Abstract
Tissue engineered vascular grafts (TEVGs) represent a promising therapeutic option for emergency vascular intervention. Although the application of small-diameter TEVGs using patient-specific primary endothelial cells (ECs) to prevent thrombosis and occlusion prior to implantation could be hindered by the long time course required for in vitro endothelialization, human induced pluripotent stem cells (hiPSCs) provide a robust source to derive immunocompatible ECs (hiPSC-ECs) for immediate TEVG endothelialization. To achieve clinical application, hiPSC-ECs should be derived under culture conditions without the use of animal-derived reagents (xenogeneic-free conditions), to avoid unwanted host immune responses from xenogeneic reagents. However, a completely xenogeneic-free method of hiPSC-EC generation has not previously been established. Herein, we substituted animal-derived reagents used in a standard method of xenogeneic hiPSC-EC differentiation with functional counterparts of human origin. As a result, we generated xenogeneic-free hiPSC-ECs (XF-hiPSC-ECs) with similar marker expression and function to those of human primary ECs. Furthermore, XF-hiPSC-ECs functionally responded to shear stress with typical cell alignment and gene expression. Finally, we successfully endothelialized decellularized human vessels with XF-hiPSC-ECs in a dynamic bioreactor system. In conclusion, we developed xenogeneic-free conditions for generating functional hiPSC-ECs suitable for vascular tissue engineering, which will further move TEVG therapy toward clinical application.
Keywords: Human induced pluripotent stem cells, endothelial cells, xenogeneic-free, vascular tissue engineering
Introduction
Readily available vascular grafts are urgently needed for treating acute vascular injuries. Native vessels and synthetic prosthesis are widely used as vascular grafts in the clinic, though their application can be significantly hindered by the restricted access to suitable native vessel segments and the risks of infection and thrombosis. Alternatively, tissue engineered vascular grafts (TEVGs) offer a non-synthetic approach for readily available vascular therapy. To develop TEVGs, primary or human induced pluripotent stem cell (hiPSC)-derived cells are cultured on a bioabsorbable scaffold within dynamic bioreactors to form vessel-like tissue engineered conduits [1,2]. TEVGs can be further decellularized as “off-the-shelf” acellular vascular grafts for emergency vascular intervention [1–3]. To date, acellular, large diameter (6 mm of inner diameter) TEVGs have led to promising results in phase 2 clinical trials for hemodialysis access [4]. However, for small diameter (< 6 mm) application, endothelial cells (ECs) are required to cover the luminal surface of acellular TEVGs to prevent blood clotting and thrombogenesis [1]. To endothelialize TEVGs using autologous primary ECs, an average of 23 days is required to complete the process from obtaining patient ECs to finalizing TEVG endothelization [1], which makes this approach for small diameter acellular TEVGs less practical for urgent interventions. Additionally, previous studies described that primary EC seeding onto the lumen of decellularized TEVGs resulted in an average percent coverage of 64% or lower with a wide variation [1,5], indicating that previously described methods of TEVG endothelialization may need further optimization for more effective therapeutic efficacy.
Fortunately, human induced pluripotent stem cell (hiPSC) technology may offer a unique opportunity to address the above issue. hiPSCs can be derived from somatic cells via the ectopic expression of essential stem cell transcription factors [6]. hiPSCs are self-renewable and can differentiate into various types of somatic cells including ECs (hiPSC-ECs), which make hiPSCs an unlimited reservoir for obtaining vascular cells. More importantly, by modulating the expression of human leukocyte antigens (HLAs), it is possible to establish minimal- or non-immunogenic “universal” hiPSCs [7–10], which when differentiated into hiPSC-ECs could be immuno-compatible to any patient. Further, these cells could be mass produced and cryopreserved as a robust cell source for TEVG endothelialization. Therefore, future application of hiPSC-ECs could eliminate the need for autologous primary ECs to endothelialize TEVGs, effectively expedite the endothelialization process, and dramatically enhance the readiness of TEVG therapy for emergency vascular treatments.
A growing body of research has described approaches to derive functional hiPSC-ECs [11–18]. To meet clinical requirements, hiPSC-ECs should be generated under xenogeneic-free conditions, since the use of animal-derived reagents may cause xenogeneic immune responses in human recipients and transmit life-threatening zoonotic diseases [19]. Establishment and expansion of hiPSCs has been accomplished under xenogeneic-free conditions [20], while a completely xenogeneic-free approach for EC differentiation from hiPSCs has yet to be established (Table S1). It is worth noting that chemically-defined methods for generating ECs from human pluripotent stem cells have been reported [11,21]. However, reagents with animal-derived components (mouse tissue-derived Matrigel, mTeSR1 medium, StemPro-34 etc.) were commonly employed in these protocols [11,21]. Therefore, it is essential to establish a completely xenogeneic-free approach to obtain functional hiPSC-ECs for endothelializing vascular grafts for future therapeutic applications.
Herein, we aimed to establish a xenogeneic-free method to derive ECs from hiPSCs for acellular vascular graft endothelialization. Based on a standard xenogeneic protocol for obtaining functional hiPSC-ECs, we have replaced animal-derived reagents with their functional counterparts of human origin, including serum supplements, serum albumin, recombinant growth factors, and extracellular matrix proteins (Fig. 1A). By using our approach, we derived xenogeneic-free hiPSC-ECs (XF-hiPSC-ECs) readily responsive to shear stress and suitable for endothelializing decellularized human vessels. Our studies have established a robust approach to attain xenogeneic-free hiPSC-ECs suitable for vascular graft endothelialization, which sets the stage for future production of promptly available endothelialized vascular grafts for potential human clinical application.
Figure 1.
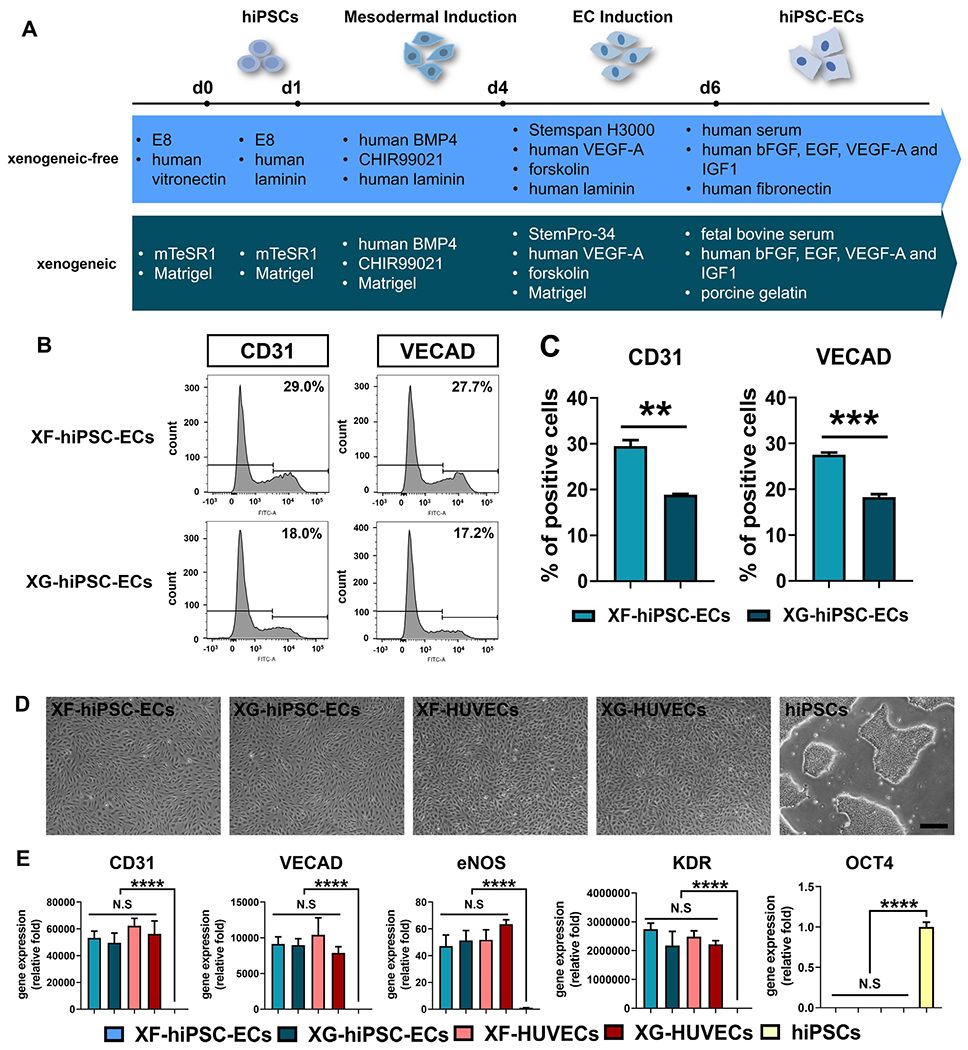
Derivation of hiPSC-ECs under xenogeneic-free conditions. (A) Schematic illustration of EC differentiation of hiPSCs under xenogeneic-free or xenogeneic conditions. Essential reagents utilized in both methods are listed. See Materials and Methods and Table S2 for details of the methods. BMP4: bone morphogenetic protein 4, 25 ng/mL for xenogeneic-free and xenogeneic mesodermal induction (day1-4); CHIR99021: glycogen synthase kinase (GSK) 3 inhibitor, 6 μM for xenogeneic-free and xenogeneic mesodermal induction (day1-4); VEGF-A: vascular endothelial growth factor A, 200 ng/mL for xenogeneic-free and xenogeneic EC induction (day 4-6) and 50 ng/mL for xenogeneic-free and xenogeneic hiPSC-EC expansion (day 6 and later); forskolin: 2 μM for xenogeneic-free and xenogeneic EC induction (day 4-6); FGF2: fibroblast growth factor 2, 10 ng/mL for xenogeneic-free hiPSC-EC expansion (day 6 and later); EGF: epithelial growth factor, 5 ng/mL for xenogeneic-free hiPSC-EC expansion (day 6 and later); IGF1: insulin growth factor 1, 20 ng/mL for xenogeneic-free hiPSC-EC expansion (day 6 and later). (B) Representative plots of percentage of CD31-positive or VE-cadherin (VECAD)-positive XF-hiPSC-ECs or XG-hiPSC-ECs by flow cytometry analysis. (C) Quantification of percentage of CD31-positive or VE-cadherin (VECAD)-positive XF-hiPSC-ECs or XG-hiPSC-ECs by flow cytometry analysis. (Two-tailed unpaired Student’s T-test; Mean values and S.E.M indicated by the error bars are shown; n=3; N.S: not significant). (D) Bright field images of hiPSC-ECs and human primary ECs cultured under xenogeneic-free or xenogeneic conditions (XF-hiPSC-ECs, XG-hiPSC-ECs, XF-human umbilical venous endothelial cells [XF-HUVECs] and XG-HUVECs), and hiPSCs cultured under xenogeneic-free conditions. Scale bar: 200 μm. (E) Relative mRNA transcript amounts of EC markers (CD31, VECAD, eNOS and KDR) and pluripotency marker OCT4 in XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs, XG-HUVECs, and hiPSCs cultured under xenogeneic-free conditions. Values in the y axis represent fold changes relative to human GAPDH expression. Gene expression in each group was normalized to that of hiPSCs (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; ****: p<0.0001; N.S: not significant).
Materials and Methods
Cultivation of human induced pluripotent stem cells (hiPSCs)
As previously described [22], unedited, wildtype human neonatal fibroblasts derived from a healthy female donor were reprogrammed into hiPSCs using non-integrative Sendai viral particles that encode the OCT4, KLF4, SOX2, and c-MYC genes. A human leukocyte antigen-C (HLA-C) retained (HLA-A-, HLA-B-, CIITA-knockout) hiPSC line (HLA-C-retained hiPSCs; named as 585A1 hiP-SCs in previously published study) was derived from peripheral blood mononuclear cells isolated from a male donor via episomal vector-mediated cellular reprogramming as previously described [9].
For xenogeneic culture, both wildtype and HLA-C-retained hiP-SCs were maintained in mTeSR1 medium (STEMCELL Technologies) on Growth Factor Reduced (GFR)-Matrigel (10 μg/cm2, Corning; xenogeneic reagent derived from mouse tissues) coated surfaces at 37°C and 5% CO2 with daily medium changes. Cells were passaged at a 1:6 ratio every 5-7 days by 0.5 mM ethylenediaminetetraacetic acid (EDTA; ThermoFisher) when they reached 80% confluency. To cryopreserve the xenogeneic hiPSCs, cell suspension in mTeSR1 medium was mixed with xenogeneic cryopreservation medium [80% (v/v) fetal bovine serum (FBS, Sigma-Aldrich) and 20% (v/v) dimethyl sulfoxide (DMSO; Sigma-Aldrich)] at 1:1 ratio (v:v) and cryopreserved in liquid nitrogen.
For xenogeneic-free culture, both wildtype and HLA-C-retained hiPSCs were maintained in xenogeneic-free Essential 8 Medium (E8 medium; ThermoFisher) on 1 μg/cm2 recombinant human Vitronectin XF™ (STEMCELL Technologies) coated surfaces at 37°C and 5% CO2 with daily medium changes [20]. Cells were passaged at a 1:6 ratio every 5-7 days by 0.5 mM EDTA when they reached 80% confluency. To cryopreserve the xenogeneic-free hiP-SCs, cells were resuspended in xenogeneic-free cryopreservation medium (CryoStor® CS2, STEMCELL Technologies) and cryopreserved in liquid nitrogen.
Cultivation of human primary endothelial cells
Human primary endothelial cells, including human umbilical venous endothelial cells (HUVECs) and human aortic endothelial cells (HAECs), were purchased from Lonza and cultured under either xenogeneic or xenogeneic-free conditions.
For xenogeneic culture, primary ECs within passage six were expanded in xenogeneic primary EC expansion medium (complete Endothelial Growth Medium, EGM-2; Lonza; medium composition listed in Table S2) on surfaces coated with 0.1% (w/v) porcine skin-derived gelatin (Sigma-Aldrich) at 37°C and 5% CO2 with medium changes every other day. Xenogeneic primary ECs (XG-primary ECs, including XG-HUVECs or XG-HAECs) were passaged by 0.05% trypsin-EDTA at a 1:3 ratio (ThermoFisher; xenogeneic reagent derived from porcine pancreas) upon reaching 80% confluency. To cryopreserve the XG-primary ECs, cell suspension in xenogeneic primary EC expansion medium was mixed with xenogeneic cryopreservation medium at 1:1 ratio (v:v) and cryopreserved in liquid nitrogen.
For xenogeneic-free culture, primary ECs within passage six were expanded in xenogeneic-free primary EC expansion medium (medium composition listed in Table S2) on surfaces coated with 2 μg/cm2 human fibronectin (Sigma-Aldrich) at 37°C and 5% CO2 with medium changes every other day. Xenogeneic-free primary ECs (XF-primary ECs, including XF-HUVECs or XF-HAECs) were passaged by xenogeneic-free, chemically defined TrypLE (ThermoFisher) at a 1:3 ratio upon reaching 80% confluency. To cryopreserve XF-primary ECs, cells were resuspended in xenogeneic-free cryopreservation medium and cryopreserved in liquid nitrogen.
Generation of ECs from hiPSCs under xenogeneic conditions
Xenogeneic hiPSC-ECs were obtained via monolayer-directed differentiation based on a previously established method [11]. After 5 days of culture in mTeSR1 medium, hiPSCs were dissociated into single cells with 0.5 mM EDTA on day 0, plated into 10 μg/cm2 Matrigel-coated 6-well plates at a density of 37,000-47,000 cells/cm2, and cultured with mTeSR1 medium containing 10 μM ROCK inhibitor Y-27632 (Calbiochem). On day 1, mTeSR1 medium was replaced with xenogeneic mesoderm induction medium (medium composition listed in Table S2), and cells were cultured for 3 days without changing media. On day 4, the cells were cultured with xenogeneic EC induction medium (medium composition listed in Table S2) with daily medium changes. On day 6 of differentiation, cells were subjected to magnetic-activated cell sorting (MACS) to isolate a homogenous cell population with expression of endothelial surface marker CD31 in general, dissociated with 0.05% trypsin-EDTA, collected and screened through a 70 μm cell strainer (Corning) to obtain single cell suspensions. Cells were then resuspended in plain Dulbecco’s Modified Eagle Medium (DMEM; ThermoFisher), mixed with pre-washed CD31 Endothelial Cell Dynabeads (ThermoFisher), incubated at 4°C for 20 minutes, and CD31 positive cells were isolated by magnetic attraction. CD31-positive, xenogeneic hiPSC-ECs were then cultured in xenogeneic hiPSC-ECs expansion medium (medium composition listed in Table S2) on surfaces coated with 0.1% porcine gelatin. Medium was changed every other day. When cells reached 80% confluency, xenogeneic hiPSC-ECs (XG-hiPSC-ECs) were dissociated with 0.05% trypsin-EDTA and subcultured at a density of 10,000 cells/cm2 on porcine gelatin-coated plates. To cryopreserve the XG-hiPSC-ECs, cell suspensions in xenogeneic hiPSC-ECs expansion medium was mixed with xenogeneic cryopreservation medium at 1:1 ratio (v:v) and cryopreserved in liquid nitrogen.
Generation of ECs from hiPSCs under xenogeneic-free conditions
The xenogeneic-free method for obtaining hiPSC-ECs was established based on the standard xenogeneic method described above. After 5 days of culture in E8 medium on a Vitronectin-coated plate, hiPSCs were dissociated with 0.5 mM EDTA on day 0, plated on surfaces coated with 0.5 μg/cm2 human recombinant laminin 511 (iMatrix, Amsbio) at a density of 37,000-47,000 cells/cm2, and cultured with E8 medium containing 10 μM Y-27632. On day 1, E8 medium was replaced with xenogeneic-free mesoderm induction medium (medium composition listed in Table S2), and cells were cultured for 3 days without changing media. On day 4, cells were cultured with xenogeneic-free EC induction medium (medium composition listed in Table S2) with medium refreshed every day. After 6 days of differentiation, homogenous xenogeneic-free hiPSC-EC population were isolated using Dynabeads-mediated MACS under xenogeneic-free conditions. In general, cells were dissociated with TrypLE and filtered through a 70 μm cell strainer to obtain single-cell suspensions. Cells were then resuspended in plain DMEM, mixed with pre-washed CD31 Endothelial Cell Dynabeads, incubated at 4°C for 20 minutes, and CD31 positive cells were isolated by magnetic attraction. Note that all the reagents for MACS assay, including TrypLE, plain CTS KnockOut DMEM and Dynabeads, were xenogeneic-free. Isolated CD31-positive hiPSC-ECs were resuspended in xenogeneic-free hiPSC-EC expansion medium (medium composition listed in Table S2) and plated on 2 μg/cm2 human fibronectin (Sigma-Aldrich)-coated plates with medium changed every other day. When cells reached 80% confluency, xenogeneic-free hiPSC-ECs (XF-hiPSC-ECs) were dissociated with TrypLE and subcultured at a density of 10,000 cells/cm2 on human fibronectin-coated plates. To cryopreserve the XF-hiPSC-ECs, cells were resuspended in xenogeneic-free cryopreservation medium and cryopreserved in liquid nitrogen.
Flow cytometry analysis
Single cell suspensions were fixed with 2% paraformaldehyde (PFA; Electron Microscopy Sciences) for 10 minutes at room temperature (RT) and then blocked with 5% bovine serum albumin (BSA) in PBST (PBS containing 0.3% Triton X-100 [Sigma-Aldrich]) for 30 minutes at RT. Samples were then incubated with FITC-conjugated primary antibody for 30 minutes at 4°C according to the manufacturer specifications. Mouse IgG isotype controls (ThermoFisher) were also applied. Fluorescence of the stained cells was measured using LSRII flow cytometer (BD biosciences) and analyzed by Flow-Jo software (Treestar). Three independent batches of cells were subjected to flow cytometry analysis for each gene. Antibody information is listed in Table S4.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was isolated using the TRIzol RNA Isolation Kit (ThermoFisher) and then reversely transcribed to cDNA using an iScript cDNA Synthesis Kit (Bio-Rad). Quantitative polymerase chain reaction (qPCR) was performed using IQ SYBR green supermix (BioRad) with human GAPDH used as an endogenous housekeeping control. Three biological replicates were completed for each candidate gene. Table S3 lists the primer sequences for the qPCR assay.
Immunostaining of cultured cells
Cells were rinsed with phosphate-buffered saline (PBS, ThermoFisher), fixed in 4% PFA for 10 minutes at room temperature (RT), permeabilized in PBST buffer for 20 minutes at RT, and blocked in 10% normal goat serum (NGS; ThermoFisher) in PBST for 30 minutes at RT. Then the cells were stained with primary antibodies in PBST containing 1% NGS overnight at 4°C. Subsequently, cells were washed with PBS, incubated with secondary antibody (1:1000 in PBST with 1% NGS) for one hour at RT in the dark, and washed three times with PBS. Cell nuclei were counterstained with DAPI (ThermoFisher). Fluorescent images were taken on a Leica fluorescence microscope. ImageJ software was used to quantify the percentage of fluorescently positive cells. Antibody information is listed in Table S4.
Tube formation assay
A layer of GFR-Matrigel (non-diluted) was coated on the surface of 24 well-culture plates and incubated at 37°C for 60 min to allow for polymerization. 100,000 XF-hiPSC-ECs (cultured in xenogeneic-free hiPSC-EC expansion medium), XG-hiPSC-ECs (cultured in xenogeneic hiPSC-EC expansion medium), XF-HUVECs (cultured in xenogeneic-free primary EC expansion medium), or XG-HUVECs (cultured in xenogeneic primary EC expansion medium) were respectively seeded onto polymerized Matrigel surface in 500 μL medium. After a 24-hour incubation, capillary-like networks were observed and imaged using a phase-contrast microscope. Quantification of networks, tube length and number of nodes per field was evaluated by ImageJ software. Three independent batches of cells were subjected to this tube formation assay for each cell type, and at least three randomly selected areas in each batch for each cell type were recorded and analyzed, respectively.
Acetylated-low density lipoprotein uptake assay
XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs, or XG-HUVECs in serum-free medium (EBM-2 Basal Medium, Lonza) were incubated with fluorescently-labeled Dil acetylated-low density lipoproteins (ac-LDL, ThermoFisher) at 37°C for 4 hours according to the manufacturer specifications. Cells were then rinsed twice with PBS and then imaged on a Leica fluorescence microscope. Undifferentiated hiPSCs cultured under xenogeneic-free conditions were included as a negative control. ImageJ software was used for quantification. Three independent batches of cells were subjected to ac-LDL treatment for each cell type, and at least three randomly selected areas in each batch for each cell type were recorded and analyzed, respectively.
Shear stress assay
To characterize the endothelial phenotype under flow, XF-hiPSC-ECs or XF-primary ECs (including XF-HUVECs and XF-HAECs) were allowed to adhere in the μ-Slide I0.4 luer (ibidi, slide surface coated with 2 μg/cm2 human fibronectin) at a density of 175,000 cells/slide with xenogeneic-free hiPSC-EC or primary EC expansion medium for 24 hours, respectively (Fig. 5A). On the next day, slide chambers were connected to the circuit with medium flowing to generate laminar shear stress at 15 dyne/cm2. After two days of dynamic culture, cells were subjected to bright field imaging, fixed for fluorescent staining or collected for qRT-PCR analysis. Cells cultured in chamber slides under static culture condition (without connecting to the flow circuit) were used as control. Three independent batches of cells were subjected to dynamic culture for both cell types.
Figure 5. Endothelialization of decelluiarized vessels using XF-hiPSC-ECs.
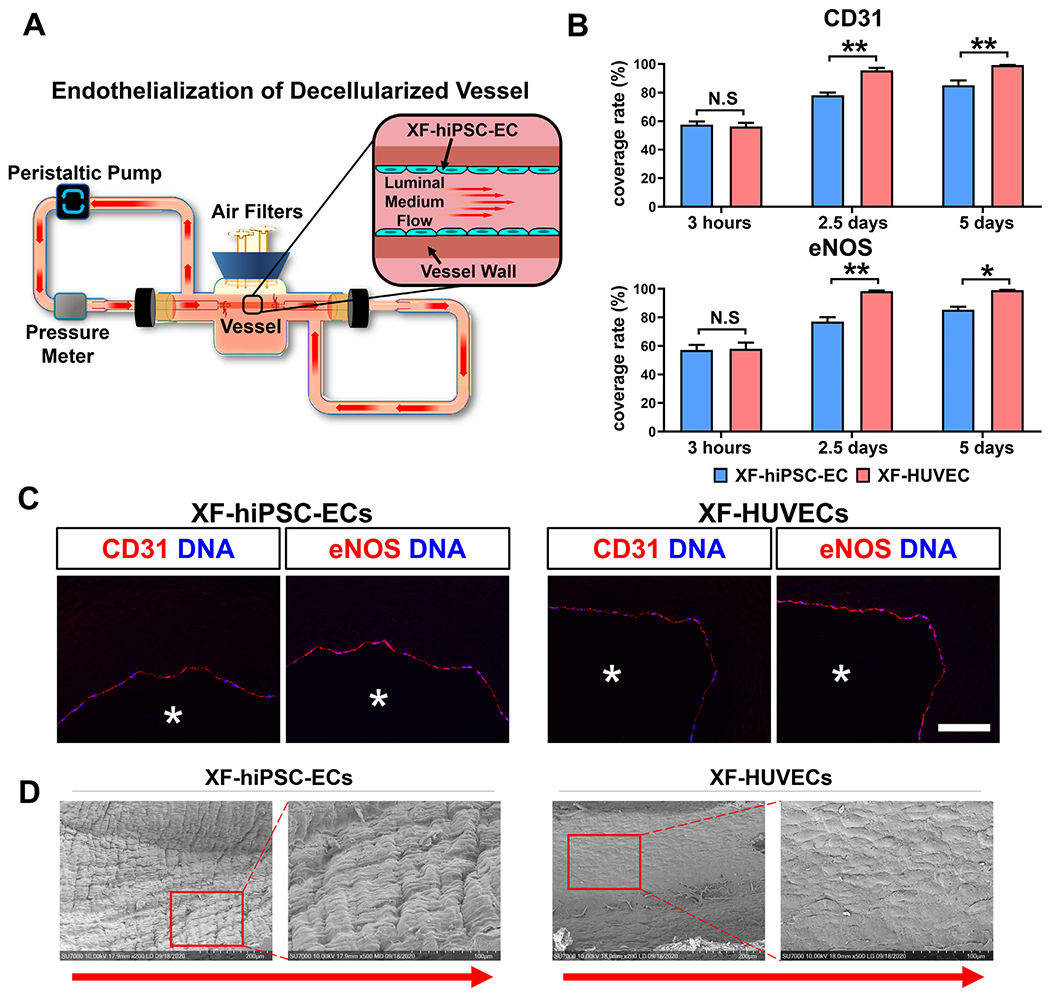
(A) Schematic illustration of the dynamic bioreactor system for endothelializing deceiluiarized human vessels using XF-hiPSC-ECs. (B) Percentage of the luminal surface of decelluiarized vessels covered by the XF-hiPSC-ECs or XF-HUVECs 3 hours (prior to adding shear stress), 2.5 days and 5 days after cell seeding based on the staining in Fig. S11. Quantification was performed based on three representative sections in each endothelialized vessel, and three vessels were independently endothelialized using each cell type (Two-way ANOVA showed that there was significant interaction between the time point and cell type/culture conditions [CD31: p<0.01; eNOS: p<0.05]. Mean values and S.E.M indicated by the error bars are shown; n=3; *: p<0.05; **: p<0.01; N.S: not significant). (C) Immunofluorescence staining of EC markers (CD31 and eNOS) of decelluiarized vessels after endotheiialization using XF-hiPSC-ECs or XF-HUVECs for 5 days (original magnification: 20x). DNA (nuclear) was counterstained by DAPI in immunostaining. Asterisk indicates the vessel lumen. Scale bar: 100 μm. (D) Representative images of luminal surface of decellularized human vessel endothelialized with XF-hiPSC-ECs or XF-HUVECs for 5 days via scanning electron microscopy. The right panels for each cell type (magnification 500X) indicate the magnified areas within the red frames in left images (magnification: 200X). Direction of arrows indicates the direction of luminal medium flow.
Quantification of cell alignment, cell surface area and level of elongation
To quantify the alignment, surface area and elongation of the cells cultured in the presence or absence of shear stress, samples were fixed in 4% PFA and immunostained with VE-cadherin to confirm the normal EC marker expression. Moreover, nuclei were counterstained with DAPI for cell alignment quantification, and filamentous actin was fluorescently labeled with Phalloidin (ThermoFisher) to further confirm the alignment. Images of cell nuclei were used to determine the nuclear centroid and orientation angle relative to the direction of medium flow. Cells with nuclei orientation within ±23° of the axis of flow were quantified as aligned using ImageJ software [23]. Surface area of cells was also evaluated using ImageJ as previously described [24]. To measure the level of elongation, the length of long axis and short axis of each cell was measured using ImageJ, and the ratio of long axis and short axis was calculated. Three independent batches of cells were subjected to a shear stress assay for each cell type, and at least three randomly selected areas with at least 60 cells in each batch for each cell type were recorded and analyzed, respectively.
Decellularization of human vessels
Human umbilical cords were collected from Yale–New Haven Hospital (New Haven, CT) with all patient identification information removed before the acquisition by the Yale Vascular Biology and Therapeutics Tissue Culture Core Facility under the approval by Yale Institutional Review Boards (IRBs) and available for research use at Yale. Human umbilical cords were delivered at 4°C and processed to obtain human umbilical arteries (HUAs), as previously described [3]. In general, HUAs were isolated from the umbilical cords (20-30 cm in length) using sharp dissection under sterile conditions. Wharton’s jelly surrounding the HUAs were removed using a pair of Metzenbaum scissors. The newly isolated HUAs (5-8 cm in length and 1-1.5 mm in inner diameters) were then gently washed with PBS containing penicillin 100U/mL and streptomycin 100μg/mL (ThermoFisher) to remove blood clots and were immediately subjected to decellularization. To perform tissue decellularization [25], HUA segments were incubated in 250mL CHAPS buffer (8mM CHAPS, 1M NaCl, and 25mM EDTA in PBS) for 24 hours, followed by washes with PBS. Next, HUAs were incubated in 250mL sodium dodecyl sulfate (SDS) buffer (1.8mM SDS, 1M NaCl, and 25mM EDTA in PBS) for 24 hours, followed by washes with PBS. Finally, HUAs were incubated at 37°C for another 24 hours in PBS containing 20% serum (v/v), followed by washes with PBS. All decellularization steps were completed at 37°C with high-speed agitation under sterile conditions. Decellularized vessels were stored in PBS at 4°C until endothelialization. Decellularized HUAs were further cut into segments of 2-3 cm in length for subsequent bioreactor assays.
Endothelialization of decellularized human vessels
Decellularized human vessels were endothelialized using XF-hiPSC-ECs or XF-HUVECs in a dynamic bioreactor system with luminal flow of xenogeneic-free hiPSC-EC or HUVEC expansion medium, respectively (Fig. 5A). The method for endothelializing decellularized vessels was established according to previous reports [1,5,26]. Both ends of the decellularized vessel were sutured to the glass arms, then assembled to the bioreactor chamber with a medium circulatory circuit. Next, the luminal surface of decellularized vessels was coated with 1.5 mL human fibronectin at 100 μg/mL for 1 hour at 37°C, and seeded with either XF-hiPSC-ECs or XF-HUVECs. Two rounds of cell seeding were completed for each vessel, and the vessels were rotated with 180° after the first round of cell seeding. In total, 5 × 105 XF-hiPSC-ECs or XF-HUVECs per cm2 were introduced into the lumen and allowed to adhere to the surface for 30 minutes. Then medium was refreshed and the vessels were cultured under static condition for another 2 hours. Xenogeneic-free hiPSC-EC or HUVEC expansion medium supplemented with dextran (30 mg/mL, Sigma) to adjust medium viscosity was applied to the culture with XF-hiPSC-ECs or XF-HUVECs, respectively. Dynamic medium flow shear stress was calculated based on Poiseuille’s equation: τ = 4 μQ/πr3 , where τ is shear stress, μ is fluid viscosity, Q is medium flow rate, and r is the radius of the vessel. Shear stress was initially maintained at 1 dyne/cm2 for 48 hours and gradually elevated to 15 dynes/cm2 in 36 hours. Eventually shear stress was maintained at 15 dynes/cm2 for another 24 hours. The endothelialized vessels were immediately isolated from the bioreactors for further processing for histological analysis.
Histological analysis of vessel tissues
Vessel tissues were fixed in 10% Neural Buffered Formalin (ThermoFisher) overnight and sent to Yale Pathology Tissue Services for sample sectioning based on standard protocols. For immunostaining, slides with tissue sections were processed as previously described [24]. Slides were dewaxed in xylene, boiled for 20 minutes in citrate buffer (10 mM, pH 6.0) for antigen retrieval, and then rehydrated. Next, slides were washed in PBS for 10 minutes, blocked in PBST with 10% NGS for 30 minutes at RT, and incubated with primary antibody in PBST with 1% NGS at 4°C overnight in a humidified chamber. On the next day, sections were incubated with secondary antibody (1:1000 in PBST with 1% NGS) for one hour at RT. Cell nuclei were stained with DAPI (ThermoFisher). Fluorescent images were taken on a Leica fluorescence microscope. ImageJ software was used to quantify the percentage of fluorescent cells. Antibody information is listed in Table S4.
Scanning electronic microscopy
Human decellularized vessels with or without endothelialization were examined via scanning electronic microscopy (SEM; model S-4700, Hitachi) as previously described [27]. SEM samples were fixed with 2.5% glutaraldehyde overnight, dehydrated through a series of graded alcohol solutions (50%, 70%, 90% and 100% alcohol), each solution for 15 minutes, air-dried overnight, and cut longitudinally to expose the luminal surface of the conduits. Next, the samples were sputter-coated with carbon and observed under the SEM at an accelerating voltage of 20 kV. Representative images at 200x and 500x were taken for each specimen.
Statistical analysis
GraphPad Prism6 software was used for all graphic illustrations and statistical analyses. All experiments and measurements were performed at least three times, and values were represented as mean ± S.E.M. Unpaired, two-tailed Student’s t test was completed to determine the significance of difference between control and experimental groups. One-way or two-way ANOVA was used for comparison among multiple groups. A p value lower than 0.05 was considered significant.
Results
Endothelial differentiation of hiPSCs under xenogeneic-free conditions
A method for endothelial differentiation from hiPSCs using reagents derived from animals (e.g. mTeSR1 medium and Matrigel) has been previously established [11]. However, the inclusion of animal-derived reagents during EC derivation may trigger xenogeneic immune responses and convey severe zoonotic diseases [19], thereby hampering the potential therapeutic application of hiPSC-ECs. To obtain hiPSC-ECs under xenogeneic-free conditions (XF-hiPSC-ECs), we significantly modified the previous approach [11] and substituted all the animal-derived reagents with new reagents of human origin (Fig. 1A; Details see Materials and Method and Table S2). Briefly, hiPSCs were cultured on a human vitronectin-coated plate with Essential 8 (E8) medium (XF-hiPSCs; Fig. 1A). To initiate EC differentiation, hiPSCs were plated on surfaces coated with 0.5 μg/cm2 human recombinant laminin, and cultured in the presence of E8 medium containing 10 μM Y-27632 for one day. A mesodermal lineage was then induced via treatment with 6 μM CHIR99021, a WNT signaling pathway agonist, and 25 ng/mL human recombinant BMP4 for three days. Next, mesodermal progenitors were committed to the EC lineage via treatment of 2 μM forskolin and 200 ng/mL human recombinant VEGF-A in the xenogeneic-free Stemspan H3000 medium for an additional period of two days. Subsequently, a CD31-positive endothelial cell population was specifically isolated by magnetic activated cell sorting under xenogeneic-free conditions. Isolated endothelial cells were immediately seeded onto human fibronectin-coated plates and cultured with xenogeneic-free EC expansion medium containing 5% (v/v) human serum (HuS), 50 ng/mL human VEGF-A, 10 ng/mL human FGF2, 5 ng/mL human EGF and 20 ng/mL human IGF-1 (Detailed medium components in Table S2). It is worth mentioning that we initially utilized human recombinant vitronectin to support both hiPSC expansion and initial stage of EC differentiation to replace the rodent-derived Matrigel which plays similar roles in xenogeneic protocols. Interestingly, we found that human recombinant laminin, a potent ECM component that supports endothelial functions and proliferation [28,29], appeared to lead to more efficient induction of CD31-positive XF-hiPSC-ECs than human vitronectin (Fig. S1). Therefore, we determined to use human vitronectin to support XF-hiPSC maintenance and human laminin for endothelial induction before cell sorting. To ensure our EC derivation approach free of any animal-derived reagents, we further replaced the growth factor carrier protein, bovine serum albumin (BSA), with human serum albumin (HSA) (Fig. 1A). Additionally, fetal bovine serum (FBS), often used in the previous hiPSC-EC differentiation [11,12,14] expansion studies, was substituted by HuS (Fig. 1A), commonly employed in human clinical applications [4,30]. Subsequently, EC differentiation efficiency was evaluated by analyzing the percentages of CD31+ or VE-cadherin+ cells on day 6 of differentiation under xenogeneic-free or xenogeneic conditions. Interestingly, the xenogeneic-free EC differentiation approach led to significantly higher percentages of cells with endothelial markers (CD31: 29.5 % ± 1.3%; VE-cadherin: 27.6 % ± 0.5 %) on day 6 than the original xenogeneic protocol (CD31: 18.8 % ± 0.2 %; VE-cadherin: 18.2 % ± 0.7 %) (Figs. 1B–C and S2). These results suggested that the xenogeneic-free approach of deriving hiPSC-ECs was effective and that laminin was able to enhance the EC differentiation efficiency of hiPSCs.
XF-hiPSC-ECs displayed cobblestone-like cell morphology similar to those of hiPSC-ECs derived from standard, xenogeneic methods (XG-hiPSC-ECs) and of human primary ECs (human umbilical venous endothelial cells [HUVECs]) cultured under xenogeneic-free and xenogeneic culture conditions (XF-HUVECs and XG-HUVECs) (Fig. 1D). Compared to those of undifferentiated XF-hiPSCs, XF-hiPSC-ECs presented upregulated expression levels of EC-specific genes (CD31, VE-cadherin [VECAD], eNOS, von Willebrand factor [vWF], and Kinase insert domain receptor [KDR]) and decreased expression levels of pluripotency gene OCT4 (Fig. 1E). The gene expression profile of XF-hiPSC-ECs was highly comparable to those of XG-hiPSC-ECs and HUVECs (Fig. 1E). Moreover, XF-hiPSC-ECs on passage 0, 3 and 6 displayed comparable expression levels of endothelial genes (Fig. S3), indicating that these XF-hiPSC-ECs appeared to be capable of maintaining their endothelial phenotype along with cell expansion. In addition, XF-hiPSC-ECs within passage 3 after sorting appeared to proliferate at a slightly higher rate than XG-hiPSC-ECs and HUVECs (Fig. S4). These data suggested that our xenogeneic-free approach could effectively induce EC differentiation of hiPSCs.
Characterization of XF-hiPSC-ECs
XF-hiPSC-ECs were immunostained to evaluate the presence of essential markers. The majority of XF-hiPSC-ECs exhibited representative EC markers (CD31, VECAD, eNOS and vWF), basal membrane markers (collagen type IV [COLIV] and laminin), and the absence of pluripotency marker OCT4, which paralleled the expression profiles of XG-hiPSC-ECs as well as human primary ECs cultured under either XG or XF conditions which included HUVECs and human aortic endothelial cells (HAECs) (Figs. 2A–B, S5 and S6). In contrast, hiPSCs were positive for OCT4 and negative for EC markers (Fig. 2A–B). Additionally, morphological features, including cell surface area and elongation, of the XF-hiPSC-ECs, XF-HUVECs and XF-HAECs were assessed (Fig. S7). We noticed that XF-hiPSC-ECs were appreciably larger than XF-HUVECs while being comparable to XF-HAECs. In addition, XF-hiPSC-ECs appeared to be more elongated than either XF-HUVECs or XF-HAECs, which could be in part attributed to the continuous exposure to the highly stiff plastic culture surfaces during the derivation of xenogeneic-free ECs from hiPSCs, while primary HAECs and HUVECs grew on the vascular tissue substrates with physiological stiffness prior to cell seeding for culture in vitro. Consistent with this notion, it was previously reported that stiffer substrate could contribute to increased cell size and elongation [31,32].
Figure 2. Marker expression of XF-hiPSC-ECs.
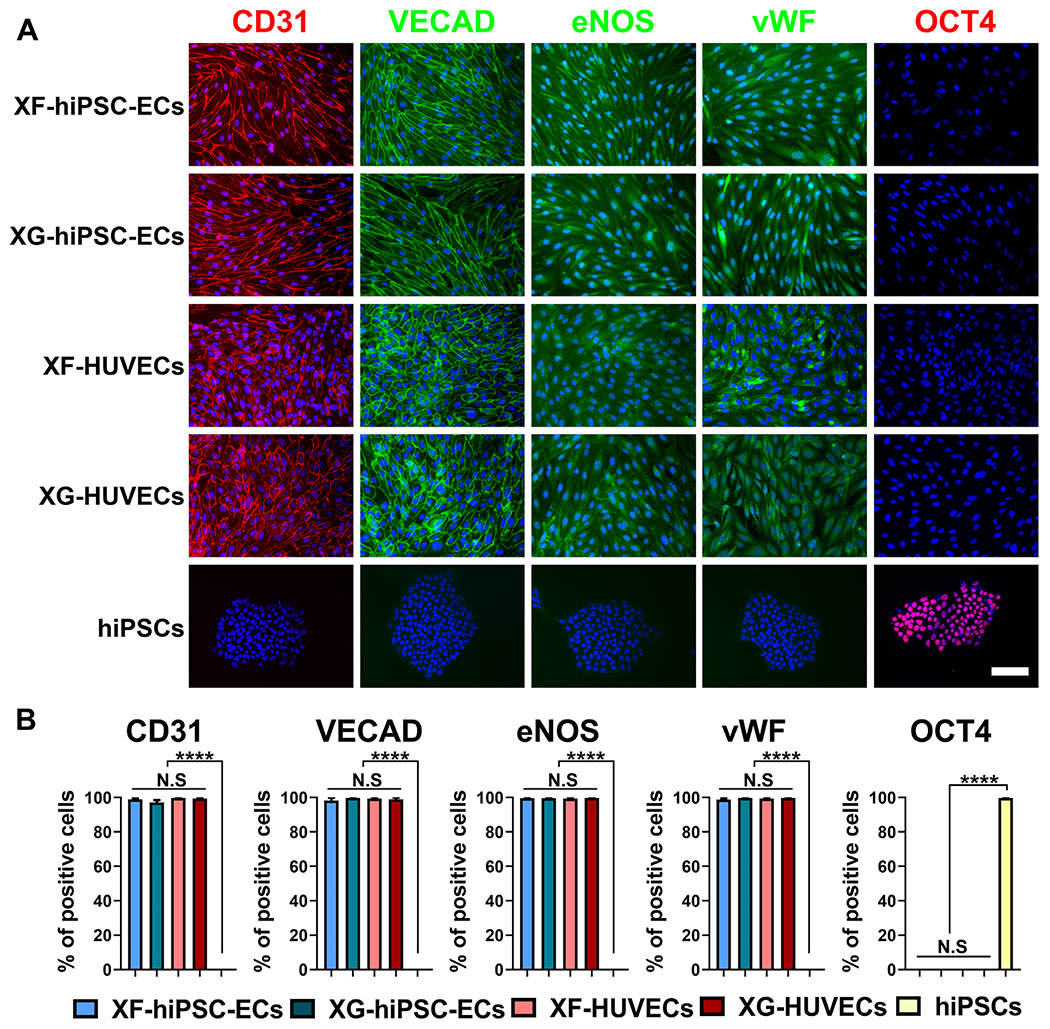
(A) Immunostaining of EC markers (CD31, VECAD, eNOS and vWF) and pluripotency marker OCT4 in XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs, XG-HUVECs, and undifferentiated hiPSCs under xenogeneic-free conditions. DNA (nuclear) was counterstained by DAPI. Scale bar: 200 μm. (B) Percentage of cells (XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs, XG-HUVECs, and xenogeneic-free hiPSCs) positive for EC markers (CD31, VECAD, eNOS and vWF) and pluripotency marker OCT4 from immunostaining (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by error bars are shown; n=3; ****: p<0.00 01; N.S: not significant).
We next examined cellular functions of XF-hiPSC-ECs. First, we performed tube formation assays on Matrigel to determine the angiogenic potential of XF-hiPSC-ECs. All hiPSC-ECs or HU-VECs derived under either xenogeneic-free or xenogeneic conditions were able to form capillary network-like structures in 24 hours with comparable capillary-like tubule length and number of nodes per field (Fig. 3A and 3B). Next, we assessed lipid uptake capacity of XF-hiPSC-ECs using fluorescently labelled acetylated low-density lipoproteins (ac-LDL). XF-hiPSC-ECs presented comparable efficiency of ac-LDL uptake to that of XG-hiPSC-ECs and HUVECs, while the uptake of ac-LDL by undifferentiated hiPSCs was not detectable (Fig. 3C and 3D). Collectively, the results above suggested that XF-hiPSC-ECs displayed representative endothelial functions.
Figure 3.
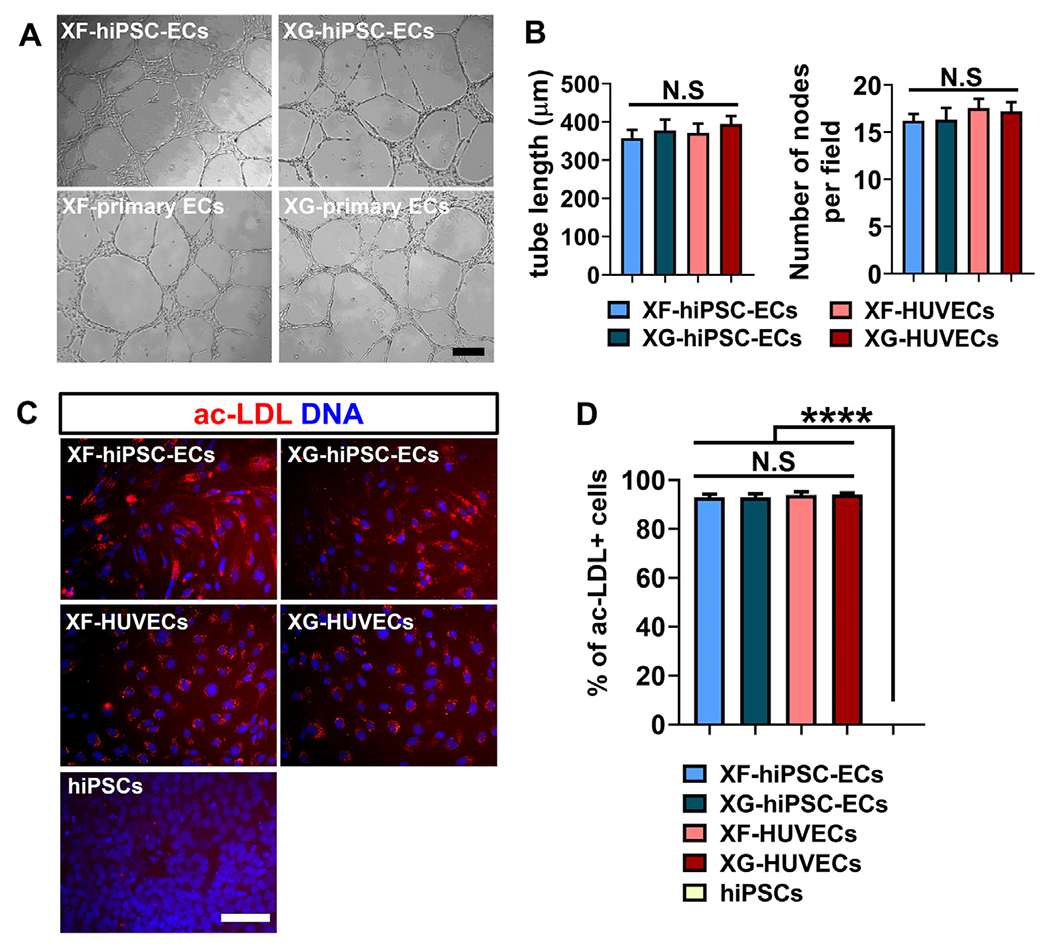
Endothelial functional analysis of XF-hiPSC-ECs. (A) Representative phase contrast images of capillary-like networks by hiPSC-ECs or HUVECs derived under xenogeneic-free or xenogeneic conditions (XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs or XG-HUVECs) on Matrigel-coated culture plates 24 hours after cell seeding. Scale bar: 200 μm. (B) Quantification of tube length and number of nodes per field of cellular networks formed by XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs or XG-HUVECs (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; N.S: not significant). (C) Uptake of fluorescently labelled acetylated low density lipoprotein (ac-LDL) in XF-hiPSC-ECs, XG-hiPSC-ECs, XF-HUVECs, XG-HUVECs or undifferentiated hiPSCs cultured under xenogeneic-free conditions. DNA (nuclear) was counterstained by DAPI. Scale bar: 200μm. (D) Quantification of percentage of cells positive for ac-LDL uptake in C (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; ****: p<0.0001; N.S: not significant).
Additionally, we applied our xenogeneic-free EC differentiation approach to the allogeneic, hypoimmunogenic, human leukocyte A (HLA)-C-retained hiPSC line (HLA-C-retained hiPSC), in which both HLA class I (-A and -B) alleles and HLA class II are disrupted while the expression of HLA-C with relatively low heterogeneity is retained [9], and generated XF-hiPSC-ECs. Similar to wildtype XF-hiPSC-ECs as described above, these HLA-C-retained-XF-hiPSC-ECs displayed expression of typical endothelial markers, angiogenic potential, and uptake of ac-LDL (Fig. S8). These results suggested allogeneic, hypoimmunogenic XF-hiPSC-ECs could be derived via our current approach.
Functional response of XF-hiPSC-ECs to shear stress
To endothelialize decellularized vascular grafts, ECs should be cultured in the presence of shear stress to simulate the condition of arterial hemodynamics (15 dyne/cm2) [33]. Therefore, we investigated the biological response of XF-hiPSC-ECs to shear stress. XF-hiPSC-ECs or XF-primary ECs (including XF-HUVECs and XF-HAECs) were seeded into the human fibronectin-coated surface of μ-Slides (Ibidi) which were connected to a system circulating xenogeneic-free medium (Fig. 4A). Thus XF-hiPSC-ECs or XF-primary ECs were cultured in the presence or absence of laminar shear stress at 15 dyne/cm2 for 48 hours. In contrast to those cultured under static condition, both XF-hiPSC-ECs and XF-primary ECs were largely oriented in parallel to the direction of medium flow when exposed to shear stress, as indicated by the cell morphologies and the fluorescent staining of nuclei, actin fibers and VE-cadherin, (Fig. S9, 4B and S10A). We further quantified the percentage of cells aligned to the direction of medium flow via image analysis, where cells with nuclei orientation within ±23° of the direction of the medium flow were considered as aligned [23]. Similar to XF-primary ECs, XF-hiPSC-ECs cultured with shear stress presented a significantly higher percentage of aligned cells than of those under static conditions, and there was no significant difference between the levels of cell alignment of XF-hiPSC-ECs and XF-primary ECs cultured with shear stress (Fig. 4C and S10B).
Figure 4. Response of XF-hiPSC-ECs to shear stress.
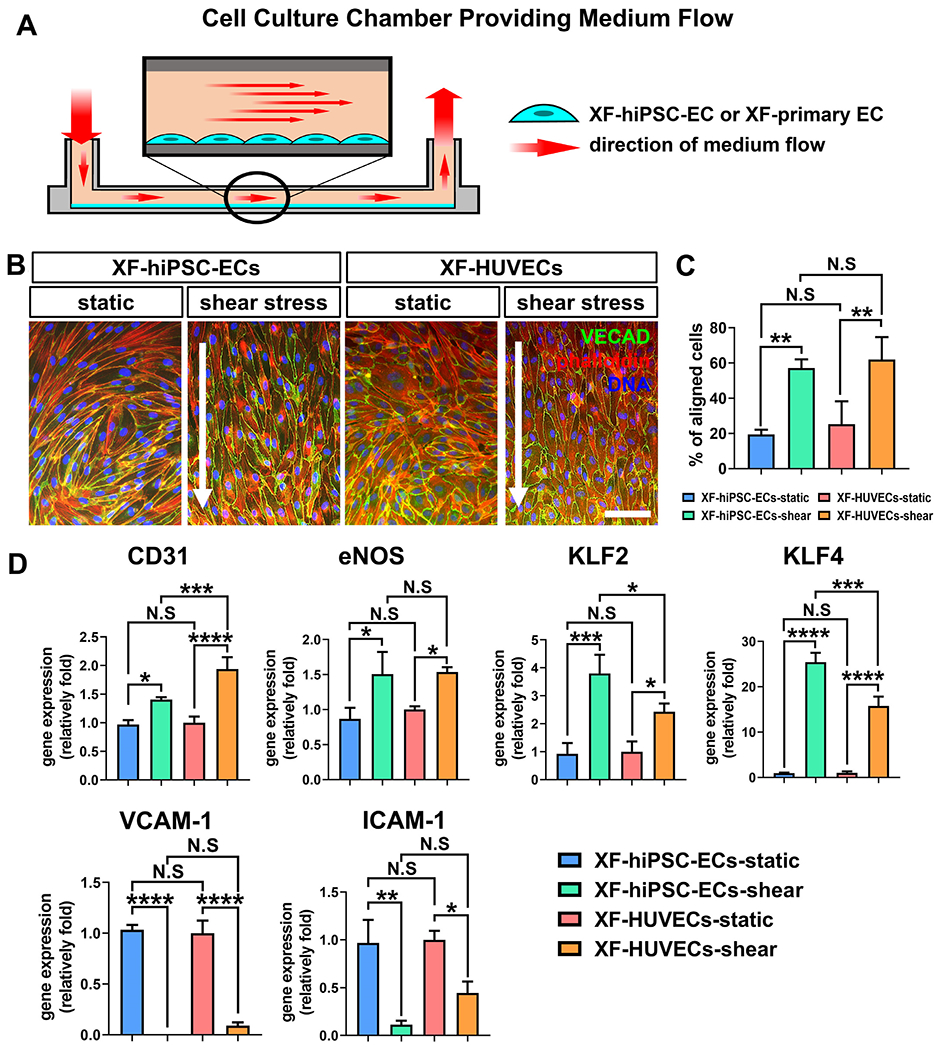
(A) Schematic illustration of the dynamic system for culturing adherent endothelial cells in the presence of shear stress at 15 dyne/cm2 for 48 hours. (B) Fluorescence staining of filamentous actin fibers (phalloidin) and VE-cadherin (VECAD) in XF-hiPSC-ECs or XF-HUVECs cultured with or without shear stress. DNA (nuclei) was counterstained with DAPI. The arrows indicate the direction of flow. Scale bar: 200 μm. (C) Percentage of cells aligned to the direction of flow in B. Cell nuclei with orientation in the direction of flow (±23°) were quantified as aligned. (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; **: p<0.01; N.S: not significant). (D) Relative mRNA transcript amounts of EC markers (CD31 and eNOS), anti-thrombotic markers (KLF2 and KLF4) and adhesion molecules (VCAM-1 and ICAM-1) in XF-hiPSC-ECs or XF-HUVECs cultured with or without shear stress. Values in the y-axis represent fold changes relative to human GAPDH expression. Gene expression in each group was normalized to that of XF-HUVECs under static culture conditions (One-way ANOVA with Tukey’s multiple comparisons test; Mean values and S.E.M indicated by the error bars are shown; n=3; *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001; N.S: not significant).
Laminar shear stress plays a critical role in maintaining endothelial phenotype and preventing vascular inflammation and thrombogenesis [14]. Therefore, we investigated whether exposure to shear stress would alter gene expression of endothelial markers (CD31 and eNOS), anti-thrombotic markers (KLF2 and KLF4) and adhesion molecules (VCAM-1 and ICAM-1) in XF-hiPSC-ECs. Shear stress significantly upregulated the expression levels of endothelial and anti-thrombotic markers while dramatically reducing the expression of endothelial adhesion molecules in both XF-hiPSC-ECs and XF-primary ECs (Figs. 4D and S10C). These data further revealed that XF-hiPSC-ECs could functionally respond to shear stress.
Endothelialization of human decellularized arteries with XF-hiPSC-ECs
We next utilized XF-hiPSC-ECs to endothelialize the luminal surface of decellularized vessels. As a proof-of-principle study, we obtained small-diameter (~1.5 mm inner diameter), decellularized human vessels (human umbilical arteries, HUAs), as previously described [25], and endothelialized their luminal surface using XF-hiPSC-ECs or XF-HUVECs under xenogeneic-free conditions (Fig. 5A). We optimized our previous method of endothelialization of vascular grafts [1,5], and mounted the decellularized vessels into a recently reported perfusion bioreactor [26,34]. The luminal surface of the decelluiarized vessels was coated with 1.5 mL human recombinant fibronectin at 100 μg/mL for 1 hour to promote EC adhesion. A total of 5 × 105 XF-hiPSC-ECs or XF-HUVECs per cm2 were then seeded onto the luminal surface of decellularized vessels in two rounds under a static condition in a 3-hour period. It is worth mentioning that the cell density applied to vessel endothelialization was 2.5 folds of that in previous study [5]. Subsequently, intraluminal flow of xenogeneic-free medium was introduced, and the shear stress was initially set at 1 dyne/cm2 for 48 hours, gradually increased to 15 dynes/cm2 in 36 hours, and maintained at 15 dynes/cm2 for an additional 24 hours to simulate arterial hemodynamics.
Decellularized vessels endothelialized with XF-hiPSC-ECs or XF-HUVECs were harvested 3 hours, 2.5 days and 5 days after cell seeding and subjected to histological analysis (Figs. 5B–C and S11). Immunostaining of endothelial markers CD31 and eNOS revealed that XF-hiPSC-ECs and XF-HUVECs adhered to the luminal surface of decellularized vessels at similar efficiency 3 hours after cell seeding. On day 2.5, vessels endothelialized using both XF-hiPSC-ECs and XF-HUVECs showed increased EC coverage rates compared with those of vessels 3 hours post cell seeding. Interestingly, it appeared that vessels endothelialized using XF-HUVECs started to show higher EC coverage rates than those endothelialized using XF-hiPSC-ECs, suggesting that XF-HUVECs were likely to be more proliferative in the presence of shear stress on the luminal surface of decellularized vessels compared with XF-hiPSC-ECs. On day 5, vessel endothelialization using XF-hiPSC-ECs led to an average coverage of 85.1%~86.2%. Although at a slightly lower coverage rate compared with that using XF-HUVECs (average coverage: 99.1%~99.3%), the efficiency of endothelialization using XF-hiPSC-ECs represented a marked improvement when compared with a previous method for endothelializing decellularized vascular grafts using autologous primary ECs (14-64% with a wide variation) [1,5]. Moreover, we examined the luminal surface of decellularized vessels 5 days after endothelialization via scanning electron microscopy (SEM). Results showed that both adhered XF-hiPSC-ECs and XF-HUVECs formed the cobblestone-like pattern on the luminal surface of decellularized vessels and aligned to the direction of medium flow, which was in sharp contrast to the non-endothelialized, naked luminal surface of decellularized vessels (Figs. 5D and S12). In summary, the above studies suggested that XF-hiPSC-ECs were capable of efficiently endothelializing the decellularized vessels.
Discussion
In this study, we established a xenogeneic-free method based on the culture conditions including growth factors and small molecules in a previous xenogeneic hiPSC-EC differentiation approach, and derived functional XF-hiPSC-ECs suitable for endothelializing vascular grafts [11]. We have replaced all the materials of animal origin in the original protocol with new reagents of human origin, and hiPSC-ECs derived under such xenogeneic-free conditions presented typical EC marker expression profiles, capillary network formation, and ac-LDL uptake comparable to XG-hiPSC-ECs, XG-primary ECs, and XF-primary ECs. Additionally, XF-hiPSC-ECs and XF-primary ECs showed a similar upregulation of endothelial and anti-thrombotic markers but downregulation of endothelial adhesion molecules in response to shear stress. Moreover, we successfully endothelialized the luminal surface of small-caliber decellularized human vessels using XF-hiPSC-ECs. To our knowledge, our research outcomes represent the establishment of the first completely xenogeneic-free method of generating functional hiPSC-ECs that are suitable for endothelializing decellularized blood vessels. Coupled with the application of immunocompatible “universal” hiPSCs [7–10], our study will set the foundation for deriving decellularized TEVGs with efficient endothelialization as a promptly available vascular therapy for future therapeutic use.
To our knowledge, xenogeneic reagents have been applied in a majority of previously reported methods for deriving hiPSC-ECs (Fig. 1A). Notably, a xenogeneic-free method for deriving hiPSC-ECs was previously claimed and reported [18]. However, the EC culture medium contained components derived from porcine intestine and ovine wool grease, and the cryopreservation conditions in this method included fetal bovine serum. Therefore, the culture conditions in this method were not fully xenogeneic-free. In our xenogeneic-free method of hiPSC-EC differentiation, it is worth noting that we used vitronectin and laminin to support pluripotency maintenance and endothelial induction, respectively. Similar to the role of Matrigel in standard xenogeneic methods, we originally employed human vitronectin for both hiPSC expansion and EC differentiation under xenogeneic-free conditions. However, vitronectin might lead to poor EC proliferation via the induction of plasminogen activator inhibitor-1 [35]. In comparison, laminin has been reported to enhance endothelial tight junctions and cellular proliferation [28,29]. As a result, we observed that human laminin led to a higher yield of XF-hiPSC-ECs than human vitronectin as indicated by the result of flow cytometric analysis, which was consistent with previous reports.
Moreover, we found that XF-hiPSC-ECs readily responded to shear stress by upregulating endothelial and anti-thrombotic markers and downregulating endothelial adhesion molecules, suggesting that XF-hiPSC-ECs were functionally appropriate for vessel endothelialization via dynamic culture [5]. Consistent with this notion, we effectively endothelialized the luminal surface of decellularized human vessels using XF-hiPSC-ECs under xenogeneic-free conditions based on our improved method. We noticed that luminal endothelialization using XF-hiPSC-ECs via our improved method led to an efficient EC coverage rate (85.1%~86.2%). Notably, vessel endothelialization using primary ECs based on previous methods displayed less effective coverage of the luminal surface with a large variation (14-64%) [1,5]. In comparison, the coverage rate of XF-hiPSC-ECs achieved by our current optimized method has been markedly improved with reduced variation. In the future, more efforts, such as optimizing lumen surface coating, cell seeding density and duration, and shear stress training regimen, will be made to further enhance the efficacy of vascular graft endothelialization using XF-hiPSC-ECs.
There are other limitations remaining to be addressed in this study. As human serum has been commonly utilized in clinical trials [4,30], our current approach incorporates human serum for the expansion of XF-hiPSC-ECs, which potentially sets the stage for future clinical applications. However, there exists a low probability event of patient immune reactivity to circulating HLA molecules present in the human serum. To avoid any potential allogeneic immune response induced by the human serum, ELISA assays could be utilized to measure the concentrations of circulating HLA molecules in different batches of human serum, and those batches with the lowest HLA level could be selected for clinical use. It is also possible to use HLA-specific antibodies to deplete the circulating HLA molecules in pooled human serum before culturing XF-hiPSC-ECs. Alternatively, a completely chemically defined, xenogeneic-free EC expansion medium could be developed in the future, provided that differentiated cells maintain the functions required for effective tissue engineering. Moreover, it is critical to understand if the XF-hiPSC-ECs can maintain their phenotype and functions when exposed to in vivo hemodynamics for extended period of time. To evaluate the performance of XF-hiPSC-ECs during long-term exposure to hemodynamic stress, decellularized vascular grafts derived in future studies could be endothelialized with XF-hiPSC-ECs and implanted into immunodeficient small animal model [3] or large animal models with necessary immune modulation [1,5]. It’s likely that blood flow-triggered shear force and physiological vascular graft substrate could help to further mature endothelial function of XF-hiPSC-ECs in vivo.
Our work is in line with current good manufacturing practice (cGMP)-compliant protocols [36], and future efforts will be made to establish “universal” hiPSCs under xenogeneic-free conditions to efficiently generate immunocompatible XF-hiPSC-ECs for therapeutic application. Additionally, current efforts are ongoing in our research group to obtain hiPSC-derived vascular smooth muscle cells for the development of decellularized TEVGs under xenogeneic-free conditions. In the future, prior to clinical application of small-diameter (< 6 mm) decellularized TEVGs for peripheral and coronary artery repair, previously cryopreserved “universal” XF-hiPSC-ECs could be thawed and applied to endothelialize these TEVGs for five days, which will be significantly more efficient than endothelializing decellularized TEVGs using patient’s autologous primary ECs (23 patient wait days on average) [1]. To further shorten the patient wait time, endothelialized TEVGs using XF-hiPSC-ECs could be maintained in a short-term via dynamic, xenogeneic-free culture conditions in flow bioreactors according to the evaluation of local clinical needs, multi-site production and logistical coordination. Based on our findings, these decellularized TEVGs could be endothelialized with “universal” XF-hiPSC-ECs to generate immunocompatible, hiPSC-based TEVGs as a promptly available therapy for emergency vascular intervention.
Conclusion
To our knowledge, we have established the first xenogeneic-free method for deriving functional hiPSC-ECs suitable for endothelializing decellularized human vessels. By substantially modifying a standard xenogeneic hiPSC-EC derivation approach and replacing all animal-derived materials with new reagents of human origin, we generated XF-hiPSC-ECs with effective endothelial marker expression and function comparable to those of human primary ECs and XG-hiPSC-ECs. These XF-hiPSC-ECs were readily responsive to shear stress by modulating endothelial, anti-thrombotic, and endothelial adhesion activities. Furthermore, we applied the XF-hiPSC-ECs to decellularized human vessels and successfully endothelialized their luminal surface at a high coverage rate. Our method of generating xenogeneic-free hiPSC-ECs sets the stage for the future development of endothelialized TEVGs of human clinical grade as a promptly available strategy for acute vascular intervention.
Supplementary Material
Statement of significance.
Small-diameter, decellularized tissue engineered vascular grafts (TEVGs) represent a promising vascular therapy, though their clinical application has be hindered by the time duration for endothelialization using a patient’s autologous endothelial cells (ECs). Alternatively, human induced pluripotent stem cell derived ECs (hiPSC-ECs) may expedite TEVG endothelialization. Clinical grade hiPSC-ECs should be generated without animal-derived reagents (xenogeneic-free conditions), although such a method has not yet been reported. Herein, we establish a method to produce XF-hiPSC-ECs with comparable marker expression, endothelial function and response to shear stress as those from standard xenogeneic protocols. Moreover, we successfully endothelialized decellularized vessels using XF-hiPSC-ECs in a dynamic bioreactor. Our research thus paves the way for xenogeneic-free hiPSC-TEVGs as a readily available clinical therapy in the future.
Acknowledgments
We thank the members of Qyang group for their feedback for this research. We thank the supports from DOD W81XWH1910557, R01HL116705, and R01HL150352 (all to YQ), The American Heart Association (AHA) Postdoctoral Fellowships 19POST34450100 (to JL), 20POST35210709 (to YY), and 19POST34381048 (to MHK), China Scholarships Council 201706370156 (to XS) and 201806280209 (to YL), Ruth L. Kirschstein Predoctoral Individual National Research Service Award NIH 5F31HL143928-03 (to CWA) and NIH Grants F31-HL143924 and T32-GM0007324 (to MWE).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.actbio.2020.11.007.
References
- [1].Dahl SL, Kypson AP, Lawson JH, Blum JL, Strader JT, Li Y, Manson RJ, Tente WE, DiBernardo L, Hensley MT, Carter R, Williams TP, Prichard HL, Dey MS, Begelman KG, Niklason LE, Readily available tissue-engineered vascular grafts, Sci Transl Med 3 (68) (2011) 68ra9. [DOI] [PubMed] [Google Scholar]
- [2].Syedain ZH, Graham ML, Dunn TB, O’Brien T, Johnson SL, Schumacher RJ, Tranquillo RT, A completely biological “off-the-shelf” arteriovenous graft that recellularizes in baboons, Sci Transl Med 9 (414) (2017) eaan4209. [DOI] [PubMed] [Google Scholar]
- [3].Luo J, Qin L, Zhao L, Gui L, Ellis MW, Huang Y, Kural MH, Clark JA, Ono S, Wang J, Yuan Y, Zhang SM, Cong X, Li G, Riaz M, Lopez C, Hotta A, Campbell S, Tellides G, Dardik A, Niklason LE, Qyang Y, Tissue-Engineered Vascular Grafts with Advanced Mechanical Strength from Human iPSCs, Cell Stem Cell 26 (2) (2020) 251–261 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, Szmidt J, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Niklason LE, Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials, Lancet 387 (10032) (2016) 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE, Decellularized tissue-engineered blood vessel as an arterial conduit, Proc Natl Acad Sci U S A 108 (22) (2011) 9214–9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors, Cell 131 (5) (2007) 861–872. [DOI] [PubMed] [Google Scholar]
- [7].Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, Prunkard D, Colunga AG, Hanafi LA, Clegg DO, Turtle C, Russell DW, HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells, Nat Biotechnol 35 (8) (2017) 765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Deuse T, Hu X, Gravina A, Wang D, Tediashvili G, De C, Thayer WO, Wahl A, Garcia JV, Reichenspurner H, Davis MM, Lanier LL, Schrepfer S, Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients, Nat Biotechnol 37 (3) (2019) 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xu H , Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, Ueda T, Gee P, Nishikawa M, Nomura M, Kitaoka F, Takahashi T, Okita K, Yoshida Y, Kaneko S, Hotta A, Targeted Disruption of HLA Genes via CRISPR-Cas9 Generates iPSCs with Enhanced Immune Compatibility, Cell Stem Cell 24 (4) (2019) 566–578 e7. [DOI] [PubMed] [Google Scholar]
- [10].Han X, Wang M, Duan S, Franco PJ, Kenty JH, Hedrick P, Xia Y, Allen A, Ferreira LMR, Strominger JL, Melton DA, Meissner TB, Cowan CA, Generation of hypoimmunogenic human pluripotent stem cells, Proc Natl Acad Sci U S A 116 (21) (2019) 10441–10446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Patsch C, Challet-Meylan L, Thoma EC, Urich E, Heckel T, O’Sullivan JF , Grainger SJ, Kapp FG, Sun L, Christensen K, Xia Y, Florido MH, He W, Pan W, Prummer M, Warren CR, Jakob-Roetne R, Certa U, Jagasia R, Freskgard PO, Adatto I, Kling D, Huang P, Zon LI, Chaikof EL, Gerszten RE, Graf M, Iacone R, Cowan CA, Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells, Nature cell biology 17 (8) (2015) 994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lian X, Bao X , Al-Ahmad A, Liu J, Wu Y, Dong W, Dunn KK, Shusta EV, Palecek SP, Efficient differentiation of human pluripotent stem cells to endothelial progenitors via small-molecule activation of WNT signaling, Stem Cell Reports 3 (5) (2014) 804–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Prasain N, Lee MR, Vemula S, Meador JL, Yoshimoto M, Ferkowicz MJ, Fett A, Gupta M, Rapp BM, Saadatzadeh MR, Ginsberg M, Elemento O, Lee Y, Voytik-Harbin SL, Chung HM, Hong KS, Reid E, O’Neill CL, Medina RJ, Stitt AW, Murphy MP, Rafii S, Broxmeyer HE, Yoder MC, Differentiation of human pluripotent stem cells to cells similar to cord-blood endothelial colony-forming cells, Nat Biotechnol 32 (11) (2014) 1151–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sivarapatna A, Ghaedi M, Le AV, Mendez JJ, Qyang Y, Niklason LE, Arterial specification of endothelial cells derived from human induced pluripotent stem cells in a biomimetic flow bioreactor, Biomaterials 53 (2015) 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y, Zhang J, Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells, Cell Stem Cell 15 (6) (2014) 750–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paik DT, Tian L, Lee J, Sayed N, Chen IY, Rhee S, Rhee JW, Kim Y, Wirka RC, Buikema JW, Wu SM, Red-Horse K, Quertermous T, Wu JC, Large-Scale Single-Cell RNA-Seq Reveals Molecular Signatures of Heterogeneous Populations of Human Induced Pluripotent Stem Cell-Derived Endothelial Cells, Circ Res 123 (4) (2018) 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee SJ, Sohn YD, Andukuri A, Kim S, Byun J, Han JW, Park IH, Jun HW, Yoon YS, Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell-Derived Endothelial Cells Encapsulated in a Nanomatrix Gel, Circulation 136 (20) (2017) 1939–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bao X, Lian X, Dunn KK, Shi M, Han T, Qian T, Bhute VJ, Canfield SG, Palecek SP, Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells, Stem Cell Res 15 (1) (2015) 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Astori G, Amati E, Bambi F, Bernardi M, Chieregato K, Schafer R, Sella S, Rodeghiero F, Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future, Stem Cell Res Ther 7 (1) (2016) 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA, Chemically defined conditions for human iPSC derivation and culture, Nature methods 8 (5) (2011) 424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Qian T, Maguire SE, Canfield SG, Bao X, Olson WR, Shusta EV, Palecek SP, Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells, Sci Adv 3 (11) (2017) e1701679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dash BC, Levi K, Schwan J, Luo J, Bartulos O, Wu H, Qiu C, Yi T, Ren Y, Campbell S, Rolle MW, Qyang Y, Tissue-Engineered Vascular Rings from Human iPSC-Derived Smooth Muscle Cells, Stem Cell Reports 7 (1) (2016) 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coon BG, Baeyens N, Han J, Budatha M, Ross TD, Fang JS, Yun S, Thomas JL, Schwartz MA, Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex, The Journal of cell biology 208 (7) (2015) 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Luo J, Qin L, Kural MH, Schwan J, Li X, Bartulos O, Cong XQ, Ren Y, Gui L, Li G, Ellis MW, Li P, Kotton DN, Dardik A, Pober JS, Tellides G, Rolle M, Campbell S, Hawley RJ, Sachs DH, Niklason LE, Qyang Y, Vascular smooth muscle cells derived from inbred swine induced pluripotent stem cells for vascular tissue engineering, Biomaterials 147 (2017) 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Park J, Anderson CW, Sewanan LR, Kural MH, Huang Y, Luo J, Gui L, Riaz M, Lopez CA, Ng R, Das SK, Wang J, Niklason L, Campbell SG, Qyang Y, Modular design of a tissue engineered pulsatile conduit using human induced pluripotent stem cell-derived cardiomyocytes, Acta Biomater 102 (2020) 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kural MH, Dai G, Niklason LE, Gui L, An Ex Vivo Vessel Injury Model to Study Remodeling, Cell Transplant 27 (9) (2018) 1375–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dimitrievska S, Wang J, Lin T, Weyers A, Bai HL, Qin LF, Li GX, Cai C, Kypson A, Kristofik N, Gard A, Sundaram S, Yamamoto K, Wu W, Zhao LP, Kural MH, Yuan YF, Madri J, Kyriakides TR, Linhardt RJ, Niklason LE, Glycocalyx-Like Hydrogel Coatings for Small Diameter Vascular Grafts, Advanced Functional Materials 30 (23) (2020). [Google Scholar]
- [28].Okumura N, Kakutani K, Numata R, Nakahara M, Schlotzer-Schrehardt U, Kruse F, Kinoshita S, Koizumi N, Laminin-511 and -521 enable efficient in vitro expansion of human corneal endothelial cells, Invest Ophthalmol Vis Sci 56 (5) (2015) 2933–2942. [DOI] [PubMed] [Google Scholar]
- [29].Song J, Zhang X, Buscher K, Wang Y, Wang H, Di Russo J, Li L, Lutke-Enking S, Zarbock A, Stadtmann A, Striewski P , Wirth B, Kuzmanov I, Wiendl H, Schulte D, Vestweber D, Sorokin L, Endothelial Basement Membrane Laminin 511 Contributes to Endothelial Junctional Tightness and Thereby Inhibits Leukocyte Transmigration, Cell Rep 18 (5) (2017) 1256–1269. [DOI] [PubMed] [Google Scholar]
- [30].Perez-Simon JA, Lopez-Villar O, Andreu EJ, Rifon J, Muntion S, Diez Campelo M, Sanchez-Guijo FM, Martinez C, Valcarcel D, Canizo CD, Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: results of a phase I/II clinical trial, Haematologica 96 (7) (2011) 1072–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Soumya SS, Sthanam LK, Padinhateeri R, Inamdar MM, Sen S, Probing cellular mechanoadaptation using cell-substrate de-adhesion dynamics: experiments and model, PLoS One 9 (9) (2014) e106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xue C, Zhang T, Xie X, Zhang Q, Zhang S, Zhu B, Lin Y, Cai X, Substrate stiffness regulates arterial-venous differentiation of endothelial progenitor cells via the Ras/Mek pathway, Biochim Biophys Acta Mol Cell Res 1864 (10) (2017) 1799–1808. [DOI] [PubMed] [Google Scholar]
- [33].Quint C, Arief M, Muto A, Dardik A, Niklason LE, Allogeneic human tissue-engineered blood vessel, J Vasc Surg 55 (3) (2012) 790–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kural MH, Wang J, Gui L, Yuan Y, Li G, Leiby KL, Quijano E, Tellides G, Saltzman WM, Niklason LE, Fas ligand and nitric oxide combination to control smooth muscle growth while sparing endothelium, Biomaterials 212 (2019) 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Underwood PA, Bean PA, Cubeddu L, Human endothelial cells grow poorly on vitronectin: role of PAI-1, J Cell Biochem 82 (1) (2001) 98–109. [DOI] [PubMed] [Google Scholar]
- [36].Baghbaderani BA, Tian X, Neo BH, Burkall A, Dimezzo T, Sierra G, Zeng X, Warren K , Kovarcik DP, Fellner T, Rao MS, cGMP-Manufactured Human Induced Pluripotent Stem Cells Are Available for Pre-clinical and Clinical Applications, Stem Cell Reports 5 (4) (2015) 647–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


