Abstract
To evaluate the clinical characteristics and liver injury in coronavirus disease 2019 (COVID-19) patients, and analyze the differences between suspected and confirmed COVID-19 patients, this retrospective study was performed on 157 COVID-19 patients and 93 suspected patients who were ultimately excluded from COVID-19 (control patients). Differences in clinical characteristics and liver injury between suspected and confirmed COVID-19 patients were analyzed. Age, male sex, fever, chest tightness and dyspnea were related to the severity of COVID-19. C-reactive protein (CRP) and D-dimer may be predictors of the severity of COVID-19. Computed tomography (CT) played an important role in the screening of COVID-19 and the evaluation of disease severity. Multiple factors may cause liver injury in COVID-19 patients. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may be more likely to cause liver injury than common respiratory infectious diseases. Age, temperature (T), white blood cell (WBC), lymphocytes (LY), hematocrit (HCT), CRP, and finger pulse oxygen saturation (SpO2) may correlate with liver function impairment and may predict the occurrence and severity of liver function impairment. Some therapeutic drugs (like glucocorticoid) may be involved in the liver function impairment of COVID-19 patients. Most liver function indices improved significantly after active treatment. Although COVID-19 and other common respiratory infectious diseases share some clinical characteristics, COVID-19 has its own characteristics.
Keywords: coronavirus disease 2019, liver function, liver injury, severe acute respiratory syndrome coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory tract infection caused by a newly emergent coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first recognized in Wuhan, China, in December 2019. Genetic sequencing of the virus suggests that SARS-CoV-2 is a beta-coronavirus that is closely linked to the SARS virus. SARS-CoV-2 has become a global threat to human health. The WHO declared the outbreak of SARS-CoV-2 infection an international public health emergency.[1–9]
Lung lesions are the major damage caused by SARS-CoV-2 infection.[10–12] The clinical features and outcomes of Chinese patients with COVID-19 were widely reported. Increasing evidence showed the frequent incidence of liver injury in COVID-19 patients, and it often manifests as a transient elevation of serum aminotransferases. The underlying mechanisms of liver injury in cases of COVID-19 include psychological stress, systemic inflammatory response, drug toxicity, and progression of preexisting liver diseases. However, there is insufficient evidence of SARS-CoV-2-infected hepatocytes or virus-related liver injury in COVID-19. The clinical, pathological and laboratory characteristics and the underlying pathophysiology and etiology of liver injury in COVID-19 are largely unclear.[4,6,8,13–16]
There are a few articles about the clinical characteristics of COVID-19 infection that simply mention damage to liver function, but there are no detailed analyses about the relationship between liver injury and COVID-19. The current research investigated the clinical characteristics of all hospitalized COVID-19 patients and all suspected patients who were ultimately excluded from COVID-19 (control patients), including differences between COVID-19 patients and control patients, to reveal the clinical characteristics of COVID-19 and the relationship between liver function and the clinical and laboratory characteristics of SARS-CoV-2 infection.
2. Methods
2.1. Study design and patients
For this retrospective, single-center study, we reviewed the medical records from all hospitalized patients with COVID-19 and all suspected patients who were ultimately excluded from COVID-19 (control patients) and admitted to the First Hospital of Yangtze University. Diagnosis of COVID-19 was based on the New Coronavirus Pneumonia Prevention and Control Program (7th edition) published by the National Health Commission of China and the interim guidance from the World Health Organization.[17,18] All patients with COVID-19 tested positive for SARS-CoV-2 using quantitative RT-PCR (qRT-PCR) on samples from the respiratory tract or had serum antibodies for SARS-CoV-2. The Medical Ethical Committee of First Hospital of Yangtze University reviewed and approved this study.
2.2. Data collection
The personal data and clinical data of patients included in the study were collected. Personal data included sex, age, history of smoking and drinking, epidemiological history and comorbidities (e.g., chronic obstructive pulmonary disease, asthma, hypertension and/or diabetes, viral hepatitis, fatty liver, etc). Clinical data included initial symptoms, clinical presentation, temperature (T), computed tomography (CT) imaging presentations, vital signs, therapeutic drugs used, and disease outcomes.
2.3. Specimen collection, etiology, and biochemical tests
SARS-CoV-2 was detected using real-time fluorescence RT-PCR of samples collected from nasopharyngeal swabs and the presence of serum antibodies for COVID-19. Relevant laboratory indicators were tested using conventional methods, including routine blood tests (white blood cell [WBC], lymphocytes [LY], platelets [PLT], hematocrit [HCT]), liver function (alanine transaminase [ALT], aspartate aminotransferase [AST], prealbumin [PA], albumin [ALB], alkaline phosphatase [ALP], total bilirubin [TB],γ-glutamyl transpeptidase [GGT], lactate dehydrogenase [LDH] and cholinesterase [CHE]), and infection index (C-reactive protein [CRP]), D-dimer, finger pulse oxygen saturation (SpO2), International Normalized Ratio [INR], and plasma prothrombin time[PT].
2.4. Statistical analysis
SPSS software version 22.0 (IBM Inc., Chicago, IL) was used for statistical analyses. Categorical variables were presented as numbers (percentages) and were analyzed using the Chi-Squared test or Fisher exact test. Continuous variables with normal distribution were expressed as the means ± standard deviation and were analyzed using independent samples t-test or ANOVA. Pearson's correlation was used to measure the degree of association between the parametric variables. A two-sided P < .05 was considered statistically significant.
3. Results
3.1. Epidemiological and clinical characteristics of COVID-19 and control patients
Strict precautionary measures were implemented in Hubei Province, including the creation of fever clinics that exclusively received patients with suspected SARS-CoV-2 infection, which was defined as presenting with a fever or any respiratory symptoms, including dry cough, suspicious imaging manifestations of COVID-19 on lung CT, especially in patients with a history of travel to Wuhan or exposure to infected people within 2 weeks before the onset of illness since January 2020. By March 27, 2020, a total of 250 hospitalized patients, including 157 patients with laboratory confirmed SARS-Cov-2 and 93 patients who were ultimately excluded from COVID-19 (control patients) and admitted to the First Hospital of Yangtze University, were recruited. The control patients included upper respiratory tract infection, influenza, common pneumonia, and infectious diseases outside the lung. The demographic and clinical characteristics are shown in Table 1. The average ages of the COVID-19 patients and the control patients were 51.49 ± 17.39 and 52.53 ± 16.24 years, respectively. Among the COVID-19 patients, 81 patients (51.6%) were males, 17 patients (10.8%) had a smoking history, 11 patients (7.0%) had a drinking history, and 126 patients (80.3%) had exposure history. Eighty one patients (51.6%) had at least 1 underlying comorbidity, the most common of which was chronic disease, such as hypertension (19.7%) and diabetes (5.1%). Only 4 viral hepatitis patients and 4 fatty liver patients were identified. Fortysix patients (49.5%) in the control group were males, 12 patients (12.9%) had a smoking history and 12 patients (12.9%) had a drinking history. Fifty-8 patients (62.4%) had at least 1 underlying comorbidity, the most common was hypertension, which was the same as the COVID-19 patients. There were no significant differences in gender, age, smoking and drinking history, or comorbidity between the COVID-19 patients and the control patients (P > .05). The COVID-19 patients were closely related to the exposure history, which was significantly higher than the control patients (χ2 = 9.667, P = .002 < .01). Fever, cough, fatigue, chest tightness/dyspnea, muscle soreness and anorexia were the most common symptoms and signs in the COVID-19 patients. Fever, fatigue, muscle soreness, diarrhea and anorexia in the COVID-19 patients were significantly higher compared to the control patients (P < .05). There were 11 deaths in the COVID-19 patients, and no deaths in the control patients. Therefore, the mortality of the COVID-19 patients was significantly higher than the control patients (χ2 = 0.008, P = .005 < .01).
Table 1.
Epidemiological and clinical characteristics of the COVID-19 patients and the control patients.
| COVID-19 patients | control patients | t/χ2 | P | |
| N | 157 | 93 | ||
| Gender | ||||
| male | 81 (51.6%) | 46 (49.5%) | 0.106 | .745 |
| female | 76 (48.4%) | 47 (50.5%) | ||
| Age (yr) | 51.49 ± 17.39 | 52.53 ± 16.24 | 1.767 | .641 |
| Smoking history | 0.245 | .620 | ||
| Never smoked | 140 (89.2%) | 81 (87.1%) | ||
| Smoked | 17 (10.8%) | 12 (12.9%) | ||
| Drinking history | 2.431 | .119 | ||
| Never drank | 146 (93.0%) | 81 (87.1%) | ||
| drank | 11 (7.0%) | 12 (12.9%) | ||
| Exposure history | 9.667 | .002 | ||
| Living in Wuhan or recently visited Wuhan | 54 (34.4%) | 23 (24.7%) | ||
| Contacted with confirmed patients | 30 (19.1%) | 17 (18.3%) | ||
| Contacted with Wuhan residents | 32 (20.4%) | 10 (10.8%) | ||
| Familiar/cluster infections | 13 (8.3%) | 0 (0.0%) | ||
| Comorbidity | ||||
| Hypertension | 31 (19.7%) | 22 (23.4%) | 0.545 | .461 |
| Coronary heart disease | 4 (2.5%) | 4 (4.3%) | 0.476 | .342 |
| COPD | 1 (0.6%) | 2 (2.2%) | 0.558 | .313 |
| Asthma | 0 (0.0%) | 3 (3.2%) | 0.050 | .050 |
| Pulmonary tuberculosis | 1 (0.6%) | 3 (3.2%) | 0.147 | .147 |
| Chronic bronchitis | 3 (1.9%) | 0 (0.0%) | 0.296 | .246 |
| Viral hepatitis | 4 (2.5%) | 0 (0.0%) | 0.300 | .153 |
| Fatty liver | 4 (2.5%) | 0 (0.0%) | 0.300 | .153 |
| Chronic renal insufficiency | 2 (1.3%) | 6 (6.5%) | 3.521 | .061 |
| Diabetes mellitus | 8 (5.1%) | 3 (3.2%) | 0.541 | .343 |
| Other | 23 (14.6%) | 15 (16.1%) | 1.808 | .179 |
| Signs and symptoms | ||||
| Fever | 118 (75.2%) | 42 (45.2%) | 22.811 | .000 |
| Cough | 94 (59.9%) | 45 (48.4%) | 3.121 | .077 |
| Fatigue | 39 (24.8%) | 13 (14.0%) | 4.183 | .041 |
| Chest tightness/dyspnea | 28 (17.8%) | 12 (12.1%) | 1.057 | .304 |
| Sore throat | 13 (8.3%) | 8 (8.6%) | 0.008 | .929 |
| Headache | 6 (3.8%) | 3 (3.2%) | 1.000 | .553 |
| Muscle soreness | 22 (14.0%) | 5 (5.4%) | 4.522 | .033 |
| Nausea or vomiting | 7 (4.5%) | 8 (8.6%) | 1.778 | .182 |
| Diarrhea | 16 (10.2%) | 2 (2.2%) | 5.651 | .017 |
| Anorexia | 28 (17.8%) | 5 (5.4%) | 7.911 | .005 |
| T (°C) | 37.8 ± 0.93 | 38.0 ± 6.4 | 2.955 | .633 |
| Mortality | 11 (7.0%) | 0 (0.0%) | 0.008 | .005 |
3.2. Laboratory characteristics of the COVID-19 and control patients
The SpO2 values of the COVID-19 and control patients were 92.5 ± 15.0 and 96.21 ± 2.51, respectively. The level SpO2 of the COVID-19 patients was significantly lower than the control patients (t = 16.335, P = .019 < .05). For routine blood tests, no differences were identified for white cell count, lymphocyte count, platelet count or hematocrit between the COVID-19 patients and the control patients (P > .05). There were 111 COVID-19 patients (70.7%) with increased CRP and 104 COVID-19 patients (66.2%) with increased D-dimer, and both levels were greater than the control patients (P < .05). For coagulation function, no differences were for INR (P > .05), but PT in the COVID-19 patients was reduced compared to the control patients (t = 3.908, P = .0001 < .05). For liver function, the PA, ALB, ALT, AST, and LDH abnormalities in the COVID-19 patients were significantly higher than that the control patients (P < .05). There were no significant differences in TB, GGT, ALP or CHE between the COVID-19 patients and the control patients (P > .05) (see Table 2).
Table 2.
Laboratory characteristics of the COVID-19 patients and the control patients.
| COVID-19 patients | control patients | t/χ2 | P | |
| N | 157 | 93 | ||
| SpO2 (%) | 92.5 ± 15.0 | 96.21 ± 2.51 | 16.335 | .019 |
| Routine blood test | ||||
| White-cell count, E+09/L | 1.655 | .437 | ||
| ≥10 | 19 (12.1%) | 10 (10.8%) | ||
| ≥4, <10 | 125 (79.6%) | 79 (84.9%) | ||
| <4 | 13 (8.3%) | 4 (4.3%) | ||
| Lymphocyte count, E+09/L | 1.546 | .462 | ||
| ≥4 | 2 (1.3%) | 3 (3.2%) | ||
| ≥0.8, <4 | 97 (61.8%) | 60 (64.5%) | ||
| <0.8 | 58 (36.9%) | 30 (32.3%) | ||
| Platelet count, E+09/L | 5.381 | .068 | ||
| ≥300 | 4 (2.5%) | 4 (4.3%) | ||
| ≥100, <300 | 112 (71.3%) | 76 (81.7%) | ||
| <100 | 41 (26.1%) | 13 (14.0%) | ||
| Hematocrit (%) | 0.457 | .499 | ||
| <40 (male), <35 (female) | 98 (62.4%) | 62 (66.7%) | ||
| 40–50 (male), 35–45 (female) | 59 (37.6%) | 31 (33.3%) | ||
| C-reactive protein (mg/L) | 9.192 | .002 | ||
| >8 | 111 (70.7%) | 48 (51.6%) | ||
| ≤8 | 46 (29.3%) | 45 (48.4%) | ||
| D-dimer (mg/L) | 6.022 | .014 | ||
| >0.55 | 104 (66.2%) | 47 (50.5%) | ||
| ≤0.55 | 53 (22.8%) | 46 (49.5%) | ||
| PT (second) | 11.20 ± 1.68 | 8.23 ± 0.95 | 3.908 | .000 |
| INR | 1.00 ± 0.15 | 0.99 ± 0.20 | 0.109 | .231 |
| Liver function | ||||
| PA (mg/L) | 16.897 | .000 | ||
| <200 | 128 (83.1%) | 56 (59.6%) | ||
| ≥200 | 29 (16.9%) | 37 (40.4%) | ||
| ALB (g/L) | 18.406 | .000 | ||
| <35 | 65 (42.4%) | 15 (16.0%) | ||
| ≥35 | 92 (57.6%) | 78 (84.0%) | ||
| TB (umol/L) | 2.718 | .096 | ||
| >20.4 | 53 (34.4%) | 23 (24.5%) | ||
| ≤20.4 | 104 (65.6%) | 70 (75.5%) | ||
| ALT (U/L) | 21.963 | .000 | ||
| >40 | 79 (51.3%) | 20 (21.3%) | ||
| ≤40 | 78 (48.7%) | 73 (78.7%) | ||
| AST (U/L) | 7.803 | .005 | ||
| >40 | 59 (38.3%) | 20 (21.3%) | ||
| ≤40 | 98 (61.7%) | 73 (78.7%) | ||
| LDH (U/L) | 9.701 | .002 | ||
| >240 | 72 (45.5%) | 25 (26.6%) | ||
| ≤240 | 85 (54.5%) | 68 (73.4%) | ||
| GGT (U/L) | 3.661 | .056 | ||
| >50 | 61 (39.6%) | 26 (27.7%) | ||
| ≤50 | 96 (60.4%) | 67 (62.3%) | ||
| ALP (U/L) | 0.008 | .929 | ||
| >128 | 13 (6.5%) | 8 (8.5%) | ||
| ≤128 | 144 (93.5%) | 85 (91.5%) | ||
| CHE (U/L) | 0.934 | .334 | ||
| <3700 | 23 (14.9%) | 10 (10.6%) | ||
| ≥3700 | 134 (85.1%) | 83 (89.4%) | ||
3.3. Symptomatic, laboratory and radiological characteristics of the clinical classifications of COVID-19 patients
Among the 157 COVID-19 patients, there were 2 mild type cases, 110 common type cases, 30 severe type cases and 15 critical type cases. By comparing the age distribution with the clinical classifications, we found that clinical classifications correlated with age, and the older patients experienced the more serious clinical classifications (χ2 = 17.217, P = .000 < .01). Clinical classifications were also related to gender (χ2 = 5.724, P = .017 < .05). The proportion of male patients increased with the aggravation of clinical classifications. For signs and symptoms, only fever and chest tightness and dyspnea were significantly related to the severity of clinical type (P < .01). The rate of fever and chest tightness and dyspnea increased with the aggravation of the disease. The SpO2 gradually decreased from the mild type to critical type cases, and it was related to the severity of clinical classifications (t = 49.588, P = .000 < .01). There was a correlation between the severity of pulmonary CT manifestations and the severity of clinical classifications (t = 17.958, P = .000 < .01). For the laboratory examination, an increase in white cell count, decrease in lymphocyte count, decrease in hematocrit, and increases in CRP and D-dimer were related to the severity of clinical type (P < .05). However, there was no correlation between platelet count and the severity of clinical classifications (χ2 = 0.191, P = .662 > .05). (See Table 3).
Table 3.
Symptomatic, laboratory, and radiological characteristics of clinical classifications in the COVID-19 patients.
| N | Mild | Common | Severe | Critical | t/χ2 | P | |
| N | 157 | 2 | 110 | 30 | 15 | ||
| Age | 51.49 ± 17.39 | 26.00 ± 1.41 | 46.60 ± 15.56 | 62.70 ± 15.82 | 68.27 ± 10.85 | 17.217 | .000 |
| Gender | 5.724 | .017 | |||||
| Male | 81 (51.6%) | 0 (0.0%) | 53 (48.2%) | 16 (53.3%) | 12 (80.0%) | ||
| Female | 76 (48.4%) | 2 (100.0%) | 57 (51.8%) | 14 (46.7%) | 3 (20.0%) | ||
| Signs and symptoms | |||||||
| Fever | 118 (75.2%) | 1 (50.0%) | 76 (69.1%) | 26 (86.7%) | 15 (100.0%) | 9.814 | .002 |
| Cough | 94 (59.9%) | 0 (0.0%) | 68 (61.8%) | 17 (56.7%) | 9 (60.0%) | 0.004 | .947 |
| Fatigue | 39 (24.8%) | 0 (0.0%) | 24 (21.8%) | 9 (30.0%) | 6 (40.0%) | 3.277 | .070 |
| Chest tightness&dyspnea | 28 (17.8%) | 0 (0.0%) | 11 (10.0%) | 10 (33.3%) | 7 (46.7%) | 17.918 | .000 |
| Sore throat | 13 (8.3%) | 0 (0.0%) | 10 (9.1%) | 3 (10.0%) | 0 (0.0%) | 0.602 | .438 |
| Headache | 6 (3.8%) | 0 (0.0%) | 6 (5.5%) | 0 (0.0%) | 0 (0.0%) | 1.882 | .170 |
| Muscle soreness | 22 (14.0%) | 0 (0.0%) | 14 (12.7%) | 8 (26.7%) | 0 (0.0%) | 0.002 | .965 |
| Nausea or vomiting | 7 (4.5%) | 0 (0.0%) | 5 (4.5%) | 1 (3.3%) | 1 (6.7%) | 0.057 | .812 |
| Diarrhea | 16 (10.2%) | 0 (0.0%) | 15 (13.6%) | 1 (3.3%) | 0 (0.0%) | 3.710 | .054 |
| Anorexia | 28 (17.8%) | 0 (0.0%) | 19 (17.3%) | 6 (20.0%) | 3 (20.0%) | 0.264 | .608 |
| SpO2 | 92.5 ± 15.0 | 98.5 ± 0.70 | 96.7 ± 2.4 | 92.4 ± 6.0 | 60.7 ± 33.8 | 49.588 | .000 |
| CT | 17.958 | .000 | |||||
| Ground-glass opacity | 30 (19.4) | 0 (0.0%) | 26 (23.6%) | 2 (6.7%) | 2 (13.3%) | ||
| Local patchy shadowing | 15 (9.7%) | 0 (0.0%) | 15 (13.6%) | 0 (0.0%) | 0 (0.0%) | ||
| Bilateral patchy shadowing | 92 (59.4%) | 0 (0.0%) | 65 (59.1%) | 22 (73.3%) | 5 (33.3%) | ||
| consolidation | 18 (11.6%) | 0 (0.0%) | 4 (3.6%) | 6 (20.0%) | 8 (53.3%) | ||
| Routine blood test | |||||||
| White-cell count, E+09/L | 33.555 | .000 | |||||
| ≥10 | 19 (12.1%) | 0 (0.0%) | 4 (3.6%) | 4 (13.3%) | 11 (73.3%) | ||
| ≥4, <10 | 125 (79.6%) | 2 (100.0%) | 93 (84.5%) | 26 (86.7%) | 4 (26.7%) | ||
| <4 | 13 (8.3%) | 0 (0.0%) | 13 (11.8%) | 0 (0.0%) | 0 (0.0%) | ||
| Lymphocyte count, E+09/L | 12.133 | .000 | |||||
| ≥4 | 2 (1.3%) | 0 (0.0%) | 1 (0.9%) | 1 (3.3%) | 0 (0.0%) | ||
| ≥0.8, <4 | 97 (61.8%) | 1 (50.0%) | 78 (70.9%) | 14 (46.7%) | 4 (26.7%) | ||
| <0.8 | 58 (36.9%) | 1 (50.0%) | 31 (28.2%) | 15 (50.0%) | 11 (73.3%) | ||
| Platelet count, E+09/L | 0.191 | .662 | |||||
| ≥300 | 4 (2.5%) | 0 (0.0%) | 4 (3.6%) | 0 (0.0%) | 0 (0.0%) | ||
| ≥100, <300 | 112 (71.3%) | 1 (50.0%) | 81 (73.6%) | 21 (70.0%) | 9 (60.0%) | ||
| <100 | 41 (26.1%) | 1 (50.0%) | 25 (22.7%) | 9 (30.0%) | 6 (40.0%) | ||
| Hematocrit | 4.644 | .031 | |||||
| <40 (male), <35 (female) | 98 (62.4%) | 0 (0.0%) | 64 (58.2%) | 23 (76.7%) | 11 (73.3%) | ||
| 40–50 (male), 35–45 (female) | 59 (37.6%) | 2 (100.0%) | 46 (41.8%) | 7 (23.3%) | 4 (26.7%) | ||
| C-reactive protein (mg/L) | 15.280 | .000 | |||||
| >8 | 111 (70.7%) | 0 (0.0%) | 70 (63.6%) | 26 (86.7%) | 15 (100.0%) | ||
| ≤8 | 46 (29.3%) | 2 (100.0%) | 40 (36.4%) | 4 (13.3%) | 0 (0.0%) | ||
| D-dimer (mg/L) | 26.665 | .000 | |||||
| >0.55 | 104 (66.2%) | 0 (0.0%) | 60 (54.5%) | 29 (96.7%) | 15 (100.0%) | ||
| ≤0.55 | 53 (22.8%) | 2 (100.0%) | 50 (45.5%) | 1 (3.3%) | 0 (0.0%) | ||
3.4. Relationship between liver function indices and clinical classifications of the COVID-19 patients
The mean values of ALT in the different clinical classifications were 10.50 ± 4.95 (Mild), 49.35 ± 49.75 (Common), 154.50 ± 134.72 (Severe), and 132.27 ± 227.91 (Critical). The mean value of ALT increased significantly with the severity of clinical classifications (F = 10.677, P = .000 < .01). The mean values of AST, PA, ALB, GGT, LDH, and CHE were significantly abnormal with the severity of clinical classifications, and these differences were statistically significant (P < .01). TB and ALP had no correlation with the clinical classifications (P > .05). (See Fig. 1).
Figure 1.
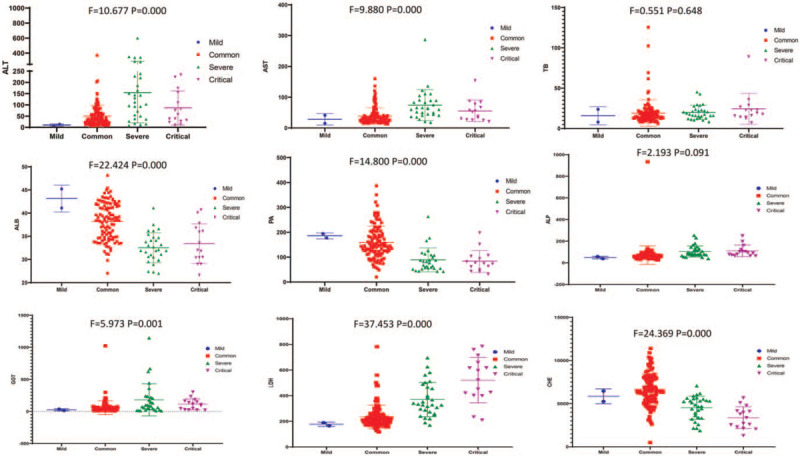
Relationship between liver function indices and clinical classifications of the COVID-19 patients (Each dot represents the value of liver function indices in all clinical classifications of the COVID-19 patients).
3.5. Relationship between the levels of ALT and AST and other clinical characteristics in COVID-19 patients
Analyses of the relationship between ALT, AST and other clinical data, such as age, temperature, SpO2, WBC, LY, PLT, HCT, CRP, and D-dimer revealed that the level of ALT positively correlated with age, temperature, WBC, and CRP, and negatively correlated with LY and SpO2, which all were statistically significant (P < .05), but these levels were not correlated with HCT, PLT, or D-dimer (See Fig. 2). The level of AST positively correlated with age, WBC and CRP and negatively correlated with LY, HCT, and SpO2, which all were statistically significant (P < .05). There was no correlation between AST and temperature, PLT, or D-dimer (P > .05). (See Fig. 3).
Figure 2.
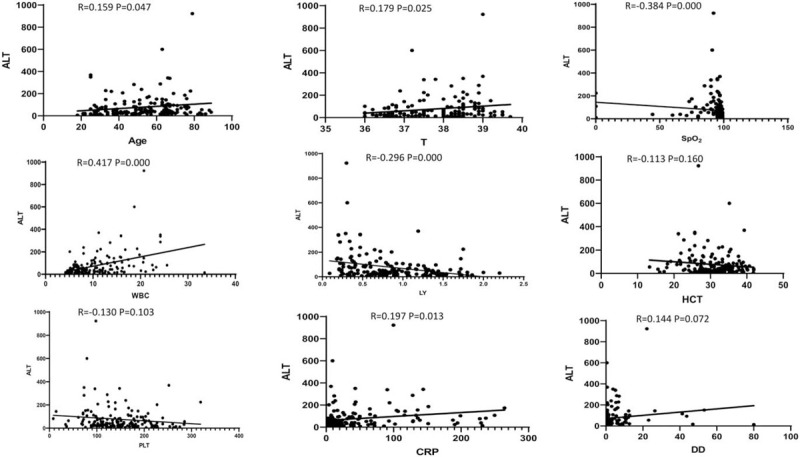
Relationship between ALT and other clinical characteristics in the COVID-19 patients (Each dot represents the value of ALT of the COVID-19 patients).
Figure 3.
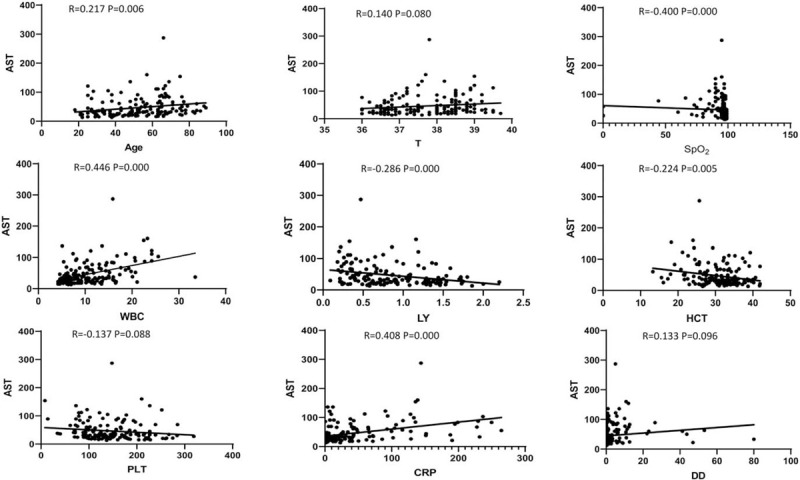
Relationship between AST and other clinical characteristics in the COVID-19 patients (Each dot represents the value of AST of the COVID-19 patients).
3.6. The effects of therapeutic drugs on liver function in COVID-19 patients
The ALT levels in COVID-19 patients treated with glucocorticoids (n = 89) were significantly higher than COVID-19 patients without glucocorticoid treatment (n = 68) (102.73 ± 133.78 vs 43.03 ± 44.053, F = 15.684, P = .001 < .01). However, there were no significant differences in ALT levels between the use of arbidol (n = 139), lopinavir/ritonavir (n = 75), oseltamivir (n = 35), ribavirin (n = 43), antibiotics (n = 144), chloroquine (n = 32), Lianhua Qingwen Capsule (n = 105) and traditional Chinese medicine (n = 131) and the nonuse of these drugs (P > .05). Similarly, the AST levels of COVID-19 patients treated with glucocorticoid was significantly higher than COVID-19 patients without glucocorticoid treatment (52.55 ± 40.51 vs 38.66 ± 26.95, F = 7.021, P = .016 < .05). There were no significant differences between the use of arbidol, lopinavir/ritonavir, oseltamivir, ribavirin, antibiotics, chloroquine, Lianhua Qingwen Capsule and traditional Chinese medicine and the nonuse of these drugs (P > .05) (See Fig. 4).
Figure 4.
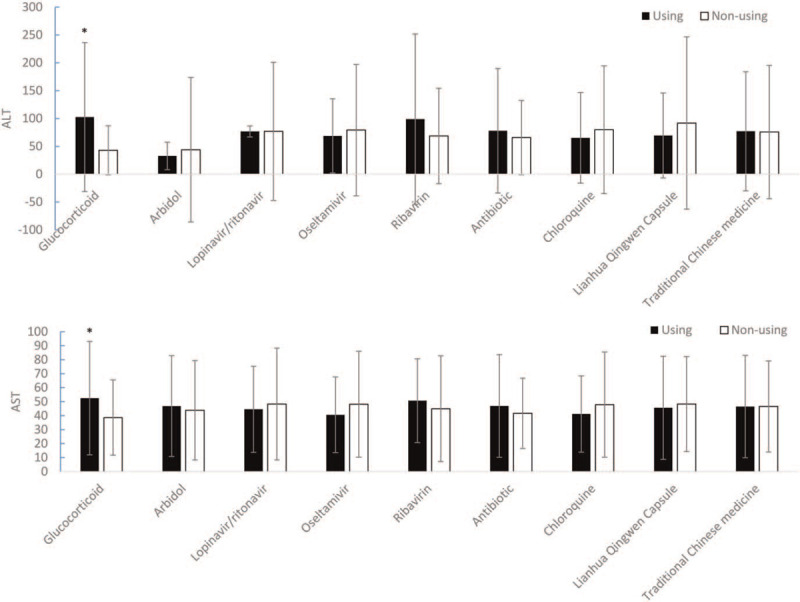
The effect of therapeutic drugs on liver function in the COVID-19 patients (∗P < .05).
3.7. Changes in liver function indices before and after routine liver-protecting treatment
In COVID-19 patients with abnormal liver function, drugs for routine liver protecting, such as reduced glutathione and polyene phosphatidylcholine, were used. We compared liver function indices (ALB, PA, TB, ALT, AST, GGT, ALP, LDH, and CHE) before and after treatment. The levels of ALB, PA, ALT, AST, GGT, ALP, LDH, and CHE before treatment were 26.98 (30.55–33.95) g/L, 114.1 (77.26–145.2) mg/L, 39.00 (57.00–147.0) U/L, 68.00 (50.00–91.00) U/L, 114.0 (79.75–190.5) U/L, 165.0 (139.0–240.0) U/L, 353.5 (278.5–502.8) U/L and 2610 (2138–3211) U/L, respectively. The levels of ALB, PA, ALT, AST, GGT, ALP, LDH, and CHE after treatment were 36.90 (34.60–38.90) U/L, 215.2 (166.1–270.6) mg/L, 39.00 (24.00–56.00) U/L, 28.00 (24.00–43.00) U/L, 83.00 (55.5–122.0) U/L, 80.0 (86–136.5) U/L, 210.0 (177.5–325.5) U/L, and 3491 (3093–4529) U/L, respectively. We found that ALB (t = 9.736, P = .000 < .01), PA (t = 13.08 P = .000 < .01) and CHE (t = 3.344, P = .0017 < .01) levels in the blood increased significantly after treatment, and 67.70% (44/65), 70% (71/128), and 47.83% (11/23) of the patients returned to normal, respectively. ALT (t = 3.984, P = .0001 < .01), AST (t = 6.930, P = .000 < .01) and GGT (t = 2.345, P = .0206 < .05) levels were reduced significantly, with 66.67% (48/72), 74.14% (43/58), and 16.67% (11/61) of the patients returned to normal, respectively. There was no significant change in LDH (P = .4802 > .05) or ALP (P = .0956 > .05) after treatment (See Fig. 5). The results indicated that most liver function indices improved significantly after active treatment.
Figure 5.
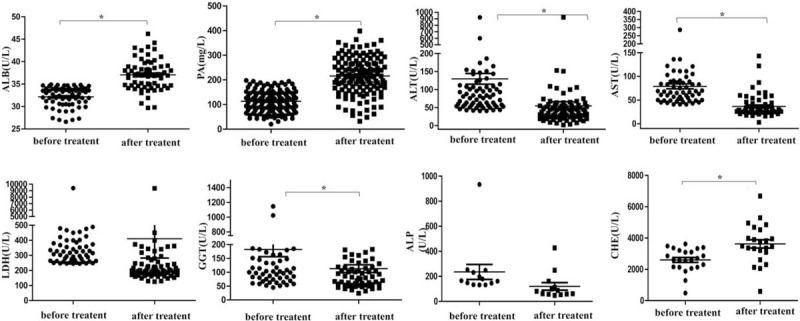
Changes of liver function indices before and after routine liver protecting treatment (Each dot represents the value of liver function indices before and after routine liver protecting treatment in the COVID-19 patients. ∗P < .05).
4. Discussion
SARS-CoV-2 is the pathogen of the 2019 novel coronavirus disease (COVID-19), and it has posed a serious threat to global public health.[19–22] To determine useful indices for COVID-19 diagnosis, we compared the clinical characteristics, laboratory test results and outcomes of the 157 COVID-19 patients with the 93 control patients (the suspected patients who were ultimately excluded from COVID-19). From our data, there were no differences in gender, age, smoking or drinking history between the COVID-19 patients and control patients. However, the present study found that the clinical classifications correlated with age and gender. Older, male patients were more likely to develop severe or critical classification. Most of the COVID-19 patients had an exposure history, which was significantly higher than the control patients. There was no significant difference in the comorbidity of the COVID-19 patients compared with the control patients. The rates of these symptoms, such as fever, fatigue, muscle soreness, diarrhea and anorexia, were significantly higher in the COVID-19 patients than the control patients. However, fever, chest tightness and dyspnea were related to the severity of clinical classification. In terms of outcome, 11 of the COVID-19 patients died, which was significantly higher than the control patients.
SpO2 decreased gradually with the severity of clinical classifications. Our data found that the lung's blood oxygen exchange ability was more likely affected in the COVID-19 patient than other common respiratory infectious diseases. There were no significant differences in routine blood parameters, such as WBC, LY, PLT, HCT, and INR between the COVID-19 patients and control patients. Analysis of the relationship between the routine blood parameters and clinical classifications found that the clinical classification was more serious with increasing leukocytes and decreasing lymphocyte count and hematocrit. CRP in the blood is a marker of inflammation. Recent research examined the correlation between D-dimer and severity of inflammation; D-dimer may also reflect an inflammatory condition.[23–26] The levels of CRP and D-dimer in the COVID-19 patients were significantly higher than the control patients, and it was related to the severity of clinical classification. Therefore, COVID-19 patients had a greater inflammatory response than patients with common respiratory tract infection, and CRP and D-dimer may be predictors of COVID-19 severity. The common characteristics of CT included ground glass nodules, patchy shadows and consolidation. We found that the more serious lung injuries were associated with aggravation of the disease, and more serious CT manifestations were observed. Because the nucleic acid detection of SARS-CoV-2 had false negative results and the ability of nucleic acid detection was not sufficient, CT diagnosis had been used as the clinical diagnostic standard in China to screen out suspected COVID-19 patients as much as possible.[27–30]
Lung lesions are the major damage caused by SARS-CoV-2 infection.[12] Recent studies on COVID-19 reported incidents of liver injury, but the main purposes of these studies were to describe the clinical characteristics of COVID-19, and only a simple description of abnormal liver function, but no in-depth research and analysis, was provided.[9,14–16,28,31–34] The present retrospective study used suspected patients who were ultimately excluded from COVID-19 as controls and systematically analyzed the characteristics of abnormal liver function of patients with COVID-19 and its possible mechanism. Multiple factors, such as exposure to toxins, excessive alcohol consumption, bile duct obstruction, and viral infections, cause liver damage. The pathological features of COVID-19 greatly resemble SARS and Middle Eastern respiratory syndrome (MERS) coronavirus infection. Liver biopsy specimens from patients with COVID-19 showed moderate microvesicular steatosis and mild lobular and portal activity, which indicates that SARS-CoV-2 infection or drugs could have caused the liver injury.[35] Bile duct cells express angiotensin-converting enzyme 2 receptor with high specificity, and SARS-CoV and SARS-CoV-2 enter human cells via the angiotensin-converting enzyme 2 receptor.[13,36–38] The damage to liver function may be due to dysfunction of the bile duct cells, drugs, systemic inflammation and other causes.
Comparison of the liver function parameters of the COVID-19 patients with the control patients revealed that the levels of PA, ALB, ALT, AST and LDH were significantly higher in the COVID-19 patients than the control patients, and PT were reduced significantly. We could infer that SARS-CoV-2 was more likely to cause liver damage than the common respiratory infectious diseases. We also compared the liver function parameters of different clinical types of COVID-19 patients. Most of the liver function parameters, such as ALT, AST, ALB, PA, GGT, LDH, and CHE, were more abnormal with aggravation of the disease. The inflammatory reaction was often more serious with more serious disease, which was more likely to cause damage to various organs. Therefore, the occurrence of liver function damage in patients with COVID-19 was related to the disease severity. More serious liver function damage was found in COVID-19 patients with more serious type.
To predict liver function damage in the COVID-19 patients, we analyzed the correlation between ALT, AST and other clinical factors. We found that ALT and AST positively correlated with age, WBC, and CRP, and negatively correlated with SpO2 and LY. ALT positively correlated with T, and AST negatively correlated with HCT. Therefore, age, T, WBC, LY, HCT, CRP, and SpO2 may correlate with liver function impairment, which may predict the occurrence and severity of liver function impairment.
Because COVID-19 is a new infectious disease, there is no specific treatment. Similar to SARS, antibiotics, antivirals and steroids are widely used for the treatment of COVID-19. These drugs are all potential causes of liver injury during COVID-19, but their impact is not evident. Our center commonly used glucocorticoids, arbidol, lopinavir/ritonavir, oseltamivir, ribavirin, antibiotics, chloroquine, Lianhua Qingwen Capsule and traditional Chinese medicine as therapeutic agents. Whether some of these drugs caused liver function damage was not known. Therefore, we compared the levels of ALT and AST between patients who received common therapeutic drugs with patients who did not receive drug treatment. The levels of ALT and AST in patients using glucocorticoids were significantly higher than the nonuse of glucocorticoids. The other drugs had no effect on liver function. Our study could not fully analyze the effects of all drugs on liver function due to the limited number of cases in our center, and some drugs were not used for a long time. We also could not analyze the effects of combined drugs on liver function. However, some drug affected liver function in our study. Therefore, some therapeutic drugs may be involved in the liver function impairment of COVID-19 patients. Our data indicated that most liver function indices were significantly improved after active treatment.
In conclusion, the liver injury in the COVID-19 patients may be caused by multiple factors, such as direct viral infection of hepatocytes, immune-related injury, or drug hepatotoxicity. The liver injury in patients with SARS-CoV-2 infection was higher than patients without SARS-CoV-2 infection. The occurrence of liver function damage in patients with COVID-19 was related to the severity of the disease. Age, T, WBC, LY, HCT, CRP, and SpO2 may correlate with liver function impairment, which may predict the occurrence and severity of liver function impairment. Some therapeutic drugs, for example, glucocorticoids, may also be involved in the liver function impairment of COVID-19 patients. However, most liver function indices were significantly improved after active treatment.
Acknowledgments
We thank the Guangdong medical team for their assistance during the outbreak of COVID-19.
Author contributions
Conceptualization: Qing Zhang, Xiaoping Tan.
Data curation: Qing Zhang, Jie Li, Yan Zhang, Jie Gao, Peixue Wang, Minghua Ai, Wen Ding.
Formal analysis: Qing Zhang, Jie Gao, Wen Ding.
Investigation: Jie Li, Peixue Wang.
Methodology: Qing Zhang, Jie Li, Xiaoping Tan.
Software: Yan Zhang, Minghua Ai.
Writing – original draft: Qing Zhang, Jie Li, Xiaoping Tan.
Writing – review & editing: Qing Zhang, Xiaoping Tan.
Footnotes
Abbreviations: ALB = albumin, ALP = alkaline phosphatase, ALT = alanine transaminase, AST = aspartate aminotransferase, CHE = cholinesterase, COVID-19 = coronavirus disease 2019, CRP = C-reactive protein, CT = computed tomography, GGT = γ-glutamyl transpeptidase, HCT = hematocrit, INR = International Normalized Ratio, LDH = lactate dehydrogenase, LY = lymphocytes, PA = prealbumin, PLT = platelets, PT = plasma prothrombin time, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, SpO2 = finger pulse oxygen saturation, T = temperature, TB = total bilirubin, WBC = white blood cell.
How to cite this article: Zhang Q, Li J, Zhang Y, Gao J, Wang P, Ai M, Ding W, Tan X. Differences in clinical characteristics and liver injury between suspected and confirmed COVID-19 patients in Jingzhou, Hubei Province of China. Medicine. 2021;100:19(e25913).
QZ and JL contributed equally to this work.
This study was reviewed and approved by the Medical Ethical Committee of First Hospital of Yangtze University.
The authors have no conflicts of interests to disclose.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
References
- [1].WHO. World Health Organization. Coronavirus disease (COVID-19) outbreak. Available at: http://www.who.int. [Google Scholar]
- [2].Chen ATC, Coura-Filho GB, Rehder MHH. Clinical characteristics of Covid-19 in China. N Engl J Med 2020;382:1859–62. [DOI] [PubMed] [Google Scholar]
- [3].Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368: 1-10,m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Henry BM, Vikse J. Clinical characteristics of Covid-19 in China. N Engl J Med 2020;382:1860–1. [DOI] [PubMed] [Google Scholar]
- [6].Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rolf JD. Clinical characteristics of Covid-19 in China. N Engl J Med 2020;382:1860. [DOI] [PubMed] [Google Scholar]
- [8].Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- [10].Wang T, Du Z, Zhu F, et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 2020;395:950,e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Salome B, Magen A. Dysregulation of lung myeloid cells in COVID-19. Nat Rev Immunol 2020;20:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Committee GOoNH. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial seventh edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.sshtm. [Google Scholar]
- [18].WHO. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Available at: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. [Google Scholar]
- [19].Dilcher M, Werno A, Jennings LC. SARS-CoV-2: a novel deadly virus in a globalised world. N Z Med J 2020;133:06–11. [PubMed] [Google Scholar]
- [20].Palacios Cruz M, Santos E, Velazquez Cervantes MA, et al. COVID-19, a worldwide public health emergency. Rev Clin Esp 2020;221:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J Autoimmun 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ling W. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect 2020;50:332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shi Y, Wang Y, Shao C, et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ 2020;27:1451–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wannamethee SG, Whincup PH, Lennon L, et al. Associations between fibrin D-dimer, markers of inflammation, incident self-reported mobility limitation, and all-cause mortality in older men. J Am Geriatr Soc 2014;62:2357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lowe GD, Yarnell JW, Rumley A, et al. C-reactive protein, fibrin D-dimer, and incident ischemic heart disease in the Speedwell study: are inflammation and fibrin turnover linked in pathogenesis? Arterioscler Thromb Vasc Biol 2001;21:603–10. [DOI] [PubMed] [Google Scholar]
- [27].Committee GOoNH. Notice on the issuance of a program for the diagnosis and treatment of novel coronavirus (2019-nCoV) infected pneumonia (trial fivth edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/d4b895337e19445f8d728fcaf1e3e13a.shtml. [Google Scholar]
- [28].Mungmungpuntipantip R, Wiwanitkit V. Clinical features and chest CT manifestations of coronavirus disease (COVID-19). AJR Am J Roentgenol 2020;215:13. [DOI] [PubMed] [Google Scholar]
- [29].Shi H, Han X, Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology 2020;295:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun Z. Diagnostic value of chest CT in coronavirus disease 2019 (COVID-19). Curr Med Imaging 2020;16:274–5. [DOI] [PubMed] [Google Scholar]
- [31].Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with corona virus disease 2019 in Wuhan, China. Clin Infect Dis 2020;71:748–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cao Y, Liu X, Xiong L, et al. Imaging and clinical features of patients with 2019 Novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol 2020;92:1449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID-19) in Wuhan, China: a retrospective study. Chin Med J (Engl) 2020;133:1261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China. Clin Infect Dis 2020;70:ciaa270. [Google Scholar]
- [35].Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].South AM, Diz D, Chappell MC. COVID-19, ACE2 and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 2020;318:1084–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sun P, Lu X, Xu C, et al. CD-sACE2 inclusion compounds: an effective treatment for corona virus disease 2019 (COVID-19). J Med Virol 2020;92:1721–3. [DOI] [PubMed] [Google Scholar]


