Abstract
Background:
In 2019, Mexico became the first Latin American country committed to hepatitis C virus (HCV) elimination, but the amount of intervention scale-up required is unclear. In Tijuana, HCV among people who inject drugs (PWID) is high; yet there is minimal and intermittent harm reduction, and involuntary exposure to compulsory abstinence programs (CAP) occurs which is associated with increased HCV risk. We determined what combination intervention scale-up can achieve HCV elimination among current and former PWID in Tijuana.
Methods:
We constructed a dynamic, deterministic model of HCV transmission, disease progression, and harm reduction among current and former PWID parameterized to Tijuana (~10,000 current PWID, 90% HCV seropositive, minimal opiate agonist therapy [OAT] or high coverage needle/syringe programs [HCNSP]). We evaluated the number of direct-acting antiviral (DAA) treatments needed from 2019 to achieve elimination targets (80% incidence reduction, 65% mortality reduction by 2030) with: (a) DAAs alone, (b) DAAs plus scale-up of OAT+HCNSP (up to 50% coverage of OAT and HCNSP separately, producing 25% of PWID receiving both), (c) DAAs plus CAP scale-up to 50%. Scenarios examined the number of DAAs required if prioritized to current PWID or provided regardless of current injection status, and impact of harm reduction interruptions.
Results:
Modeling suggests among ~30,000 current and former PWID in Tijuana, 16,160 (95%CI: 12,770–21,610) have chronic HCV. DAA scale-up can achieve the incidence target, requiring 770 treatments/year (95%CI: 640–970) if prioritized to current PWID. 40% fewer DAAs are required with OAT+HCNSP scale-up to 50% among PWID, whereas more are required with involuntary CAP scale-up. Both targets can only be achieved through treating both current and former PWID (1,710 treatments/year), and impact is reduced with harm reduction interruptions.
Conclusions:
Elimination targets are achievable in Tijuana through scale-up of harm reduction and DAA therapy, whereas involuntary CAP and harm reduction interruptions hamper elimination.
Keywords: Hepatitis C elimination, people who inject drugs, modeling
INTRODUCTION
Hepatitis C virus (HCV) is a blood-borne infection which, if untreated, can result in cirrhosis, liver cancer, and death. Of the estimated 71 million chronic HCV infections globally, 80% occur in low to middle income countries (LMIC) (Graham & Swan, 2015; Mohd Hanafiah, Groeger, Flaxman, & Wiersma, 2013; Polaris Observatory, 2017). Globally, people who inject drugs (PWID) are a main group at risk for HCV transmission, with an estimated 52% of all PWID having a history of HCV infection (Degenhardt et al., 2017). In addition, an estimated 43% of incident HCV infections could be prevented from 2018–2030 if the risks of unsafe injecting practices were removed (Trickey et al., 2019). In 2016, in an effort to focus resources on reducing the high global burden of HCV and aim for global elimination, the World Health Organization (WHO) set elimination targets to be achieved by 2030 (WHO, 2016). These elimination targets include an 80% reduction in HCV incidence and 65% reduction in HCV-related mortality in 2030 compared to 2015 (WHO, 2016). In 2019, Mexico became the first country in Latin America to launch an HCV elimination strategy, with the first phase including purchase of 12,500 direct-acting antiviral (DAA) treatments, among an estimated 450–550,000 people with HCV infection in Mexico (Ochoa, 2019; Secretaría de Salud, 2019). Yet the intervention scale-up required to achieve the WHO elimination targets among PWID is unknown, particularly in settings with very high HCV prevalence and limited harm reduction as in Mexico.
Tijuana, Mexico, a border city with the United States, is situated on a major drug trafficking route. Within Mexico, Tijuana has had the highest rates of illicit drug use and the most recent estimate suggests that approximately 10,000 current PWID reside there (Magis-Rodriguez et al., 2005). HCV is highly prevalent among PWID in Mexico, with HCV seroprevalence being reported among PWID as high as 92% in Ciudad Juarez and 79% in San Luis Rio Colorado (Fleiz-Bautista et al., 2019). In Tijuana, seroprevalence exceeds 90% (Fleiz-Bautista et al., 2019; White et al., 2007). Despite this, access to harm reduction is minimal. After a brief increase in needle and syringe exchange program (NSP) services resulting from the Global Fund support in 2011–2013, withdrawal of this funder has severely limited NSP provision (Bórquez et al., 2019; Cepeda et al., 2019) and there is no access to High Coverage NSP [HCNSP] (as defined as receiving one or more sterile needles/syringes for each injection (Platt et al., 2018)) in Tijuana. These services are critical given the evidence they reduce recent HIV transmission (Aspinall et al., 2014), and could reduce HCV transmission, particularly if provided in combination with opiate agonist therapy (OAT) (Platt et al., 2018). Access to evidence-based OAT among PWID is <5% (Robertson et al., 2014). Instead, beginning in 2014, government funds in Tijuana were allocated to compulsory drug abstinence programs (CAP), which remain the main type of drug rehabilitation program available (Juridicción Sanitaria, 2016). In Tijuana, CAPs entail involuntary physical restraint of individuals in non-medically supervised centers for approximately 3–6 months, with non-evidence-based detoxification and abstinence interventions (Rafful et al., 2019). PWID who had been brought involuntarily by friends/family or police to CAP were more likely to engage in receptive syringe sharing, which if this association is causal, could mean involuntary exposure to CAPs fuel HIV transmission, and therefore could serve to disseminate HCV and hamper elimination progress (Bórquez et al., 2018).
Previous mathematical models of HCV transmission amongst PWID found modest scale-up of HCV antiviral treatment, especially coupled with harm reduction interventions such as OAT and HCNSP could be used as prevention (Martin, Hickman, Hutchinson, Goldberg, & Vickerman, 2013; Martin, Vickerman, et al., 2013). Yet only a few modeling studies have examined what combination intervention scale-up is required to achieve the WHO elimination targets among PWID, and these were limited to high income settings (Fraser et al., 2018). We used dynamic modeling to determine the level of combination intervention scale-up necessary to achieve WHO hepatitis C virus elimination goals of 80% incidence reduction and 65% mortality reduction by 2030 among PWID in Tijuana, and to assess the potential impact of involuntary CAP expansion or interruptions to harm reduction services.
METHODS
Mathematical model
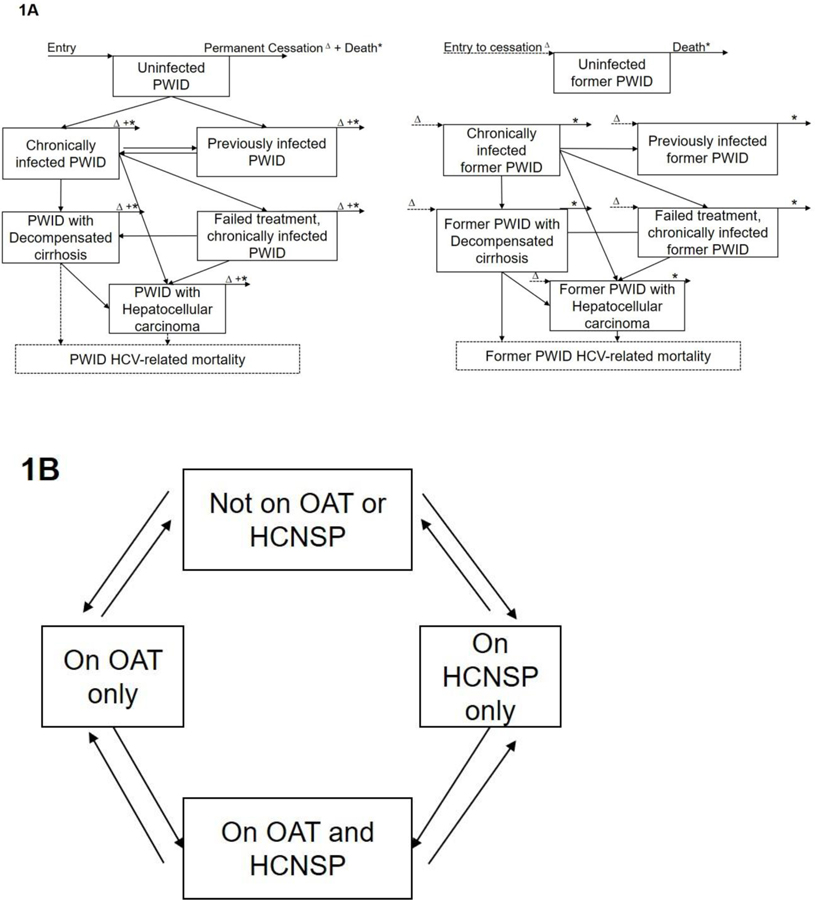
We constructed a dynamic, deterministic model of HCV transmission and progression among current and former (permanently cessated) PWID. We assumed transmission only occurs among current PWID, but continue to track HCV disease progression and mortality among former PWID. We used this model to evaluate changes in prevalence, incidence, and mortality with varying levels of scale-up of DAA treatment and harm reduction interventions (Figure 1). We modeled an open population where individuals continually enter due to initiation of drug use and exit the model due to death. Additionally, we assumed random mixing among PWID and that PWID continuously transition between evidence-based harm reduction intervention compartments: (a) no intervention, (b) OAT only, (c) HCNSP only, and (d) OAT + HCNSP simultaneously. A proportion of PWID may become chronically infected with HCV, with their risk being proportional to the background prevalence of disease and to their intervention exposure. Those with chronic infection who remain untreated can progress to decompensated cirrhosis (DC), hepatocellular carcinoma (HCC), and death. Individuals with chronic infection can receive DAAs. For those who received DAAs, a proportion may fail treatment and remain chronically infected, and progress through the natural history of disease stages. PWID who are successfully treated, progress to the previously infected compartment, where they are at risk of re-infection. After permanent injecting cessation, former PWID are tracked in our model for disease progression. For the baseline scenario, we assumed that PWID with DC or HCC are not provided DAA treatment as this is unlikely to occur currently given health services in Tijuana. We assumed no coverage of HCV treatment at baseline given the lack of availability in Mexico. At baseline, we also assume no coverage of harm reduction (OAT or HCNSP), as <5% of PWID in Tijuana report recent access to OAT, and there is no access to HCNSP. For an alternative scenario analysis, we redefine the OAT compartment to represent involuntary receipt of CAP, and parameterize the relative risks accordingly to examine the impact of scale-up of CAP instead of OAT. Analyses were conducted in Matlab version R2018b (The Mathworks, Inc., Natick, MA).
Figure 1. Model schematics showing (A) HCV disease progression by liver disease states and (B) stratification by harm reduction interventions.
PWID: People who inject drugs; OAT: Opiate agonist therapy; HCNSP: High coverage needle/syringe exchange program (receiving ≥1 sterile syringes per injection). *PWID exiting the model due to death; ∆Current PWID transitioning to former PWID model, resulting from permanent cessation.
Model parameterization
The model was parameterized to Tijuana, Mexico, with an estimated 10,000 current PWID (Magis-Rodriguez et al., 2005). Model parameters were obtained from published literature and data from cross-sectional and longitudinal cohorts of PWID in Tijuana (Table 1). HCV seroprevalence (90%) was obtained from PWID recruited from drug consumption sites in Tijuana between 2017–18 (Fleiz-Bautista et al., 2019). Similar to other modeling analyses (Martin, Hickman, et al., 2013), we estimate chronic prevalence from seroprevalence, based on a 26% spontaneous clearance rate (95% CI: 0.22–0.30) from a systematic review of longitudinal studies (Micallef, Kaldor, & Dore, 2006), consistent with other studies evaluating spontaneous clearance among PWID (Kaberg, Naver, Hammarberg, & Weiland, 2018) as well as region-specific viraemic rates (74% for Latin America) (Petruzziello, Marigliano, Loquercio, Cozzolino, & Cacciapuoti, 2016). Data including proportion of individuals accessing harm reduction interventions (OAT, HCNSP), the average duration of injecting, and PWID mortality rate were obtained from the El Cuete IV study in Tijuana (Bórquez et al., 2018; Robertson et al., 2014; West et al., 2019). Average duration of injecting by sex was obtained from the El Cuete IV study and weighted by the distribution of PWID in Tijuana by sex (85% males, 15% females, (Strathdee et al., 2008), as women were oversampled in El Cuete IV.
Table 1. Model parameters and their distributions.
Distribution ranges: Uniform: minimum, maximum; Beta: alpha, beta; Lognormal: shape, scale. DC: decompensated cirrhosis; HCC: Hepatocellular carcinoma; OAT: Opiate agonist therapy; HCNSP: High coverage needle and syringe program.
| Definition | Mean sampled value (95% CI) | Sample distribution | Unit | Reference |
|---|---|---|---|---|
| Rate of new PWID initiations | Fit to 10,000 PWID | - | Individuals per year | -- |
| Rate of infection | Fit to HCV chronic prevalence among current PWID | Per year | ||
| HCV seroprevalence among current PWID | 0.90 (0.85–0.95) | Beta (alpha= 141.74, Beta=14.48); | -- | (Fleiz-Bautista et al., 2019) |
| Proportion of infections that spontaneously clear | 0.26 (0.22–0.30) | Uniform (min=0.22, max=0.30) | -- | (Micallef, Kaldor, & Dore, 2006) |
| Sustained viral response | 0.95 (0.91–0.99) | Uniform (min=0.903, max=0.998) | -- | (Dore et al., 2016; Grebely et al., 2018) |
| Average duration of injecting until permanent cessation | 17.5 | Uniform (min=11, max=24) | Years | Weighted average assumed (15% female; 85% male) (Bórquez et al., 2018; Strathdee et al., 2008) |
| Mortality rate among PWID | 0.02 (0.016, 0.024) | Uniform (min=0.016, max=0.024) | Per year | (Bórquez et al., 2018) |
| OAT recruitment rate | Varied to fit to target proportion PWID on OAT | - | Per year | -- |
| Leaving rate from OAT | 1.5 | Uniform (min=1, max=2) | Per year | (Cornish, Macleod, Strang, Vickerman, & Hickman, 2010; Martin, Hickman, Hutchinson, Goldberg, & Vickerman, 2013; Vickerman, Martin, Turner, & Hickman, 2012) |
| HCNSP recruitment | Varied to fit to target proportion on HCNSP | - | Per year | -- |
| Leaving rate from HCNSP | Assumed to be the same as OAT (1.5) | Uniform (min=1, max=2) | Per year | Assumed same as OAT (Martin, Vickerman, et al., 2013) |
| Relative risk of HCV transmission on OAT only compared to no OAT | 0.50 (0.39, 0.64) | Lognormal (ln( 0.50), 95%CI: 0.40–0.63) | -- | (Platt et al., 2018) |
| Relative risk of HCV transmission on HCNSP only compared to no HCNSP | 0.79 (0.38, 1.60) | Lognormal (ln(0.79), 95% CI: 0.39–1.61) | -- | (Platt et al., 2018) |
| Relative risk of HCV acquisition on OAT and HCNSP compared to none | 0.23 (0.09, 0.62) | Lognormal (ln(0.24), 95% CI: 0.09–0.62) | -- | (Platt et al., 2018) |
| Relative risk of HCV transmission on involuntary CAP compared to not on involuntary CAP | 1.14 (1–1.3) | Lognormal (mean 1.14, 95% CI: 1.0–1.3) | -- | Implemented through a relative change in syringe sharing as in (Brquez et al., 2018) |
| Disease transition probabilities | ||||
| Chronic – DC | 0.016 (0.013, 0.019) | Uniform (min=0.0128, max=0.0192) | Per year | Calculated from fibrosis progression rates in (Thein, Yi, Dore, & Krahn, 2008) |
| Chronic—HCC | 0.009 (0.007, 0.01) | Uniform (min=0.0072, max=0.0108) | Per year | Calculated from fibrosis progression rates in (Thein, Yi, Dore, & Krahn, 2008) |
| DC – HCC | 0.012 (0.002, 0.04) | Beta (alpha=1.193, beta=136.107) | Per year | (Shepherd et al., 2007) |
| Excess HCV-related mortality rate from DC | 0.14 (0.11, 0.17) | Uniform (min=0.11, max=0.17) | Per year | (Shepherd et al., 2007) |
| Excess HCV-related mortality rate from HCC | 0.55 (0.31, 0.79) | Uniform (min=0.3, max=0.8) | Per year | (El-Serag et al., 2006; Shepherd et al., 2007) |
Intervention effect estimates:
HCV DAA sustained virological response (SVR) rates (approximately 95%) were obtained from published data for PWID (Dore et al., 2016; Grebely et al., 2018). Effectiveness of evidence-based OAT and HCNSP (defined as receiving one or more sterile syringe per each injection) on reducing HCV acquisition was obtained from a Cochrane systematic review and meta-analysis, with OAT reducing the risk of HCV transmission by 50% (sampled from the 95% confidence interval (95%CI) of the relative risk (RR): 0.40–0.63), and with HCNSP reducing the risk of HCV transmission by 23% (sampled from the 95%CI of the RR 0.38–1.54) (Platt et al., 2018). In combination, both HCNSP and OAT reduce the risk of acquiring HCV by an estimated 71% (sampled from the 95%CI of the RR: 0.13–0.65) (Platt et al., 2018). Data from El Cuete IV cohort among PWID in Tijuana indicate that, compared to PWID never exposed to involuntary CAP, PWID with a history of involuntary CAP had an elevated relative risk of recent receptive syringe sharing (RR 1.14 [95%CI: 1.00–1.30]) (Bórquez et al., 2018). We note that it is unclear from the epidemiological data whether this elevation in syringe sharing occurs during and/or after CAP exposure, or whether this association is truly causal. However, evidence from other custodial settings (such as prisons) where injecting drug use is prohibited indicates that although a lower proportion of PWID continue to inject drugs while detained, injecting risk is greatly enhanced due to elevated rates of syringe sharing (Cunningham et al., 2018; Dolan et al., 2010). Accordingly, we implemented the effect of CAP exposure in the model by assuming a 14% increase (varying from 0 to 30%) in receptive syringe sharing among PWID while in CAP. As such, our model neglects any potential negative effects of syringe sharing on CAP after release.
Model calibration
To account for uncertainty in these model parameters, we sampled 1000 parameter sets from each parameter’s associated uncertainty distribution (Table 1). For each parameter set, the model was calibrated to HCV chronic prevalence among PWID in Tijuana in 2018, through varying the HCV transmission rate (calibrated fits shown in Supplementary Figure S1). Since historic estimates show stable HCV prevalence among PWID in Tijuana (Fleiz-Bautista et al., 2019; Frost et al., 2006; White et al., 2007), we assumed HCV is at steady-state. We then calibrated fixed recruitment rates onto OAT and HCNSP starting in 2019 required to achieve scale-up to 20%, 40%, and 50% coverage by 2030 of each intervention among PWID for each intervention scenario (producing 10%, 20%, and 25% of PWID receiving both OAT and HCNSP). Model calibration was achieved by minimizing the least squares fit to the prevalence data using a global optimization solver (lsqnonlin with multistart in MATLAB).
Intervention scenarios
We evaluated various strategies to achieve the previously-referenced WHO HCV elimination goals. We examined the impact on incidence, chronic prevalence, and HCV-related mortality of the following intervention scenarios:
Status quo: no treatment or harm reduction.
DAAs only: DAA treatment scale-up only beginning in 2019 to achieve the elimination goals.
DAAs combined with evidence-based harm reduction: DAA treatment scale-up in combination with scaled-up OAT+HCNSP from 2019 to reach 20%, 40%, or 50% coverage levels of each intervention among PWID by 2030 (producing 10%, 20%, and 25% of PWID receiving both OAT and HCNSP). Supplementary Figure S2 shows coverage scale-up over time.
DAAs combined with involuntary CAP: DAA treatment scale-up in combination with scaled-up CAP to 50% among PWID beginning in 2019.
Additionally, to evaluate the potential impact of interruptions in harm reduction coverage as historically observed, we examined the impact of scale-up of combination prevention required to achieve the incidence target (DAAs and 50% OAT+HCNSP among PWID) but interruption/removal of harm reduction from 2025–2030.
Sensitivity analyses
Due to parameter uncertainty, we performed sensitivity analyses examining the impact on numbers of treatments (inclusive of retreatments of reinfection) required to achieve both elimination goals if DAAs are allocated to both current and former PWID with the following scenarios: lower or higher number of current PWID (5,000 or 15,000 compared to 10,000 at baseline), lower chronic prevalence among current PWID (50%, compared to 67% at baseline), lower SVR (90% vs 95% at baseline), and differing injecting duration (5 years or 25 years, compared to 17.5 at baseline).
RESULTS
Model calibration
The calibrated model estimated 10,000 current PWID, with an associated 19,500 former PWID, (95% CI: 12,700–30,200) in Tijuana. Among the approximately 30,000 individuals with a history of injection drug use (IDU), we estimated there were 16,160 (95% CI: 12,770–21,610) chronic HCV infections in 2019. In 2019, HCV incidence among PWID was estimated to be 20 per 100 person years (/100py) (95% CI: 14–29/100py), equating to 650 new infections per year (95% CI: 510–850). An estimated 430 current PWID (95% CI: 310–560) and 1,350 former PWID (95% CI: 910–1,970) had decompensated cirrhosis and approximately 100 current PWID (95% CI: 60–160) and 280 former PWID (95% CI: 150–500) had hepatocellular carcinoma. Among both current and former PWID, there were an estimated 330 (95% CI: 250–440) HCV-related deaths in 2019.
WHO Goal: Achieving 80% incidence reduction among PWID by 2030
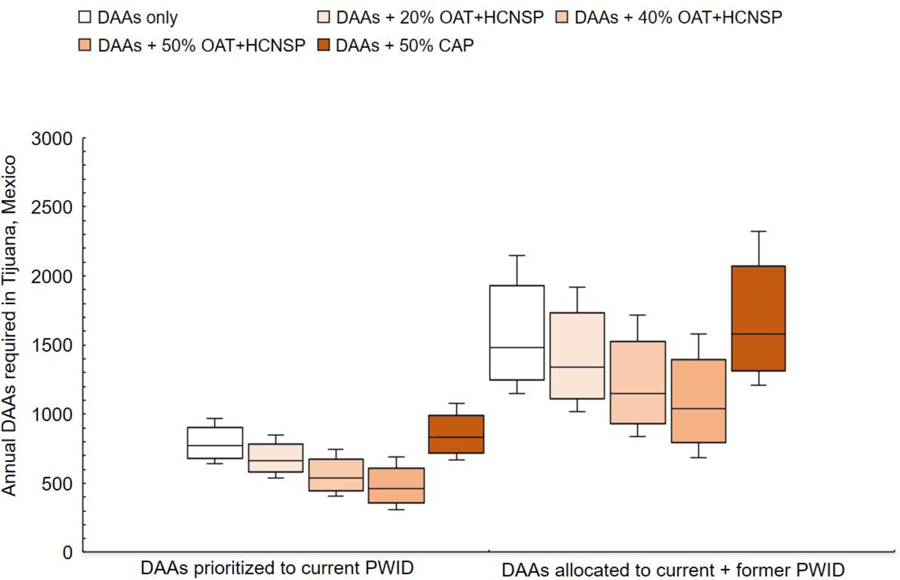
Combination intervention strategies needed to achieve 80% incidence reduction (from 20/100py to 4/100py) in Tijuana among PWID by 2030 are shown in Figure 2. Without any DAA treatment, harm reduction alone was unable to achieve the 80% incidence target (Supplementary Figures S3–S4). If DAAs were prioritized to PWID, DAAs alone, at a median rate of 770 annually [95% CI: 640–970] (equivalent to 11% of chronic infections among PWID being treated the first year) could achieve the HCV incidence target by 2030. Moderate scale-up of OAT+HCNSP to coverage levels of 20% or 40% among PWID, in addition to DAA scale-up, reduced the annual number DAAs needed to 670 PWID annually (95%CI: 540–850) or 540 PWID annually (95% CI: 410–750), respectively. Scaling up OAT+HCNSP to 50% coverage among PWID reduced the DAAs required by 40% (460 PWID annually [95% CI: 310–690]). Conversely, if involuntary exposure to CAP were scaled-up to 50% coverage among PWID, 7% more DAAs would be required compared to DAAs alone because of the increase in syringe sharing (and therefore HCV) associated with CAP (830 annually [95% CI: 670–1080]).
Figure 2. Annual DAA treatment numbers (B) required (with or without combination scale-up) to achieve an 80% incidence reduction among PWID by 2030 in Tijuana, Mexico, if treatment is prioritized to current PWID or allocated to current and former PWID. Scenarios examine combination scale-up of DAAs with evidence-based harm reduction (OAT+HCNSP) or involuntary exposure to compulsory drug abstinence programs (CAP).
DAAs: HCV direct-acting antiviral treatment; OAT: Opiate agonist therapy; HCNSP: High coverage needle/syringe exchange program (receiving ≥1 sterile syringes per injection).
If DAAs were provided without regard to injecting status, nearly double the DAA treatment numbers would be required to achieve the incidence target, compared to if DAAs were prioritized to current PWID (1,480/year [95%CI: 1,150–2,150], Figure 2). Without DAA prioritization, combination scale-up of OAT+HCNSP to current PWID could substantially reduce the DAAs required by 30% if harm reduction was scaled-up to 50% coverage among current PWID. As before, scale-up of CAP to 50% coverage would require more DAAs to achieve the WHO target (Figure 2).
Achieving both 80% incidence reduction and 65% HCV-related mortality reduction by 2030
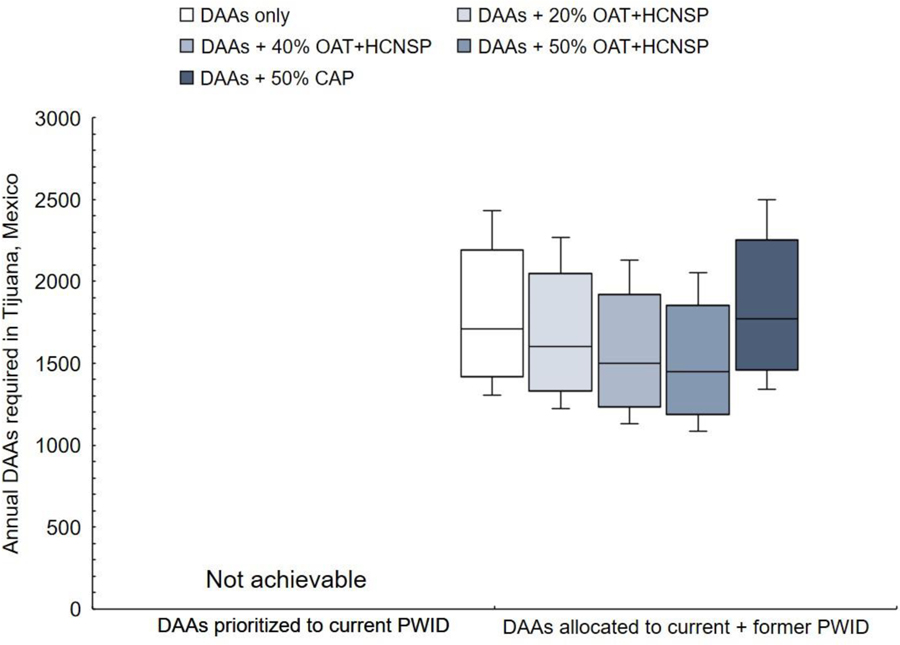
To achieve the 65% HCV-related mortality reduction, total HCV-related deaths among both current and former PWID would need to decrease from an estimated 330/year in 2018 to <110/year by 2030. More DAAs would be required to reach both WHO targets compared to the incidence target alone (Figure 3). To achieve the HCV-related mortality target, treatment must also be provided to former PWID. Even if current PWID were treated each year, HCV-related mortality could only be reduced by a maximum of 55% by 2030. If DAAs were provided to current and former PWID, approximately 1,710 (95%CI: 1,310–2,430) would need to be treated annually to reach both targets (13% greater than the incidence target). Scaling up evidence-based harm reduction reduces DAAs required compared to giving DAAs only; scaled-up OAT+HCNSP to 50% coverage among PWID requires 15% fewer treatments per year to reach both targets (1,450 [95%CI: 1,090–2,060]).
Figure 3. Annual DAA treatment numbers required (with or without combination scale-up) to achieve both WHO targets (80% incidence reduction and 65% reduction in HCV-related mortality) by 2030 in Tijuana, Mexico, if treatment is prioritized to current PWID or allocated to current and former PWID.
Scenarios examine combination scale-up of DAAs with evidence-based harm reduction (opiate agonist therapy and high coverage needle/syringe programs, OAT+HCNSP) or involuntary exposure to compulsory drug abstinence programs (CAP). DAAs: HCV direct-acting antiviral treatment; OAT: Opiate agonist therapy; HCNSP: High coverage needle/syringe exchange program (receiving ≥1 sterile syringes per injection).
****Treating only current PWID cannot achieve the 65% mortality goal by 2030.
Impact of interruptions in harm reduction coverage
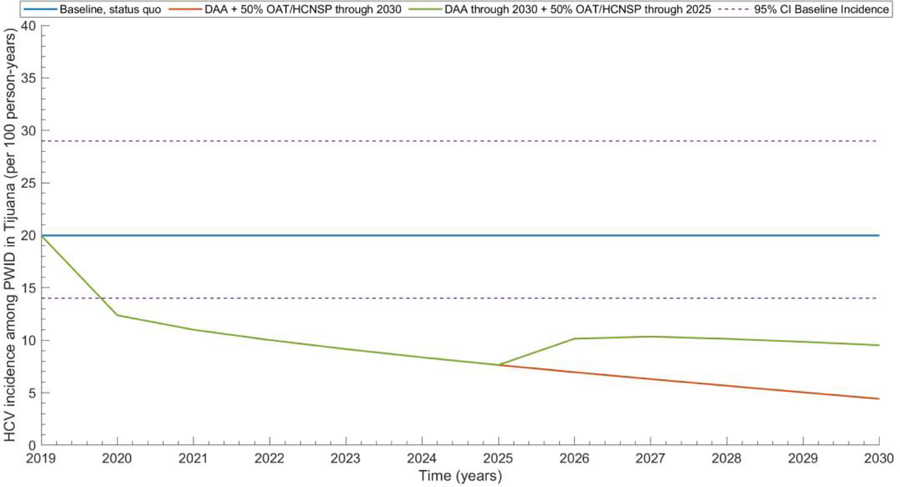
Even if combination intervention scale-up is achieved from 2020–2025, HCV incidence can rebound if harm reduction (OAT+HCNSP) availability is stopped at 2025 increasing by a mean relative 18% from 2025–2030 (Figure 4).
Figure 4. Future projections of HCV incidence among PWID in Tijuana with: status quo scenario, combination harm reduction elimination scenario (DAAs plus OAT+HCNSP at 50% coverage), and combination intervention but harm reduction removal in 2025.
Lines present mean values from 1,000 simulations. Dashed lines show 2.5 and 97.5% centile predictions from the baseline scenario. DAA: Direct-acting antiviral treatment; OAT: Opiate agonist therapy; HCNSP: Needle/syringe exchange program.
Sensitivity analysis
Sensitivity analyses evaluating the numbers of DAAs required to achieve both elimination goals when allocated randomly are shown for various scenarios in Supplementary Figure S5. For both elimination goals, more treatments (median to reach incidence: 1,590 [95%CI: 1,230–2,300]; median to reach mortality: 1,870 [95%CI: 1,420–2,700]) were required with a lower SVR rate (90%), and more (median to reach incidence: 1,850 [95%CI: 1,350–2,910]; median to reach mortality: 2,170 [95%CI: 1,560–3,310]) were required when the average duration of injection was short (5 years). Fewer treatments were also needed if the baseline chronic prevalence was 50% (median to reach incidence: 970 [95%CI: 800–1,320]; median to reach mortality: 1,150 [95%CI: 920–1,610]) and the average duration of injection was longer (25 years; median to reach incidence: 600 [95%CI: 440–910]; median to reach mortality: 720 [95%CI: 520–1,070]). Estimates of numbers of treatments required scaled linearly with analyses assuming greater (50% more; median: 2,220–2,570) or fewer (50% fewer; median: 740–860) PWID.
DISCUSSION
There is a high burden of HCV among people with a history of IDU in Tijuana, Mexico, where harm reduction and HCV treatment access is low. Our modeling indicates that even in very high burden settings such as Tijuana, HCV elimination could be achieved provided the forthcoming DAAs are widely available, scaled-up among both current and former PWID, and there is concomitant scale-up of harm reduction services. The incidence target could be achieved through treating approximately 770 (95%CI: 640–970) current PWID annually, but 40% fewer would be required if combination harm reduction is scaled-up among 50% of PWID. If DAAs were allocated at the same level needed to achieve the WHO elimination goals using treatment alone, the goals could be met earlier if combination harm reduction is scaled-up. Over double the number of treatments (median 1,710) would be required to achieve both the incidence and mortality targets, and these would need to reach both current and former PWID. Allocation of the currently purchased 12,500 DAAs across Mexico is unclear, but if allocated in proportion to estimated burden, then approximately 355–600 total treatments would be allocated to Tijuana, indicating treatment investment needs to be increased for WHO elimination. However, the incidence target may be achievable with expansion of harm reduction. As such, our findings highlight the critical role harm reduction can play in achieving elimination and reducing the number of DAAs required, and that sustained harm reduction is important to maintaining elimination. Furthermore, there are multiple sites in Mexico with concentrated HCV epidemics among PWID, including in other U.S. border cities of Ciudad Juarez and San Luis Rio Colorado (Fleiz-Bautista et al., 2019), further highlighting the need for DAAs to be allocated to the PWID within these communities to halt transmission.
There are numerous challenges which curtail the possibility of achieving HCV elimination in Tijuana. Despite indication that HCV treatment will become available under the Mexican public health care system, and that it will be available or PWID, DAAS are not currently available for PWID in Tijuana. Additionally, even if technically available, many PWID do not access or receive basic health care (Davidson et al., 2012; Smith et al., 2016), in part due to negative experiences and stigma (Biancarelli et al., 2019; von Hippel, Brener, & Horwitz, 2018), both internalized and expressed by healthcare providers (Brener et al., 2019; Paquette, Syvertsen, & Pollini, 2018). Additionally, PWID often lack the documentation required to register for Mexico’s national health care system. Compounding this lack of access is also a lack of awareness of HCV. Estimates of the proportion of HCV-infected individuals who are diagnosed in Tijuana are unknown, but in a recent cross-sectional survey among PWID in treatment centers in Tijuana, despite the vast majority of PWID harboring HCV infection, only 25% of all respondents self-reported having ever been diagnosed with HCV, and none had received treatment (unpublished data from (Fleiz-Bautista et al., 2019). However, once diagnosed and initiated on treatment, other studies have found high HCV treatment adherence rates among PWID, which supports feasibility of implementing these interventions in Tijuana (Dore et al., 2016).
In addition to barriers to treatment scale-up, expansion of evidence-based harm reduction is challenging in Tijuana. Existing OAT provision is expensive, and unaffordable for most PWID (Burgos et al., 2018). Capacity is also a considerable issue, as OAT is only offered by a few providers, so expansion of providers would be required. As mentioned, government funding has been provided for CAPs, which based on our model should be reoriented towards evidence-based treatment, including OAT provision to enhance HCV elimination strategies. Expansion in the quantity of HCNSPs and their provision is also urgently needed, particularly as the withdrawal of the Global Fund in late 2013 resulted in a dramatic reduction in the number of syringes and ancillary harm reduction components provided, as well as the geographical scope of the service, leading to increases in syringe sharing (Bórquez et al., 2019). The intermittent and fluctuating provision of harm reduction and recent cut to harm reduction funding in Mexico will hamper efforts to control HCV (Lopez, 2019); our simulations indicate that even if scaled-up to achieve WHO targets, removal of these programs after 2030 will allow HCV infections to rebound to their pre-treatment levels. We recognize that within government administrations, there is uncertainty in securing, directing, and continuing funding for such programs, spanning all levels from municipal, state, to federal, particularly with frequent turnover of elected officials. Our analyses support the need to provide long-term funding opportunities as to prevent a rebound in HCV transmission both before and after HCV elimination goals have been met. Importantly, there are additional benefits of implementing the proposed harm reduction strategies which are not represented in this model which might have a broader impact on the health of the PWID community in Tijuana. For example, improved harm reduction coverage could additionally reduce HIV transmission (Aspinall et al., 2014), fatal overdoses (Sordo et al., 2017) and reincarceration (Larney, Toson, Burns, & Dolan, 2012), which itself is associated with HIV and HCV risk (Stone et al., 2018).
Strengths and limitations
To our knowledge this is the first modeling analysis to project the level of combination interventions required to achieve the WHO HCV global elimination goals among PWID in a low-middle income setting. Other studies have examined country-level elimination strategies, such as in Pakistan, which also highlight the benefit of prioritization of PWID and of concomitant scale-up of harm reduction on achieving elimination (Lim et al., 2018). Our results also support findings from other studies that combination harm reduction and treatment strategies are a key component of HCV epidemic control (Gountas et al., 2017; Heffernan, Cooke, Nayagam, Thursz, & Hallett, 2019). Additionally, our results support literature showing that scaling-up harm reduction interventions reduces the number of treatments required and is cost-effective in the United States (Fraser et al., 2018). Finally, this analysis supports our previous modeling work indicating that scale-up of involuntary exposure to CAP could increase infectious diseases among PWID, such as HIV (Bórquez et al., 2018).
Our modeling study has a number of limitations, most notably related to uncertainty in parameterization. To account for these, we incorporated these uncertainties in our analysis and performed additional sensitivity analyses. First, there is substantial uncertainty in the number of PWID in Tijuana, with the estimate of 10,000 PWID arising from a Centro Nacional para la Prevención y Control del VIH/SIDA (CENSIDA) cross-sectional survey of 35 zones in Tijuana (Magis-Rodriguez et al., 2005). Our sensitivity analyses indicate uncertainty could strongly impact the number of DAAs needed. More robust, current estimates of PWID population sizes in Tijuana would help improve intervention planning and resource allocation.
Second, there is always considerable uncertainty in the average duration of injecting until final cessation as injecting drug use is a chronic relapsing condition. Cohort estimates of average duration of injection are often used to infer average time to cessation, yet these data are both right censored (participants have not cessated yet) and left censored (people who inject for a very brief period of time are unlikely to be captured by the cohort). As such, we used Tijuana data to inform this estimate but account for wide uncertainty in this parameter within our analyses. More generally, we note that our analysis characterizes the PWID population between those who have permanently cessated from injecting and those who have not, and as such do not explicitly simulate periods of temporary cessation. Thus, the current PWID prioritization strategies we examine incorporate prioritization to PWID who may be in a period of temporary cessation (such as those receiving OAT), but who are at high risk of relapse, and therefore future transmission.
Third, as HCV treatment is unavailable in Tijuana, we were unable to obtain local estimates of SVR rates and relied on estimates from other populations. However, studies of PWID indicate SVR among those with a history of IDU or who continue to inject after treatment, are high and exceed 90% (Grebely et al., 2018).
Fourth, we assumed exposure to involuntary CAP is causally associated with increased receptive syringe sharing while exposed to CAP, however causality has not been established and even if causal it is unclear when syringe sharing is elevated. It is possible that PWID who are involuntarily brought to CAPs may represent a population with higher baseline injecting risk. Alternatively, the observed increased odds in syringe sharing could potentially result from poor mental health and consequences of diminished self-care following physical or psychological abuse occurring in CAPs (Bórquez et al., 2018). Additionally, it is possible individuals who relapse after CAP release may share more to avoid being caught in trying to obtain clean syringes. Further, even if exposure to CAP causes syringe sharing it is unclear whether risk is elevated during and/or after exposure. Indeed, recent incarceration has been associated with elevated HIV and HCV incidence, but there is evidence this effect persists long-term (Stone et al., 2018). As duration in CAP is relatively short (6–12 months) compared to an overall injecting career, if there is a long-term excess risk associated with CAP these would not be included in our results, and would mean CAP has even more of a negative effect than we predict. Future work examining causal pathways and periods of heightened risk associated with CAP is warranted.
Furthermore, we neglected to account for HIV among PWID in Tijuana in our model as previous studies have shown it to be relatively low (3–7%) compared to HCV prevalence (Fleiz-Bautista et al., 2019; Strathdee et al., 2008). HIV infection would act as a competing risk for death; however, the same intensity of interventions would be required to reach the same relative reduction in HCV related mortality regardless of HIV status.
Finally, our analyses explored DAA provision to PWID with chronic infection, as the current published strategy highlights PWID as a priority population, and does not restrict by disease stage. Yet, we recognize that given the current health system reform in Mexico, the prioritization and allocation of treatment may change in the future, which would merit further modeling to assess the population impact and cost-effectiveness of these strategies. As the current strategy focuses highlights PWID and prisoners (many of whom are PWID) as priority populations, strategies to improve case finding and linkage to care among these groups will be an important focus. Future studies should examine these issues in further detail.
CONCLUSION
In conclusion, WHO global elimination goals can be achieved by 2030 in Tijuana, Mexico, through combination scale-up of evidence-based harm reduction and DAA treatment. Access to affordable HCV treatment and sustained evidence-based harm reduction is critical to achieving these goals, and existing compulsory abstinence programs could hamper elimination efforts.
Supplementary Material
Acknowledgments
Funding: LM was supported by the UC San Diego Frontiers of Innovation Graduate Fellowship (FISP/CRES), San Diego, CA. NM acknowledges funding from the National Institute for Drug Abuse (R01 DA03773 and R01 AI147490), the National Institute for Allergy and Infectious Diseases (R01 AI147490), and the University of San Diego Center for AIDS Research (CFAR), a NIH funded program (P30 AI036214). CR was supported by the CIHR Postdoctoral Research Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
Declaration of competing interest: Natasha Martin has received unrestricted research grants from Gilead and Merck unrelated to this work.
References
- Asher AK, Zhong Y, Garfein RS, Cuevas-Mota J, & Teshale E (2019). Association of Self-Reported Abscess With High-Risk Injection-Related Behaviors Among Young Persons Who Inject Drugs. J Assoc Nurses AIDS Care, 30(2), 142–150. doi: 10.1097/JNC.0000000000000016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspinall EJ, Nambiar D, Goldberg DJ, Hickman M, Weir A, Van Velzen E, … Hutchinson SJ (2014). Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol, 43(1), 235–248. doi: 10.1093/ije/dyt243 [DOI] [PubMed] [Google Scholar]
- Biancarelli DL, Biello KB, Childs E, Drainoni M, Salhaney P, Edeza A, … Bazzi AR (2019). Strategies used by people who inject drugs to avoid stigma in healthcare settings. Drug Alcohol Depend, 198, 80–86. doi: 10.1016/j.drugalcdep.2019.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bórquez A, Abramovitz D, Cepeda J, Rangel G, González-Zúñiga P, Martin NK, … Strathdee SA (2019). Syringe sharing among people who inject drugs in Tijuana: before and after the Global Fund. Salud mental, 42(4), 149–156. doi: 10.17711/sm.0185-3325.2019.020 [DOI] [Google Scholar]
- Bórquez A, Beletsky L, Nosyk B, Strathdee SA, Madrazo A, Abramovitz D, … Martin NK (2018). The effect of public health-oriented drug law reform on HIV incidence in people who inject drugs in Tijuana, Mexico: an epidemic modelling study. Lancet Public Health, 3(9), e429–e437. doi: 10.1016/S2468-2667(18)30097-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener L, Cama E, Broady T, Hopwood M, de Wit J, & Treloar C (2019). Predictors of health care workers’ support for discriminatory treatment and care of people who inject drugs. Psychol Health Med, 24(4), 439–445. doi: 10.1080/13548506.2018.1546018 [DOI] [PubMed] [Google Scholar]
- Burgos JL, Cepeda JA, Kahn JG, Mittal ML, Meza E, Lazos RRP, … Martin NK (2018). Cost of provision of opioid substitution therapy provision in Tijuana, Mexico. Harm Reduct J, 15(1), 28. doi: 10.1186/s12954-018-0234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda JA, Burgos JL, Kahn JG, Padilla R, Meza Martinez PE, Segovia LA, … Martin NK (2019). Evaluating the impact of global fund withdrawal on needle and syringe provision, cost and use among people who inject drugs in Tijuana, Mexico: a costing analysis. BMJ Open, 9(1), e026298. doi: 10.1136/bmjopen-2018-026298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish R, Macleod J, Strang J, Vickerman P, & Hickman M (2010). Risk of death during and after opiate substitution treatment in primary care: prospective observational study in UK General Practice Research Database. BMJ, 341, c5475. doi: 10.1136/bmj.c5475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham EB, Hajarizadeh B, Amin J, Bretana N, Dore GJ, Degenhardt L, … Investigators, H. I.-p. (2018). Longitudinal injecting risk behaviours among people with a history of injecting drug use in an Australian prison setting: The HITS-p study. Int J Drug Policy, 54, 18–25. doi: 10.1016/j.drugpo.2017.12.013 [DOI] [PubMed] [Google Scholar]
- Davidson PJ, Lozada R, Rosen PC, Macias A, Gallardo M, & Pollini RA (2012). Negotiating access: social barriers to purchasing syringes at pharmacies in Tijuana, Mexico. Int J Drug Policy, 23(4), 286–294. doi: 10.1016/j.drugpo.2012.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, … Larney S (2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Health, 5(12), e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan K, Teutsch S, Scheuer N, Levy M, Rawlinson W, Kaldor J, … Haber P (2010). Incidence and risk for acute hepatitis C infection during imprisonment in Australia. Eur J Epidemiol, 25(2), 143–148. doi: 10.1007/s10654-009-9421-0 [DOI] [PubMed] [Google Scholar]
- Dore GJ, Altice F, Litwin AH, Dalgard O, Gane EJ, Shibolet O, … Group, C. E. C.-S. S. (2016). Elbasvir-Grazoprevir to Treat Hepatitis C Virus Infection in Persons Receiving Opioid Agonist Therapy: A Randomized Trial. Ann Intern Med, 165(9), 625–634. doi: 10.7326/M16-0816 [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Siegel AB, Davila JA, Shaib YH, Cayton-Woody M, McBride R, & McGlynn KA (2006). Treatment and outcomes of treating of hepatocellular carcinoma among Medicare recipients in the United States: a population-based study. J Hepatol, 44(1), 158–166. doi: 10.1016/j.jhep.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Fleiz-Bautista C, Domínguez-García M, Villatoro-Velázquez JA., Vázquez-Quiroz F, Zafra-Mora E, Sánchez-Ramos R, Resendiz-Escobar E, Bustos-Gamiño M, Medina-Mora ME Cuqueando la Chiva: Contextos del consumo de heroína en la frontera norte de México… Ciudad de México, México: INPRFM; 2019. [Google Scholar]
- Fraser H, Zibbell J, Hoerger T, Hariri S, Vellozzi C, Martin NK, … Vickerman P (2018). Scaling-up HCV prevention and treatment interventions in rural United States-model projections for tackling an increasing epidemic. Addiction, 113(1), 173–182. doi: 10.1111/add.13948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SD, Brouwer KC, Firestone Cruz MA, Ramos R, Ramos ME, Lozada RM, … Strathdee SA (2006). Respondent-driven sampling of injection drug users in two U.S.-Mexico border cities: recruitment dynamics and impact on estimates of HIV and syphilis prevalence. J Urban Health, 83(6 Suppl), i83–97. doi: 10.1007/s11524-006-9104-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gountas I, Sypsa V, Anagnostou O, Martin N, Vickerman P, Kafetzopoulos E, & Hatzakis A (2017). Treatment and primary prevention in people who inject drugs for chronic hepatitis C infection: is elimination possible in a high-prevalence setting? Addiction. doi: 10.1111/add.13764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham CS, & Swan T (2015). A path to eradication of hepatitis C in low- and middle-income countries. Antiviral Research, 119, 89–96. doi: 10.1016/j.antiviral.2015.01.004 [DOI] [PubMed] [Google Scholar]
- Grebely J, Dalgard O, Conway B, Cunningham EB, Bruggmann P, Hajarizadeh B, … Group, S. S. (2018). Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol, 3(3), 153–161. doi: 10.1016/S2468-1253(17)30404-1 [DOI] [PubMed] [Google Scholar]
- Heffernan A, Cooke GS, Nayagam S, Thursz M, & Hallett TB (2019). Scaling up prevention and treatment towards the elimination of hepatitis C: a global mathematical model. Lancet, 393(10178), 1319–1329. doi: 10.1016/S0140-6736(18)32277-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanitaria Juridicción. (2016). Report of the total of people censed at treatment centers in Tijuana during the operativo. Tijuana, Mexico. [Google Scholar]
- Kaberg M, Naver G, Hammarberg A, & Weiland O (2018). Incidence and spontaneous clearance of hepatitis C virus (HCV) in people who inject drugs at the Stockholm Needle Exchange-Importance for HCV elimination. J Viral Hepat, 25(12), 1452–1461. doi: 10.1111/jvh.12969 [DOI] [PubMed] [Google Scholar]
- Larney S, Toson B, Burns L, & Dolan K (2012). Effect of prison-based opioid substitution treatment and post-release retention in treatment on risk of re-incarceration. Addiction, 107(2), 372–380. doi: 10.1111/j.1360-0443.2011.03618.x [DOI] [PubMed] [Google Scholar]
- Lim AG, Qureshi H, Mahmood H, Hamid S, Davies CF, Trickey A, … Vickerman P (2018). Curbing the hepatitis C virus epidemic in Pakistan: the impact of scaling up treatment and prevention for achieving elimination. Int J Epidemiol, 47(2), 550–560. doi: 10.1093/ije/dyx270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez O (2019). Thousands feared at risk after Mexico reforms HIV+ regime [Press release]. Retrieved 21st October 2019 from https://www.reuters.com/article/us-mexico-health-aids/thousands-feared-at-risk-after-mexico-reforms-hiv-regime-idUSKCN1RT1FC
- Magis-Rodriguez C, Brouwer KC, Morales S, Gayet C, Lozada R, Ortiz-Mondragon R,…Strathdee SA (2005). HIV prevalence and correlates of receptive needle sharing among injection drug users in the Mexican-U.s. border city of Tijuana. J Psychoactive Drugs, 37(3), 333–339. doi: 10.1080/02791072.2005.10400528 [DOI] [PubMed] [Google Scholar]
- Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, & Vickerman P (2013). Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis, 57 Suppl 2, S39–45. doi: 10.1093/cid/cit296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, … Hickman M (2013). Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology, 58(5), 1598–1609. doi: 10.1002/hep.26431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micallef JM, Kaldor JM, & Dore GJ (2006). Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat, 13(1), 34–41. doi: 10.1111/j.1365-2893.2005.00651.x [DOI] [PubMed] [Google Scholar]
- Mohd Hanafiah K, Groeger J, Flaxman AD, & Wiersma ST (2013). Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology, 57(4), 1333–1342. doi: 10.1002/hep.26141 [DOI] [PubMed] [Google Scholar]
- Ochoa M (2019). México se compromete a eliminar la Hepatitis C para 2030. Retrieved 21st October 2019 from https://www.unotv.com/noticias/portal/nacional/detalle/mxico-se-compromete-eliminar-hepatitis-c-609911/
- Paquette CE, Syvertsen JL, & Pollini RA (2018). Stigma at every turn: Health services experiences among people who inject drugs. Int J Drug Policy, 57, 104–110. doi: 10.1016/j.drugpo.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, & Cacciapuoti C (2016). Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol, 22(34), 7824–7840. doi: 10.3748/wjg.v22.i34.7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt L, Minozzi S, Reed J, Vickerman P, Hagan H, French C, … Hickman M (2018). Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: findings from a Cochrane Review and meta-analysis. Addiction, 113(3), 545–563. doi: 10.1111/add.14012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polaris Observatory HCVC (2017). Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol, 2(3), 161–176. doi: 10.1016/S2468-1253(16)30181-9 [DOI] [PubMed] [Google Scholar]
- Rafful C, Medina-Mora ME, Gonzalez-Zuniga P, Jenkins JH, Rangel MG, Strathdee SA, & Davidson PJ (2019). “Somebody Is Gonna Be Hurt”: Involuntary Drug Treatment in Mexico. Med Anthropol, 1–14. doi: 10.1080/01459740.2019.1609470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafful C, Orozco R, Rangel G, Davidson P, Werb D, Beletsky L, & Strathdee SA (2018). Increased non-fatal overdose risk associated with involuntary drug treatment in a longitudinal study with people who inject drugs. Addiction, 113(6), 1056–1063. doi: 10.1111/add.14159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson AM, Garfein RS, Wagner KD, Mehta SR, Magis-Rodriguez C, Cuevas-Mota J, … Stahr II (2014). Evaluating the impact of Mexico’s drug policy reforms on people who inject drugs in Tijuana, B.C., Mexico, and San Diego, CA, United States: a binational mixed methods research agenda. Harm Reduct J, 11, 4. doi: 10.1186/1477-7517-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secretaría de Salud. (2019). México establecerá una estrategia de tratamiento y eliminación de la hepatitis C [Press release]. Retrieved 18th October 2019 from https://www.gob.mx/salud/prensa/239-mexico-tendra-disponible-nuevo-tratamiento-que-cura-la-hepatitis-c
- Shepherd J, Jones J, Hartwell D, Davidson P, Price A, & Waugh N (2007). Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess, 11(11), 1–205, iii. [DOI] [PubMed] [Google Scholar]
- Smith LR, Patterson TL, Magis-Rodriguez C, Ojeda VD, Burgos JL, Rojas SA, … Strathdee SA (2016). Engagement in the HIV Care Continuum among Key Populations in Tijuana, Mexico. AIDS Behav, 20(5), 1017–1025. doi: 10.1007/s10461-015-1186-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, … Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ, 357, j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Fraser H, Lim AG, Walker JG, Ward Z, MacGregor L, … Vickerman P (2018). Incarceration history and risk of HIV and hepatitis C virus acquisition among people who inject drugs: a systematic review and meta-analysis. Lancet Infect Dis, 18(12), 1397–1409. doi: 10.1016/S1473-3099(18)30469-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Lozada R, Pollini RA, Brouwer KC, Mantsios A, Abramovitz DA, … Patterson TL (2008). Individual, social, and environmental influences associated with HIV infection among injection drug users in Tijuana, Mexico. J Acquir Immune Defic Syndr, 47(3), 369–376. doi: 10.1097/QAI.0b013e318160d5ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thein HH, Yi Q, Dore GJ, & Krahn MD (2008). Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology, 48(2), 418–431. doi: 10.1002/hep.22375 [DOI] [PubMed] [Google Scholar]
- Trickey A, Fraser H, Lim AG, Peacock A, Colledge S, Walker JG, … Vickerman P (2019). The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol Hepatol, 4(6), 435–444. doi: 10.1016/S2468-1253(19)30085-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman P, Martin N, Turner K, & Hickman M (2012). Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction, 107(11), 1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x [DOI] [PubMed] [Google Scholar]
- von Hippel C, Brener L, & Horwitz R (2018). Implicit and explicit internalized stigma: Relationship with risky behaviors, psychosocial functioning and healthcare access among people who inject drugs. Addict Behav, 76, 305–311. doi: 10.1016/j.addbeh.2017.08.036 [DOI] [PubMed] [Google Scholar]
- West BS, Abramovitz DA, Gonzalez-Zuniga P, Rangel G, Werb D, Cepeda J, … Strathdee SA (2019). Drugs, discipline and death: Causes and predictors of mortality among people who inject drugs in Tijuana, 2011–2018. Int J Drug Policy, 75, 102601. doi: 10.1016/j.drugpo.2019.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EF, Garfein RS, Brouwer KC, Lozada R, Ramos R, Firestone-Cruz M, … Strathdee SA (2007). Prevalence of hepatitis C virus and HIV infection among injection drug users in two Mexican cities bordering the U.S. Salud Publica Mex, 49(3), 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. (2016). Global Health Sector Strategy on Viral Hepatitis 2016–2021. Retrieved 10 June 2019 from https://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.