Abstract
The coexistence of hemicrania‐continua and trigeminal neuralgia is called HC‐tic syndrome. We describe a case of an elderly man who suffered both types of headache related to fungal sphenoiditis. This is the first case to be reported, to the best of our knowledge.
Keywords: fungal sphenoiditis, hemicrania continua, hemicrania continua‐tic syndrome, trigeminal neuralgia
The coexistence of hemicrania‐continua and trigeminal neuralgia is called HC‐tic syndrome. We describe a case of an elderly man who suffered both types of headache related to fungal sphenoiditis. This is the first case to be reported, to the best of our knowledge.

1. INTRODUCTION
Hemicrania continua (HC)‐tic syndrome is a rare disorder characterized by the overlap of HC and trigeminal neuralgia (TN) or TN‐like paroxysms. We describe a case of secondary HC‐tic syndrome related to fungal sphenoiditis. Less common causes including sphenoiditis should be considered when headache presents with a refractory and complicated form.
Trigeminal neuralgia (TN) or TN‐like paroxysms can coexist with various forms of trigeminal autonomic cephalalgia (TAC), such as cluster headache, hemicrania continua (HC), and paroxysmal hemicrania. TN can coexist with both primary headache disorders and secondary TAC, and the initial onset of TAC and TN may be simultaneous or separated by months or years. 1 The co‐occurrence of TN and HC is a rare condition known as HC‐tic syndrome, 2 and HC and TN may occur concurrently or at different times. Several reports have described the association of TN or HC with intracranial lesions, 3 , 4 but no previous report has described such an association in a case of HC‐tic syndrome. In this report, we describe a case of secondary HC‐tic syndrome related to fungal sphenoiditis.
2. CASE REPORT
A 71‐year‐old man presented with a 7‐month history of right‐sided head pain and was referred to our department. He described his unilateral headache as very severe and sudden in onset. He reported experiencing episodes of electric‐like and stabbing pain that each lasted for only a few seconds and affected the right orbital, retro‐orbital, and maxillary areas. The pain was usually precipitated by tooth‐brushing or the chewing or eating of food. He experienced such headaches one to five times per day. He did not report any other accompanying symptoms, such as fever, mucopurulent discharges, nasal stuffiness, or cranial autonomic symptoms.
He had been tentatively diagnosed with TN at another center, and his symptoms had improved considerably after he started taking 200‐mg doses of carbamazepine thrice daily. Three months later, he developed another type of right‐sided headache affecting the retro‐orbital region. He experienced continuous pain of mild‐to‐moderate intensity with some episodes of exacerbation. Most such episodes of exacerbation had durations of 5 minutes‐4 hours, and the longer episodes were accompanied by ipsilateral forehead and facial sweating, lacrimation, rhinorrhea, right‐sided conjunctival injection, and eyelid edema but not ptosis. The patient felt agitated and could not fall asleep. The previously prescribed carbamazepine provided no relief. Various combinations of drugs, including gabapentin, lamotrigine, and somedon, also provided no benefit.
Physical and neurological examinations conducted at our department revealed no abnormalities. He had no sensory losses in the trigeminal nerve region or local tenderness over his sinuses. Routine biochemical and hematological tests returned unremarkable results. The patient did not report any history of headache, facial pain, vomiting, nausea, head injury, or nasal discharge prior to the onset of head pain 7 months earlier.
We considered HC to be a probable diagnosis, and we prescribed indomethacin at an initial dose of 25 mg twice daily. Our patient's headache completely resolved within 12 hours, but it returned after only a week. We changed the indomethacin dose to 25 mg thrice daily, but this did not ameliorate our patient's headache.
A computed tomography (CT) scan revealed a hypodense shadow in the right ethmoidal and sphenoid sinuses but no marked contrast enhancement (Figure 1A,B). A subsequent nasal endoscopic examination revealed some drainage of brown mucous from the right sphenoethmoidal recesses. These signs suggested that the patient's pain could be associated with sphenoiditis. We therefore referred him to our otorhinolaryngology department, where he underwent transnasal sphenoidotomy under general anesthesia. Pathological analysis of the extracted tissues confirmed the presence of fungal sphenoid sinusitis (Figure 2). Over 6 years of postoperative follow‐up, the patient has remained fully pain‐free without additional treatment, and a postoperative follow‐up CT scan revealed no abnormalities (Figure 3).
FIGURE 1.
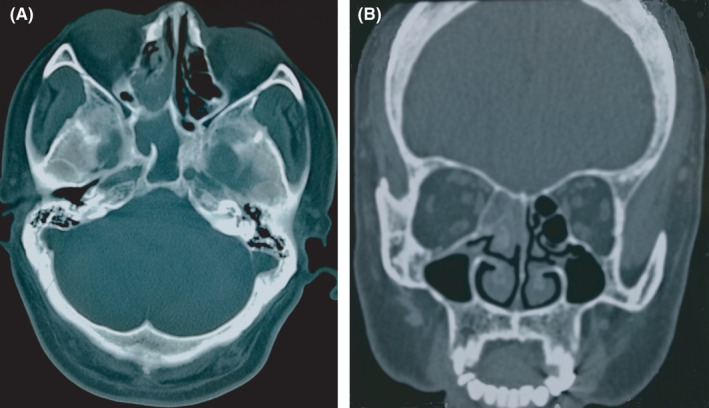
Computed tomography findings. A, A hypodense shadow in the right ethmoidal and sphenoid sinuses. B, No marked contrast enhancement in the coronal plane
FIGURE 2.
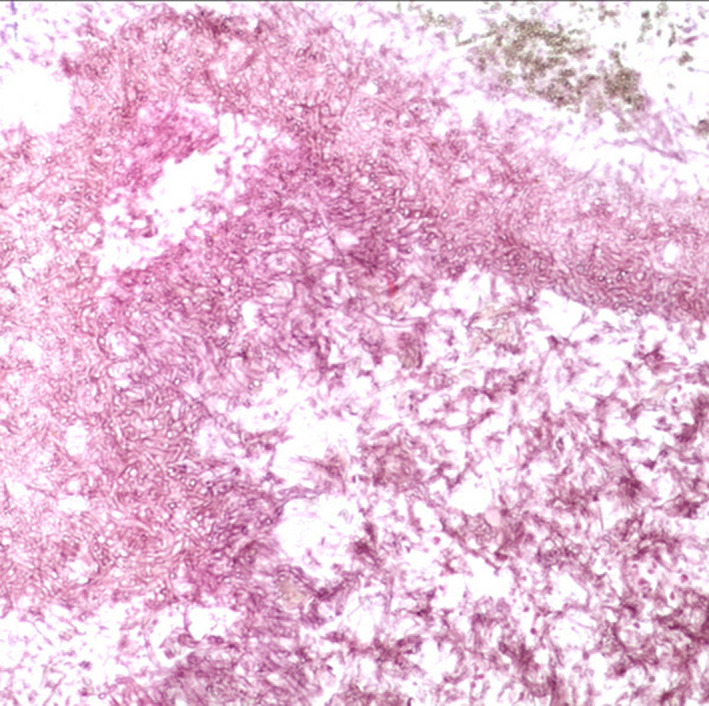
Histopathological examination results. Periodic acid‐Schiff staining showing fungal hyphae (×100 magnification)
FIGURE 3.
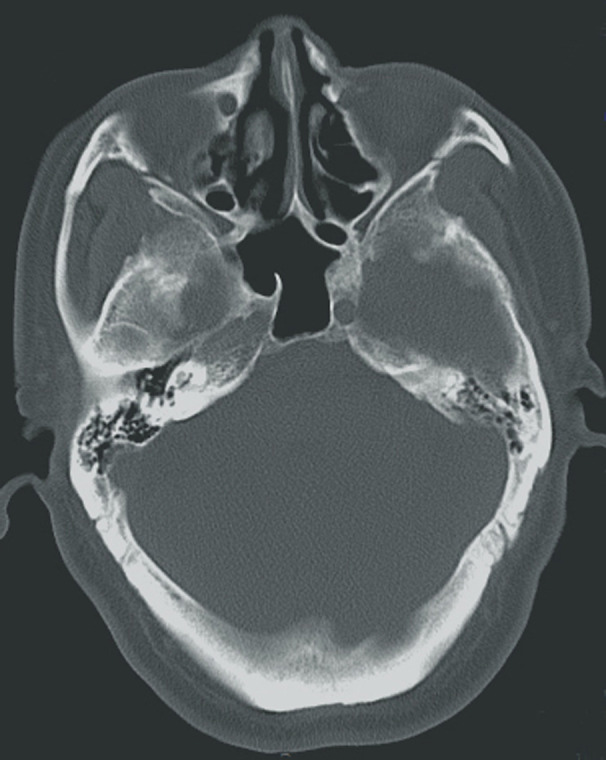
Follow‐up computed tomography scan performed after surgery
3. DISCUSSION
Our patient experienced two different types of headache: TN and HC‐like pain. He described his initial pain syndrome as involving severe and sudden episodes of electric‐like and stabbing pain, usually triggered by eating or chewing of food, in the areas innervated by the first and second branches of the right trigeminal nerve. He later developed another form of headache that presented as continuous background pain with episodes of exacerbation and ipsilateral autonomic symptoms. These clinical features met the International Headache Association's diagnostic criteria for both TN and HC apart from responding absolutely to therapeutic doses of indomethacin, 5 and his clinical symptoms were similar to those described in patients with HC‐tic syndrome.
Prakash et al 2 described two cases of primary HC‐tic syndrome. In case 1, the patient first developed TN and then developed HC 5 years later. In case 2, the patient developed TN and HC at the same time. Both patients developed TN and HC on the same side of the body, and both responded well to treatment with indomethacin and carbamazepine. In our patient's case, carbamazopine and indomethacin provided temporary relief from TN and HC‐like pain.
We believe that sphenoiditis caused our patient to experience HC‐tic syndrome, but it is difficult to definitively establish a causal relationship. The existing literature contains only one reported case of HC related to sphenoid sinusitis 6 and five cases of TN associated with paranasal sinusitis. 7 , 8 , 9 In all of these cases, successful treatment of the sinusitis resulted in complete resolution of neuralgia symptoms. In our patient's case, we considered HC‐tic syndrome reasonably attributable to sphenoid sinusitis for the following reasons: (1) He had no history of headache before the onset of TN, (2) both TN and HC occurred on the side of the body ipsilateral to the sphenoid sinusitis location, and (3) his headache symptoms completely disappeared immediately after surgery for sinusitis and never returned over the subsequent 6 years.
The exact pathophysiology of HC‐tic syndrome remains unclear. Considering the close anatomical relationship between the sphenoidal sinus and the V1 and V2 divisions of the trigeminal nerve, we speculate that inflammation of the sphenoidal sinus can spread to the area of the V1 and V2 trigeminal nerve branches. Such spreading inflammation would activate the trigeminal nerve and trigeminal‐autonomic reflexes, ultimately leading to the development of neuralgia symptoms.
Our patient experienced a delay of more than 7 months between the date of symptom onset and the date of receiving a diagnosis of secondary HC‐tic syndrome. There were several reasons for this delay, but a major reason is that headache was the sole initial symptom. He also did not experience typical sinonasal symptoms such as nasal discharges, congestion, or fever.
Several additional points relevant to secondary HC‐tic syndrome should be noted. First, a nasoendoscopy examination is necessary to confirm a causal relationship between sinusitis and headache, especially in the cases of patients without sinonasal symptoms but with imaging evidence of paranasal sinus disease. 10 Second, the efficacy of indomethacin at relieving headache symptoms does not provide a clear indication as to whether HC is primary or secondary. Of 26 patients with secondary HC mentioned in a literature review, 11 two were partially responsive to indomethacin, and four others experienced temporary relief of symptoms as in our patient's case. A final point worth noting is that neuroimaging should be recommended for elderly and immunocompromised patients with HC‐like pain or TN‐like pain who respond poorly to standard treatments.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
CW and DW: drafted the manuscript. XZ and YY: contributed to subsequent revisions of the paper. All authors read and approved the manuscript.
ETHICAL APPROVAL
The consent has been obtained from patient for publication of this manuscript.
Wang C, Zhang X, Yang Y, Wan D. Secondary hemicrania continua‐tic syndrome associated with fungal sphenoiditis: A case report. Clin Case Rep. 2021;9:e04297. 10.1002/ccr3.4297
DATA AVAILABILITY STATEMENT
Information related to this article is available from the corresponding author upon reasonable request.
REFERENCES
- 1. Wöber C. Tics in TACs: A step into an avalanche? Systematic literature review and conclusions. Headache. 2017;57(10):1635‐1647. [DOI] [PubMed] [Google Scholar]
- 2. Prakash S, Rathore C. Two cases of hemicrania continua‐trigeminal neuralgia syndrome: expanding the spectrum of trigeminal autonomic cephalalgia‐tic (TAC‐TIC) syndrome. Headache. 2017;57(3):472‐477. [DOI] [PubMed] [Google Scholar]
- 3. Prakash S, Patel P. Hemicrania continua: clinical review, diagnosis and management. J Pain Res. 2017;10:1493‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maarbjerg S, Di Stefano G, Bendtsen L, et al. Trigeminal neuralgia ‐ diagnosis and treatment. Cephalalgia. 2017;37(7):648‐657. [DOI] [PubMed] [Google Scholar]
- 5. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1‐211. [DOI] [PubMed] [Google Scholar]
- 6. Meckling SK, Becker WJ. Sphenoid sinusitis presenting as indomethacin responsive “hemicrania continua”: a case report. Cephalalgia. 1997;17:303. [Google Scholar]
- 7. Chun MK, Eom TH, Lim GY, et al. Secondary trigeminal neuralgia attributed to paranasal sinusitis in a pediatric patient. Childs Nerv Syst. 2017;33(3):397‐398. [DOI] [PubMed] [Google Scholar]
- 8. Maschio M, Mengarelli A, Girmenia C, et al. Trigeminal neuralgia as unusual isolated symptom of fungal paranasal sinusitis in patients with haematological malignancies. Neurol Sci. 2012;33(3):647‐652. [DOI] [PubMed] [Google Scholar]
- 9. Sawaya RA. Trigeminal neuralgia associated with sinusitis. ORL J Otorhinolaryngol Relat Spec. 2000;62(3):160‐163. [DOI] [PubMed] [Google Scholar]
- 10. Jones NS. Sinus headaches: avoiding over‐ and mis‐diagnosis. Expert Rev Neurother. 2009;9(4):439‐444. [DOI] [PubMed] [Google Scholar]
- 11. Prakash S, Shah ND, Soni RK. Secondary hemicrania continua: case reports and a literature review. J Neurol Sci. 2009;280(1–2):29‐34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Information related to this article is available from the corresponding author upon reasonable request.


