Abstract
Background
There are no effective systemic therapies for chordoma. The recent successes of immunotherapeutic strategies in other cancers have resulted in a resurgence of interest in using immunotherapy in chordoma. These approaches rely on a functional interaction between the host’s immune system and the expression of tumor peptides via the human leukocyte antigen (HLA) Class I antigen. It is not known whether chordoma cells express the HLA Class I antigen.
Questions/purposes
(1) Do chordoma tumors exhibit defects in HLA Class I antigen expression? (2) What is the pattern of lymphocyte infiltration in chordoma tumors?
Methods
Patients with chordoma treated at Massachusetts General Hospital between 1989 and 2009 were identified with permission from the institutional review board. Of the 75 patients who were identified, 24 human chordoma tumors were selected from 24 distinct patients based on tissue availability. Histology slides from these 24 formalin-fixed paraffin-embedded chordoma tissue samples were deparaffinized using xylene and ethanol and underwent heat-induced antigen retrieval in a citrate buffer. Samples were incubated with monoclonal antibodies directed against HLA Class I antigen processing machinery components. Antibody binding was detected via immunohistochemical staining. Staining intensity (negative, weakly positive, strongly positive) was assessed semiquantitatively and the percentage of chordoma cells stained for HLA Class I antigen subunits was assessed quantitatively. Hematoxylin and eosin-stained histology slides from the same 24 chordoma samples were assessed qualitatively for the presence of tumor-infiltrating lymphocytes and histologic location of these lymphocytes. Immunohistochemical staining with monoclonal antibodies directed against CD4 and CD8 was performed in a quantitative manner to identify the lymphocyte subtype present in chordoma tumors. All results were scored independently by two investigators and were confirmed by a senior bone and soft tissue pathologist.
Results
Seven of 24 chordoma samples exhibited no staining by the anti-HLA-A heavy chain monoclonal antibody HC-A2, two had weak staining intensity, and eight had a heterogeneous staining pattern, with fewer than 60% of chordoma cells exhibiting positive staining results. Four of 24 samples tested were not stained by the anti-HLA-B/C heavy chain monoclonal antibody HC-10, five had weak staining intensity, and 11 displayed a heterogeneous staining pattern. For the anti-β-2-microglobulin monoclonal antibody NAMB-1, staining was detected in all samples, but 11 had weak staining intensity and four displayed a heterogeneous staining pattern. Twenty-one of 24 samples tested had decreased expression in at least one subunit of HLA Class I antigens. No tumors were negative for all three subunits. Lymphocytic infiltration was found in 21 of 24 samples. Lymphocytes were primarily found in the fibrous septae between chordoma lobules but also within the tumor lobules and within the fibrous septae and tumor lobules. Twenty-one of 24 tumors had CD4+ T cells and 11 had CD8+ T cells.
Conclusion
In chordoma tissue samples, HLA Class I antigen defects commonly were present, suggesting a mechanism for escape from host immunosurveillance. Additionally, nearly half of the tested samples had cytotoxic CD8+ T cells present in chordoma tumors, suggesting that the host may be capable of mounting an immune response against chordoma tumors. The resulting selective pressure imposed on chordoma tumors may lead to the outgrowth of chordoma cell subpopulations that can evade the host’s immune system.
Clinical Relevance
These findings have implications in the design of immunotherapeutic strategies for chordoma treatment. T cell recognition of tumor cells requires HLA Class I antigen expression on the targeted tumor cells. Defects in HLA Class I expression may play a role in the clinical course of chordoma and may account for the limited or lack of efficacy of T cell–based immunity triggered by vaccines and/or checkpoint inhibitors.
Introduction
Chordoma, a primary malignant bone tumor that almost always arises in the axial skeleton, is thought to originate from notochordal remnants or benign notochordal cell tumors [3]. Chordomas are locally aggressive and have a high rate of local recurrence when complete en bloc surgical resection is not performed [58]. Unfortunately, the morbidity associated with en bloc resection can lead to poor function and quality of life [54], and in the case of sacral chordoma, can lead to loss of bowel, bladder, and sexual function [60]. The morbidity associated with sacral resection has led some centers to abandon surgery in favor of charged-particle radiation therapies such as carbon ion and proton radiation [35, 46]. The favorable off-target effect profile associated with these therapies allows for higher doses to be used. However, a recent report on the use of definitive carbon ion therapy without surgery revealed a local recurrence rate of 50% after 10 years, indicating that standalone therapy may not be appropriate in all patients [31]. Furthermore, disease-free survival is particularly poor once chordomas recur locally, and there are no effective treatments for metastatic chordomas [48]. Because of the morbidity of en bloc resection and the lack of an alternative with proven efficacy to control chordomas locally or systemically, there is a need for different treatment strategies.
Recent studies using an immune checkpoint blockade have been successful in treating various types of cancer [4, 5, 38], and these strategies have been suggested for patients with chordoma based on reports of checkpoint inhibitor molecule expression in chordoma tissues [20, 43, 66]. These immunotherapeutic strategies require that the host mounts an immune response against the tumor. A marker of a host immune response is the presence of tumor-infiltrating lymphocytes [24, 32]. The presence of tumor-infiltrating lymphocytes has been associated with improved prognoses in numerous malignancies, including melanoma [16, 17, 39, 59], breast cancer [2, 40, 42, 44], lung cancer [1, 19, 30, 33, 37], colorectal cancers [19-40], and ovarian cancer [29, 53, 64]. Recently, tumor-infiltrating lymphocytes have been shown to be present in chordomas; however, the lymphocyte infiltration has not been characterized [20, 66].
Immunosurveillance requires tumor cells to express human leukocyte antigen (HLA) Class I antigens loaded with tumor antigen-derived peptides. These are recognized by T cell receptors on cognate CD8+ T lymphocytes [7]. T cell–based immunotherapies such as checkpoint inhibitors rely on a functional interaction between CD8+ T cells and HLA Class I antigen/tumor antigen–derived peptide complexes that are expressed on the surface of tumor cells. A growing body of evidence indicates that a major obstacle to the success of immunotherapy is represented by the many escape mechanisms used by tumor cells to avoid recognition and destruction by the host’s immune system [13, 14, 23]. Among these are abnormalities in the expression and function of the HLA Class I antigen processing machinery subunits, resulting in defective recognition of tumor antigen–derived peptides by cognate CD8+ T cells. Most, if not all, malignancies have been shown to have downregulation or loss of HLA Class I antigen processing machinery components, as recently reviewed by our group [8-15, 21-23, 41, 55]. Chordoma is one of the few solid tumors for which no information is available about the HLA Class I antigen processing machinery component expression on tumor cells. The lack of this information affects our ability to assess the role of immunosurveillance in the clinical course of chordoma and to design the most effective immunotherapy to treat this malignancy.
To overcome this limitation, in this study, we therefore asked: (1) Do chordoma tumors exhibit defects in HLA Class I antigen expression? (2) What is the pattern of lymphocyte infiltration in chordoma tumors?
Patients and Methods
Human Chordoma Samples
Patients with chordoma treated at Massachusetts General Hospital between 1989 and 2009 were identified from our orthopaedic oncology database with approval from the hospital’s institutional review board. Seventy-five patients were identified. Of these, 24 human chordoma tumors were selected from 24 distinct patients based on tissue availability. All chordoma tissue slides were reviewed in tandem with a senior bone and soft tissue pathologist (GPN, VD) to confirm the diagnosis of chordoma and ensure that a sufficient amount of tumor tissue for analysis was present on each slide.
Monoclonal Antibodies
Monoclonal antibodies specifically directed against the subunits of HLA Class I antigens were used. The mAb HC-A2, which recognizes β-2-microglobulin-free HLA-A (excluding -A24), -B7301, and -G heavy chains [56, 57]; the mAb HC-10, which recognizes β-2-microglobulin-free HLA-A3, -A10, -A28, -A29, -A30, -A31, -A32, -A33, and –B (excluding -B5702, -B5804, and -B73) heavy chains [50, 56, 57]; and the β-2-microglobulin-specific mAb NAMB-1 [49] were developed and characterized as described. Antibodies were purified from ascitic fluid by affinity chromatography based on Protein G. The purity and activity of the purified monoclonal antibodies were monitored by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by Western blotting with lymphoid cell lysate [47, 61]. For our study, we used antibodies that recognize the gene products of the different HLA Class I loci instead of an antibody that recognizes all of the HLA Class I antigen. It has been our experience that the use of antibodies specific for the gene products of HLA Class I loci increases the possibility to identify selective loss of HLA Class I antigen subunits [6, 45]. Some studies have reported that selective HLA Class I antigen defects are not detected with anti-pan HLA Class I antibodies [36, 62].
To analyze lymphocyte populations in chordoma tissue sections, we purchased human CD4- and CD8-specific mAbs from Dako North America Inc (Carpinteria, CA, USA).
Immunohistochemical Staining of Tissue Samples with Monoclonal Antibodies
We obtained formalin-fixed paraffin-embedded human chordoma tissue samples. Four-micron-thick tissue sections were deparaffinized in xylene and ethanol and subjected to heat-induced antigen retrieval in citrate buffer. We incubated the slides in 3% hydrogen peroxide for 20 minutes and then rinsed in tris-buffered saline with Tween 20 (TBST). After incubation with a blocking buffer, the slides were incubated with mAbs overnight at 4°C. After being rinsed with TBST, slides were incubated for 45 minutes at room temperature with EnVision™ + Dual Link System-horseradish peroxidase (HRP) goat anti-mouse immunoglobulin (Dako). Antibody binding was detected with the diaminobenzidine (DAB) + Peroxidase Substrate Kit (Dako), and the slides were counterstained with hematoxylin. Slides were then dehydrated and mounted with coverslips.
We examined the slides throughout the tissue section at high-power (200x) magnification. For HLA Class I expression in chordoma cells, the staining intensity and percentage of stained tumor cells were assessed. Staining intensity was scored semiquantitatively as 0 (negative), 1+ (weakly positive), or 2+ (strongly positive). We considered cytoplasmic and membranous staining positive for the HLA-A heavy chain, HLA-B/C heavy chain, and β-2-microglobulin staining. The following controls were used: mouse skin as an external negative control, melanoma xenograft as an external positive control, and normal muscle and fat as an internal positive control when present adjacent to chordoma tissue. For CD4 and CD8 staining of lymphocytes, four high-power fields were randomly chosen on slides, and the number of stained lymphocytes was counted. We considered membranous staining positive for CD4 and CD8 staining. The following controls were used: mouse skin as an external negative control and human spleen as an external positive control. Staining results were independently scored by two investigators (SSP, SPN), and the results were confirmed by a senior bone and soft tissue pathologist (GPN, VD). There were no disagreements regarding the staining intensity of samples. For the quantitative analysis of both the percentage of chordoma cells stained for HLA Class I antigen subunits and lymphocytes stained for CD4 and CD8, disagreements were rare. In the event of a different count between investigators, the pathologist settled the disagreement.
Assessment of Lymphocytic Infiltration
Hematoxylin and eosin-stained human chordoma tissue slides from the 24 patients were qualitatively assessed for the presence of lymphocytes and histologic location. Slides were evaluated to ensure that a sufficient amount of intact tumor tissue was reviewed for lymphocytic infiltration and localization. Lymphocytes were present in the tumor lobule, the fibrous septae between tumor lobules, both the tumor lobules and fibrous septae, or absent throughout a tumor sample. Slides were independently reviewed by two investigators (SSP, SPN), and results were confirmed by a senior bone and soft tissue pathologist (GPN, VD). There were no disagreements among the investigators.
Results
HLA Class I Expression in Chordoma Tumors
Chordoma tumors exhibited defects in HLA Class I antigen expression at a high frequency. Twenty-four samples were tested. In seven samples, no staining by the HLA-A heavy chain-specific mAb HC-A2 was detected (Table 1). Nine samples were homogeneously stained and eight exhibited a heterogeneous staining pattern, meaning that fewer than 60% of chordoma cells were stained. When analyzing staining intensity, two of the positive samples exhibited a weak staining intensity (Fig. 1).
Table 1.
HLA Class I antigen defects in chordoma
| HLA Class I component | |||
| HLA-A (n = 24) | HLA-B/C (n = 24) | β-2-microglobulin (n = 24) | |
| Staining intensity | |||
| Negative | 7 | 4 | 0 |
| Weakly positive | 2 | 5 | 11 |
| Strongly positive | 15 | 15 | 13 |
| Staining pattern | |||
| Homogeneous negative | 7 | 4 | 0 |
| Heterogeneous (fewer than 60% of tumor cells stain positive) | 8 | 11 | 4 |
| Homogeneous positive | 9 | 9 | 20 |
Fig. 1.
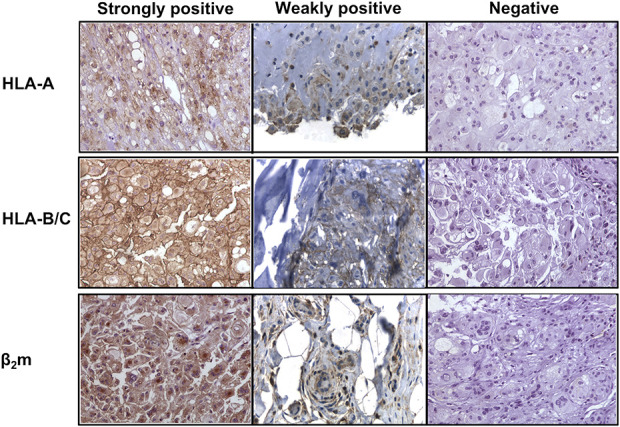
Human chordoma tissue sections were stained with monoclonal antibodies directed against the HLA class I antigen components: HLA-A heavy chain, HLA-B/C heavy chain, and β2m. These representative sections demonstrate staining intensity (strongly positive, weakly positive, and negative). Scoring was performed independently by at least two investigators and was confirmed independently by a senior bone and soft tissue pathologist; β2m = beta-2-microglobulin.
In 4 of 24 samples, we detected no staining by the HLA-B/C heavy chain-specific mAb HC-10. Nine samples were homogeneously stained, and 11 samples were heterogeneously stained. Five of the positive samples exhibited a weak staining intensity.
In all samples, we detected staining by the β-2-microglobulin-specific mAb NAMB-1. Specifically, four of 24 samples exhibited a heterogeneous staining pattern and 20 were homogeneously stained. Eleven samples exhibited a weak staining intensity.
Twenty-one of 24 tumors tested exhibited decreased expression of at least one of the HLA Class I antigen subunits. This included nine samples with no staining by at least one mAb specific to components of the HLA Class I antigen and an additional 12 samples with weak staining intensity or a heterogeneous staining pattern. No tumor samples were negative for all three HLA Class I antigen subunits (HLA-A, HLA-B/C, and β-2-microglobulin). Although both HLA-A and HLA-B/C mAbs ranged in staining from completely negative to strongly positive, the β-2-microglobulin mAb was positive in all samples, although with different intensities.
Lymphocyte Infiltration in Chordoma Tumors
Lymphocyte infiltration in chordoma tumors was present at a high frequency, primarily within the fibrous septae. Lymphocyte infiltration was detected in 21 of 24 tumors (Table 2). The lymphocyte infiltrates were distributed in the fibrous septae surrounding the tumor lobules, within the tumor lobules themselves, or both (Fig. 2). In one sample, lymphocytes were found in the tumor lobules only. In 17 samples, lymphocytes were found only within the fibrous septae between tumor lobules. Lymphocytes were found throughout the tumor specimens, in both the tumor lobules and fibrous septae, in three samples. In contrast, in three samples, lymphocytes were not detected in any of the slides analyzed for each sample.
Table 2.
Lymphocytic infiltration in chordoma
| Histologic pattern | Number of samples |
| Absent in tumor sample | 3 |
| In tumor lobule only | 1 |
| In fibrous septae only | 17 |
| Present in tumor lobule and fibrous septae | 3 |
Fig. 2.
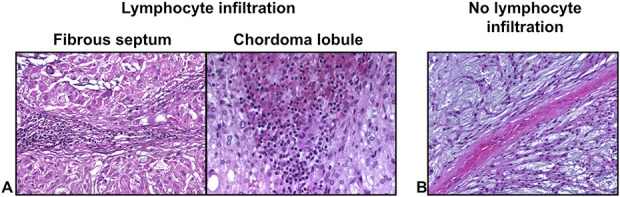
A-B Hematoxylin and eosin staining of human chordoma tissue depicts (A) the histologic distribution of lymphocytes within the tumor microenvironment compared with (B) human chordoma tissue without lymphocytic infiltration. The more common location for lymphocytic infiltration was in the fibrous septae.
All 21 tumors with lymphocytic infiltrates had CD4+ lymphocytes (Fig. 3). The number of CD4+ T cells per high-power field was not uniform in the chordoma tumors, ranging from one to 300 lymphocytes per high-power field. CD8+ T cells were present in 11 of 24 tumors. Similar to the CD4+ T cells, the number of CD8+ T cells was not uniform in the chordoma tumors, and ranged from one to 39 per high-power field.
Fig. 3.
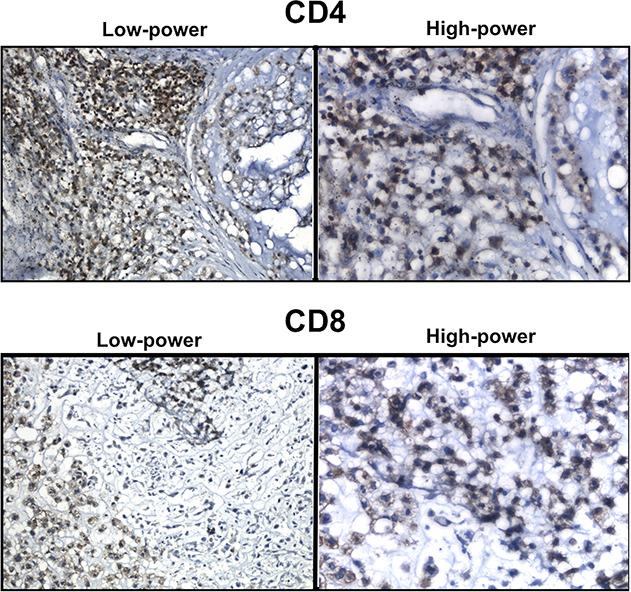
Both CD4+ and CD8+ T lymphocytes were noted in chordoma specimens. Low-power images were taken at 200x total magnification. High-power images were taken at 400x total magnification. Interpretation of immunohistochemical staining was confirmed by a senior bone and soft tissue pathologist.
Discussion
Chordoma is a solid tumor with limited treatment options. In the growing era of immunotherapy as a treatment for a variety of cancers, it remains to be seen whether immunotherapeutic strategies may have a role in the treatment of chordoma. Although many malignancies have previously been reported to have absent or decreased expression of HLA Class I antigen [8-15, 21-23, 41, 55], it was unknown whether the same holds true for chordoma. Additionally, even though lymphocytic infiltration has recently been reported in chordoma tumors [20, 66], their histologic distribution and subtype analysis remained unknown. In this study, we found a high frequency of defects in HLA Class I antigen expression in chordoma tumors. Additionally, we confirmed a high frequency of lymphocyte infiltration in chordoma, described their histologic pattern, and characterized their subtypes within chordoma tumors. Taken together, these findings suggest a mechanism of immune escape for chordoma tumors, potentially in part due to selective pressure imposed by the host immune response. This information will be critical when designing and implementing immunotherapeutic strategies for chordoma since a functional interaction between the host immune system and a patient’s tumor is requisite for these treatments to be effective.
Limitations
This study has several limitations. First, we had a limited sample size. Our group has extensive experience treating chordoma clinically, and we are a tertiary care referral center for the treatment of chordoma; however, despite this, chordoma is a rare malignancy. We were limited by tissue availability, which not only restricted our tumor sample size but also limited our ability to perform further analysis. For example, when characterizing the tumor-infiltrating lymphocytes, it would be interesting to evaluate whether regulatory T cells (Tregs) were present within chordoma tumors. Tregs are a subset of CD4+ T cells that are immunosuppressive and have been suggested to play a role in tumor cell escape from immunosurveillance. We theorize that tumor infiltration by Tregs could be a mechanism for poor prognosis in chordoma. Although we were limited by our sample size and ability to perform further subtype analysis of the lymphocytic infiltration in chordoma tumors, our study is the first to describe HLA Class I antigen defects in chordoma and to begin to characterize the tumor-infiltrating lymphocytes in terms of their histologic location and subtype. Another limitation of our study is the heterogeneity of prior treatments that the patients were exposed to before their chordoma tumor was resected at our institution. Even though this is to be expected when studying a rare disease, it precludes any substantive clinical correlations between our basic science findings presented here and the outcomes of the patients. However, our study furthers the current understanding of the interplay between chordoma tumors and the host immune response, which is crucial when designing and trialing immunotherapy for the treatment of chordoma.
HLA Class I Expression in Chordoma Tumors
The expression of HLA Class I subunits was defective in 21 of 24 chordoma tumors we studied. This frequency is consistent with that found in other malignancies that have been characterized for HLA Class I antigen expression, including the mechanisms underlying their defective expression and clinical importance [7, 51]. Interest in HLA Class I antigen expression by malignant cells is generated by the crucial role they play in the interactions of tumor cells with the host’s immune system. Specifically, they present tumor antigen–derived peptides to cognate cytotoxic T cells. As a result, HLA Class I antigens are involved in tumor immunosurveillance and respond to T cell–based immunotherapy. The high percentage of chordoma tumors with defects in HLA Class I antigen expression is particularly relevant in light of the current interest in using vaccines to induce T cell immunity [18] and immune checkpoint inhibitors [20, 43, 66] to treat chordoma. These immunotherapeutic strategies rely on a functional interaction between T cell receptors and HLA Class I antigens loaded with tumor antigen–derived peptides on tumor cells. Both strategies have had limited success. The results we present in this study suggest that the efficacy of both therapeutic strategies may be improved by approaches that correct defects in the expression of HLA Class I antigens, provided that they are generated by epigenetic mechanisms. In this regard, one might consider combining T cell–based immunotherapy with radiation therapy because the latter is part of a multidisciplinary therapeutic strategy used to treat chordoma. In previous preclinical studies, we showed that radiotherapy can enhance the expression of HLA Class I antigen processing machinery components in malignant cells [26, 27]. These phenotypic changes have functional relevance because they are associated with an increased susceptibility of tumor cells to recognition and destruction by cognate cytotoxic T cells. The total dose and fractionation schedule required to upregulate HLA Class I antigen processing machinery components in chordoma cells are likely to be different from those required to eradicate chordoma cells when used as a single agent or as an adjuvant to surgery.
Lymphocyte Infiltration in Chordoma Tumors
In agreement with other studies, we showed that lymphocytes are present in most chordoma tumors [20, 25, 65, 66]. In addition, we analyzed, for the first time that we know of, the distribution of lymphocytes within chordoma tumors. Histologically, chordomas are arranged in lobules that contain tumor cells. The lobules are separated by fibrous septae. These septae contain collagen, fibroblasts, and blood vessels. Tumor-infiltrating lymphocytes are found primarily in the fibrous septae. However, 17% of our samples had lymphocytes within the lobules surrounding tumor cells. The mechanism underlying this finding remains to be elucidated. Nevertheless, our findings suggest that most patients with chordomas mount a T cell immune response against their own tumors. The selective pressure applied by the host’s immune response on the chordoma tumor cells plays a role in the generation of tumors with HLA Class I antigen defects [15]. Specifically, selective pressure facilitates the overgrowth of chordoma cells that can escape the host’s immune response because of defects in the expression of HLA Class I antigens.
Several studies have demonstrated the presence of tumor-infiltrating lymphocytes in chordoma [20, 25, 65, 66], and two have shown that the tumor expression of programmed death-ligand 1 (PD-L1) correlated with increased tumor-infiltrating lymphocytes [20, 66]. Furthermore, the expression of PD-1 in tumor-infiltrating lymphocytes has been correlated with reduced survival [66]. These findings have led to interest in using immune checkpoint inhibitors in patients with metastatic or unresectable chordomas. However, one of the mechanisms by which previously responsive tumors develop resistance to checkpoint inhibitors is by losing HLA Class I antigen expression [28, 63]. In addition, chordoma cells have been shown to express PD-L1, yet PD-L1 expression has not been correlated with worse survival [20, 66]. This finding resembles what was found in intrahepatic cholangiocarcinoma, where PD-L1 expression and lack of HLA Class I antigen expression, when analyzed separately, did not correlate with a worse oncologic outcome [52]. However, when the expression of PD-L1 was analyzed in the context of loss of HLA Class I antigen expression, it portended a worse prognosis [52].
Furthermore, the efficacy of immune checkpoint inhibitors relies on “releasing the brakes” on normal tumor surveillance. The normal presentation of tumor antigens is not possible without fully functional HLA Class I antigen processing machinery. This was shown in esophageal carcinoma, where responses to anti-PD-1 monoclonal antibodies were documented only in cases in which HLA Class I antigens were highly expressed on malignant cells [34]. This finding suggests that the high frequency of defects of HLA Class I antigen expression in chordoma tumors provides malignant cells with an escape mechanism from the cognate cytotoxic T cells unleashed by checkpoint inhibitors. As a result, this therapy is expected to have a low efficacy unless HLA Class I antigen expression is restored on malignant cells.
Conclusion
Our findings provide a useful background for the rational design of combinatorial strategies that take advantage of the major progress made recently in the immunotherapy of solid tumors. These strategies may have an effect on the treatment of chordoma in combination with local strategies such as surgery and radiation therapy. By understanding that chordoma tumors have a high frequency of defects in HLA Class I antigen expression, we may be able to better understand why some chordoma tumors may or may not respond to certain types of immunotherapy. This in turn could also explain why some tumors display a more aggressive clinical phenotype than others. This information could potentially help prognosticate patient outcomes based on their tumor’s expression of HLA Class I antigen subunits. The high frequency of lymphocyte infiltration in chordoma tumors and lymphocyte subtype analysis we present suggests that the host is able to mount an immune response against the tumor. Further studies investigating the presence of Tregs in chordoma tumors could also point to mechanisms by which chordoma pathogenesis occurs. Future prospective translational studies that track patient outcomes and analyze their tumor specimens for HLA class I antigen expression and lymphocyte infiltration could broaden our understanding of the clinical consequences of immune escape mechanisms in chordoma. Additionally, information about a tumor’s expression of HLA Class I antigen could identify which patients would be ideal candidates for immunotherapy. Further investigation into the effects of adjuvant treatments, such as radiation, on upregulating expression of HLA Class I antigen in chordoma tumors would be relevant in further elucidating methods to prime these tumors for more successful attempts at immunotherapy.
Footnotes
Two of the authors certify that they (SF, JHS) have received research funding, during the study period, in an amount of USD 50,000 from the Chordoma Foundation, which supported this work.
Two of the authors certify that they (SF, JHS) have received research funding, during the study period, in an amount of USD 100,000 from the National Cancer Institute, grant R03 CA253319, which supported this work.
One of the authors certifies that he (VD) has received research funding, during the study period, in an amount of less than USD 10,000 from Agios Pharmaceuticals Inc; has received research funding, during the study period, in an amount of less than USD 10,000 from Advanced Cell Diagnostics Inc; has received payments or benefits, during the study period, in an amount of less than USD 10,000 from Incyte Corp; and has received payments of benefits, during the study period, in an amount of less than 10,000 from Viela Bio Inc, all outside of the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220-5227. [DOI] [PubMed] [Google Scholar]
- 2.Alexe G, Dalgin GS, Scanfeld D, et al. High expression of lymphocyte-associated genes in node-negative HER2+ breast cancers correlates with lower recurrence rates. Cancer Res. 2007;67:10669-10676. [DOI] [PubMed] [Google Scholar]
- 3.Arain A, Hornicek FJ, Schwab JH, Chebib I, Damron TA. Chordoma arising from benign multifocal notochordal tumors. Skeletal Radiol. 2017;46:1745-1752. [DOI] [PubMed] [Google Scholar]
- 4.Bordon Y. Immunotherapy: checkpoint parley. Nat Rev Cancer. 2015;15:3. [DOI] [PubMed] [Google Scholar]
- 5.Bordon Y. Tumour immunology: checkpoint parley. Nat Rev Immunol. 2015;15:5. [DOI] [PubMed] [Google Scholar]
- 6.Cai L, Michelakos T, Deshpande V, et al. Role of tumor-associated macrophages in the clinical course of pancreatic neuroendocrine tumors (PanNETs). Clin Cancer Res. 2019;25:2644-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai L, Michelakos T, Yamada T, et al. Defective HLA class I antigen processing machinery in cancer. Cancer Immunol Immunother. 2018;67:999-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(suppl 4):A40-45. [DOI] [PubMed] [Google Scholar]
- 9.Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res. 2010;16:11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campoli M, Ferrone S. Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72:321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campoli M, Ferrone S. HLA antigen and NK cell activating ligand expression in malignant cells: a story of loss or acquisition. Semin Immunopathol. 2011;33:321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campoli M, Ferrone S, Zea AH, Rodriguez PC, Ochoa AC. Mechanisms of tumor evasion. Cancer Treat Res. 2005;123:61-88. [DOI] [PubMed] [Google Scholar]
- 14.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189-234. [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark WH, Jr, Elder DE, Guerry D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893-1904. [DOI] [PubMed] [Google Scholar]
- 17.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303-1310. [DOI] [PubMed] [Google Scholar]
- 18.ClinicalTrials.gov. QUILT-3.011 Phase 2 Yeast-Brachyury Vaccine Chordoma. Available at: https://clinicaltrials.gov/ct2/show/NCT02383498. Accessed 27 Mar 2020.
- 19.Dieu-Nosjean MC, Antoine M, Danel C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410-4417. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Shen J, Gao Y, et al. Expression of programmed cell death ligand 1 (PD-L1) and prevalence of tumor-infiltrating lymphocytes (TILs) in chordoma. Oncotarget. 2015;6:11139-11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390-4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12:3890-3895. [DOI] [PubMed] [Google Scholar]
- 23.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755-774. [DOI] [PubMed] [Google Scholar]
- 24.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717-734. [DOI] [PubMed] [Google Scholar]
- 25.Fujii R, Friedman ER, Richards J, et al. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7:33498-33511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gameiro SR, Malamas AS, Bernstein MB, et al. Tumor cells surviving exposure to proton or photon radiation share a common immunogenic modulation signature, rendering them more sensitive to T cell-mediated killing. Int J Radiat Oncol Biol Phys. 2016;95:120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gettinger S, Choi J, Hastings K, et al. Impaired HLA class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov. 2017;7:1420-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res. 2006;12:5423-5434. [DOI] [PubMed] [Google Scholar]
- 31.Imai R, Kamada T, Araki N, Working Group for Bone and, Soft Tissue Sarcomas. Carbon ion radiation therapy for unresectable sacral chordoma: an analysis of 188 Cases. Int J Radiat Oncol Biol Phys. 2016;95:322-327. [DOI] [PubMed] [Google Scholar]
- 32.Ino Y Yamazaki-Itoh R Shimada K, et al. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito N, Suzuki Y, Taniguchi Y, Ishiguro K, Nakamura H, Ohgi S. Prognostic significance of T helper 1 and 2 and T cytotoxic 1 and 2 cells in patients with non-small cell lung cancer. Anticancer Res. 2005;25:2027-2031. [PubMed] [Google Scholar]
- 34.Ito S, Okano S, Morita M, et al. Expression of PD-L1 and HLA class I in esophageal squamous cell carcinoma: prognostic factors for patient outcome. Ann Surg Oncol. 2016;23:508-515. [DOI] [PubMed] [Google Scholar]
- 35.Kabolizadeh P, Chen YL, Liebsch N, et al. Updated outcome and analysis of tumor response in mobile spine and sacral chordoma treated with definitive high-dose photon/proton radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97:254-262. [DOI] [PubMed] [Google Scholar]
- 36.Kageshita T, Wang Z, Calorini L, et al. Selective loss of human leukocyte class I allospecificities and staining of melanoma cells by monoclonal antibodies recognizing monomorphic determinants of class I human leukocyte antigens. Cancer Res. 1993;53:3349-3354. [PubMed] [Google Scholar]
- 37.Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008;113:1387-1395. [DOI] [PubMed] [Google Scholar]
- 38.Lipowska-Bhalla G, Gilham DE, Hawkins RE, Rothwell DG. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunol Immunother. 2012;61:953-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mackensen A, Ferradini L, Carcelain G, et al. Evidence for in situ amplification of cytotoxic T-lymphocytes with antitumor activity in a human regressive melanoma. Cancer Res. 1993;53:3569-3573. [PubMed] [Google Scholar]
- 40.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29:1949-1955. [DOI] [PubMed] [Google Scholar]
- 41.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181-273. [DOI] [PubMed] [Google Scholar]
- 42.Marrogi AJ, Munshi A, Merogi AJ, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-beta as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492-501. [DOI] [PubMed] [Google Scholar]
- 43.Mathios D, Ruzevick J, Jackson CM, et al. PD-1, PD-L1, PD-L2 expression in the chordoma microenvironment. J Neurooncol. 2015;121:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menegaz RA, Michelin MA, Etchebehere RM, Fernandes PC, Murta EF. Peri- and intratumoral T and B lymphocytic infiltration in breast cancer. Eur J Gynaecol Oncol. 2008;29:321-326. [PubMed] [Google Scholar]
- 45.Michelakos T, Cai L, Villani V, et al. Tumor microenvironment immune response in pancreatic ductal adenocarcinoma patients treated with neoadjuvant therapy. J Natl Cancer Inst. 2020. Published online June 4, 2020. DOI: 10.1093/jnci/djaa073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nishida Y, Kamada T, Imai R, et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys. 2011;79:110-116. [DOI] [PubMed] [Google Scholar]
- 47.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385-393. [DOI] [PubMed] [Google Scholar]
- 48.Palthe O, Tromp I, Ferreira A, et al. Sacral chordoma: a clinical review of 101 cases with 30-year experience in a single institution. Spine J. 2019;19:869-879. [DOI] [PubMed] [Google Scholar]
- 49.Pellegrino MA, Ng AK, Russo C, Ferrone S. Heterogeneous distribution of the determinants defined by monoclonal antibodies on HLA-A and B antigens bearing molecules. Transplantation. 1982;34:18-23. [DOI] [PubMed] [Google Scholar]
- 50.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918-1926. [DOI] [PubMed] [Google Scholar]
- 51.Sabbatino F, Schwab JH, Ferrone S, Ferrone CR. Evolution of studies of HLA class I antigen processing machinery (APM) components in malignant cells. Clin Transpl. 2013:453-463. [PubMed] [Google Scholar]
- 52.Sabbatino F, Villani V, Yearley JH, et al. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22:470-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538-18543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwab JH, Janssen SJ, Paulino Pereira NR, et al. Quality of life after resection of a chordoma of the mobile spine. Bone Joint J. 2017;99:979-986. [DOI] [PubMed] [Google Scholar]
- 55.Seliger B, Dressler SP, Massa C, et al. Identification and characterization of human leukocyte antigen class I ligands in renal cell carcinoma cells. Proteomics. 2011;11:2528-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sernee MF, Ploegh HL, Schust DJ. Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol. 1998;35:177-188. [DOI] [PubMed] [Google Scholar]
- 57.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299-2306. [PubMed] [Google Scholar]
- 58.Sun X, Hornicek F, Schwab JH. Chordoma: an update on the pathophysiology and molecular mechanisms. Curr Rev Musculoskelet Med. 2015;8:344-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tefany FJ, Barnetson RS, Halliday GM, McCarthy SW, McCarthy WH. Immunocytochemical analysis of the cellular infiltrate in primary regressing and non-regressing malignant melanoma. J Invest Dermatol. 1991;97:197-202. [DOI] [PubMed] [Google Scholar]
- 60.van Wulfften Palthe OD, Houdek MT, Rose PS, et al. How does the level of nerve root resection in en bloc sacrectomy influence patient-reported outcomes? Clin Orthop Relat Res. 2017;475:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Campoli M, Cho HS, et al. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods. 2005;299:139-151. [DOI] [PubMed] [Google Scholar]
- 62.Wang Z, Marincola FM, Rivoltini L, Parmiani G, Ferrone S. Selective histocompatibility leukocyte antigen (HLA)-A2 loss caused by aberrant pre-mRNA splicing in 624MEL28 melanoma cells. J Exp Med. 1999;190:205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375:819-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203-213. [DOI] [PubMed] [Google Scholar]
- 65.Zou MX, Guo KM, Lv GH, et al. Clinicopathologic implications of CD8(+)/Foxp3(+) ratio and miR-574-3p/PD-L1 axis in spinal chordoma patients. Cancer Immunol Immunother. 2018;67:209-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zou MX, Peng AB, Lv GH, et al. Expression of programmed death-1 ligand (PD-L1) in tumor-infiltrating lymphocytes is associated with favorable spinal chordoma prognosis. Am J Transl Res. 2016;8:3274-3287. [PMC free article] [PubMed] [Google Scholar]


