75.8% of the patients who received abdominal ultrasound. Rare symptoms, including hemorrhagic stools8 and intussusception,8,14 were documented in other studies but were not observed in our study. These symptoms are easily misdiagnosed as other more common diseases, such as gastroenteritis, appendicitis, pancreatitis, or kidney problems, which leads to unnecessary surgical procedures.
Currently, no reliable maker is provided to confirm gastrointestinal angioedema on presentation of symptoms.12 Detailed medical and family histories are important for the diagnosis of HAE; however, notably, de novo mutations account for ∼25% of the cases.6 Gastrointestinal edema was not observed in four patients with type II HAE in our survey but was reported in other research,15 possibly due to the low number enrolled (4/107 [3.7%]). As for the diagnosis of patients with type II HAE, C1-INH functional testing can only be realized in few hospitals currently in China, which may explain the low percentage of patients with type II than expected. If HAE is suspected in patients in an emergency situation, then a blood test of the C4 level is recommended for initial screening,12,15,16 which can exclude HAE with C1-INH deficiency with an accuracy of > 95%,15 and research of a reduced level of C4 in 95% of cases during edema remission and virtually 100% during the attack.12 A genetic test is not necessarily required for the diagnosis but can be useful with the sporadic cases and helpful to understand the pathogenesis.
Epinephrine, corticosteroids, and antihistamines are not efficacious for the treatment of HAE.17 Currently, C1 inhibitor concentrate, ecallantide (kallikrein inhibitor) and icatibant (bradykinin-receptor antagonist), are considered best for on-demand treatment, and the C1 inhibitor concentrate and ecallantide are also recommended for self-administration during home therapy.18 However, no drugs for acute HAE attacks have been provided in the Chinese market to date. If the above drugs are not available, then FFP can also be used for the attacks, but very few patients have ever received FFP for on-demand treatment. Because many physicians in China still lack the knowledge of this disease due to its rarity; therefore, when patients need emergency treatment, it is usually difficult for physicians in nearby hospitals to provide immediate accurate treatment. Also, the supply of FFP is not available in all hospitals at any time.
Most patients have adopted danazol for long-term prophylaxis in China. The results show that danazol can significantly reduce the frequency of gastrointestinal edema and alleviate the abdominal symptoms. Adverse effects are also obvious, mostly accompanied by weight gain, menstrual disorder, and arthralgia. Plasma-derived C1 inhibitor concentrate, as the preferred long-term prophylaxis, has been observed with rare adverse events18,19 but also has not been available in China. Efforts are required to increase global access to medications both for on-demand treatment and long-term prophylaxis.
Our study had several limitations. First, the data were based on a survey of a modest number of patients and mainly relied on participants' memories, which were subject to recall bias. Second, changes in abdominal symptoms during one attack have been observed in our clinical practice and also revealed by other studies.8 However, the participants in our survey did not recall the details of the whole process of gastrointestinal angioedema, and, therefore, we were not able to summarize the clinical course of abdominal attacks. Third, for patients with known HAE, imaging examinations were not routinely required for diagnosis during an abdominal attack, and they counted one episode of HAE, mainly according to characteristic symptoms. Abdominal pain and diarrhea due to other causes may also have been thought of as gastrointestinal angioedema.
CONCLUSION
To our knowledge, this was the first study to summarize the abdominal attacks of patients with HAE as well as their conditions of diagnosis and treatment in China. Abdominal symptoms are common and important clinical features of HAE but are easily confused with other gastrointestinal diseases, which lead to unnecessary invasive procedures and a heavier mental and economic burden on patients. ED physicians and gastroenterologists should consider HAE when patients experience recurrent and unexplained abdominal pain, especially when ascites is reported by ultrasound and disappears after pain relief. Proper medical treatment should be administered in a timely manner if an HAE diagnosis is confirmed, and, in China, efforts are required to increase access to medications both for on-demand treatment and long-term prophylaxis.
ACKNOWLEDGEMENTS
We thank all the patients with HAE who participated in this study.
Footnotes
This project was supported by the National Natural Science Foundation of China (grant 81472870), the CAMS (Chinese Academy of Medical Sciences)
Innovation Fund for Medical Sciences (2016-I2M-1-002), and the National Key Research and Development Program of China (2016YFC0901501)
The authors have no conflicts of interest to declare pertaining to this article
The datasets generated and analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request
REFERENCES
- 1. Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med. 2008; 359:1027–1036. [DOI] [PubMed] [Google Scholar]
- 2. Liu S, Xu Y, Liu Y, et al. Hereditary angioedema: a Chinese perspective. Eur J Dermatol. 2019; 29:14–20. [DOI] [PubMed] [Google Scholar]
- 3. Liu S, Wang X, Xu Y, et al. Risk factors for diagnostic delay in Chinese patients with hereditary angioedema. Allergy Asthma Proc. 2019; 40:343–349. [DOI] [PubMed] [Google Scholar]
- 4. Walford HH, Zuraw BL. Current update on cellular and molecular mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol. 2014; 112:413–418. [DOI] [PubMed] [Google Scholar]
- 5. Csuka D, Veszeli N, Varga L, et al. The role of the complement system in hereditary angioedema. Mol Immunol. 2017; 89:59–68. [DOI] [PubMed] [Google Scholar]
- 6. Pappalardo E, Cicardi M, Duponchel C, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol. 2000; 106:1147–1154. [DOI] [PubMed] [Google Scholar]
- 7. Xu Y-Y, Jiang Y, Zhi Y-X, et al. Clinical features of hereditary angioedema in Chinese patients: new findings and differences from other populations. Eur J Dermatol. 2013; 23:500–504. [DOI] [PubMed] [Google Scholar]
- 8. Bork K, Staubach P, Eckardt AJ, et al. Symptoms, course, and complications of abdominal attacks in hereditary angioedema due to C1 inhibitor deficiency. Am J Gastroenterol. 2006; 101:619–627. [DOI] [PubMed] [Google Scholar]
- 9. Zanichelli A, Magerl M, Longhurst H, et al. Hereditary angioedema with C1 inhibitor deficiency: delay in diagnosis in Europe. Allergy Asthma Clin Immunol. 2013; 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jolles S, Williams P, Carne E, et al. A UK national audit of hereditary and acquired angioedema. Clin Exp Immunol. 2014; 175:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christiansen SC, Davis DK, Castaldo AJ, et al. Pediatric hereditary angioedema: onset: diagnostic delay, and disease severity. Clin Pediatr (Phila). 2016; 55:935–942. [DOI] [PubMed] [Google Scholar]
- 12. Rubinstein E, Stolz LE, Sheffer AL, et al. Abdominal attacks and treatment in hereditary angioedema with C1-inhibitor deficiency. BMC Gastroenterol. 2014; 14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bork K, Meng G, Staubach P, et al. Treatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedema. Transfusion. 2005; 45:1774–1784. [DOI] [PubMed] [Google Scholar]
- 14. Witschi A, Krähenbühl L, Frei E, et al. Colorectal intussusception: an unusual gastrointestinal complication of hereditary angioedema. Int Arch Allergy Immunol. 1996; 111:96–98. [DOI] [PubMed] [Google Scholar]
- 15. Abuzakouk M, AlMahmeed N, Memisoglu E, et al. Hereditary angioedema type II: first presentation in adulthood with recurrent severe abdominal pain. Case Reports Immunol. 2018: 2018; 7435870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zanichelli A, Arcoleo F, Barca MP, et al. A nationwide survey of hereditary angioedema due to C1 inhibitor deficiency in Italy. Orphanet J Rare Dis. 2015; 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zuraw BL, Bernstein JA, Lang DM, et al. A focused parameter update: hereditary angioedema, acquired C1 inhibitor deficiency, and angiotensin-converting enzyme inhibitor-associated angioedema. J Allergy Clin Immunol. 2013; 131:1491–1493. [DOI] [PubMed] [Google Scholar]
- 18. Maurer M, Magerl M, Ansotegui I, et al. The international WAO/EAACI guideline for the management of hereditary angioedema-the 2017 revision and update. Allergy. 2018; 73:1575–1596. [DOI] [PubMed] [Google Scholar]
- 19. Riedl MA, Grivcheva-Panovska V, Moldovan D, et al. Recombinant human C1 esterase inhibitor for prophylaxis of hereditary angio-oedema: a phase 2, multicentre, randomised, double-blind, placebo-controlled crossover trial. Lancet. 2017; 390:1595–1602. [DOI] [PubMed] [Google Scholar]