Abstract
Pre-operative status of axillary lymph node (ALN) in early breast cancer is usually initially assessed by pre-operative ultrasound, followed by ultrasound-guided needle biopsy (UNB) confirmation. Patients with positive nodal status will undergo axillary lymph node dissection (ALND), while those with negative nodal status will have sentinel lymph node biopsy. ALND is associated with higher morbidity than Sentinel lymph node biopsy. The objective of this study is to determine if axillary ultrasound alone without UNB is predictive enough to assign patients to ALND and to identify ultrasound features that are significantly associated with pathologically positive ALN.
383 newly diagnosed primary breast cancer patients between 2012 and 2014, and who had undergone pre-operative axillary ultrasound in University Malaya Medical Centre with a complete histopathology report of the axillary surgery were retrospectively reviewed. ALN was considered positive if it had any of these features: cortical thickening > 3 mm, loss of fatty hilum, hypoechoic solid node, mass-like appearance, round shape and lymph node size > 5 mm. Post-operative histopathological reports were then analyzed for nodal involvement.
The overall sensitivity, specificity, and accuracy of pre-operative axillary ultrasound in detecting diseased nodes were 45.5%, 80.7%, and 60.3% respectively. The positive (PPV) and negative predictive values were 76.5% and 51.8%. Round shape, loss of fatty hilum and mass-like appearance had the highest PPVs of 87%, 83% and 81.6% respectively and significant odds ratios (ORs) of 5.22 (95% confidence interval [CI]: 1.52 - 17.86), ORs of 4.77 (95% CI: 2.62 - 8.70) and ORs of 4.26 (95% CI: 2.37 - 7.67) respectively (P-value < .05). Cortical thickness of > 3 mm was identified to have low PPV at 69.1%, ORs of 1.71 (95% CI: 0.86 - 3.41, P = .126).
There are features on axillary ultrasound that confer high PPV for axillary involvement i.e. round shape, loss of fatty hilum, and mass-like appearance. In a low resource setting, these features may benefit from ALND without further pre-operative biopsies. However, pre-operative UNB for features with low PPV that is, cortical thickness > 3 mm should be considered to obviate the unnecessary morbidity associated with ALND.
Keywords: axillary node, breast cancer, lymph node dissection, surgery, ultrasound
1. Introduction
In early stages of breast cancer, pre-operative status of axillary lymph node (ALN) is usually initially assessed by pre-operative ultrasound, followed by ultrasound-guided needle biopsy (UNB) confirmation. Patients with positive nodal status will undergo axillary lymph node dissection (ALND), while those with negative nodal status will have sentinel lymph node biopsy (SLNB). ALND is associated with higher morbidity than SLNB. However, the requirement for dual modalities to confirm nodal status is costly and time consuming for both patient and physician. ALND plays an important role in the management of breast cancer. Apart from reducing the risk of axillary recurrence,[1–3] it provides information for risk stratification of patients[4] and guide adjuvant treatment decisions.[5] While ALND is a standard surgical procedure in patients with positive ALN pre-operatively, it is frequently associated with complications including lymphedema, seroma, sensory loss and motion impairment.[6,7] In contrast, SLNB is the management of choice in clinically negative nodal status,[8] which is associated with less complications rate compared to ALND. However, SLNB is resource intensive as it requires added human resource, increased cost of using radiotracer and blue dye and increased cost in the case of re-operations. Thus, proper selection of patients for ALND or SLNB is greatly dependent upon accurate pre-operative assessment of the ALN status. Due to lack of breast cancer awareness and late detection of breast cancer as there are no available breast screening programs in the low and middle resource setting,[9] women generally present with symptomatic disease which may result in ALND being the only option of treatment for axillary staging. Furthermore, limited resources and expertise; and shortage of nuclear medicine facilities and equipment for SLNB are identified to be contributing factors to the widespread use of ALND.[10]
Clinical examination and mammography alone are not sufficiently accurate in detecting ALN metastases. Physical examination for palpable lymphadenopathy is accurate in approximately 50% of patients.[11] The accuracy, will however be increased significantly by imaging. Pathological lymph nodes can be found on mammograms, ultrasound and magnetic resonance imaging. Magnetic resonance imaging is the best technique for the assessment of lymph node status. Yet, ultrasound is the most common modality for axillary evaluation given that it is cheap, easily accessible and safe.
In this regard, pre-operative evaluation of ALN by ultrasound have been extensively investigated and increasingly recognized as a useful diagnostic tool in staging of the axilla. In fact, the National Institute of Health and Care Excellence guideline recommends pre-treatment ultrasound evaluation of the axilla to be performed for all patients being investigated for early invasive breast cancer.[12] In a study of 178 patients Rautiainen et al observed that the combination of ultrasound and biopsy in the event of a suspicious lymph node improves sensitivity (88% versus 61%), specificity (100% versus 85%), positive predictive value (PPV) (91% versus 73%) and negative predictive value (NPV) (100% versus 77%) compared to ultrasound alone. This study also showed that histological samples can reduce the number of sentinel nodes that require a second dissection by 20 to 25%, thereby enabling patients to avoid having two surgical interventions.[13] A meta-analysis, further suggested that routine pre-operative axillary ultrasound combined with lymph node biopsy in the diagnostic work-up of breast cancer patients will identify axillary metastatic disease in 50% of patients with axillary involvement.[14] Of the many features of ALN metastasis on ultrasound, the findings of hypoechoic rounded shape of the lymph node and loss of the fatty hilum have been shown to have high specificity for malignancy (80%).[15] Cortical thickening, whether diffuse or focal, is a more non-specific feature for malignancy, as it can be a reactive feature in various disease processes. However, the presence of a normal cortex (< 3 mm in diameter) has a high NPV (96%) for the absence of metastatic disease.[15]
According to National Comprehensive Cancer Network Guideline Version 1.2018, in patients who are clinically node positive at the time of breast cancer diagnosis, UNB of suspected lymph node should be performed.[16] In the case of positive lymph node post UNB, patient is subjected for ALND. Comparative studies looking at the performance of ultrasound alone vs combination of pre-operative axillary ultrasound and UNB in detecting metastatic ALN suggests the former to be inferior to that of the latter in terms of its sensitivity, specificity and accuracy in determining nodal status. To this effect, a sizeable number of patients (in the false positive [FP] group) could potentially be falsely identified to have a positive metastatic lymph node on pre-operative ultrasound alone and hence subjected to unnecessary ALND and its potential associated morbidities. On the other hand, patients with high nodal volume false negative status will have to undergo completion ALND after a positive SLNB.
In University Malaya Medical Centre (UMMC), the type of axillary surgery is dependent on axillary ultrasound to predict ALN involvement. We do not routinely perform UNB in positive lymph node patients, in view of increasing diagnostic and work up time for the patient and ultimately most of them will be triaged directly into ALND. Hence, re-evaluation of our protocol is carried out to guide future practice on the evidence of current clinical practice should be highlighted.
The primary objective of this study is to determine if axillary ultrasound alone without UNB is predictive enough to assign patients to ALND. The secondary objective is to identify ultrasound features significantly associated with pathologically positive ALN.
2. Method
A total of 831 patients who were newly diagnosed with breast cancer and undergoing primary surgery from January 1, 2012 to December 31, 2014 at UMMC were prospectively registered in the UMMC Breast Cancer Registry. Data on basic demography, clinical characteristics and histopathology were obtained from the registry. The registry has received approval from the Ethical Review Committee of UMMC. Details of the registry have been previously described.[17]
2.1. Study population
Women of all ages diagnosed with primary breast cancer who had undergone pre-operative axillary ultrasound in UMMC with a complete history, clinical examination and post-operative axillary histopathology record were included in this study. Patients who had pre-operative ultrasound done outside of UMMC were excluded from this study. Out of 831, only 518 patients met the inclusion criteria. Among the 518, 135 patients did not have ultrasound of the axilla were also excluded from the study. A final total of 383 patients were enrolled in the study.
Breast and axillary ultrasound examinations were performed using Philips iU22 unit (Philips Medical Systems, Bothell, WA) utilizing a 12-mHz or 17-mHz linear array high frequency ultrasound probe. All ultrasounds were performed by experienced radiologists as part of routine clinical practice and standard of care.
For the purpose of this study, all the axillary ultrasonography images of these 383 patients were retrospectively reviewed by two certified breast radiologists by consensus method who were blinded to the final outcome and nodal involvement. The ultrasound images were reviewed and the axillary node size and morphological features were assessed. Particular features were recorded such as cortical thickness of > 3 mm, loss of fatty hilum, hypoechoic solid node, mass-like appearance, round shape and lymph node size > 5 mm (short axis diameter). ALN was considered as abnormal at the time of examination if it had any one of the first three features. SLNB was only performed in patients who were clinically and radiologically node negative.
2.2. Procedures
Both ALND and SLNB were done under general anesthesia. These procedures were carried out via mastectomy incision or separate incision in the axilla for breast conserving surgery patients. The extent of ALND defined as level I – lateral to pectoralis minor muscle, level II – extending posterior to the muscle and level III – extending to medial to pectoralis minor muscle (apex of the axilla). Routine level II ALND was performed in all cases unless gross disease was present, where a level III would be performed. Suspicious inter-pectoral nodes were dissected if present. Axillary vein and nerves (long thoracic nerve and thoracodorsal nerve) were preserved. Intercostal brachial nerves were preserved at the discretion of the surgeon.
Identification of the sentinel lymph node (SLN) was performed using dual techniques, using radioactive colloid (99m technetium - sulfur colloid) and blue dye (patent blueTM). The tracer was injected at the subareolar region on the morning of surgery. Scintigraphic images of the axilla and breast were taken. Patent blue was injected in a similar fashion when patient was under general anesthesia. Light massage was performed for about 5 minutes. Skin incision was made similar to ALND. SLNs were identified using hand-held gamma probe and visual inspection for blue nodes.
2.3. Histopathological examination
Lymph nodes were grossly identified and numbers of lymph nodes were recorded. All tissue specimens were fixed with formalin and paraffin embedded. Small nodes were bivalved or 2 mm cut sections and stained with hematoxylin and eosin. Immunohistochemistry was not routinely performed in our center.
2.4. Outcomes
ALNs were deemed positive from pathological reports of macrometastases. The patient underwent either ALND or SLNB.
2.5. Analysis
The sensitivity, specificity, PPV, NPV, accuracy, as well as the positive and negative likelihood ratios of the axillary ultrasound in diagnosing nodal involvement were determined using the formulas as in Table 1. All statistical analyses were performed using SPSS software (version 24) and Microsoft Excel 2011. Odds ratios with 95% confidence interval were estimated using logistic models. p-value refers to the comparison of pathologically positive node in each ultrasound feature studied which was considered significant if less than 0.05.
Table 1.
Calculation table to determine diagnostic value of axillary ultrasound.
| SLNB /ALND | ||
| Axillary Ultrasound | Pathological Node Positive | Pathological Node Positive |
| Positive | True Positive (TP) | False Positive (FP) |
| Negative | False Negative (FN) | True Negative (TN) |
| Sensitivity | = TP / TP + FN | |
| Specificity | = TN / TN + FP | |
| Positive Predictive Value | = TP / TP + FP | |
| Negative predictive value | = TN / TN + FN | |
| Accuracy | = TP + TN / TP + FP + TN + FN | |
ALND = axillary lymph node dissection, FN = false negative, FP = false positive, SLNB = sentinel lymph node biopsy, TN = true negative, TP = true positive.
3. Results
Table 2 shows the sociodemographic and clinicopathological characteristics of the sample population. More than half of the patients were 55 years or more (54.6%) and were postmenopausal (66.3%) with a median age of 57 years. Estrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 (HER2) status were positive in 68.1%, 49.1% and 29.2% of cases. Lymphovascular invasion was present in 29% of all cases. Median tumor size was 3 cm. Most patients (75.5%) presented with tumor size less than or 5 cm (T1-T2) and a majority (58%) were node positive based on post-operative histopathological evaluation (N1- N3).
Table 2.
Patient demographics and clinicopathologic characteristics (n = 383).
| Variables | Number (%) of cases |
| Age | |
| <55 | 174 (45.4) |
| ≥55 | 209 (54.6) |
| ∗Median age: 57 | |
| Ethnicity | |
| Chinese | 159 (41.5) |
| Malay | 129 (33.7) |
| Indian | 81 (21.1) |
| Others | 14 (3.7) |
| Menopause | |
| No | 129 (33.7) |
| Yes | 254 (66.3) |
| ER status | |
| Negative | 113 (29.5) |
| Positive | 261 (68.1) |
| PR status | |
| Negative | 185 (48.3) |
| Positive | 188 (49.1) |
| HER 2 status | |
| Negative | 160 (41.8) |
| Positive | 112 (29.2) |
| Lymphovascular invasion | |
| No | 239 (62.4) |
| Yes | 111 (29.0) |
| Tumour Size Stage | |
| T1 (0–2cm) | 126 (32.9) |
| T2 (>2 and ≤5cm) | 163 (42.6) |
| T3 (>5cm) | 56 (14.6) |
| T4 (involvement of skin or chest wall) | 38 (9.9) |
| ∗Median tumour size: 3cm | |
| Axillary Lymph Node Stage | |
| N0 (nil) | 161 (42) |
| N1 (1–3 involved nodes) | 149 (38.9) |
| N2 (4–9 involved nodes) | 42 (11) |
| N3 (≥10 involved nodes) | 31 (8.1) |
ER = estrogen receptor, HER 2 = human epidermal growth factor 2, PR = progesterone receptor.
Out of the 383 cases studied, there were 251 patients (65.5%) with normal axillary ultrasound results and 132 patients with suspicious ultrasound findings. 222 patients (121 + 101, 58.0%) had positive pathologic ALN involvement whilst 161 of them (130 + 31, 42.04%) had no nodal involvement (Fig. 1). The results of the axillary ultrasound were compared with the data on surgically resected ALNs, followed by calculation of the false negative rate (FNR). FNR was revealed to be 54.5%, reflecting the proportion of pathological metastatic lymph nodes that had normal ultrasound images (121/121 + 101). Conversely, 31 cases were found to be FP as no pathologic metastatic lymph node was discovered post axillary surgery. The FP rate was calculated to be 19.25% (31/ (130 + 31) x 100). Sensitivity, specificity, accuracy, PPV and NPV of axillary ultrasound in the detection of lymph node involvement were 45.5%; 80.7%; 60.3%; 76.5% and 51.8% respectively.
Figure 1.
Comparison of axillary lymph node status as assessed by axillary ultrasound and histopathology (n = 383). FN = False negative, FP = false positive, LN = lymph node, TF = true positive, TN = true negative.
The PPV of each pre-operative axillary ultrasound feature in detecting positive ALN are presented in Table 3. Three ultrasound features which are round shape, loss of fatty hilum and mass-like appearance were identified to have high PPVs of 87%, 83% and 81.6% respectively. Cortical thickness of > 3 mm was identified to have the lowest PPV of 69.1% (n = 42). The PPV of hypoechoic solid node was 76.5% whilst PPV of lymph node size > 5 mm was 76.9%.
Table 3.
Positive predictive values of individual axillary ultrasound features in detecting pathologically positive axillary lymph nodes.
| Ultrasound features (n = 383) | N | TP | FP | PPV % |
| Cortical thickening > 3mm | 42 | 29 | 13 | 69.1 |
| Loss of fatty hilum | 88 | 73 | 15 | 83.0 |
| Hypoechoic solid node | 132 | 101 | 31 | 76.5 |
| Mass Like Appearance | 87 | 71 | 16 | 81.6 |
| Round shape | 22 | 19 | 3 | 87.0 |
| Lymph node size > 5 mm | 130 | 100 | 30 | 76.9 |
FP = false positive, PPV = positive predictive value, TP = true positive.
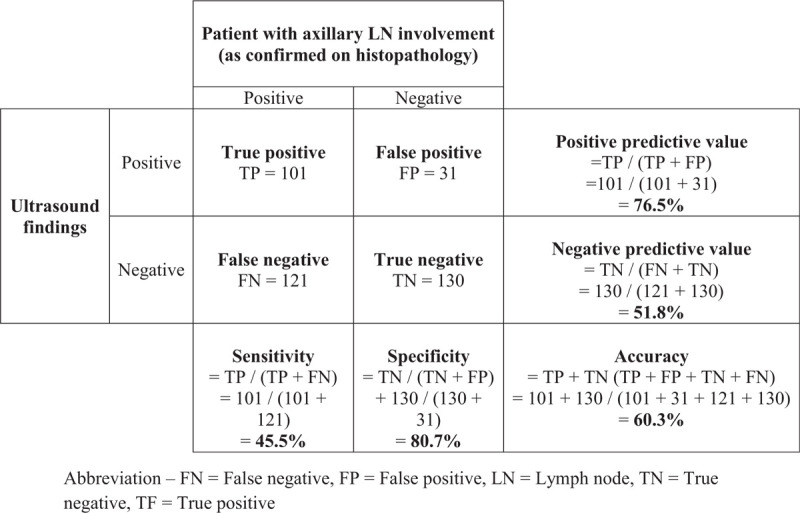
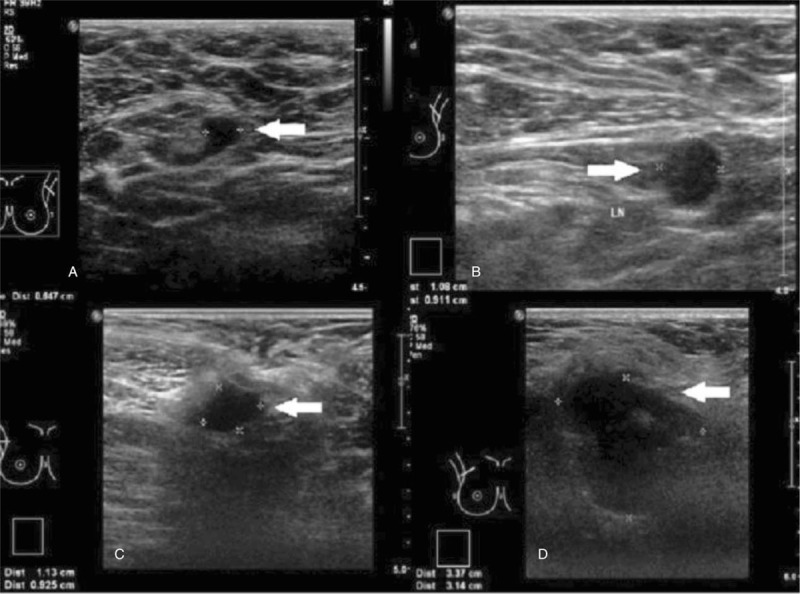
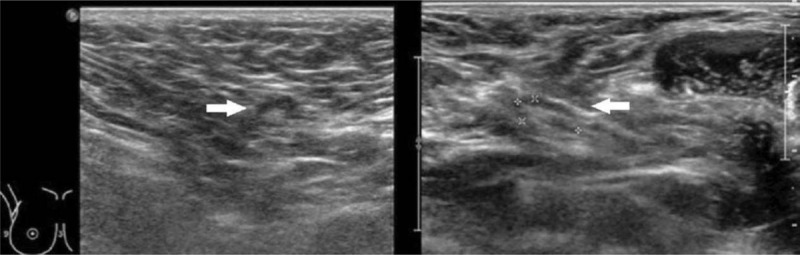
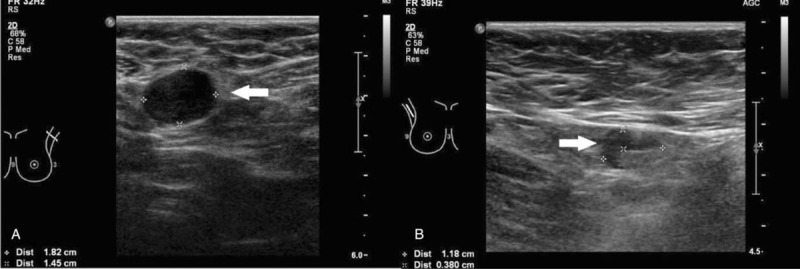
Figure 2 illustrates enlarged ALN metastases with thickened cortex (A), loss of fatty hilum (B), hypoechoic round shape (C) and irregular mass-like appearance (D). Figure 3 shows histologically proven normal ALN with preserved fatty hilum and normal cortical thickness. Figures 4 and 5 illustrate FP and false negative ultrasound images of ALN respectively.
Figure 2.
Ultrasound images of metastatic axillary nodes. Ultrasound images of metastatic axillary nodes A) with thickened cortex, B) round hypoechoic node, C) irregular node with loss of fatty hilum and D) enlarged node with mass-like appearance.
Figure 3.
Ultrasound images showing normal axillary nodes. Ultrasound images showing normal axillary nodes with ovoid shape, thin uniform cortices and preserved hyperechoic fatty hilum.
Figure 4.
Ultrasound images of false positive axillary nodes. Ultrasound images of false positive axillary nodes A) round hypoechoic node with loss of fatty hilum B) with thickened cortex > 3 mm.
Figure 5.
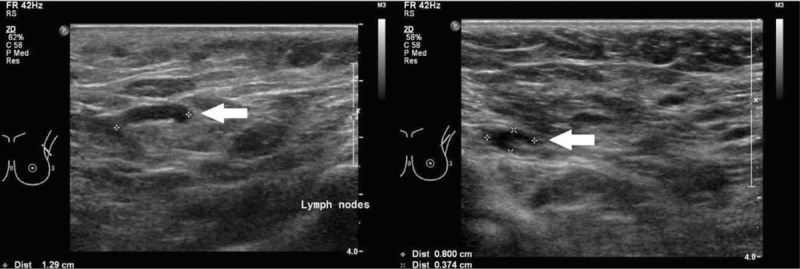
Ultrasound images of false negative axillary lymph nodes. Ultrasound images of false negative axillary lymph nodes showing normal looking axillary nodes with ovoid shape, thin uniform cortices and preserved hyperechoic fatty hilum.
With the exception of cortical thickness of > 3 mm, all the other ultrasound features including
-
(1)
round shape,
-
(2)
loss of fatty hilum,
-
(3)
mass-like appearance,
-
(4)
lymph node size > 5 mm, and
-
(5)
hypoechoic solid node on pre-operative ultrasound were significantly associated with pathologically positive ALN (Table 4).
Table 4.
Univariate logistic regression predicting pathological lymph node involvement according to individual ultrasound features.
| Ultrasound features (n = 383) | n | Pathologically Positive node, n (%) | P-value | OR (95% CI) |
| Cortical thickening | .126 | |||
| Not present | 341 | 193 (56.6%) | 1 | |
| Present | 42 | 29 (69%) | 1.71 (0.86–3.41) | |
| Loss of fatty hilum | <.05 | |||
| Not present | 295 | 149 (50.5%) | 1 | |
| Present | 88 | 73 (83%) | 4.77 (2.62–8.70) | |
| Hypoechoic solid node | <.05 | |||
| Not present | 251 | 121 (48.2%) | 1 | |
| Present | 132 | 101 (76.5%) | 3.50 (2.18–5.62) | |
| Mass-like appearance | <.05 | |||
| Not present | 296 | 151 (51%) | 1 | |
| Present | 87 | 71 (81.6%) | 4.26 (2.37–7.67) | |
| Round shape | <.05 | |||
| Not present | 361 | 202 (56%) | 1 | |
| Present | 22 | 20 (90.9%) | 5.22 (1.52–17.86) | |
| Lymph node size > 5mm | <.05 | |||
| Not present | 253 | 122 (48.2%) | 1 | |
| Present | 130 | 100 (76.9%) | 3.58 (2.22–5.77) |
CI = confidence interval, OR = odds ratio.
P-value refers to the comparison of pathologically positive node in each ultrasound feature studied.
4. Discussion
In this study, we found that the overall sensitivity, specificity and accuracy of pre-operative axillary ultrasound in detecting diseased nodes were 45.5%, 80.7%, and 60.3% respectively. The PPV and NPVs were 76.5% and 51.8% respectively.
Various studies have reported a NPV of 49 to 90.7% for the pre-operative axillary ultrasound.[18,19] Similar to our findings, previous studies concluded that exclusion of the axillary node metastases must not be solely rely on negative axillary ultrasound findings.[20–22] The images from ultrasound are closely related to the pathogenesis of tumor metastases to the affected lymph node. The morphological changes of the cortex, hilum as well as the shape occurs when tumor infiltration begins at the periphery of the lymph node. The tumor metastasizes via afferent lymphatic ducts in the subcapsular sinus with the tumor growing within the sinus. As the tumor grows, the peripheral neoagiogenesis starts and tumor infiltration progresses to the medullary sinus and the perinodal fat.[23–26]
There are many factors that contribute to the low NPV of axillary ultrasound alone in detecting nodal metastasis, namely small ALN size of ≤ 5 mm, skill of operator, neoadjuvant chemotherapy and primary tumor histopathological type where invasive lobular carcinoma is more likely to be falsely negative compared to invasive ductal carcinoma by nature of the infiltration by this subtype.[27–29] The low NPV result in our study could be due to the differences in skill and experience of the operator and resolution of the ultrasound that limit the examination. Another limiting factors for the lymph node detection in our study is the location of the diseased node that may be too deep to be visualized in the ultrasound plane. Patient characteristics like obesity with increased axillary fat mass possibly limits the detection of the ALN. Due to the low NPV, axillary ultrasound alone is not sufficient in determining node negativity and obviating SLNB for axillary staging. In 25% of the patients with negative sonographic assessment, a positive node may still be detected through SLNB.[14] Indeed, given its low sensitivity with FNR of more than 50%, SLNB still remains an important staging procedure for a negative axillary ultrasound.
In our study round shape, loss of fatty hilum and mass-like appearance had the highest PPVs of 87%, 83%, and 81.6% respectively. The PPV of hypoechoic solid node was 76.5% whilst PPV of lymph node size > 5 mm was 76.9%. Detection of lymph nodes < 5 mm may be affected by the quality of the ultrasound images and skill of the operator. Thus the sensitivity and specificity range widely.[30,31] Some studies report that normal lymph nodes have similar echogenicity with the surrounding fat tissue hence, any visible lymph nodes by ultrasonography may be considered as malignant.[32,33]
Some studies did not find significant correlation between size and lymph node involvement, in fact they suggested that the visibility of the pathological lymph nodes on ultrasonography is due to the alteration of the lymph nodes morphology or structure rather than the size of the ALNs itself.[15,34]
If the positivity of the lymph node was based on morphology i.e. loss of fatty hilum, round shape, hypoechoic and eccentric cortical hypertrophy, the specificity range became narrower at 88.4% to 98.1%.[22] The analysis on loss of fatty hilum in various studies demonstrated it to be the most strongly associated morphological feature of malignant ALN and the single best finding in detecting nodal metastasis.[35–37] The PPV varies from 93% to 100%.[35,37] Theoretically, these specific sign would denote higher volume disease as the tumor had invaded into the hilum. Apart from the loss of fatty hilum, a round node is reported to have higher PPV and the finding is always seen in more advanced nodal involvement.[15,22] The results of our study showed similar findings for round shape and mass-like appearance with PPV at 87% and 81.6% respectively.
As mentioned previously, metastases in lymph nodes commences in the periphery thus, cortical thickness would be at the early stage of nodal metastasis as compared to loss of fatty hilum, round shape and mass-like appearance. Based on previous studies, there were various cortical thickness cut-offs used to predict suspicious lymph nodes.[25,37–42] However, cortical thickness of > 3 mm in our study had a low PPV of 69.1% ORs of 1.71 (95% confidence interval: 0.86 -3.41, P = .126). 31% of patients with cortical thickness of > 3 mm were node negative. Other studies also reported low NPV and sensitivity for cortical thickness of > 3.8 mm at 53% and 56% respectively.[21] Univariate analysis in our study, failed to demonstrate the association of cortical thickness of > 3 mm with pathological positive ALN (P = .126). Out of 42 patients with thickened LN cortex, 40 were subjected to ALND and only 2 patients underwent SLNB, and one of them had further ALND. Thus, in resource constrained setting, cortical thickness of > 3 mm should be biopsied or SLNB rather than ALND, in view of the expected low volume disease and low PPV.
Emerging imaging modality like shear wave elastography (SWE) has been recognized as one of the non-invasive and useful method in examining the elasticity characteristics of a lesion. It has the ability in differentiating reactive from metastatic ALN. Previously, shear wave has been used to assess cervical lymph nodes and several organs include liver, prostate and pancreas.[43–47] Sensitivity and specificity of the SWE for diagnosing malignant cervical lymph nodes were reported as 81% and 85% in a systematic review and meta-analysis.[48] A more recent study by Luo et al, revealed that the qualitative SWE classifications of ALNs is more accurate in detecting metastatic ALNs than quantitative SWE and conventional ultrasound.[49] Undoubtedly, additional cost of the equipment and specialized training remain major barriers in the low and middle resource settings.
More importantly, mature outcome studies on low volume nodal disease (2 or less nodal involvement) for example, American College of Surgeons Oncology Group Z0011[50] and After Mapping of the Axilla: Radiotherapy or Surgery? trial,[51] have recommended that no further axillary dissection is needed in this subgroup of patients, hence there may be a less prescriptive view of biopsy of low nodal involvement by ultrasound and a direct ALND in grossly involved lymph nodes. Careful selection in low volume disease, breast cancer patients with clinical T1–T2 and N0 with negative axillary ultrasound should be offered SLNB with adequate oncological safety. The main challenge is to identify a subgroup of patients with low volume nodal disease that may benefit from SLNB alone without ALND.
Given the morbidity on both SLNB and ALND, the role of not performing surgical axillary staging is being investigated in low volume disease. There are three on-going randomized trials comparing axillary ultrasound with SLNB. One of on-going large scale multi-centric randomized controlled trial was Sentinel Node versus Observation after Axillary Ultrasound. It was designed to compare SLNB versus observation when axillary ultrasound is negative with small breast cancer candidates to breast conserving surgery.[52] Likewise, at Washington University School of Medicine, breast cancer patients with clinical T1–T2, N0 M0 and have a negative axillary ultrasound are being subjected to the study on either axillary ultrasound alone or SLNB.[53] Another similar called Intergroup-Sentinel-Mamma trial is conducted in Germany.[54] Having said that, considering the FNR of axillary ultrasound alone, some researchers have raised the question about the reliability of these trials. In contrary, axillary staging may gradually become less important with the emerging of molecular profiling tests, however, at the moment prognostic and predictive tests such as Oncotype DX still require information on nodal status to estimate risk of recurrence and selectively identify patient who would benefit from chemotherapy.
4.1. Limitation
We acknowledge that there are several limitations to this study. This includes its retrospective nature that may compromise the internal and external validation control numbers compared with a prospective study. It must be taken into consideration that the caveat of this study also includes SLNB false negative rates, which in a study by Martin et al was reported to be 3.1%.[55] For the purpose of future studies, a multivariate analysis of clinicopathological factors and ultrasound features combined would be useful in fine-tuning the criteria to determine the group of patients in whom ALND is really indicated.
5. Conclusion
SLNB remains an important staging procedure given the low NPV of more than 50% on axillary ultrasound. However, there are features on axillary ultrasound that confer high PPV for axillary involvement i.e. round shape, loss of fatty hilum, and mass-like appearance. In a low resource setting, these features may benefit from ALND without further pre-operative biopsies. However, pre-operative ultrasound guided biopsy for features with low PPV that is, cortical thickness > 3 mm should be consider to obviate the unnecessary morbidity associated with ALND.
Author contributions
Conceptualization: Kartini Rahmat, Nirmala Bhoo-Pathy, Nur Aishah Mohd Taib.
Data curation: Suniza Jamaris, Jazree Jamaluddin, Tania Islam, Mee Hoong See, Farhana Fadzli, Kartini Rahmat, Nur Aishah Mohd Taib.
Formal analysis: Jazree Jamaluddin, Tania Islam.
Funding acquisition: Nur Aishah Mohd Taib.
Investigation: Suniza Jamaris, Jazree Jamaluddin, Farhana Fadzli, Kartini Rahmat, Nur Aishah Mohd Taib.
Methodology: Suniza Jamaris, Jazree Jamaluddin, Farhana Fadzli, Nur Aishah Mohd Taib.
Project administration: Suniza Jamaris, Nur Aishah Mohd Taib.
Resources: Tania Islam, Farhana Fadzli, Kartini Rahmat, Nur Aishah Mohd Taib.
Supervision: Kartini Rahmat, Nirmala Bhoo-Pathy, Nur Aishah Mohd Taib.
Validation: Jazree Jamaluddin, Kartini Rahmat, Nur Aishah Mohd Taib.
Visualization: Suniza Jamaris, Tania Islam, Kartini Rahmat, Nur Aishah Mohd Taib.
Writing – original draft: Suniza Jamaris.
Writing – review & editing: Suniza Jamaris, Jazree Jamaluddin, Tania Islam, Mee Hoong See, Farhana Fadzli, Kartini Rahmat, Nirmala Bhoo-Pathy, Nur Aishah Mohd Taib.
Footnotes
Abbreviations: ALN = axillary lymph node, ALND = axillary lymph node dissection, CI = confidence interval, FNR = false negative rate, FP = false positive, NPV = negative predictive value, OR = odds ratio, PPV = positive predictive value, SLNB = sentinel lymph node biopsy, SWE = shear wave elastography, UMMC = University Malaya Medical Centre, UNB = ultrasound-guided biopsy needle.
How to cite this article: Jamaris S, Jamaluddin J, Islam T, See MH, Fadzli F, Rahmat K, Bhoo-Pathy N, Taib NAM. Is pre-operative axillary ultrasound alone sufficient to determine need for axillary dissection in early breast cancer patients? Medicine. 2021;100:19(e25412).
This study was financially supported by the Ministry of Education Malaysia (High Impact Research Grant [UM.C/HIR/MOHE/06]).
The authors have no conflicts of interest to disclose.
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
- [1].Graversen H, Blichert-Toft M, Andersen J, et al. Breast cancer: risk of axillary recurrence in node-negative patients following partial dissection of the axilla. Eur J Surg Oncol 1988;14:407–12. [PubMed] [Google Scholar]
- [2].Kissin M, Price A, Thompson E, et al. The inadequacy of axillary sampling in breast cancer. Lancet 1982;319:1210–2. [DOI] [PubMed] [Google Scholar]
- [3].Cady B, Sears H. Usefulness and technique of axillary dissection in primary breast cancer. J Clin Oncol 1986;4:623–4. [DOI] [PubMed] [Google Scholar]
- [4].Fisher B, Slack N, Obstetrics. Number of lymph nodes examined and the prognosis of breast carcinoma. Surg Gynecol Obstet 1970;131:79–88. [PubMed] [Google Scholar]
- [5].Yarnold J. Selective avoidance of lymphatic irradiation in the conservative management of breast cancer. Radiother Oncol 1984;2:79–92. [DOI] [PubMed] [Google Scholar]
- [6].Shim VC. How much is enough? the continuing debate on the axillary lymph node dissection in breast cancer. Perm J 2007;11:77–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ivens D, Hoe A, Podd T, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer 1992;66:136–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fraile M, Rull M, Julian F, et al. Sentinel node biopsy as a practical alternative to axillary lymph node dissection in breast cancer patients: an approach to its validity. Ann Oncol 2000;11:701–5. [DOI] [PubMed] [Google Scholar]
- [9].Gutnik LA, Matanje-Mwagomba B, Msosa V, et al. Breast cancer screening in low-and middle-income countries: a perspective from Malawi. J Glob Oncol 2016;2:04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Brand NR, Wasike R, Makhdomi K, et al. Sentinel lymph node biopsy pathology and 2-year postsurgical recurrence of breast cancer in kenyan women. J Glob Oncol 2017;4:01–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Black D. Axillary ultrasound: for all, for none, to diagnose positive nodes, or to support avoiding sentinel lymph node biopsy altogether. Ann Surg Oncol 2017;24:64–9. [DOI] [PubMed] [Google Scholar]
- [12].Harnett A, Smallwood J, Titshall V, et al. Diagnosis and treatment of early breast cancer, including locally advanced disease—summary of NICE guidance. Bmj 2009;338:b438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rautiainen S, Masarwah A, Sudah M, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology 2013;269:54–60. [DOI] [PubMed] [Google Scholar]
- [14].Diepstraten SC, Sever AR, Buckens CF, et al. Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 2014;21:51–9. [DOI] [PubMed] [Google Scholar]
- [15].Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol 2008;191:646–52. [DOI] [PubMed] [Google Scholar]
- [16]. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Surgical Axillary staging. Breast cancer (Version 2018). https://www2.tri-kobe.org/nccn/guideline/archive/breast2018/english/breast_v1.pdf. [Google Scholar]
- [17].Pathy NB, Yip CH, Taib NA, et al. Breast cancer in a multi-ethnic Asian setting: results from the Singapore–Malaysia hospital-based breast cancer registry. Breast 2011;20:S75–80. [DOI] [PubMed] [Google Scholar]
- [18].Jung J, Park H, Park J, et al. Accuracy of preoperative ultrasound and ultrasound-guided fine needle aspiration cytology for axillary staging in breast cancer. ANZ J Surg 2010;80:271–5. [DOI] [PubMed] [Google Scholar]
- [19].Rajesh Y, Ellenbogen S, Banerjee B. Preoperative axillary ultrasound scan: its accuracy in assessing the axillary nodal status in carcinoma breast. Breast 2002;11:49–52. [DOI] [PubMed] [Google Scholar]
- [20].Leenders M, Kramer G, Belghazi K, et al. Can we identify or exclude extensive axillary nodal involvement in breast cancer patients preoperatively?Journal of oncology 2019;2019:8404035. 10.1155/2019/8404035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee B, Lim AK, Krell J, et al. The efficacy of axillary ultrasound in the detection of nodal metastasis in breast cancer. AJR Am J Roentgenol 2013;200:W314–20. [DOI] [PubMed] [Google Scholar]
- [22].Alvarez S, Añorbe E, Alcorta P, et al. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: a systematic review. AJR Am J Roentgenol 2006;186:1342–8. [DOI] [PubMed] [Google Scholar]
- [23].Maxwell F, De Margerie Mellon C, Bricout M, et al. Diagnostic strategy for the assessment of axillary lymph node status in breast cancer. Diagn Interv Imaging 2015;96:1089–101. [DOI] [PubMed] [Google Scholar]
- [24].Ecanow JS, Abe H, Newstead GM, et al. Axillary staging of breast cancer: what the radiologist should know. Radiographics 2013;33:1589–612. [DOI] [PubMed] [Google Scholar]
- [25].Choi YJ, Ko EY, Han B-K, et al. High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast 2009;18:119–22. [DOI] [PubMed] [Google Scholar]
- [26].Mainiero MB. Regional lymph node staging in breast cancer: the increasing role of imaging and ultrasound-guided axillary lymph node fine needle aspiration. Radiol Clin North Am 2010;48:989–97. [DOI] [PubMed] [Google Scholar]
- [27].Stachs A, Göde K, Hartmann S, et al. Accuracy of axillary ultrasound in preoperative nodal staging of breast cancer-size of metastases as limiting factor. SpringerPlus 2013;2:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tucker NS, Cyr AE, Ademuyiwa FO, et al. Axillary ultrasound accurately excludes clinically significant lymph node disease in patients with early stage breast cancer. Ann Surg 2016;264:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Boughey JC, Middleton LP, Harker L, et al. Utility of ultrasound and fine-needle aspiration biopsy of the axilla in the assessment of invasive lobular carcinoma of the breast. Am J Surg 2007;194:450–5. [DOI] [PubMed] [Google Scholar]
- [30].Bruneton JN, Caramella E, Hery M, et al. Axillary lymph node metastases in breast cancer: preoperative detection with US. Radiology 1986;158:325–6. [DOI] [PubMed] [Google Scholar]
- [31].Pamilo M, Soiva M, Lavast, et al. Real-time ultrasound, axillary mammography, and clinical examination in the detection of axillary lymph node metastases in breast cancer patients. J Ultrasound Med 1989;8:115–20. [DOI] [PubMed] [Google Scholar]
- [32].Mustonen P, Farin P, Kosunen O. Ultrasonographic detection of metastatic axillary lymph nodes in breast cancer. Ann Chir Gynaecol 1990;79:15–8. [PubMed] [Google Scholar]
- [33].De Freitas R, Jr, Costa M, Schneider S, et al. Oncology tBAoS. Accuracy of ultrasound and clinical examination in the diagnosis of axillary lymph node metastases in breast cancer. Eur J Surg Oncol 1991;17:240–4. [PubMed] [Google Scholar]
- [34].Obwegeser R, Lorenz K, Hohlagschwandtner M, et al. Axillary lymph nodes in breast cancer: is size related to metastatic involvement? World J Surg 2000;24:546–50. [DOI] [PubMed] [Google Scholar]
- [35].Abe H, Schmidt RA, Kulkarni K, et al. Axillary lymph nodes suspicious for breast cancer metastasis: sampling with US-guided 14-gauge core-needle biopsy—clinical experience in 100 patients. Radiology 2009;250:41–9. [DOI] [PubMed] [Google Scholar]
- [36].Britton P, Goud A, Godward S, et al. Use of ultrasound-guided axillary node core biopsy in staging of early breast cancer. Eur Radiol 2009;19:561–9. [DOI] [PubMed] [Google Scholar]
- [37].Mainiero MB, Cinelli CM, Koelliker SL, et al. Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. AJR Am J Roentgenol 2010;195:1261–7. [DOI] [PubMed] [Google Scholar]
- [38].Deurloo E, Tanis P, Gilhuijs K, et al. Reduction in the number of sentinel lymph node procedures by preoperative ultrasonography of the axilla in breast cancer. Eur J Cancer 2003;39:1068–73. [DOI] [PubMed] [Google Scholar]
- [39].Stachs A, Thi AT-H, Dieterich M, et al. Assessment of ultrasound features predicting axillary nodal metastasis in breast cancer: the impact of cortical thickness. Ultrasound Int Open 2015;1:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cho N, Moon WK, Han W, et al. Preoperative sonographic classification of axillary lymph nodes in patients with breast cancer: node-to-node correlation with surgical histology and sentinel node biopsy results. AJR Am J Roentgenol 2009;193:1731–7. [DOI] [PubMed] [Google Scholar]
- [41].Zhu Y, Zhou W, Zhou JQ, et al. Axillary staging of early-stage invasive breast cancer by ultrasound-guided fine-needle aspiration cytology: which ultrasound criteria for classifying abnormal lymph nodes should be adopted in the post-ACOSOG Z0011 trial era? J Ultrasound Med 2016;35:885–93. [DOI] [PubMed] [Google Scholar]
- [42].Saffar B, Bennett M, Metcalf C, et al. Retrospective preoperative assessment of the axillary lymph nodes in patients with breast cancer and literature review. Clin Radiol 2015;70:954–9. [DOI] [PubMed] [Google Scholar]
- [43].Kanamoto M, Shimada M, Ikegami T, et al. Real time elastography for noninvasive diagnosis of liver fibrosis. J Hepatobiliary Pancreat Surg 2009;16:463. [DOI] [PubMed] [Google Scholar]
- [44].Hong Y, Liu X, Li Z, et al. Real-time ultrasound elastography in the differential diagnosis of benign and malignant thyroid nodules. J Ultrasound Med 2009;28:861–7. [DOI] [PubMed] [Google Scholar]
- [45].Miyagawa T, Tsutsumi M, Matsumura T, et al. Real-time elastography for the diagnosis of prostate cancer: evaluation of elastographic moving images. Jpn J Clin Oncol 2009;39:394–8. [DOI] [PubMed] [Google Scholar]
- [46].Dietrich C, Hirche T, Ott M, et al. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy 2009;41:718–20. [DOI] [PubMed] [Google Scholar]
- [47].Alam F, Naito K, Horiguchi J, et al. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: comparison with conventional B-mode sonography. AJR Am J Roentgenol 2008;191:604–10. [DOI] [PubMed] [Google Scholar]
- [48].Suh CH, Choi YJ, Baek JH, et al. The diagnostic performance of shear wave elastography for malignant cervical lymph nodes: a systematic review and meta-analysis. Eur Radiol 2017;27:222–30. [DOI] [PubMed] [Google Scholar]
- [49].Luo S, Yao G, Hong Z, et al. Qualitative classification of shear wave elastography for differential diagnosis between benign and metastatic axillary lymph nodes in breast cancer. Front Oncol 2019;9:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Olson JA, Jr, Mccall LM, Beitsch P, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. Oncology 2007;26:3530–5. [DOI] [PubMed] [Google Scholar]
- [51].Donker M, Van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 2014;15:1303–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Gentilini O, Veronesi U. Abandoning sentinel lymph node biopsy in early breast cancer? A new trial in progress at the European Institute of Oncology of Milan (SOUND: Sentinel node vs Observation after axillary UltraSouND). Breast 2012;21:678–81. [DOI] [PubMed] [Google Scholar]
- [53].Washington USoM. Axillary ultrasound with or without sentinel lymph node biopsy in detecting the spread of breast cancer in patients receiving breast conservation therapy [ClinicalTrials.gov identifier NCT01821768]. US National Institutes of Health, ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/NCT01821768. [Google Scholar]
- [54].Reimer T, Von Minckwitz G, Loibl S, et al., Abstract OT2-04-02: Comparison of axillary sentinel lymph node biopsy versus no axillary surgery in patients with early-stage invasive breast cancer and breast-conserving surgery: A randomized prospective surgical trial. The Intergroup-Sentinel-Mamma (INSEMA)-trial. 2017, AACR. [Google Scholar]
- [55].Martin RC, Ii AC, Scoggins CR, et al. Clinicopathologic factors associated with false-negative sentinel lymph-node biopsy in breast cancer. Ann Surg 2005;241:1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


