Abstract
To analyze the clinical, serological, and imaging characteristics of patients with interstitial lung diseases (ILD) positive to different anti-aminoacyl-tRNA synthetase (anti-ARS) antibodies.
The clinical data, serological indexes, pulmonary high-resolution computed tomography (HRCT) imaging features and pulmonary functions, and bronchoalveolar lavage fluid of 84 ILD patients with anti-ARS antibody positive in Beijing Chao-yang Hospital, Capital Medical University were reviewed.
(1) Anti-ARS antibodies included anti-Jo-1 (42.86%), anti-PL-7 (26.19%), anti-PL-12 (10.71%), anti-EJ (14.29%), and anti-OJ (5.95%). (2) Nonspecific interstitial pneumonia was the main type of patients with ILD positive to antibodies of anti-Jo-1, anti-PL-7, and anti-EJ, organizing pneumonia was the main type of patients with ILD positive to anti-PL-12 antibody and usual interstitial pneumonia was the main type of patients with ILD positive to anti-OJ antibody. (3) Only 14.29% of the patients had typical “triad syndrome” (interstitial pneumonia, myositis, and non-erosive arthritis). Myositis mainly occurred in patients with ILD positive to antibodies of anti-PL-7, anti-Jo-1, and anti-EJ. The incidence of arthritis in ILD patients with anti-Jo-1 was higher than that in ILD patients with anti-PL-12 and anti-EJ (P < .05). The incidence of mechanic's hand in ILD patients with anti-Jo-1 was higher than that in ILD patients with anti-PL-12 (P < .05).
ILD positive to anti-Jo-1 antibody is associated with multiple organ involvement, mainly manifested as myositis, mechanic's hand, and arthritis. As other clinical manifestations of some ILD patients are relatively hidden, ILD patients should pay attention to the screening of the anti-ARS antibodies and guard against anti-synthetase syndrome.
Keywords: anti-aminoacyl-tRNA synthetase antibodies, interstitial pulmonary disease
1. Introduction
Interstitial lung disease (ILD) is a diffuse lung disease with varying degrees of inflammation and fibrosis in the interstitial lung region.[1] The most common cause of the disease is connective tissue disease (CTD).[2] However, a large number of ILD patients have clinical features that indicate potential autoimmune processes, but still do not meet the clear diagnostic criteria of CTD. This subset of ILD has been classified as undifferentiated CTD-associated ILD,[3] autoimmune-featured ILD,[4] and interstitial pneumonia with autoimmune features (IPAF).[5]
Although anti-synthetase syndrome (ASS) is a rare autoimmune disease, in recent years, ASS has been recognized as an important cause of ILD. ILD can seriously affect the prognosis of the disease, therefore early diagnosis and early treatment are of great significance. Anti-synthetase antibodies are specific antibodies for ASS. The clinical characteristics and prognosis of ILD patients with different anti-synthetase antibodies may be different. Therefore, it is of great significance to summarize the clinical characteristics of ILD patients with different anti-synthetase antibodies.
Anti-ARS antibodies are the most common myositis-specific antibody detected in ASS. Eleven anti-ARS antibodies have been identified so far: anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, anti-OJ, anti-KS, anti-Zo, anti-Ha, anti-JS, anti-SC, and anti-YRS.[6] ASS is a clinical syndrome characterized by “ILD, myositis, arthritis, Raynaud phenomenon, and mechanic's hand.”[7] Although patients positive to different types of anti-ARS antibodies show some unique clinical features and outcomes,[8] ILD is still the most common extramuscular manifestation in ASS, with a prevalence rate ranging from 67% to 100%.[9] CTD-ILD and IPAF[10] have been reported so far, but the clinical characteristic antibody of anti-ARS antibody positive ILD is still unclear. The aim of this study was to clarify the clinical characteristics of ILD patients with anti-ARS antibody positive and to explain the correlation between clinical, laboratory, and radiological to the serology subtypes, so as to deepen physician's understanding of these patients. Early diagnosis and preemptive treatment could be made to achieve greater clinical benefits.
2. Material and methods
2.1. Study design
This article is a retrospective study.
2.2. Study population
All patients voluntarily participated in this study, and agreed to the authors to use their clinical data, and agreed to the publication of this article, and signed relevant informed consent. This study was approved by the ethics committee of Beijing Chaoyang Hospital, and the ethical batch number was 2020-3-17-69.
2.3. Inclusion and exclusion criteria
This study collected 84 ILD patients who had anti-ARS antibody positive results admitted to the department of rheumatology of Beijing Chao-yang Hospital, Capital Medical University from January 2017 to June 2019. The patients would be excluded who had contained the following: (1) Caused by drugs. (2) Occupational exposure. (3) Overlap Syndrome. (4) ILD patients had been treated with steroid or immunosuppressants before enrollment. The diagnosis of ILD was based on the 2013 American Thoracic Society/European Respiratory Society (ATS/ERS) criteria.[11] The diagnosis of ASS was based on the diagnostic criteria published by Solomon et al[14] in 2011: patients with positive anti-ARS antibody combined with 2 primary criteria or 1 primary criterion plus 2 secondary criteria could be diagnosed ASS. Primary criteria: (1) ILD (excluding other reasons) and (2) PM/DM. Secondary criteria: arthritis, Raynaud phenomenon, and mechanic's hands. Diagnosis of myositis must have a polymyositis diagnosis that meets the Bohan/Peter recommendations in 1975. (1) Symmetric proximal muscle weakness. (2) Increased serum muscle enzymes. (3) Electromyography suggests myogenic damage. (4) Muscle biopsy supports the diagnosis of inflammatory myopathy. Meet any 3 of the 4 could diagnose myositis. The diagnosis of arthritis requires joint pain and swelling diagnosed by a rheumatologist, most of which were non-erosive arthritis.
2.4. Collection of clinical data and serological indexes
The age, sex, clinical manifestations, biochemical tests, immunological tests, pulmonary functions, imaging, and bronchoscopy results of patients were collected.
2.5. Detection of subtypes of anti-ARS antibodies
EUROLINE method was adopted for detecting the anti-ARS antibodies. The results determination was as follows: positive (++) and strong positive (+++) were determined as positive, negative (−), suspicious (±), and weak positive (+) were determined as negative.
2.6. Imaging features
Two senior imaging physicians and 1 senior rheumatologist carried out the film reading jointly. The main evaluation contents included: (1) Signs of ILD: chest diseases were evaluated according to diagnostic criteria of Fleischner Society.[12] The main evaluation signs included honeycombing opacity, reticular opacities, ground-glass opacities (GGO), consolidation, and traction bronchiectasis. Other signs included nodules, pleural effusion, and pericardial effusion. (2) Patterns of ILD: The diagnostic criteria of ATS/ERS for idiopathic ILD were applied to evaluate the types of ILD that best matched with the pulmonary lesions,[11] including usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), nonspecific interstitial pneumonia-organizing pneumonia (NSIP-OP), and unclassifiable type ILD. The diagnostic criteria of UIP were honeycombing opacity mainly distributed under the pleura of bilateral lower lungs, with or without grid shadow and traction bronchiectasis. The diagnostic criteria of NSIP were that the lesion was mainly GGO with basal distribution, with or without reticular opacities and/or traction bronchiectasis, without or with mild honeycombing opacity. The diagnostic criteria of OP were peripheral, peribronchovascular bundle, or lamellar solid shadows with air bronchogram and peripheral GGO. NSIP-OP was defined as a solid shadow on the background of large flaky GGO, with or without grid shadow or traction bronchiectasis. If the CT signs did not conform to the above 4 types of ILD, the diagnosis was uncertain.
2.7. Statistical method
SPSS 17.0 software was adopted for the statistical analysis. Normal distribution or approximate normal distribution data were expressed by .
Non-normal distribution data were represented by the median (M). Rank sum test was used for comparison among groups, chi-square test and Fisher exact probability method were adopted for comparison between 2 groups. The difference had statistical significance when P < 0.05.
3. Results
3.1. Clinical data analysis
Among 84 patients with anti-ARS antibodies positive ILD, 18 (21.43%) were male and 66 (78.57%) were female. The average age was 55 ± 9.69 years. There were 36 (42.86%) cases in anti-Jo-1 antibody positive group, 22 (26.19%) cases in anti-PL-7 antibody positive group, 9 (10.71%) cases in anti-PL-12 antibody positive group, 12 (14.29%) cases in anti-EJ antibody positive group, and 5 (5.95%) cases in anti-OJ antibody positive group. The anti-Jo-1, anti-PL-7, and anti-EJ antibody positive groups were mainly female patients, while the anti-PL-12 and anti-OJ antibody positive groups were mainly male patients. Compared with the anti-Jo-1, anti-PL-7, and anti-EJ antibody positive groups, the male incidence of the anti-OJ antibody positive group was predominant (χ2 = 15.391, P = 0.000; Fisher exact test, P = 0.001; Fisher exact test, P = 0.009).
Among 84 patients with anti-ARS antibody positive ILD, mechanic's hand (the skin on the palm and side of the finger is hyperkeratized, cracked, and rough, which is similar to a skilled hand who has been engaged in manual work for a long time, hence the name “mechanic's hand”) (32 cases, 38.1%) and fever (32 cases, 38.1%) were the most commonly combined clinical manifestations, followed by arthritis (29 cases, 34.5%), myositis (21 cases, 25.0%), and Raynaud phenomenon (19 cases, 22.6%), among which only 14.29% of the patients had typical “triad syndrome” (interstitial pneumonia, myositis, and non-erosive arthritis). The incidences of myasthenia (10/36; 7/22; 2/12) and myalgia (8/36; 8/22; 1/12) in ILD patients with positive anti-Jo-1, anti-PL-7, and anti-EJ antibodies were higher than those in ILD patients with positive anti-PL-12 and anti-OJ antibodies (myasthenia: 0/9; 0/5) (myalgia 0/9; 0/5). The incidence of myositis in ILD patients with positive anti-PL-7 antibody was 40.91% (9/22), which was significantly higher than that in ILD patients with positive anti-PL-12 antibody (0%, 0/9) (P < 0.05). The incidence of arthritis in ILD patients with anti-Jo-1 and anti-PL-7 was 47.22% and 50.0%, respectively, which was higher than that in ILD patients with anti-EJ (0%, 0/12), (χ2 = 6.831, P = 0.009; Fisher exact test, P < .05). The incidence of mechanic's hand in ILD patients with positive anti-Jo-1 antibody was higher than that in ILD patients with positive anti-PL-12 antibody (χ2 = 6.891, P < 0.05). There was no significant difference in incidences of Raynaud phenomenon and fever among groups.
In terms of physical signs: among all ARS-ILD patients, 30 cases (35.7%) were complicated with Gottron sign (macular papules involving the elbow, knee, and ankle joints may be accompanied by scaly, skin atrophy, and hypopigmentation), 18 cases (21.43%) were complicated with Gottron papules (macular papules located on the extension of the metacarpophalangeal joints and proximal interphalangeal joints may be accompanied by scaly, skin atrophy, and hypopigmentation), and only 1 case (1.19%) was complicated with shawl sign (edema purple-red rash on the back of the shoulder, aggravated by light). There was no significant difference in the incidences of Gottron papules among groups. The incidence of Gottron sign in ILD patients with positive anti-EJ antibody was higher than that in ILD patients with positive anti-Jo-1 and anti-OJ antibody (χ2 = 4.114, P < .05; Fisher exact test, P < .05) (Table 1).
Table 1.
Comparison of clinical features.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | Overall | |
| n = 36 | n = 22 | n = 9 | n = 12 | n = 5 | ||
| Age median | 57.0 | 55.5 | 60.0 | 55.5 | 55.0 | |
| Age (IQR) | (64.75–50.50) | (64.25–47.00) | (68.00–48.00) | (60.00–53.50) | (69.00–53.50) | |
| Number of males/number of females | 4/32 | 3/19 | 5/4 | 3/9 | 5/0 | |
| Myositis | 10 | 9 | 0 | 2 | 0 | 21 |
| Arthritis | 17 | 11 | 1 | 0 | 0 | 29 |
| Mechanic's hand | 20 | 9 | 0 | 3 | 0 | 32 |
| Raynaud phenomenon | 8 | 6 | 2 | 2 | 1 | 19 |
| Fever | 10 | 11 | 2 | 6 | 3 | 32 |
| Heliotrope rash | 1 | 6 | 1 | 3 | 0 | 11 |
| V sign | 0 | 1 | 0 | 2 | 0 | 3 |
| Shawl sign | 0 | 0 | 0 | 1 | 0 | 1 |
| Gottron papules | 7 | 7 | 3 | 1 | 0 | 18 |
| Gottron sign | 12 | 7 | 3 | 8 | 0 | 30 |
Gottron papules = macular papules located on the extension of the metacarpophalangeal joints and proximal interphalangeal joints may be accompanied by scaly, skin atrophy, and hypopigmentation, Gottron sign = macular papules involving the elbow, knee, and ankle joints may be accompanied by scaly, skin atrophy, and hypopigmentation, heliotrope rash = an edematous purplish-red rash on the upper eyelid or periorbital area, which can be on 1 or both sides, and is aggravated by light, IQR = inter quartile range, mechanic hand = the skin on the palm and side of the finger is hyperkeratized, cracked, and rough, which is similar to a skilled hand who has been engaged in manual work for a long time, Shawl sign = edema purple-red rash on the back of the shoulder, aggravated by light, V sign = an edematous purplish-red rash in the V-shaped area of the front chest, which is aggravated by light.
3.2. Serological index analysis
Although creatine kinase, serum albumin, and C-reactive protein were not significantly different among groups, alanine aminotransferase, aspartate aminotransferase, and lactate dehydrogenase (LDH) of ILD patients with positive anti-PL-7 antibody were higher than those of ILD patients with positive anti-OJ antibody (P < 0.05). LDH of ILD patients with positive anti-OJ antibody was the lowest in all groups (P < .05) (Table 2).
Table 2.
Comparison of laboratory data.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | P | |
| n = 36 | n = 22 | n = 9 | n = 12 | n = 5 | ||
| ALB g/l M (IQR) | 35.8 (38.45–33.08) | 35.1 (39.05–32.95) | 39.3 (41.30–28.15) | 35.7 (39.63–32.05) | 37.6 (40.10–35.80) | .781 |
| ALT u/l M (IQR) | 20.5 (37.25–14.25) | 27.0 (88.75–17.75) | 21.0 (33.50–10.00) | 7.5 (21.50–14.25) | 14.0 (17.50–10.00) | .037 |
| AST u/l M (IQR) | 23.0 (34.75–20.50) | 34.5 (90.00–20.50) | 20.0 (35.50–15.50) | 22.0 (30.50–17.25) | 15.0 (22.50–14.00) | .041 |
| CK u/l M (IQR) | 103.5 (342.75–42.75) | 76.0 (1201.00–36.00) | 53.0 (97.00–34.00) | 69.5 (163.50–33.00) | 39.0 (102.50–21.00) | .445 |
| LDH u/l M (IQR) | 236.0 (336.25–204.75) | 339.5 (499.00–213.50) | 260.0 (332.50–200.50) | 254.0 (346.00–205.00) | 167.0 (184.00–133.00) | .006 |
| CRP mg/dl M (IQR) | 0.91 (1.61–0.36) | 1.10 (1.83–0.72) | 1.09 (3.27–0.16) | 0.92 (2.54–0.42) | 0.91 (9.20–0.17) | .766 |
ALB = serum albumin, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CK = creatine kinase, CRP = C-reactive protein, M = median, IQR = inter quartile range, LDH = lactate dehydrogenase.
3.3. Pulmonary function test results
A total of 56 of 84 ILD-ARS patients completed the pulmonary function tests, including 23 cases in anti-Jo-1 antibody positive group, 17 cases in anti-PL-7 antibody positive group, 6 cases in anti-PL-12 antibody positive group, 7 cases in anti-EJ antibody positive group, and 3 cases in anti-OJ antibody positive group. Pulmonary function is mainly manifested as restrictive ventilation dysfunction accompanied by decreased diffusing capacity. The median percentage of predicted total lung capacity (TLC) was 71.25 (55.23–84.98), the median percentage of predicted forced expiratory volume (FVC) was 74.75 (59.30–88.43), the median percentage of predicted diffusing capacity of the lung for carbon monoxide (DLCO) was 54.65 (44.68–65.23), and the median percentage of predicted carbon monoxide alveolar metastasis rate (DLCO/VA) was 75.20 (66.40–87.85%). The predicted DLCO in anti-EJ positive group was lower than that in other groups (P < 0.05). Although there was no significant difference in predicted TLC, predicted FVC, and predicted DLCO/VA among groups, the anti-EJ antibody positive group had relatively lower predicted TLC, predicted FVC, predicted DLCO/VA values, and poorer pulmonary functions compared with other groups (Table 3).
Table 3.
Pulmonary function test results.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | P | |
| TLC %pred M (IQR) | 72.1 (86.3–53.1) | 73.1 (84.3–60.9) | 80.6 (86.2–64.3) | 54.3 (64.1–52.4) | 88.0 (91.1–70.3) | .53 |
| FVC %pred M (IQR) | 73.6 (88.7–58.5) | 83.0 (92.3–65.3) | 81.4 (93.4–64.8) | 58.4 (68.8–52.8) | 77.9 (98.8–75.4) | .103 |
| DLCO %pred M (IQR) | 53.9 (67.7–45.7) | 56.2 (64.6–48.1) | 64.1 (82.6–55.1) | 38.9 (47.0–34.0) | 60.6 (77.9–54.2) | .015 |
| DLCO/VA %pred M (IQR) | 74.2 (93.6–66.4) | 81.7 (89.8–67.4) | 76.4 (83.8–68.4) | 61.7 (71.7–58.3) | 78.2 (88.2–76.2) | .548 |
DLCO = diffusing capacity of the lung for carbon monoxide, DLCO/VA = carbon monoxide alveolar metastasis rate, FVC = forced expiratory volume, IQR = inter quartile range, M = median, TLC = total lung capacity.
3.4. Features on HRCT imaging and HRCT patterns
The high-resolution computed tomography (HRCT) imaging signs of 84 ILD-ARS patients were dominant by GGO (97.62%, 82/84) and reticular opacities (73.81%, 62/84), followed by consolidation (42.86%, 36/84), air bronchogram (29.76%, 25/84), and subpleural line (26.19%, 22/84). Traction bronchiectasis (22%,18/84), honeycombing opacity(14.29%, 12/84), pericardial effusion (13.10%, 11/84), and pleural effusion (11.90%, 10/84, including 1 case of unilateral pleural effusion and 9 cases of bilateral pleural effusion) were relatively rare and nodule shadow (5.95%, 5/84) was the least. The incidence of honeycombing opacity (3, 60%) in ILD patients with anti-OJ was significantly higher than that in ILD patients with anti-Jo-1 (1, 2.78%) (χ2 = 10.475, P < 0.05). The incidence of pleural effusion (3, 60%) in ILD patients with anti-OJ was higher than that of ILD patients with anti-Jo-1 (2, 5.56%) and anti-PL-7 (2, 9.09%) (χ2 = 7.601, P < 0.05; Fisher exact test, P < 0.05) (Table 4).
Table 4.
Features on HRCT imaging.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | |
| n = 36 | n = 22 | n = 9 | n = 12 | n = 5 | |
| Ground-glass opacity | 35 (97.22%) | 22 (100% | 9 (100%) | 11 (91.67%) | 5 (100%) |
| Reticular opacities | 24 (66.67%) | 20 (90.91%) | 7 (77.78%) | 8 (66.67%) | 3 (60.00%) |
| Subpleural line | 11 (30.55%) | 6 (27.27%) | 3 (33.33%) | 1 (8.33%) | 1 (20.00%) |
| Honeycombing opacity | 1 (2.78%) | 5 (22.73%) | 1 (11.11%) | 2 (16.67%) | 3 (60.00%) |
| Consolidation | 17 (47.22%) | 10 (45.45%) | 4 (44.44%) | 4 (33.33%) | 1 (20.00%) |
| Nodular | 4 (11.11%) | 1 (4.55%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Traction bronchiectasis | 9 (25.00%) | 4 (18.18%) | 1 (11.11%) | 2 (16.67%) | 2 (40.00%) |
| Pericardial effusion | 4 (11.11%) | 3 (13.64%) | 1 (11.11%) | 2 (16.67%) | 1 (20.00%) |
| Air bronchogram | 11 (30.55%) | 5 (22.73%) | 3 (33.33%) | 5 (41.67%) | 1 (20.00%) |
| Pleural effusion | 2 (5.56%) | 2 (9.09%) | 1 (11.11%) | 1 (8.33%) | 3 (60.00%) |
HRCT = high-resolution computed tomography.
NSIP (48.57%, 34/70) (Fig. 1) was the main type of ILD patients with positive anti-Jo-1, anti-PL-7, and anti-EJ antibodies. OP (33.33%, 3/9) (Fig. 2) was the main type of ILD patients with positive anti-PL-12 antibody. UIP (40%, 2/5) (Fig. 3) was the main type of ILD patients with positive anti-OJ antibody. But the typing of ILD among groups showed no statistical difference (P > 0.05) (Table 5).
Figure 1.
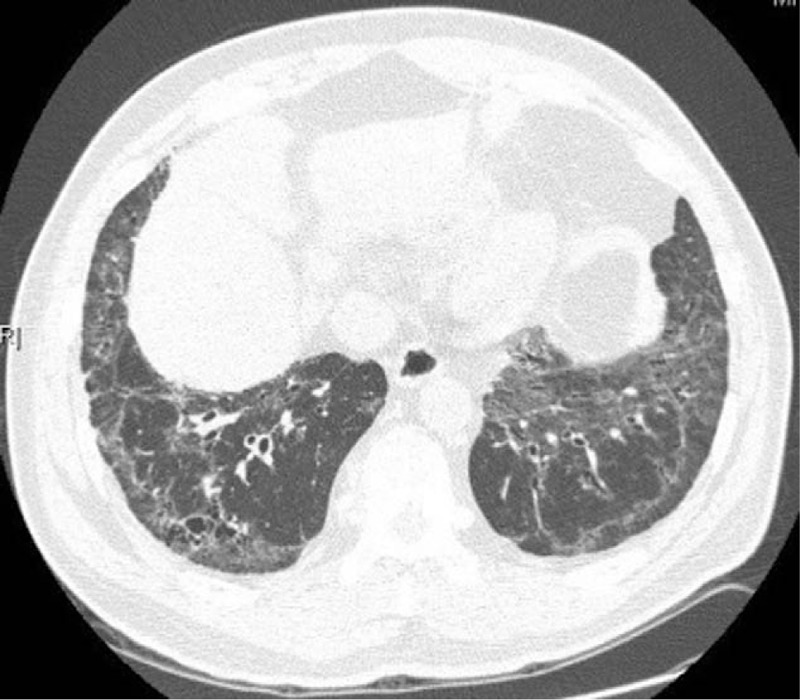
GGO and grid shadows could be seen under the pleura of bilateral lungs and around the bronchial vascular bundles, which were consistent with NSIP. GGO = ground-glassopacities, NSIP = nonspecific interstitial pneumonia.
Figure 2.
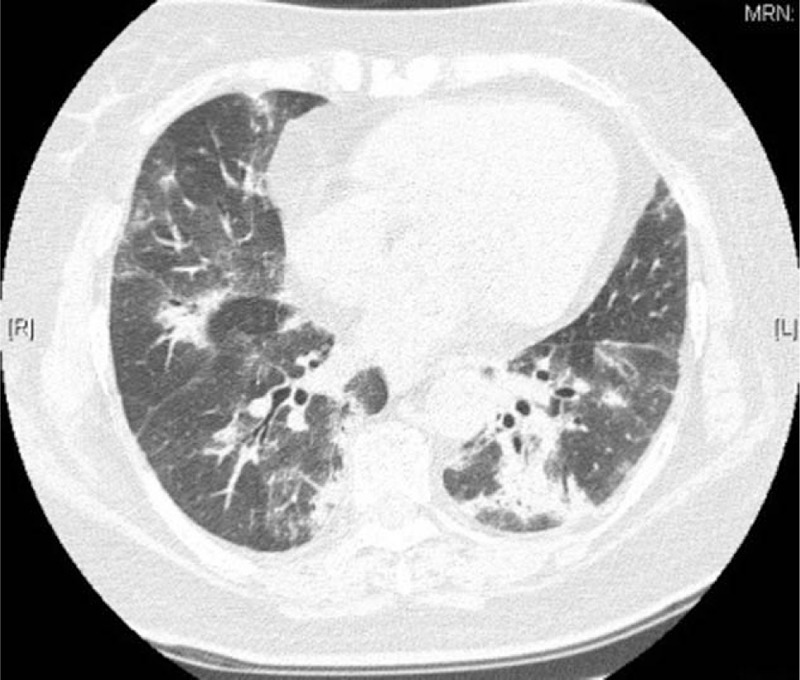
GGO and large solid shadows could be seen in bronchovascular bundles of both lungs, which were consistent with OP. GGO = ground-glassopacities, OP = organizing pneumonia.
Figure 3.
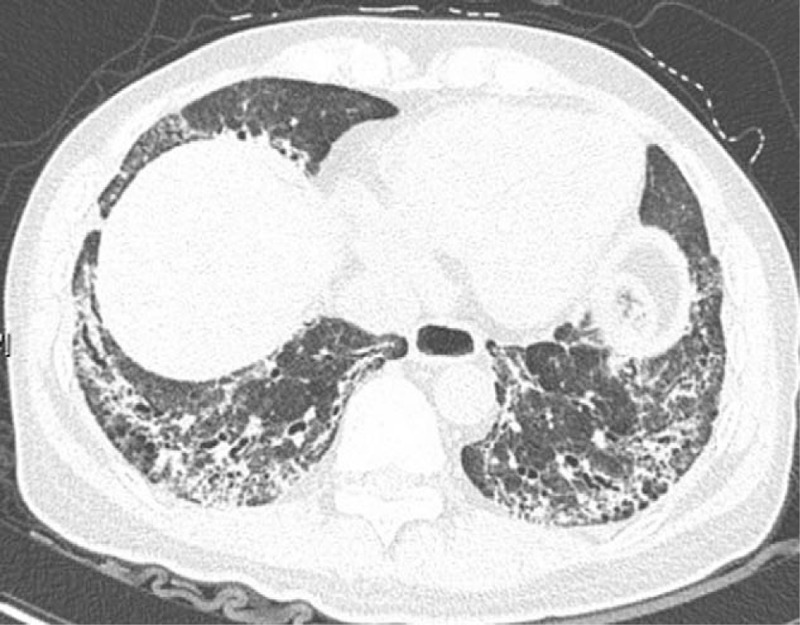
Diffuse honeycomb shadows could be seen in bilateral lower lungs, which were consistent with UIP. UIP = usual interstitial pneumonia.
Table 5.
Chest HRCT patterns.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | |
| n = 36 | n = 22 | n = 9 | n = 12 | n = 5 | |
| NSIP | 16 (44.45%) | 11 (50%) | 2 (22.22%) | 7 (58.33%) | 1 (20.00%) |
| OP | 10 (27.78%) | 4 (18.18%) | 3 (33.33%) | 4 (33.33%) | 0 (0%) |
| NSIP + OP | 7 (19.44%) | 5 (22.73%) | 2 (22.22%) | 1 (8.33%) | 1 (20.00%) |
| UIP | 0 (0%) | 2 (9.09%) | 1 (11.11%) | 0 (0%) | 2 (40.00%) |
| Unclassifiable | 3 (8.33%) | 0 (0%) | 1 (11.11%) | 0 (0%) | 1 (20.00%) |
HRCT = high-resolution computed tomography, NSIP = nonspecific interstitial pneumonia, OP = organizing pneumonia, UIP = usual interstitial pneumonia.
3.5. Bronchoalveolar lavage findings
Bronchoalveolar lavage fluid (BALF) was the use of fiberoptic bronchoscope to constrict the lung segment or sub-segment, rapid injection of sterile saline, 20 to 60 ml each time, repeat 4 to 5 times, the total amount of lavage was 100 to 300 ml, and the total resorption rate was 40% to 70%.
A total of 31 patients completed BALF tests, including 11 cases in anti-Jo-1 antibody positive group, 8 cases in anti-PL-7 antibody positive group, 4 cases in anti-PL-12 antibody positive group, 6 cases in anti-EJ antibody positive group, and 2 cases in anti-OJ antibody positive group. Macrophages were dominant in all groups and there was no statistical difference in cell contents of BALF among groups (Table 6).
Table 6.
BAL findings.
| BAL findings, median (IQR) | Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | P |
| Macrophage (%) | 51.0 (62.0–50.0) | 62.0 (66.0–45.0) | 65.5 (74.8–57.8) | 51.0 (60.5–44.8) | 46.0 (52.0–40.0) | 0.155 |
| Lymphocyte (%) | 14.0 (25.0–11.0) | 6.0 (16.8–1.5) | 5.5 (12.8–4.3) | 8.0 (17.1–5.8) | 12.0 (17.0–7.0) | 0.193 |
| Neutrophil (%) | 26.0 (36.0–23.0) | 28.0 (37.5–20.0) | 25.0 (32.0–19.5) | 36.5 (43.0–21.8) | 42.0 (53.0–31.0) | 0.478 |
| Hemosiderin cell (%) | 2.0 (3.0–0) | 3.0 (4.0–0) | 6.5 (19.5–3.3) | 5.5 (7.5–1.5) | 2.0 (4.0–0) | 0.159 |
| Eosinophil (%) | 1.0 (2.0–0) | 1.5 (3.0–0.3) | 1.0 (2.0–0) | 1.0 (1.8–0.4) | 0.0 (0–0) | 0.487 |
BAL = bronchoalveolar lavage, IQR = inter quartile range
3.6. Follow-up results
Forty-five of 84 patients had chest CT reexamination within 12 months. The median follow-up was 6 months (range, 1–12months). Thirty-two patients were treated with combination therapy, and 13 received steroid monotherapy. The most commonly used combination therapy was prednisone and cyclophosphamide in 28 patients, followed by prednisone and mycophenolate mofetil in 2 patients, prednisone and methotrexate in 1 patient, and prednisone and intravenous immunoglobulins in 1 patient. Compared with the baseline chest CT findings, regression was observed in 19 patients (42%), the disease extent on HRCT remained stable in 15 patients (33%), and deterioration was observed in 11 patients (24%) on follow-up chest CT. And the regression on chest CT mainly occurred within 3 months after treatment. Compared with Jo-1 negative patients, Jo-1 positive patients had a higher regression rate on lung CT (χ2 = 7.348, P = 0.007). During the follow-up, most patients with OP and NSIP remained stable or showed regression, while most of the patients with UIP deteriorated (Table 7).
Table 7.
Comparison of ILD pattern on HRCT.
| Anti-Jo-1 | Anti-PL-7 | Anti-PL-12 | Anti-EJ | Anti-OJ | Total | |
| Regression | 12 | 4 | 1 | 1 | 1 | 19 |
| Stability | 2 | 6 | 3 | 3 | 1 | 15 |
| Deterioration | 4 | 5 | 0 | 1 | 1 | 11 |
| Total | 18 | 15 | 4 | 5 | 3 | 45 |
HRCT = high-resolution computed tomography, ILD = interstitial lung diseases.
4. Discussion
In this study, we compared the differences between ILD patients with different anti-ARS antibodies from clinical, serological, and radiological aspects.
At present, there are still no unified diagnostic criteria for ASS. Refer to the diagnostic criteria of Connors et al[13] in 2010: ASS could be diagnosed in patients with positive anti-ARS antibody combined with 1 or more of the following clinical manifestations. Clinical manifestations include Raynaud phenomenon, arthritis, ILD, fever (excluding other reasons), and mechanic's hand. ASS could be diagnosed in all patients in this study. Refer to the diagnostic criteria published by Solomon et al[14] in 2011: ASS could be diagnosed in patients with positive anti-ARS antibody combined with 2 primary criteria or 1 primary criterion plus 2 secondary criteria. Primary criteria: (1) ILD (excluding other reasons) and (2) PM/DM. Secondary criteria: arthritis, Raynaud phenomenon, and mechanic's hands. A total of 36 cases of ASS could be diagnosed in this study by the criteria.
In Yura et al[15] study, anti-ARS antibodies were found in 6.0% of patients with idiopathic interstitial pneumonia. The prevalence of ILD among patients in ASS was from 67% to 100%.[9] And in China, the frequency of ILD in ASS reached 94.4% in Shi et al[21] study.
As a single-center study on ILD with 5 kinds of positive anti-ARS antibodies (anti-Jo-1, anti-PL-7, anti-PL-12, anti-EJ, and anti-OJ), this study had the largest number of enrolled subjects up to now. Among patients with anti-ARS antibody positive ILD in our study, anti-Jo-1 antibody was the most common, followed by anti-PL-7 antibody, which was consistent with the previous literature report of Yura et al.[15] The positive frequency of anti-OJ antibody was the lowest,[8,16] which was consistent with previous large-scale cohort study on anti-ARS antibodies [2.5% (5/202) in the United States and 4.8% (8/166) in Japan vs 5.95% in our study].
According to our data, myositis mainly occurred in ILD patients with positive anti-PL-7, anti-Jo-1, and anti-EJ antibodies. These findings were consistent with the previous reports of myositis and anti-PL-7, anti-Jo-1, and anti-EJ antibodies.[17,18] In contrast, no myositis was found in ILD patients with positive anti-PL-12 and anti-OJ antibodies.
The incidence of arthritis in ILD patients with positive anti-Jo-1 antibody was higher than that in ILD patients with positive anti-PL-12 and anti-EJ antibodies (P < 0.05), which was consistent with the findings in 828 ASS cases reported by Cavagna et al.[19] We found that the incidence of arthritis in ILD patients with positive anti-PL-7 antibody was higher than that in ILD patients with positive anti-Jo-1 antibody, but the incidence of mechanic's hand was higher in ILD groups with positive anti-Jo-1 antibody than that in ILD groups without positive anti-Jo-1 antibody, including ILD group with positive anti-PL-7 antibody. Ang et al[20] believed that the occurrence of mechanic's hand was related to the occurrence of diffuse ILD, which suggested that ILD patients should be alert to ASS, especially when mechanic's hand is combined. There was no significant difference in the incidences of fever and Raynaud phenomenon between the groups.
There are reports that NSIP is the most common type in ILD-ARS, followed by OP and UIP.[21,22] We found in our study that NSIP was the main type of ILD patients with anti-Jo-1, anti-PL-7, and anti-EJ antibodies, while OP was the main type of ILD patients with anti-PL-12 antibody and UIP was the main type of ILD patients with anti-OJ antibody. Since ILD is the first symptom before the development of myositis in 20% of inflammatory myopathy cases, ASS should be vigilant for anti-ARS antibody positive patients with these ILD types.[23]
In our study, only 14.29% of the patients had typical “triad syndrome.” Therefore, we recommended that ILD patients clinically suspected of CTD, especially middle-aged females with NSIP as the main HRCT type, should still pay attention to the screening of anti-ARS antibodies even if no definite symptoms such as myositis and arthritis are combined, which may be helpful for early diagnosis. In each subgroup, GGO was the main imaging sign, followed by reticular opacities. The incidence of honeycombing opacity was the highest in ILD patients with positive anti-OJ antibody, which was the same as that of reticular opacities. This might be related to the fact that UIP was the main type of ILD patients with positive anti-OJ antibody. In terms of pulmonary function, reduction of pulmonary diffusion function was mainly found in each group. Although there was no significant difference in predicted TLC, predicted FVC, and predicted DLCO/VA among groups, lower predicted DLCO and poorer pulmonary function were observed in anti-EJ antibody group. Macrophages were dominant in BALF of each group, followed by neutrophils.
In terms of physical signs, the manifestations of rashes were different among the anti-ARS subgroups. In this study, it was found that Gottron sign (30, 35.71%) had the highest incidence and shawl sign (1,1.19%) had the lowest incidence among ILD-ARS patients. The ILD patients with positive anti-Jo-1 antibody mainly showed Gottron sign (12, 33.33%) and Gottron papules (7, 19.44%). Heliotrope rash (an edematous purplish-red rash on the upper eyelid or periorbital area, which can be on 1 or both sides, and is aggravated by light) (1, 2.78%) was rare and the incidence of V sign (an edematous purplish-red rash in the V-shaped area of the front chest, which is aggravated by light) and shawl sign was 0. However, about 25% to 28% ILD patients with positive anti-PL-7 and anti-EJ antibodies had a heliotrope rash, which was consistent with the finding on 165 adult patients with positive anti-ARS antibody previously reported by Hamaguchi Y.[8] Skin damage did not occur in ILD patients with positive anti-OJ antibody. The incidence of Gottron sign in ILD patients with positive anti-EJ antibody was higher than that in ILD patients with positive anti-Jo-1 and anti-OJ antibodies (P < 0.05).
In our follow-up study, compared with Jo-1 negative patients, Jo-1 positive patients had a higher regression rate on lung CT. This was consistent with Aggarwal et al[16] describing that the prognosis of Jo-1 negative patients in ASS was worse than that of Jo-1 positive patients. However, no significant difference was found in each subtype of Jo-1 antibody negative group. In another study, the anti-PL-7 was associated with rapidly progressive ILD.[26] In our study, there was no significant difference between the 5 groups in frequency of rapidly progressive ILD. It requires more experimental data to confirm.
To sum up, the article summarizes the clinical characteristics of ILD with different anti-ARS antibodies, deepened physician's understanding of these patients. ILD with anti-Jo-1 antibody is associated with multiple organ involvement, with prominent manifestations of myositis, mechanic's hand, and arthritis. These results have also been confirmed in other cohort studies.[24,25] NSIP is the main type of patients with ILD positive to antibodies of anti-Jo-1, anti-PL-7, and anti-EJ, OP is the main type of patients with ILD positive to anti-PL-12 antibody and UIP is the main type of patients with ILD positive to anti-OJ antibody. As other clinical manifestations of some ILD patients are relatively hidden, ILD patients, especially middle-aged females with NSIP as the main HRCT type, should pay attention to screening of the anti-ARS antibodies and guard against ASS. The article enabled people to diagnose ASS earlier, early treatment could be achieved and greater clinical benefits could be obtained. We had observed that some patients have relieved pulmonary function, blood gas analysis, and even relieved rash, arthritis, and mechanic hands after medication. These people were still being followed up to see if these patients could be prevented from developing ASS. The limitation of this study is that it did not study whether patients who only have ILD with anti-synthetase antibody positive but cannot be diagnosed with ASS, according to the diagnostic criteria published by Solomon et al[14] in 2011, have subsequent symptoms such as myositis and arthritis, and whether they subsequently develop ASS. Follow-up study for patients in this study will be continued to observe whether these ILD-ARS patients have clinical manifestations of ASS such as myositis, arthritis, mechanic hand, and so on after the occurrence of ILD and finally develop into ASS.
Author contributions
Conceptualization: Minna Jiang.
Data curation: Xin Dong.
Formal analysis: Xin Dong, Yi Zheng.
Investigation: Yi Zheng.
Methodology: Minna Jiang.
Project administration: Minna Jiang, Xin Dong.
Resources: Xin Dong, Yi Zheng.
Writing – original draft: Minna Jiang, Xin Dong.
Writing – review & editing: Minna Jiang, Xin Dong, Yi Zheng.
Footnotes
Abbreviations: ASS = anti-synthetase syndrome, ATS/ERS = American Thoracic Society/European Respiratory Society, CTD = connective tissue disease, DLCO = diffusing capacity of the lung for carbon monoxide, DLCO/VA = carbon monoxide alveolar metastasis rate, FVC = forced expiratory volume, GGO = ground-glassopacities, HRCT = high-resolution computed tomography, ILD = interstitial lung diseases, IPAF = interstitial pneumonia with autoimmune features, NSIP = nonspecific interstitial pneumonia, NSIP-OP = nonspecific interstitial pneumonia-organizing pneumonia, OP = organizing pneumonia, UIP = usual interstitial pneumonia.
How to cite this article: Jiang M, Dong X, Zheng Y. Clinical characteristics of interstitial lung diseases positive to different anti-synthetase antibodies. Medicine. 2021;100:19(e25816).
The authors have no conflicts of interest to declare.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Demoruelle MK, Mittoo S, Solomon JJ. Connective tissue disease-related interstitial lung disease. Best Pract Res Clin Rheumatol 2016;30:39–52. [DOI] [PubMed] [Google Scholar]
- [2].Mira-Avendano I, Abril A, Burger CD, et al. Interstitial lung disease and other pulmonary manifestations in connective tissue diseases. Mayo Clin Proc 2019;94:309–25. [DOI] [PubMed] [Google Scholar]
- [3].American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed] [Google Scholar]
- [4].Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 2011;140:1292–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976–87. [DOI] [PubMed] [Google Scholar]
- [6].Witt LJ, Curran JJ, Strek ME. The diagnosis and treatment of antisynthetase syndrome. Clin Pulm Med 2016;23:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tieu J, Lundberg IE, Limaye V. Idiopathic inflammatory myositis. Best Pract Res Clin Rheumatol 2016;30:149–68. [DOI] [PubMed] [Google Scholar]
- [8].Hamaguchi Y, Fujimoto M, Matsushita T, et al. Common and distinct clinical features in adult patients with anti-aminoacyl-tRNA synthetase antibodies: heterogeneity within the syndrome. PLoS One 2013;8:e60442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hallowell RW, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies and the antisynthetase syndrome: recent advances. Curr Opin Rheumatol 2014;26:684–9. [DOI] [PubMed] [Google Scholar]
- [10].Tian MX, Huang WH, Ren FF, et al. Comparative analysis of connective tissue disease-associated interstitial lung disease and interstitial pneumonia with autoimmune features. Clin Rheumatol 2019. [DOI] [PubMed] [Google Scholar]
- [11].Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hansell DM, Bankier AA, MacMahon H, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008;246:697–722. [DOI] [PubMed] [Google Scholar]
- [13].Connors GR, Christopher-Stine L, Oddis CV, et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 2010;138:1464–74. [DOI] [PubMed] [Google Scholar]
- [14].Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol 2011;37:100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yura H, Sakamoto N, Satoh M, et al. Clinical characteristics of patients with anti-aminoacyl-tRNA synthetase antibody positive idiopathic interstitial pneumonia. Respir Med 2017;132:189–94. [DOI] [PubMed] [Google Scholar]
- [16].Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014;73:227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mahler M, Miller FW, Fritzler MJ. Idiopathic inflammatory myopathies and the anti-synthetase syndrome: a comprehensive review. Autoimmun Rev 2014;13:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Watanabe K, Handa T, Tanizawa K, et al. Detection of antisynthetase syndrome in patients with idiopathic interstitial pneumonias. Respir Med 2011;105:1238–47. [DOI] [PubMed] [Google Scholar]
- [19].Cavagna L, Trallero-Araguás E, Meloni F, et al. Influence of antisynthetase antibodies specificities on antisynthetase syndrome clinical spectrum time course. J Clin Med 2019;8:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ang CC, Anyanwu CO, Robinson E, et al. Clinical signs associated with an increased risk of interstitial lung disease: a retrospective study of 101 patients with dermatomyositis. Br J Dermatol 2017;176:231–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shi J, Li S, Yang H, et al. Clinical profiles and prognosis of patients with distinct antisynthetase autoantibodies. J Rheumatol 2017;44:1051–7. [DOI] [PubMed] [Google Scholar]
- [22].Mejía M, Herrera-Bringas D, Pérez-Román DI, et al. Interstitial lung disease and myositis-specific and associated autoantibodies: clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF). Respir Med 2017;123:79–86. [DOI] [PubMed] [Google Scholar]
- [23].De Sadeleer LJ, De Langhe E, Bodart N, et al. Prevalence of myositis-specific antibodies in idiopathic interstitial pneumonias. Lung 2018;196:329–33. [DOI] [PubMed] [Google Scholar]
- [24].Pinal-Fernandez I, Casal-Dominguez M, Huapaya JA, et al. A longitudinal cohort study of the anti-synthetase syndrome: increased severity of interstitial lung disease in black patients and patients with anti-PL7 and anti-PL12 autoantibodies. Rheumatology (Oxford) 2017;56:999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Marie I, Josse S, Decaux O, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 2012;11:739–45. [DOI] [PubMed] [Google Scholar]
- [26].Hervier B, Devilliers H, Stanciu R, et al. Hierarchical cluster and survival analyses of antisynthetase syndrome: phenotype and outcome are correlated with anti-tRNA synthetase antibody specificity. Autoimmun Rev 2012;12:210–7. [DOI] [PubMed] [Google Scholar]


