Abstract
Hirschsprung's disease (HSCR) is a common congenital defect. It occurs when bowel colonization by neural crest‐derived enteric nervous system (ENS) precursors is incomplete during the first trimester of pregnancy. Several sources of candidate cells have been previously studied for their capacity to regenerate the ENS, including enteric neural crest stem cells (En‐NCSCs) derived from native intestine or those simulated from human pluripotent stem cells (hPSCs). However, it is not yet known whether the native NCSCs other than En‐NCSCs would have the potential of regenerating functional enteric neurons and producing neuron dependent motility under the intestinal environment. The present study was designed to determine whether premigratory NCSCs (pNCSCs), as a type of the nonenteric NCSCs, could form enteric neurons and mediate the motility. pNCSCs were firstly transplanted into the colon of adult mice, and were found to survive, migrate, differentiate into enteric neurons, and successfully integrate into the adult mouse colon. When the mixture of pNCSCs and human intestinal organoids was implanted into the subrenal capsule of nude mice and grown into the mature tissue‐engineered intestine (TEI), the pNCSCs‐derived neurons mediated neuron‐dependent peristalsis of TEI. These results show that the pNCSCs that were previously assumed to not be induced by intestinal environment or cues can innervate the intestine and establish neuron‐dependent motility. Future cell candidates for ENS regeneration may include nonenteric NCSCs.
Keywords: bowel motility, enteric neuroregeneration, premigratory neural crest stem cells, tissue engineered intestine, transplantation
Schematic diagram for premigratory neural crest stem cells (pNCSCs) derivation, subrenal capsule implantation, and transplantation. pNCSCs emigrated from neural tube at E9.0 are harvested. To confirm the formation of enteric nervous system‐like neurons in natural intestine, pNCSCs are transplanted into mice colon for differentiation and electrophysiology analysis. To further study the mediation of neuron‐dependent motility, pNCSCs are implanted into tissue engineering intestine in subrenal capsule.
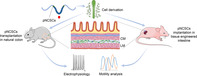
Significance statement.
The cell candidates for the enteric nervous system (ENS) regeneration and Hirschsprung's disease are mostly based on the derivation or induction of enteric neural stem cells which has been induced by the gut environment or the developmental signal. This article shows that premigratory neural crest stem cells (NCSCs), which have not yet migrated into the gut at the time of derivation, populate in the adult mouse colon, differentiate into enteric neurons, and mediate neuron dependent peristalsis in the tissue‐engineered intestine. It suggests that the cell candidates for the treatment of ENS regeneration may be expanded to include the NCSCs of nonenteric origin in the future.
Abbreviations
- CM
circular muscle
- ENS
enteric nervous system
- hESCs
human embryonic stem cells
- HIOs
human intestine organoids
- hPSCs
human pluripotent stem cells
- HSCR
Hirschsprung's disease
- ICCs
interstitial cells of Cajal
- LM
longitudinal muscle
- NCSCs
neural crest stem cells
- NSCs
neural stem cells
- Synapsin‐1
Syn1
- TEI
tissue‐engineered intestine
- WT
wild type
1. INTRODUCTION
Neural crest cells (NCCs) form transiently in the developing vertebrate embryo at the border between the neural plate and the adjacent nonneural ectoderm. 1 These cells undergo an epithelial‐mesenchymal transition 2 and migrate along different routes to many locations where they produce various neural and nonneural types of cells in adults, with remarkable multipotency. 3 Vagal and sacral NCCs produce enteric nervous system (ENS). 4 The dysregulation of some factors has the potential to cause poor migration and dysfunction of NCCs during the embryonic development phase, resulting in various diseases. 5 , 6
One of the most common neurocristopathies is Hirschsprung's disease (HSCR) which results from the absence of ENS in a variable region of the distal large intestine, but in severe cases involves the whole intestine. HSCR is a birth defect occurring in 1:5000 infants. The ENS forms from migrating and proliferating neural crest‐derived precursors during the first trimester of pregnancy, and HSCR occurs when bowel colonization by ENS precursors is incomplete. 7 , 8 Surgery is generally recommended for infants with HSCR; however, the long‐term complications and consequences of HSCR can be unpredictable. 9 , 10 , 11 Stem cell‐based replacement of missing and defective enteric neurons in the colon is a promising alternative (or complementary) therapy for restoring bowel motility that could mitigate some adverse effects of surgery. 12
Various sources of candidate cells have been studied for ENS reconstruction, including enteric neural stem cells (En‐NSCs) isolated from the native intestine and En‐NSCs induced from human pluripotent stem cells (hPSCs). 13 , 14 , 15 , 16 , 17 , 18 En‐NSCs (also referred as ENS progenitors) were derived from hPSCs including human embryonic stem cells (hESCs) or induced PSCs (iPSCs). 18 , 19 , 20 It was systematically demonstrated that hPSCs could be induced into vagal NCSCs, which then develop into En‐NSCs and finally differentiate into enteric neurons in vitro. Other reports have described that hPSCs‐derived enteric NCSCs (En‐NCSCs) could regulate the neuronal‐dependent peristalsis of the tissue‐engineered intestine (TEI) at the organ level. 21 , 22 However, there are some concerns about safety that need to be considered before thinking about the potential clinical applications of hPSCs. 23 Several reports have demonstrated that the isolated En‐NSCs from native intestines were capable of differentiating into functional enteric neurons, 24 , 25 integrating functionally in the colon of wild type (WT) mice 26 , 27 and rescue bowel motility in disease model mice with non–cell‐autonomous effects. 28 However, the limited proliferation capacity and cell quantity of postnatal En‐NSCs limited their application. 14 , 29 Thus far, with En‐NSCs harvested from the native intestine, few studies reported the neuron‐dependent motility at the organ or animal individual level. 28
Irrespective of whether the transplanted donors were derived from the intestine or induced from hPSCs, both enteric NSCs originated from the En‐NCSCs which are located in the gut or induced by intestinal development cues, such as retinoic acid and glial cell line‐derived neurotrophic factor (GDNF). 18 In addition, few cells from other native sources have generated functional enteric neurons in the intestine. 30 Considering the current limitations of cell candidates, more cellular neuroregeneration options are needed, in spite of the encouraging developments in this field. The present study aimed to determine whether the native NCSCs, other than the En‐NSCs isolated from the intestine or those simulated from hPSCs by intestinal development cues, could regenerate a functional enteric neuron and mediate neuron dependent motility.
Most premigratory NCSCs (pNCSCs) are multipotent and can differentiate into many or all the potential derivatives during embryonic development in mice 31 ; however, it is unclear whether they would be multipotent in the intestines of adult mice. To the best of our knowledge, the specific effects of pNCSCs in the mouse colon have not been investigated. We sought to determine if trunk pNCSCs that normally do not contribute significantly to ENS, is capable of generating enteric neurons and establish neuron‐dependent bowel motility.
2. MATERIALS AND METHODS
2.1. Experimental animal
WT C57BL/6J and C57BL/6‐Tg (CAG‐EGFP) (average body weight 25 ± 1.2 g) mice were purchased from the animal core facility of the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Nude and NOD/SCID mice were purchased from the Shanghai SLAC Laboratory Animal Co, Ltd. Animal experiments were conducted as per the Guidelines of the Zhejiang University Laboratory Animal Center for the care and use of laboratory animals and were approved by the Animal Care and Use Committee of the Medical School, Zhejiang University. In the 86 grafted animals, the pNCSCs were transplanted into the colon of WT C57BL/6J mice. The grafted mice were allowed to survive for 1 to 18 weeks before the intestinal tissues were harvested.
2.2. Isolation and identification of pNCSCs
Neural tubes (NT) were isolated from the trunk level using mouse embryos (9 day post coitum [dpc]), and the pNCSCs were isolated from the NT following the established procedure, with some modifications 32 (see Supplemental Methods). In order to identify the pNCSCs migrating from the NT, the NT isolated from the EGFP+ mice were cultured on glass slides, and the pNCSCs were initially identified and selected following P75NTR immunostaining (hereafter referred to P75) after 48 hours.
2.3. In vitro differentiation
In order to assess the capability of pNCSCs to differentiate into the neural lineage and mesenchyme lineage in the environment of chemical defined medium or serum, the pNCSCs that proliferated at Passage 1 (P1, approximately 8 days) and P5 (more than 20 days) were selected for differentiation experiments. The pNCSCs were seeded on coverslips coated with poly‐L‐ornithine (0.01%, 1:5 dilutions) and fibronectin (25 μg/mL) in 24‐well plates. Differentiation medium I, composed of DMEM: F12 medium (1:1, Gibco) and 10% fetal bovine serum (FBS; Gibco), was used for triple‐lineage (mesenchyme, neurons, and glia cells) differentiation. For the neuron differentiation, the pNCSCs were cultured in differentiation medium II composed of DMEM:F12 medium (1:1, Gibco), 1% N2 medium (Gibco), 2% B27 medium (Gibco), brain‐derived neurotrophic factor (BDNF, 10 ng/mL, Peprotech), GDNF (10 ng/mL, Peprotech), nerve growth factor (10 ng/mL, Peprotech), neurotrophin‐3 (NT‐3, 10 ng/mL, Peprotech), ascorbic acid (200 μM, Sigma), cAMP (0.5 mM, Sigma), and 1% Penicillin/Streptomycin (Gibco). 33 Differentiated cells were identified by the expression of markers of neural lineage and mesenchyme lineage with fluorescent immunocytochemistry.
2.4. Electrophysiology
For in vitro electrophysiology recordings, the pNCSCs were plated onto small round glass coverslips at 37°C, 5% CO2. The cells were visualized using an Olympus Optical Microscope (BX51WI, Tokyo, Japan) with differential interference contrast optics at 40× powers. Whole‐cell patch recording was performed to examine the electrophysiology properties of pNCSC‐derived neurons. The EGFP+ cells derived from the populated colon at 3 to 5 weeks after transplantation were analyzed for electrophysiology (see Supplemental Methods).
2.5. Transplantation into the adult mouse colon
The surgical procedure for transplantation followed that reported previously. 24 Briefly, pNCSCs at P3 or P4 were transplanted into the distal colon of 6‐ to 8‐weeks‐old C57BL/6J WT or NOD/SCID mice. In order to form 3D spheroids used for transplantation, the pNCSCs were dissociated and transferred onto ultra‐low attachment plates (Corning). The mice were anesthetized with Isohalothane (RWD Life Science, San Diego, California). The distal colon was exteriorized through a small abdominal incision. The pNCSCs (approximately 3 × 105 cells in 3 μL) with 50% Matrigel (BD Biosciences, Franklin Lakes, New Jersey, 354234) were transplanted in the external muscle layer of the distal colon at three sites that were each marked with 7/0 Nylon. At 1 to 18 weeks following surgery, the mice were sacrificed, and the tissue was collected for histological analysis.
2.6. Generation of the human TEI with pNCSCs
A human ES cell line H1 was maintained in mTeSR (STEMCELL Technologies, Shanghai, China, 05850) medium and routinely passaged with collagenase IV (Gibco). Human intestinal organoids (HIOs) were generated as described previously 21 , 34 (see Supplemental Method). For TEI maturation, HIOs and HIOs + pNCSCs were transplanted into the kidney capsule of BALB/c nude mice following previous protocol, 21 , 35 and allowed to grow for 6 10 weeks to in vivo maturity.
2.7. Time‐lapse video microscopy of mechanical contractility
The TEI and pNCSCs‐TEI was harvested 6 to 10 weeks following transplantation, explanted into FluoroBrite DMEM (Thermo Scientific, Grand Island, New York, A18967‐01), and then allowed to equilibrate for 30 minutes at 37°C. Cell imaging was performed using a Nikon SMZ800N and a Nikon Digital Sight DS‐Filc Camera. Explants were initially imaged to evaluate for spontaneous contractility and then treated with methylene blue (50 μM) for 4 hours before imaging for the selective inhibition of any contractility produced by interstitial cells of Cajal (ICCs). 21 , 22
For each TEI, the observation was made and recorded at least for 3 minutes. Videos were post‐processed with Apowersoft Video Editor to 15× speed to visualize the contraction and relaxation of the TEI and analyzed using the video‐analysis software Tracker, version 4.91. Automated point tracking with the position was performed to measure the movement within the explanted TEI and TEI‐pNCSCs upon different treatments.
2.8. Immunochemistry
Immunostaining on cultured cells, frozen cryosections, and whole‐mount of the intestines of each mouse or HIO was performed (see Supplemental Files). The primary and secondary antibody information is listed in Tables 1 and 2, respectively. The tissues were examined using a Nikon A1 confocal microscope (Nikon, Japan). Statistical analyses were performed for each marker using the immunostaining results of three mice or three HIOs.
TABLE 1.
Primary antibody list
| Antigen | Supplier | Cat. No. | Host | Dilution | RRID |
|---|---|---|---|---|---|
| P75NTR | Abcam | ab8875 | Rabbit | 1:200 | AB_306828 |
| Nestin | Millipore | MAB353 | Mouse | 1:200 | AB_94911 |
| AP2 | Santa Cruz | sc‐12 726 | Mouse | 1:50 | AB_667767 |
| SOX10 | Abcam | ab155279 | Rabbit | 1:250 | AB_2650603 |
| Ki67 | Abcam | ab15580 | Rabbit | 1:500 | AB_443209 |
| TuJ1 | Covance | mrb‐435p | Rabbit | 1:200 | AB_663339 |
| TuJ1 | Promega | G712A | Mouse | 1:500 | AB_430874 |
| PGP9.5 | Abcam | ab8189 | Mouse | 1:200 | AB_306343 |
| NF200 | Sigma | N4142 | Rabbit | 1:200 | AB_477272 |
| GFAP | Abcam | ab7260 | Rabbit | 1:200 | AB_305808 |
| S100β | Abcam | ab52642 | Rabbit | 1:200 | AB_882426 |
| nNOS | Abcam | ab76067 | Rabbit | 1:500 | AB_2152469 |
| VIP | Santa Cruz | sc‐20 727 | Rabbit | 1:20 | AB_2304501 |
| ChAT | Millipore | AB144p | Goat | 1:50 | AB_2079751 |
| ChAT | Proteintech | 20 747‐1‐AP | Rabbit | 1:100 | AB_10898169 |
| HuC/D | Abcam | ab184267 | Rabbit | 1:500 | AB_2864321 |
| Synapsin‐1 | CST | 5297 | Rabbit | 1:100 | AB_2616578 |
| SMA | Abcam | ab5694 | Rabbit | 1:1500 | AB_2223021 |
| C‐KIT | Abcam | ab32363 | Rabbit | 1:100 | AB_731513 |
| CDX2 | Abcam | ab76541 | Rabbit | 1:500 | AB_1523334 |
| CDH1 | R&D | AF648 | Goat | 1:20 | AB_355504 |
| FoxA2 | Abcam | ab108422 | Rabbit | 1:300 | AB_11157157 |
| Sox17 | R&D | AF1924 | Rabbit | 1:20 | AB_355060 |
TABLE 2.
Secondary antibody list
| Secondary antibody | Cat. No. | Dilution | Supplier | RRID |
|---|---|---|---|---|
| Donkey anti‐Mouse IgG (H+L), Alexa Fluor 488 | A‐21202 | 1:500 | Thermo Fisher Scientific | AB_141607 |
| Donkey anti‐Rabbit IgG (H+L), Alexa Fluor 488 | A‐21206 | 1:500 | Thermo Fisher Scientific | AB_2535792 |
| Donkey anti‐Rabbit IgG (H+L), Alexa Fluor 594 | A‐21207 | 1:500 | Thermo Fisher Scientific | AB_141637 |
| Donkey anti‐Mouse IgG (H+L), Alexa Fluor 594 | A‐21203 | 1:500 | Thermo Fisher Scientific | AB_141633 |
| Donkey anti‐Goat IgG (H+L), Alexa Fluor 594 | A‐32758 | 1:500 | Thermo Fisher Scientific | AB_2762828 |
| Donkey anti‐Rabbit IgG (H+L), Alexa Fluor 647 | A‐31573 | 1:500 | Thermo Fisher Scientific | AB_2536183 |
2.9. Statistical analyses
Data are displayed as mean ± SEM. For intestinal motility experiments, statistical significance was considered at P < .05. GraphPad Prism was used for statistical analysis of cell numbers in the culture, proliferation, and immunostaining experiments.
3. RESULTS
3.1. Isolation and characterization of NCSCs from mouse NT on embryo day 9.0
In order to study the potential of pNCSCs for ENS neuroregeneration in the intestine, the pNCSCs were first harvested using a modified version of a previously described method. 32 Trunk NT segments of embryos 9.0 dpc were isolated by microdissection. The trunk sections were enzymatically treated with collagenase IV and dispase II; they were plated on poly‐L‐ornithine coated six‐well Corning plates. The cells that migrated out of NT explants were closely monitored because the acquisition of pNCSCs was based on the morphological and behavioral differences observed between the supposed NCSCs and the surrounding cells. The NCSCs showed a high migration capability, as reported previously. 30 , 36 The supposed NCSCs were the first type of cells that migrated from NT explants within 12 hours of NT attachment.
The first batch of cells that emigrated from NT explants had a high nuclear‐to‐mass ratio, and expressed the NCSC marker P75 37 , 38 , 39 (Figure 1). These P75+ cells clung to each other and gathered in the condensed area where they were easily distinguished from other areas using a microscope (Figure 1B,C). The cells migrated out 1 to 2 days after the NT explants were attached to the plates. Thereafter, the NT explants were removed 4 days after attachment. Cells at P0 (passage 0) were cultured for 7 days in the proliferation medium before being passaged to P1. During the passage, the supposed NCSCs were purified after the mechanical removal of unrelated cell types. Premigratory NCSCs at P3 or P4 were cultured on low‐attachment plates in the proliferation medium to form spheres for transplantation and implantation. The whole process is illustrated in Figure 1A. Along with the morphological characteristics, the NCSC marker, P75, was primarily used to identify pNCSCs derived from NT (Figure 1B,C).
FIGURE 1.
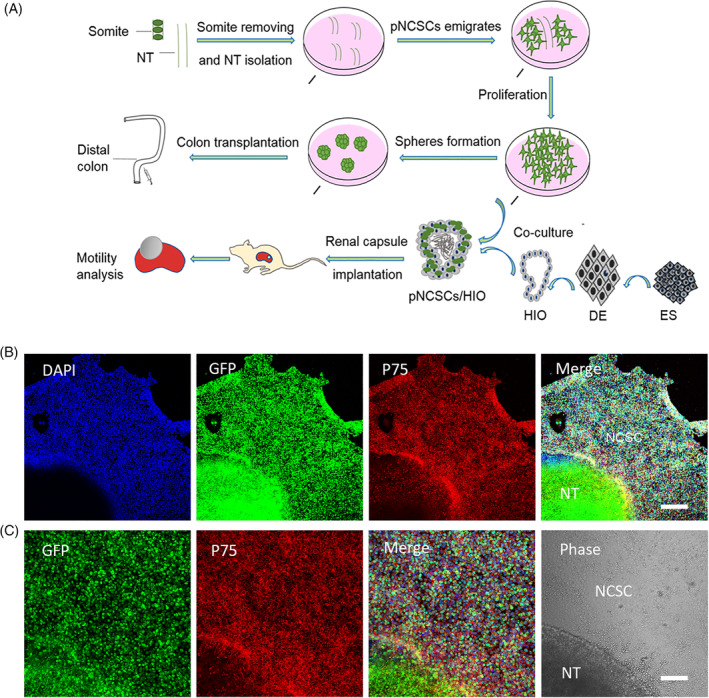
The experimental workflow for manipulating premigratory neural crest stem cells (pNCSCs) and primary characteristics of pNCSCs in culture. A, Schematic diagram of workflow for isolation of the pNCSCs, cell proliferation, subrenal capsule implantation, and transplantation. The pieces of mouse neural tubes (NT) at E9.0 are placed in the proliferation medium. The emigrated cells from NT are manually isolated and cultured as P0. These cells are proliferated to P3 or P4 before transplantation or cocultured with human intestinal organoids (HIO). With respect to the route of transplantation, spheres formed from pNCSCs are transplanted into the distal colon of mice to check the capacity to regenerate the enteric nerve. The other route is used for checking if the intestinal motility could be mediated by the tissue‐engineered intestine's (TEI) pNCSCs‐derived neurons. Human embryonic stem cells (hESCs) are first induced into the definitive endoderm (DE), and DE is further induced into the HIO. For TEI maturation, HIO and HIO + pNCSCs are transplanted into the kidney capsule of BALB/c nude mice to mature. The mature TEI is further analyzed for intestine motility. B, The immunostaining of P75, DAPI, EGFP, and the merged images of the cells migrated from NT in the first batch in sequence. The cells displaying positive P75 immunostaining are presumably NCSCs. C, Higher magnification of the inset in (B) about EGFP, P75 staining, merged image of EGFP, DAPI and P75 staining, and phase image in the sequence. The supposed pNCSCs are shown in the phase image. Scale bars = 200 μm (B), 100 μm (C)
3.2. Derived cells expressed markers of NCSCs and differentiated into peripheral neurons and glia cells
The cells that emigrated from NT in the first batch were analyzed with fluorescent immunocytochemistry to confirm their identity. Immunostaining was performed at P2, at about 12 to 16 days of NT isolation and cell culture. The culture medium was changed every other day, and the cells were passaged at 1:2 or 1:3 every 2 to 3 days. As per the results, a high proportion of these cells expressed the neural stem cell marker Nestin (93.2% ± 4.8%), the NCSC marker P75 (94.5% ± 3.5%), AP2 (93.1% ± 5.0%), and SOX10 (94.6% ± 4.1%) (Figure 2F). AP2 and SOX10 were the transcription factors that play crucial roles during NCSCs development.
FIGURE 2.
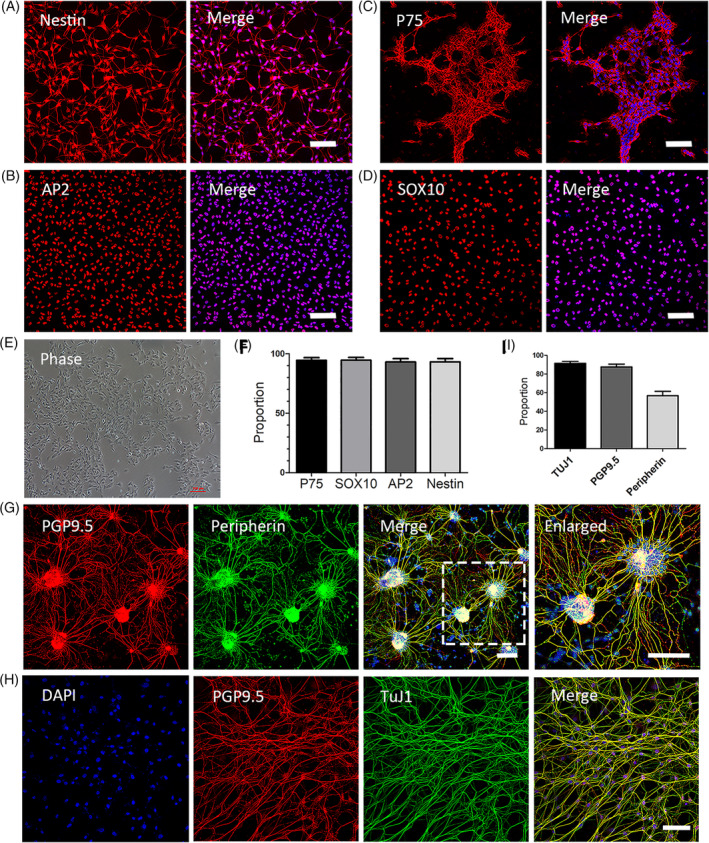
Characterization of the identity and the differentiation capability of mouse premigratory neural crest stem cells (pNCSCs). A, The supposed pNCSCs at P2 are identified with immunofluorescence staining using the neural stem cells (NSCs) marker, Nestin (A‐left image), and the NCSCs markers P75 (B, left), AP2 (C, left), and SOX10 (D, left). These cells are counterstained with DAPI in the nuclei and showed merged images (A‐D, right image). The cultured cells at P2 are shown as the phase image (E). Most cells are immunoreactive to Nestin (93.2% ± 4.8%), P75 (94.5% ± 3.5%), AP2 (93.1% ± 5.0%), and SOX10 (94.6% ± 4.1%) (n = 3 independent experiments) as showed in the cartogram (F). G,H, Immunofluorescence staining of pNCSCs following cultured in differentiation medium II for 7 to 14 days. The differentiation into neurons is confirmed by the double immunostaining of pan‐neuronal markers PGP9.5 (red), peripheral neuronal marker peripherin (green, pseudo‐color), and the merged image of DAPI, PGP9.5, and peripherin staining. TuJ1 (green, pseudo‐color) and PGP9.5 staining with DAPI are also demonstrated (H). I, The proportion of positive cells in the TuJ1 staining (TuJ1, 91.93% ± 3.7%), PGP 9.5 (87.3% ± 5.4%), and peripherin (57.3% ± 7.0%). Scale bars = 100 μm
The capability of multipotent differentiation is an important characteristic of pNCSCs. 40 , 41 In order to test whether these supposed NCSCs have the capacity to differentiate into triple lineages, these cells were cultured in differentiation medium I that contained 10% fetal bovine serum (FBS). After 10 to 14 days, the immunostaining data revealed that the pNCSCs harvested from EGFP mice had differentiated into cells stained with an antibody against GFAP (an enteric glial and astrocyte marker), TuJ1 (a neuronal marker), and smooth muscle actin (smooth muscle marker [SMA]) (Supplemental Figure 1).
To further test the capability of directed pNCSCs to differentiate into peripheral neurons, the pNCSCs were cultured in a chemically defined medium (differentiation medium II) for 7 to 14 days. Most pNCSCs differentiated into neurons that displayed robust expression of the pan‐neuronal markers, β‐III tubulin (TuJ1, 91.93% ± 3.7%), and PGP9.5 (87.3% ± 5.4%) (Figure 2G‐I). Furthermore, we found that >50% of the cells differentiated into peripheral neurons, as shown by the staining of the peripheral neuron marker, peripherin (57.3% ± 7.0%) (Figure 2G‐I). Moreover, the pNCSCs displayed the standard morphology of peripheral neurons with long fibers and nerve bundles in differentiation medium II (Figure 2G,H).
3.3. Derived pNCSCs maintained the expression of NCSC markers after proliferation
In order to ensure sufficient cells for transplantation and implantation, the migrated cells were proliferated in the proliferation medium (as described in the Material and Methods section) to P3 or P4. About 67% cells expressed proliferation marker Ki67 at P3 (Supplemental Figure 2), and the derived pNCSCs were able to maintain continuous expansion capability through at least six passages (>30 days) in proliferation medium. In order to guarantee the identity and purity of NCSCs, the immunostaining of neural stem cell and NCSC markers was performed on P2 and P4 cells. At P4, the staining results showed the expression Nestin (94.33% ± 3.37%), P75 (93.27% ± 2.58%), SOX10 (92.50% ± 2.95%), and AP2 (85.3% ± 2.10%). The staining and statistics result of P2 (Figure 2A‐F) and P4 (Supplemental Figure 3) showed that the identity and purity of NCSCs were maintained during proliferation.
3.4. Premigratory NCSCs were distributed in the muscle layer and generated a neuronal network after transplantation into the adult mouse colon
The intestinal environment of adult mice differs from that of mouse fetuses. We were unsure whether pre‐migratory cells derived from the NT of the mouse embryo, as nonenteric NCSCs, could form the enteric subtype neurons and glia cells in adult mice colon. In order to track the destiny and behavior of pNCSCs in the adult mice colon, the pNCSCs harvested from the NT of EGFP+ mice were cultured to P4 and injected into the external muscle layer of the distal colon of 6‐ to 8‐weeks‐old C57BL/6J mice (total 86 mice were injected). We found that the survival of EGFP+ pNCSCs could be tracked for about 2 months (Figures 3 and 4), 4 months (Figure 5), and up to 5 months (data not shown) after transplantation in colons. The EGFP+ cells are mainly localized in the longitudinal muscle (LM) and circular muscle (CM) layers with few EGFP+ fibers extended to the mucosa and submucosa layers 3 weeks following transplantation (Supplemental Figure 4A‐C).
FIGURE 3.
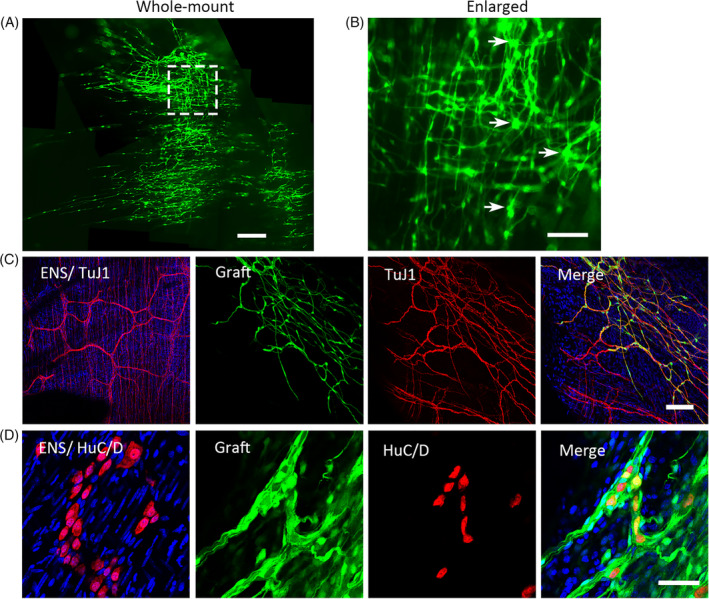
Premigratory neural crest stem cells (NCSCs)‐derived cells were distributed in the mouse colon and generated a neural network with ganglion‐like clusters at 2 months after transplantation. A, Representative image of low‐magnification views of a whole‐mount preparation shows exogenous EGFP+ cells and fibers within the distal colon. Some EGFP+ cells form ganglion‐like clusters (arrows, right image of B). The inset of image A is shown enlarged in image (B). C, Enteric nervous system (ENS) network and the network formed by exogenous EGFP+ cells with immunostaining. ENS network of TuJ1 staining is shown on the left as a control. The other three images show EGFP fluorescence, TuJ1 immunostaining, and the merged image of EGFP, TuJ1 and DAPI immunostaining in sequence. D, HuC/D staining of inherent enteric neurons is shown on the left as a control. The next three images showed EGFP+ cells, HuC/D immunostaining, and the merged image of EGFP, HuC/D, and DAPI immunofluorescence in sequence. Scale bar = 500 μM (A), 100 μm (B,C), 50 μm (D)
FIGURE 4.
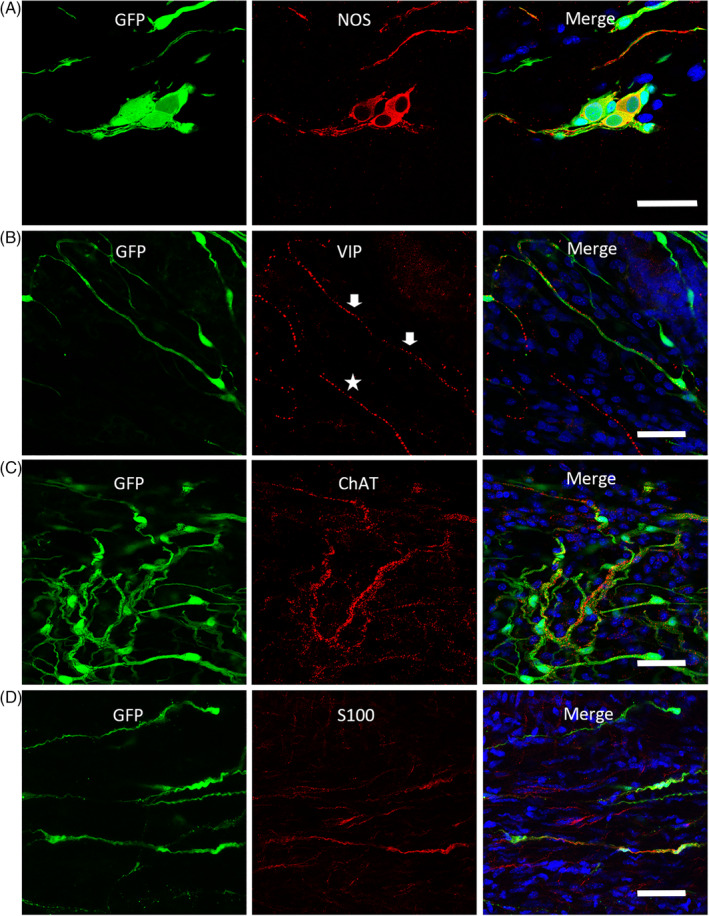
EGFP+ cells differentiated into glia cells expressing S100β, and neurons expressing nitric oxide synthase (nNOS), Vasoactive Intestinal Peptide (VIP), and choline acetyltransferase (ChAT) at 2 months after transplantation. A‐C, The colocalization of grafted EGFP+ cells with the immunofluorescence staining of enteric neurochemical markers nNOS, VIP, and ChAT. B, The arrows indicate the VIP staining with EGFP fluorescence, and the asterisks show VIP expression of endogenous neurons. D The fluorescence of some EGFP+ cells is colocalized with the immunostaining of glia cell marker, S100β. Sections of the mouse colon are counterstained in blue with DAPI. Scale bars = 50 μm
FIGURE 5.
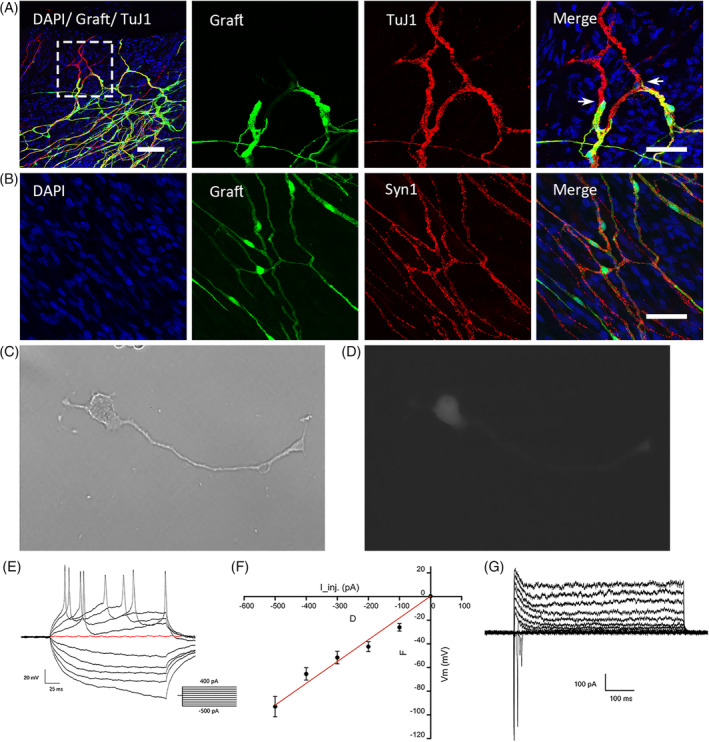
Extrinsic EGFP+ cells were integrated into the colon and displayed the electrophysiological properties of neurons. A, The whole mount immunostaining shows that EGFP+ cells expressing TuJ1 connected with the endogenous TuJ1‐expressing neurons at 4 months after transplantation. The three images on the right display the inset of the left image at a higher resolution. B, The immunostaining of Synapsin‐1 (Syn1) among EGFP+ cells and endogenous cells in the whole mount preparation. C‐G, The graft‐derived neurons are impaled 3 weeks after transplantation of premigratory neural crest stem cells (pNCSCs). These cells were isolated by trypsin and attached to the coverslips before electrophysiological examination. C,D, A derived neuronal image with a single long and axon‐like process under the lens of phase (C) and fluorescence (D) of the microscope. Passive membrane properties and AP properties of pNCSCs‐derived EGFP+ neurons. E, Representative membrane voltage traces upon step injection of currents (current‐clamp). Inset: description of current injection from −500 pA to +400 pA. The red trace indicates the membrane potential at 0 pA injection. F, The plot of membrane potential response (Vm) against injected currents (I_inj.). G, Representative whole‐cell recordings from the pNCSCs derived neurons (holding potential, −80 mV; test potentials from −100 to 100 mV at 10 mV intervals). Scale bars (A) left = 100 μm; (A) right 50 μm; (B) 50 μm
After 2 months of transplantation, exogenous EGFP+ cells migrated, and the fibers extended broadly in the colon. The EGFP+ fibers covered more than 3.55 × 4.04 mm2 from one injection site (about 1 × 105 cells). Moreover, the transplanted pNCSC cells formed neural network resembled that of the mouse ENS network (Figure 3C), some ganglion‐like clusters were also found (arrows, Figure 3B) with the expression of HuC/D (Figure 3D).
Both neurons and glial cells play crucial roles in the formation of the complete ENS. Therefore, we further examined if the pNCSCs derived from NT could differentiate into neurons and glial cells in vitro and vivo. The in vitro experiments showed that the pNCSCs differentiated into neurons and glial cells in the medium with 10% serum (Supplemental Figure 1A,B); moreover, the in vivo results showed that most grafted cells had differentiated into TuJ1‐expressing cells via the colocalization of EGFP with TuJ1 staining (Supplemental Figure 4A,B; Figure 4E).
To validate our results, transverse sections of adult mice colon were used to confirm the EGFP coexpression with the glial cell marker S100β (Supplemental Figure 4D; Figure 4D). TuJ1 was expressed in most grafted cells, whereas only a small proportion expressed S100β after transplantation. Our in vivo and in vitro studies indicated that pNCSCs could differentiate into neurons and glia cells, both of which are the basic components of ganglia. The pNCSCs survived, extended fibers, formed ganglion‐like clusters, and generated a neural network in the adult mouse colon following transplantation.
3.5. Premigratory NCSCs differentiate into neurons with the neurochemical characteristics of enteric neurons following transplantation
The capability of differentiation into enteric neural subtypes is a critical criterion for cell candidates to be chosen in enteric neuroregeneration. The colocalization of EGFP+ cells with the expression of nitric oxide synthase (nNOS) (Figure 4A), vasoactive intestinal peptide (VIP, Figure 4B), choline acetyltransferase (ChAT, Figure 4C) demonstrated that the exogenous pNCSCs had differentiated into neurons with the neurochemical characteristics of enteric neurons at 2 months after transplantation.
3.6. EGFP + cells integrated into the colon
We explored whether pNCSCs integrated into the local system in adult mice colons. The EGFP+ cells and their fibers integrated with endogenous enteric neurons and fibers, as shown by the cell morphology at 2 months after transplantation (Figure 3C). At 4 months after transplantation, the EGFP+ fibers expressing TuJ1 had not only formed connections with inherent neurons (Figure 5A), but also expressed the synaptic vesicle protein Synapsin‐1 (Syn1) (Figure 5B).
3.7. Transplanted pNCSCs differentiated into neurons with active electrophysiology
In order to confirm whether the neurons differentiated from exogenous pNCSCs exhibited electrophysiological function at 3 to 4 weeks following transplantation, small pieces of colon containing EGFP+ cells were dissected and digested into single cells, and the harvested cells were cultured in a petri dish. The electrophysiological actions were tested after the cells attached to petri dish. The results showed that the EGFP+ cells issued continuous action potentials (APs) (Figure 5C‐G; n = 3 slides, 4 cells each) following the current stimulation. The rapidly separated exogenous cells exhibited electrophysiological function.
3.8. Premigratory NCSCs differentiated into enteric neurons in the TEI
Implantation into TEI has proven an effective method to prove that exogeneous neurons can mediate peristalsis. 21 , 22 In order to test if pNCSCs could differentiate into enteric neurons and mediate intestinal peristalsis in the human TEI, pNCSCs were combined with HIO and implanted into the kidney capsule of nude mice. To make HIO, we induced hESCs (H1 line) in a stepwise differentiation protocol to definitive endoderm, confirmed by FOXA2 and SOX17 expression (Supplemental Figure 5A). Then, mid/hindgut tube spheroids were formed in vitro, as shown by immunostaining of the epithelium marker CDH1 and CDX2 (Supplemental Figure 5B). 42 These induced HIOs contained most of the epithelial and mesenchymal cells present in the developing gut; however, they lacked ENS. 43 In order to generate TEI with an ENS‐like nervous system, HIOs without or with pNCSCs were engrafted ‐into the subrenal capsule of nude mice for TEI maturation. After 6 to 10 weeks, HIOs grew into mature TEI with vessels and were harvested for the next analysis (Figure 7; Supplementary Video). In order to confirm the formation of TEI anatomy, immunostaining of TEI cryosections and whole mounts was performed. The positive SMA expression showed the formation of the myenteric layers of the smooth muscle fibers (Figure 6A,E). The epithelium formation was proved by the immunostaining of CDH1 and CDX2 (Figure 6B,D). The ICCs that were required for spontaneous pacemaker activity in the intestine were also found available in TEI, as indicated by c‐Kit expression (Figure 6C).
FIGURE 7.
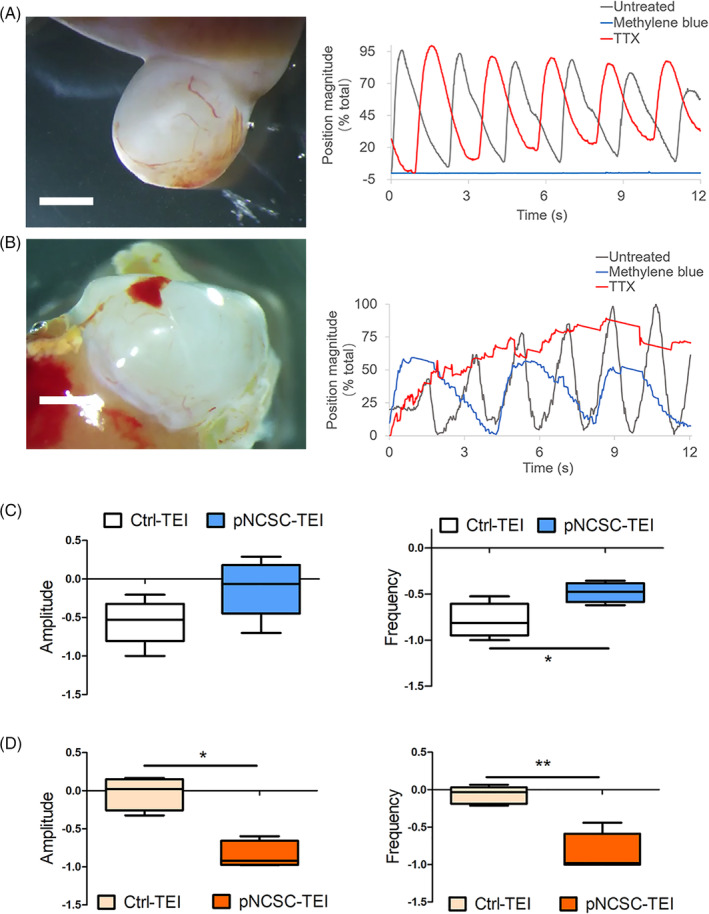
Motility analysis of control tissue‐engineered intestine and premigratory neural crest stem cells (pNCSCs‐TEI). A, In the motion analysis of Ctrl‐TEI, the gray curve represents spontaneous peristalsis and the red curve represents the peristalsis after TTX treatment. There is no obvious change compared with the treatment before (n = 5). The blue curve represents the peristalsis following methylene blue treatment, showing an obvious decline in the amplitude and frequency (n = 6) (see [Link], [Link]). B, In the motion analysis of pNCSCs‐TEI, the gray curve represents spontaneous peristalsis. The blue curve represents the peristalsis following treatment with methylene blue, showing a decreased but not blocked peristalsis. The red curve represents the peristalsis following TTX treatment, showing an obvious decline both in amplitude and frequency, especially the frequency (n = 5) (see [Link], [Link]). (A,B, right panel) X axis represents the recording time (s); the Y axis represents the position magnitude (% total). The Abscissa accompanying each curve is showed as the time (s) after 15× speed acceleration. Scale bar (A,B, left panel) = 2 mm. C, Comparison of the amplitude (left) and frequency (right) in Ctrl‐TEI and pNCSCs‐TEI after methylene blue treatment following automated point tracking. D, Comparison of the amplitude (left) and frequency (right) after TTX treatment. For box and whisker plots, the black line across the box represents the median, the box represents the interquartile range; and the whiskers represent the minimum and maximum. C,D, The Y axis represents rate of change after (Xa) and before (Xb) the treatment, that is, (Xa‐Xb)/Xb *P < .05, **P < .01, ***P < .001; Mann‐Whitney test
FIGURE 6.
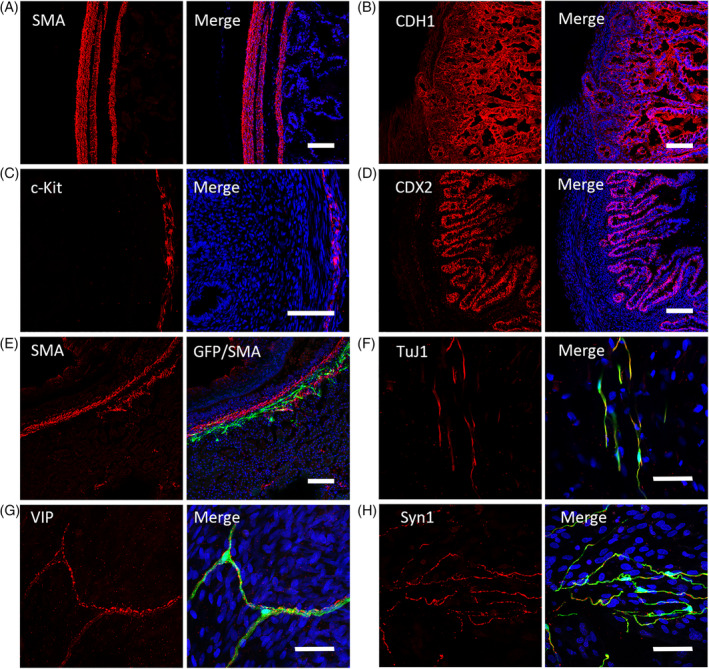
Immunofluorescent staining of tissue‐engineered intestine (TEI) and TEI + premigratory neural crest stem cells (pNCSCs). A, The positive immunostaining of smooth‐muscle marker (SMA) is found in the locations of myenteric and submucosal layers in the TEI sample. B, The positive immunostaining of the epithelium marker CDH1 is showed in TEI. C, Immunostaining of c‐Kit, a marker of interstitial cells of Cajal (ICCs). D, Immunostaining of CDX2, a marker expressed in the nuclei of epithelial cells throughout the intestine. E, The distribution of EGFP+ cells in the muscular layer (expressing SMA) of TEI. F‐H, The positive immunofluorescent staining of pan‐neural marker TuJ1 (F) and marker of enteric neuron subtype VIP (G) are available as showed by co‐expression with EGFP in the muscular layers of pNCSCs‐TEI, Synapsin‐1 was also found coexpressed with EGFP+ cells in the myenteric layers (H). Scale bars (A‐E) = 100 μm and (F‐H) 50 μm
We performed immunostaining to examine the differentiation capacity of pNCSCs into enteric neurons in the TEI environment. The results showed that EGFP+ pNCSCs had differentiated into the cells expressing neuronal markers as TuJ1 (Figure 6F), NF‐L (Supplemental Figure 6C), and also the cells expressing the glia cell marker S100β (Supplemental Figure 6D). In TEI‐Ctrl group, no positive expression of TuJ1 and S100β was detected (Supplemental Figure 6A,B). In addition, in TEI‐pNCSCs, part of EGFP+ cells expressed VIP (Figure 6G). Moreover, colocalization of Syn1 with EGFP fluorescence were detected in muscle layers, suggesting the possible connection of pNCSCs‐derived neurons with inherent cells (Figure 6H).
3.9. Premigratory NCSCs could mediate the peristalsis activity of TEI in a neuron‐dependent manner
To explore the relationship between TEI and its motility with different treatments, the motility of TEI was recorded and analyzed. A sustained wave of phasic contraction was detected during inspection in the Ctrl‐TEI group (where 31 of 36 HIOs developed into mature TEI following the subrenal implantation) and pNCSCs‐TEI group (where 37 of 55 TEIs were successfully integrated with EGFP+ cells with microscopic examination). Intestinal motility involves the coordination of the ENS and ICCs (Figure 6C) that act as the pacemaker cells of gut motor activity. 44 , 45 In order to determine whether the contractions resulted from ICC activity or were regulated by the pNCSCs‐derived neurons, we used methylene blue, a known inhibitor of ICCs, in our experiment. As individual differences such as the size of TEI and initial motion capacity (amplitude, frequency) were found to affect the results under drug treatment, statistical analysis was performed for the recording results of TEI.
We found that the TEI‐Ctrl and TEI‐pNCSCs exhibited different reactions after the application of methylene blue. In the Ctrl‐TEI group that lacked exogenous neurons inside the intestine (Supplemental Video 1), the peristalsis was evidently blocked with methylene blue treatment (n = 6) (Figure 7A,C; Supplemental Video 3). In the pNCSCs‐TEI group, the peristalsis decreased but not blocked after methylene blue treatment (n = 5) (Figure 7B,C; Supplemental Video 5). The different reactions after ICC inhibitor treatment suggested that the motility in the pNCSCs‐TEI group was controlled not only by the ICCs activity, but also by another mechanism.
Further studies that determine whether the contractions were a result of neuronal activity, treatment with tetrodotoxin (TTX), 21 which acts as a neuron inhibitor through binding to voltage‐gated Na+ channels, was performed. The phasic contractile activity in Ctrl‐TEI was slightly affected (Figure 7A,D; Supplemental Video 2). Simultaneously, that in pNCSCs‐TEI were evidently inhibited (n = 5) (Figure 7B,D; Supplemental Video 4 and 6), suggesting that the motility was mediated in a neuron‐dependent manner. The statistics result displayed evident difference between Ctrl‐TEI and pNCSCs‐TEI under TTX treatment.
In sum, these TEI anatomical and motility analyses results showed that the pNCSCs‐derived cells mediated the motility of the TEI in a neuron‐dependent manner.
4. DISCUSSION
NCCs are the ancestors of the peripheral nervous system, including the ENS that plays a crucial role in maintaining normal gastrointestinal function. Reduction or destruction of neurons in the ENS of the GI tract causes illnesses that can be severe or fatal. NCSC‐derived En‐NSCs might be direct cell candidates for ENS neuroregeneration. Various cell types have been used in HSCR studies with stem cell transplantation. These include En‐NSCs derived from the native intestine and those induced from the hPSCs; both the studies are based on the En‐NCSCs. However, in assessing the current options, En‐NSCs derived from the intestine and induced from hPSCs have their individual advantages and limitations. 12 Limitations inherent to both cell sources prompted the consideration of more possible options beyond the enteric NCSCs.
Several reports have shown that premigratory NCCs are multipotent in vivo during the embryonic development phase. 31 , 46 , 47 However, to our knowledge, no reports have demonstrated whether these cells can further differentiate into enteric neurons in the postnatal intestine. In normal embryo development, NCCs migrate to the gut and form En‐NSCs following induction by the embryonic gut environment. This developmental principle led to the use of En‐NSCs for enteric neuroregeneration for many years. The key question was whether the adult gut's environment could support the formation of functional enteric neurons from pNCSCs, similar to the environment of the embryonic gut to the migrated NCCs. The destiny of stem cells in the intestinal environment not only depends on the inherent differentiation potential, but also on the milieu where the cells locate. This essentially depends on the interaction between cells and the microenvironment. In our experiments, pNCSCs showed various multipotency and plasticity in the different environments in vitro and in vivo.
We found that pNCSCs differentiated into neurons and glia cells in the differentiation medium in vitro. When grown in the serum environment in differentiation medium I, the pNCSCs differentiated into the triple lineages (smooth muscles, neurons, and glia cells, as Supplemental Figure 1 showed). When cultured in the chemically defined medium with BDNF, GDNF, NGF, NT‐3, ascorbic acid, and cAMP, the pNCSCs mainly differentiated into neurons, particularly into peripheral neurons.
For in vivo transplantation, cell candidates for ENS regeneration and even HSCR therapy should be viable, have the ability to migrate, and feature enteric neuron differentiation and integration into the local neural circuitry after transplantation. 24 If cell candidates could regulate intestinal motility, it might become a more practical candidate for ENS neuroregeneration. 21 It has been shown that pNCSCs are multipotent during embryonic development. 31 Here, we found that in the colons of adult mouse, pNCSCs were competent to form neuronal network including ganglion‐like anatomy. Under the circumstances of the natural colon, pNCSCs survived, migrated, and differentiated into glia cells and enteric neurons. These differentiated cells exhibited the morphological characteristics and neurochemical markers' expression, including nNOS, VIP, and ChAT, similar to resident enteric neurons in the recipient mouse colon. It is worth noting that ChAT does not exclusively expressed in ENS neurons and also expressed in other identities such as parasympathetic lineage. 48 Further staining with other markers is needed to identify more subpopulation differentiated from pNCSCs next.
Intestinal motility is dependent on a complete neural circuit and the perfect connection between the functional plexus and the muscles. The pNCSC‐derived cells were mostly distributed in appropriate layers between the LM and CM, with some fibers extending into the submucosal layer. In these cases, the layers resembled those observed in normal adult mice. The EGFP+ graft cells expressed the mature presynaptic protein Syn1 after transplantation. It has been reported that En‐NSCs have been capable of differentiating into functional neurons in the gut. 24 , 27 , 30 Our results showed that the pNCSCs derived neurons in the colon and could trigger AP electrophysiological activity after their derivation and attachment.
In human TEI environment, the pNCSCs differentiated into enteric neurons that were distributed throughout the muscle layers. The intestines in TEI + pNCSCs group showed peristalsis that could not be blocked by methylene blue; however, they were significantly inhibited by TTX treatment. In human TEIs, the pNCSCs that presumably had no intestinal information could form functional enteric neurons and mediate neuron‐dependent motility. It also suggested that non–En‐NCSCs are potential cell sources for the reconstructed intestine with respect to organ transplantation.
ENS is mainly derived from the vagal (somite 1‐7) and sacral regions. 49 We previously showed that DRG‐derived NCSCs can form functional enteric neurons in the adult mice colon. 30 Here, we showed that NCSCs derived from premigratory trunk NT generated functional enteric neurons in normal mouse colon and mediated motility in TEI. Neither cell type, DRG‐derived NCSCs or pNCSCs, is considered to have been induced by the gut environment or intestinal development cues. However, they display the capability of generating functional enteric neurons in the intestine in vivo. These results suggested that the nonenteric NCSCs from two sources could differentiate into functional enteric neurons in the natural intestinal environment of adult mice. However, the potential of DRG‐derived NCSCs has not been studied in different intestinal environments, and the motility‐mediating capacity of these cells requires further investigation. Our results showed that the pNCSCs, as other nonenteric NCSCs, can respond to different environmental cues and display considerable plasticity. Furthermore, the TEI model results showed that the TEI's microenvironment would promote pNCSCs to form enteric neurons and establish neuron‐dependent motility. These results indicate that it may not be necessary to use enteric NSCs induced previously by the gut environment or cues as donor cells to form functional ENS in the recipient's intestine. The intestinal environment may provide enough cues for nonenteric NCSCs for the formation of functional enteric neurons. In addition, it has also been recently demonstrated that a subset of Schwann precursors that emigrate through trunk nerves invade the gut and also generate parts of the ENS. 50 The pNCSCs that are extracted from trunk NT and cultured through P3/P4 before transplantation likely contain a mixture of progenitor cells destined to form other lineages (such as the DRG, sympathetic ganglia), sacral ENS, and Schwann‐cell precursor cells with potential to generate ENS.
NCCs extensively migrated along different routes and persisted in individual tissues and organs. NCSCs are widely distributed in adult tissues and organs, including the skin, hair follicles, and dental pulps. 51 , 52 , 53 , 54 Cells, such as the premigratory, migratory, and tissue‐specific NCSCs, share similar developmental origins and display similar expression patterns of key transcription factors with embryonic NCCs. They might have similar developmental potentials. Autologous NCSCs from nonenteric tissues, such as skin, hair follicles, and dental pulp, may be important potential candidates for ENS neuroregeneration and treatment of GI tract motility neuropathies based on their derivation, ethic, safety, and anti‐immune rejection properties. Future exploration of nonenteric NCSCs may broaden the range of cell candidates for ENS regeneration and impact other NCSCs‐related neurological diseases.
5. CONCLUSION
One of the most common diseases associated with NCC abnormalities is HSCR, which results from the absence of the colonic ENS. Several sources of candidate cells have been studied for ENS reconstruction. However, it is unclear whether nonenteric NCSCs possess the capability of regenerating functional ENS and mediating neuron dependent motility in the intestinal environment. Here, we demonstrate that pNCSCs, as nonenteric NCSCs, can form neuronal network including enteric neurons and ganglion‐like anatomy in the natural mouse colon, and mediating neuron‐dependent peristalsis in the TEI.
CONFLICT OF INTEREST
The authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
H.Y., H.H., R.C.: experimental design, collection and assembly of data, data analysis and interpretation; W.M., L.W., Y.L.: collection and assembly of data, data analysis and interpretation; Y.X.: critical revision of the manuscript for important intellectual content, Manuscript writing; C.Y., D.X.: provision of study material; L.W., S.M., J.C.: provision of study material, data analysis and interpretation; Y.D.: data analysis and interpretation, manuscript writing, final approval of manuscript; J.T.: financial support, data analysis and interpretation, manuscript writing, final approval of manuscript; W.C.: conception and design, financial support, data analysis and interpretation, manuscript writing, study supervision, final approval of manuscript.
Supporting information
Appendix S1 Supporting Information.
Supplemental Video 1 Control TEI demonstrates spontaneous phasic contraction and relaxation.
Supplemental Video 2 TTX treatment shows no obvious change compared with the spontaneous phasic peristalsis in Ctrl‐TEI.
Supplemental Video 3 Treatment with methylene blue abolishes the peristalsis in Ctrl‐TEI.
Supplemental Video 4 Premigratory NCSCs‐TEI demonstrates spontaneous phasic contraction and relaxation.
Supplemental Video 5 Following the treatment with methylene blue, pNCSCs‐TEI shows a reduction in contractility instead of the complete blocking.
Supplemental Video 6 Treatment of pNCSCs‐TEI with Tetrodotoxin evidently inhibits the peristalsis during the inspection period.
ACKNOWLEDGMENTS
The authors thank members of the technical platform at the Institute of Translational Medicine, School of Medicine, Zhejiang University for helpful advice and comments. This research was financially supported by the National Key Research and Development Program of China (No. 2012CB967903 and No. 2014CB541705), the National Natural Science Foundation of China (No. 31171423), Zhejiang Provincial Natural Science Foundation of China (No. Z18C090002), National Key Research and Development Program of Zhejiang (2020C03113), Xiamen's two hundred talent Program (10th).
Yuan H, Hu H, Chen R, et al. Premigratory neural crest stem cells generate enteric neurons populating the mouse colon and regulating peristalsis in tissue‐engineered intestine. STEM CELLS Transl Med. 2021;10:922–938. 10.1002/sctm.20-0469
Huipu Yuan, Hui Hu, and Rui Chen contributed equally to this study.
Funding information Xiamen's two hundred talent Program; National Key Research and Development Program of Zhejiang, Grant/Award Number: 2020C03113; Zhejiang Provincial Natural Science Foundation of China, Grant/Award Number: Z18C090002; National Natural Science Foundation of China, Grant/Award Number: 31171423; National Key Research and Development Program of China, Grant/Award Numbers: 2014CB541705, 2012CB967903
Contributor Information
Yi Dong, Email: brainstein@fudan.edu.cn.
Jinfa Tou, Email: toujinfa@zju.edu.cn.
Wei Chen, Email: chenwei001@zju.edu.cn.
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7(8):1013‐1019. [DOI] [PubMed] [Google Scholar]
- 2. Duband JL, Monier F, Delannet M, Newgreen D. Epithelium‐mesenchyme transition during neural crest development. Acta Anat. 1995;154(1):63‐78. [DOI] [PubMed] [Google Scholar]
- 3. Hall BK. The neural crest and neural crest cells: discovery and significance for theories of embryonic organization. J Biosci. 2008;33(5):781‐793. [DOI] [PubMed] [Google Scholar]
- 4. Burns AJ, Thapar N. Neural stem cell therapies for enteric nervous system disorders. Nat Rev Gastroenterol Hepatol. 2014;11(5):317‐328. [DOI] [PubMed] [Google Scholar]
- 5. Wehrle‐Haller B, Weston JA. Receptor tyrosine kinase‐dependent neural crest migration in response to differentially localized growth factors. BioEssays. 1997;19(4):337‐345. [DOI] [PubMed] [Google Scholar]
- 6. Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013;10(1):43‐57. [DOI] [PubMed] [Google Scholar]
- 7. Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung's disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466‐479. [DOI] [PubMed] [Google Scholar]
- 8. McKeown SJ, Stamp L, Hao MM, et al. Hirschsprung disease: a developmental disorder of the enteric nervous system. Wiley Interdiscip Rev Dev Biol. 2013;2(1):113‐129. [DOI] [PubMed] [Google Scholar]
- 9. Collins L, Collis B, Trajanovska M, et al. Quality of life outcomes in children with Hirschsprung disease. J Pediatr Surg. 2017;52(12):2006‐2010. [DOI] [PubMed] [Google Scholar]
- 10. Granstrom AL, Danielson J, Husberg B, et al. Adult outcomes after surgery for Hirschsprung's disease: evaluation of bowel function and quality of life. J Pediatr Surg. 2015;50(11):1865‐1869. [DOI] [PubMed] [Google Scholar]
- 11. Menezes M, Puri P. Long‐term clinical outcome in patients with Hirschsprung's disease and associated Down's syndrome. J Pediatr Surg. 2005;40(5):810‐812. [DOI] [PubMed] [Google Scholar]
- 12. Burns AJ, Goldstein AM, Newgreen DF, et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies [in English]. Dev Biol. 2016;417(2):229‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metzger M, Bareiss PM, Danker T, et al. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137(6):2063‐2073.e2064. [DOI] [PubMed] [Google Scholar]
- 14. Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130(25):6387‐6400. [DOI] [PubMed] [Google Scholar]
- 15. Cheng LS, Graham HK, Pan WH, et al. Optimizing neurogenic potential of enteric neurospheres for treatment of neurointestinal diseases. J Surg Res. 2016;206(2):451‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almond S, Lindley RM, Kenny SE, Connell MG, Edgar DH. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56(4):489‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heanue TA, Pachnis V. Prospective identification and isolation of enteric nervous system progenitors using Sox2. Stem Cells. 2011;29(1):128‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fattahi F, Steinbeck JA, Kriks S, et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature. 2016;531(7592):105‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hotta R, Natarajan D, Burns AJ, Thapar N. Stem cells for GI motility disorders. Curr Opin Pharmacol. 2011;11(6):617‐623. [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni S, Becker L, Pasricha PJ. Stem cell transplantation in neurodegenerative disorders of the gastrointestinal tract: future or fiction? Gut. 2012;61(4):613‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Workman MJ, Mahe MM, Trisno S, et al. Engineered human pluripotent‐stem‐cell‐derived intestinal tissues with a functional enteric nervous system. Nat Med. 2017;23(1):49‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schlieve CR, Fowler KL, Thornton M, et al. Neural crest cell implantation restores enteric nervous system function and alters the gastrointestinal transcriptome in human tissue‐engineered small intestine. Stem Cell Rep. 2017;9(3):883‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aoi T. 10th anniversary of iPS cells: the challenges that lie ahead. J Biochem. 2016;160(3):121‐129. [DOI] [PubMed] [Google Scholar]
- 24. Hotta R, Stamp LA, Foong JP, et al. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123(3):1182‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hetz S, Acikgoez A, Voss U, et al. In vivo transplantation of neurosphere‐like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One. 2014;9(4):e93605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stamp LA, Gwynne RM, Foong JPP, et al. Optogenetic demonstration of functional innervation of mouse colon by neurons derived from transplanted neural cells. Gastroenterology. 2017;152(6):1407‐1418. [DOI] [PubMed] [Google Scholar]
- 27. Cooper JE, McCann CJ, Natarajan D, et al. In vivo transplantation of enteric neural crest cells into mouse gut; engraftment, functional integration and long‐term safety. PLoS One. 2016;11(1):e0147989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McCann CJ, Cooper JE, Natarajan D, et al. Transplantation of enteric nervous system stem cells rescues nitric oxide synthase deficient mouse colon. Nat Commun. 2017;8:15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kruger GM, Mosher JT, Bixby S, Joseph N, Iwashita T, Morrison SJ. Neural crest stem cells persist in the adult gut but undergo changes in self‐renewal, neuronal subtype potential, and factor responsiveness. Neuron. 2002;35(4):657‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hu H, Ding Y, Mu W, et al. DRG‐derived neural progenitors differentiate into functional enteric neurons following transplantation in the postnatal colon. Cell Transplant. 2019;28(2):157‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Baggiolini A, Varum S, Mateos JM, et al. Premigratory and migratory neural crest cells are multipotent in vivo. Cell Stem Cell. 2015;16(3):314‐322. [DOI] [PubMed] [Google Scholar]
- 32. Etchevers H. Primary culture of chick, mouse or human neural crest cells. Nat Protoc. 2011;6(10):1568‐1577. [DOI] [PubMed] [Google Scholar]
- 33. DuHamel KN, Mosher CE, Winkel G, et al. Randomized clinical trial of telephone‐administered cognitive‐behavioral therapy to reduce post‐traumatic stress disorder and distress symptoms after hematopoietic stem‐cell transplantation. J Clin Oncol. 2010;28(23):3754‐3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Spence JR, Mayhew CN, Rankin SA, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro [in English]. Nature. 2011;470(7332):105‐U120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Watson CL, Mahe MM, Munera J, et al. An in vivo model of human small intestine using pluripotent stem cells [in English]. Nat Med. 2014;20(11):1310‐1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le Douarin NM, Creuzet S, Couly G, et al. Neural crest cell plasticity and its limits. Development. 2004;131(19):4637‐4650. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Zer H, Apel C, Heiland M, et al. Enrichment and schwann cell differentiation of neural crest‐derived dental pulp stem cells. In Vivo. 2015;29(3):319‐326. [PubMed] [Google Scholar]
- 38. Binder E, Natarajan D, Cooper J, et al. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. PLoS One. 2015;10(3):e0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkinson DJ, Bethell GS, Shukla R, Kenny SE, Edgar DH. Isolation of enteric nervous system progenitor cells from the aganglionic gut of patients with Hirschsprung's disease. PLoS One. 2015;10(5):e0125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dupin E, Sommer L. Neural crest progenitors and stem cells: from early development to adulthood. Dev Biol. 2012;366(1):83‐95. [DOI] [PubMed] [Google Scholar]
- 41. Menendez L, Kulik MJ, Page AT, et al. Directed differentiation of human pluripotent cells to neural crest stem cells. Nat Protoc. 2013;8(1):203‐212. [DOI] [PubMed] [Google Scholar]
- 42. McCracken KW, Howell JC, Wells JM, et al. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6(12):1920‐1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sinagoga KL, Wells JM. Generating human intestinal tissues from pluripotent stem cells to study development and disease. EMBO J. 2015;34(9):1149‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Al‐Shboul OA. The importance of interstitial cells of Cajal in the gastrointestinal tract. Saudi J Gastroenterol. 2013;19(1):3‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ambache N. The electrical activity of isolated mammalian intestines. J Physiol. 1947;106(2):139‐153. [PubMed] [Google Scholar]
- 46. Bronner‐Fraser M, Fraser SE. Cell lineage analysis reveals multipotency of some avian neural crest cells. Nature. 1988;335(6186):161‐164. [DOI] [PubMed] [Google Scholar]
- 47. Stemple DL, Anderson DJ. Lineage diversification of the neural crest: in vitro investigations. Dev Biol. 1993;159(1):12‐23. [DOI] [PubMed] [Google Scholar]
- 48. Bellier JP, Kimura H. Peripheral type of choline acetyltransferase: biological and evolutionary implications for novel mechanisms in cholinergic system. J Chem Neuroanat. 2011;42(4):225‐235. [DOI] [PubMed] [Google Scholar]
- 49. Le Douarin NM, Teillet MA. The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol. 1973;30(1):31‐48. [PubMed] [Google Scholar]
- 50. Uesaka T, Nagashimada M, Enomoto H. Neuronal differentiation in schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35(27):9879‐9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3(9):778‐784. [DOI] [PubMed] [Google Scholar]
- 52. Amoh Y, Li L, Katsuoka K, Penman S, Hoffman RM. Multipotent nestin‐positive, keratin‐negative hair‐follicle bulge stem cells can form neurons. Proc Natl Acad Sci USA. 2005;102(15):5530‐5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang D, Ighaniyan S, Stathopoulos L, et al. The neural crest: a versatile organ system. Birth Defects Res C Embryo Today. 2014;102(3):275‐298. [DOI] [PubMed] [Google Scholar]
- 54. Ibarretxe G, Crende O, Aurrekoetxea M, et al. Neural crest stem cells from dental tissues: a new hope for dental and neural regeneration. Stem Cells Int. 2012;2012:103503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Supplemental Video 1 Control TEI demonstrates spontaneous phasic contraction and relaxation.
Supplemental Video 2 TTX treatment shows no obvious change compared with the spontaneous phasic peristalsis in Ctrl‐TEI.
Supplemental Video 3 Treatment with methylene blue abolishes the peristalsis in Ctrl‐TEI.
Supplemental Video 4 Premigratory NCSCs‐TEI demonstrates spontaneous phasic contraction and relaxation.
Supplemental Video 5 Following the treatment with methylene blue, pNCSCs‐TEI shows a reduction in contractility instead of the complete blocking.
Supplemental Video 6 Treatment of pNCSCs‐TEI with Tetrodotoxin evidently inhibits the peristalsis during the inspection period.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


